Anti-Microbial and Remineralizing Properties of Self-Adhesive Orthodontic Resin Containing Mesoporous Bioactive Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Mesoporous Bioactive Glass Nanoparticle Synthesis
2.2. Preparation of Experimental Self-Adhesive Orthodontic Bonding Resin
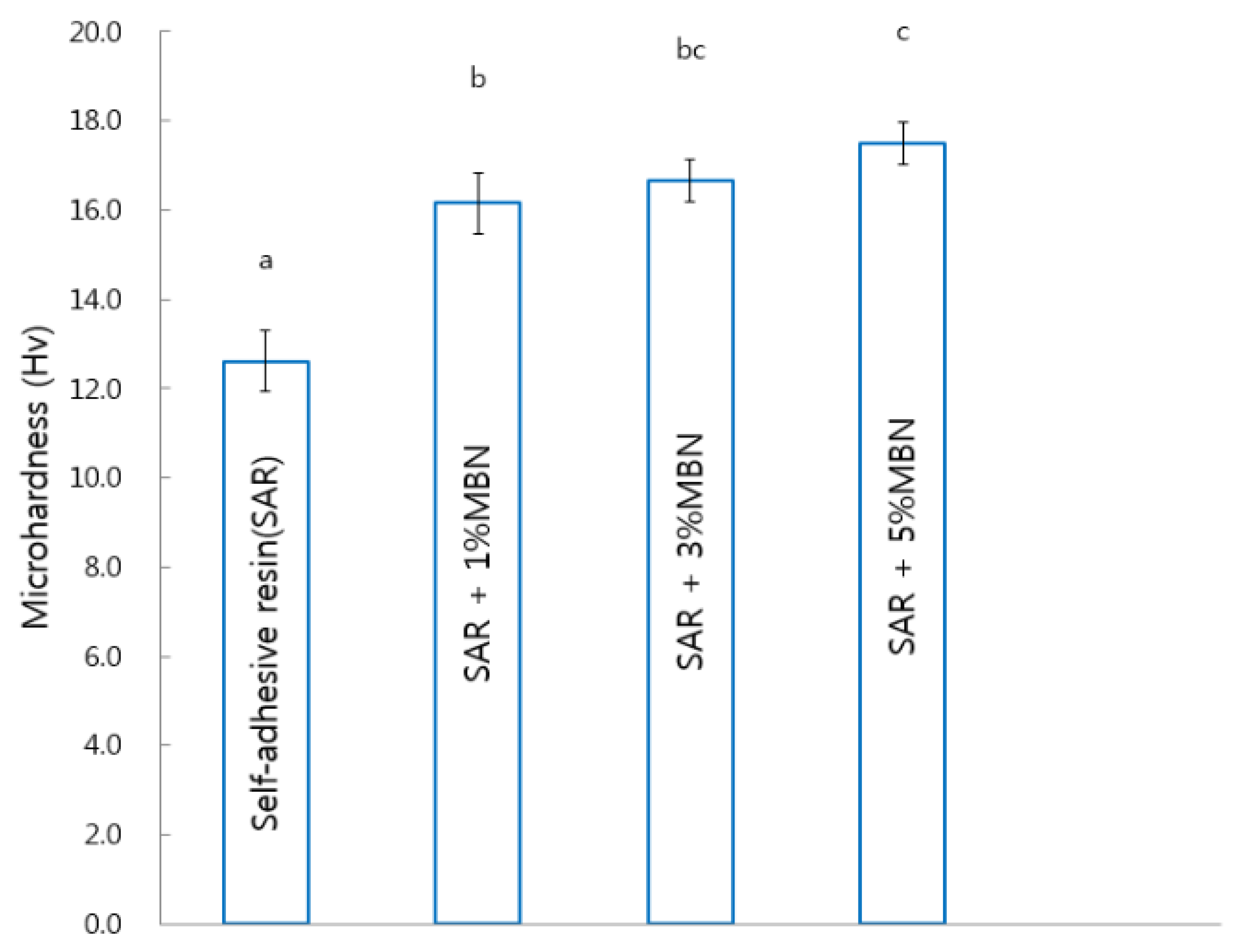
2.3. Microhardness
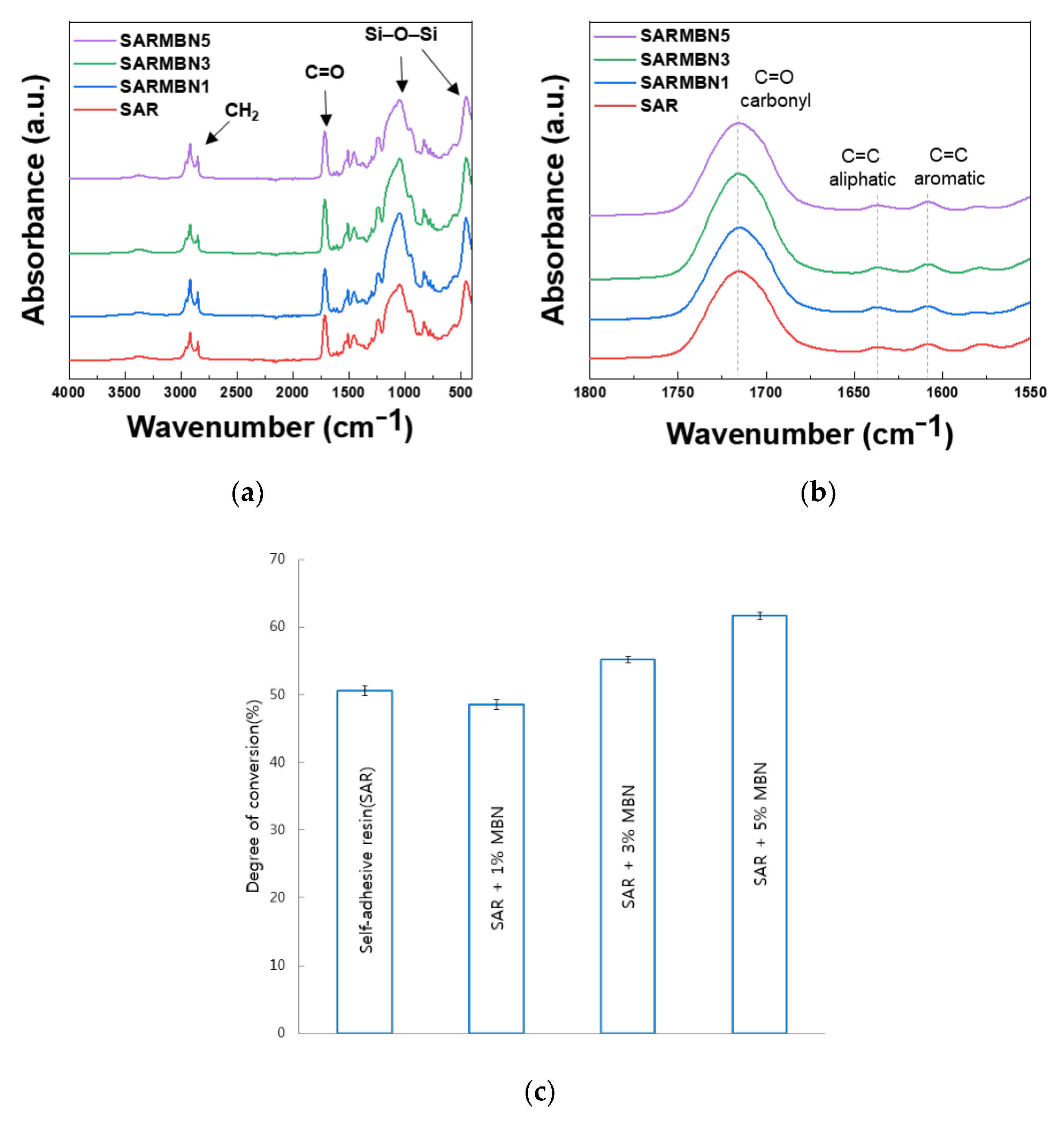
2.4. Degree of Conversion (DC)
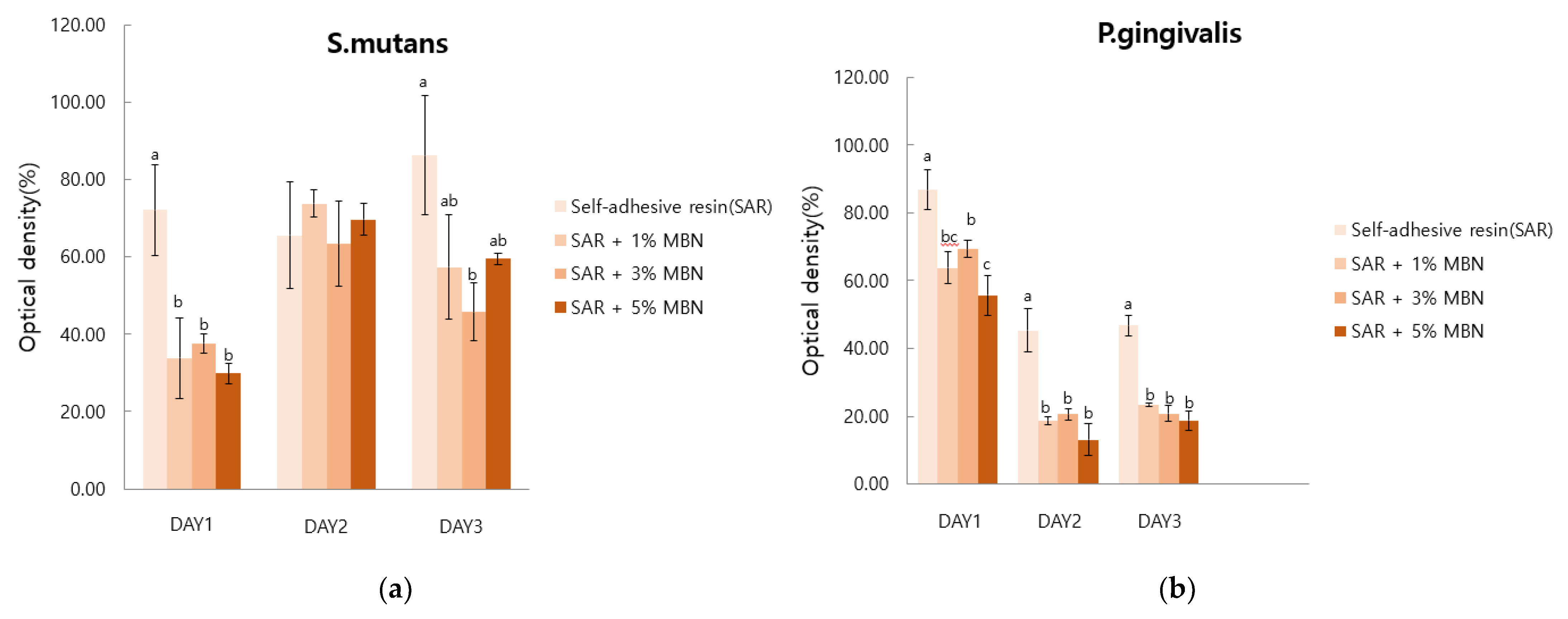
2.5. Anti-Bacterial Test
2.6. Shear Bond Strength (SBS) Test
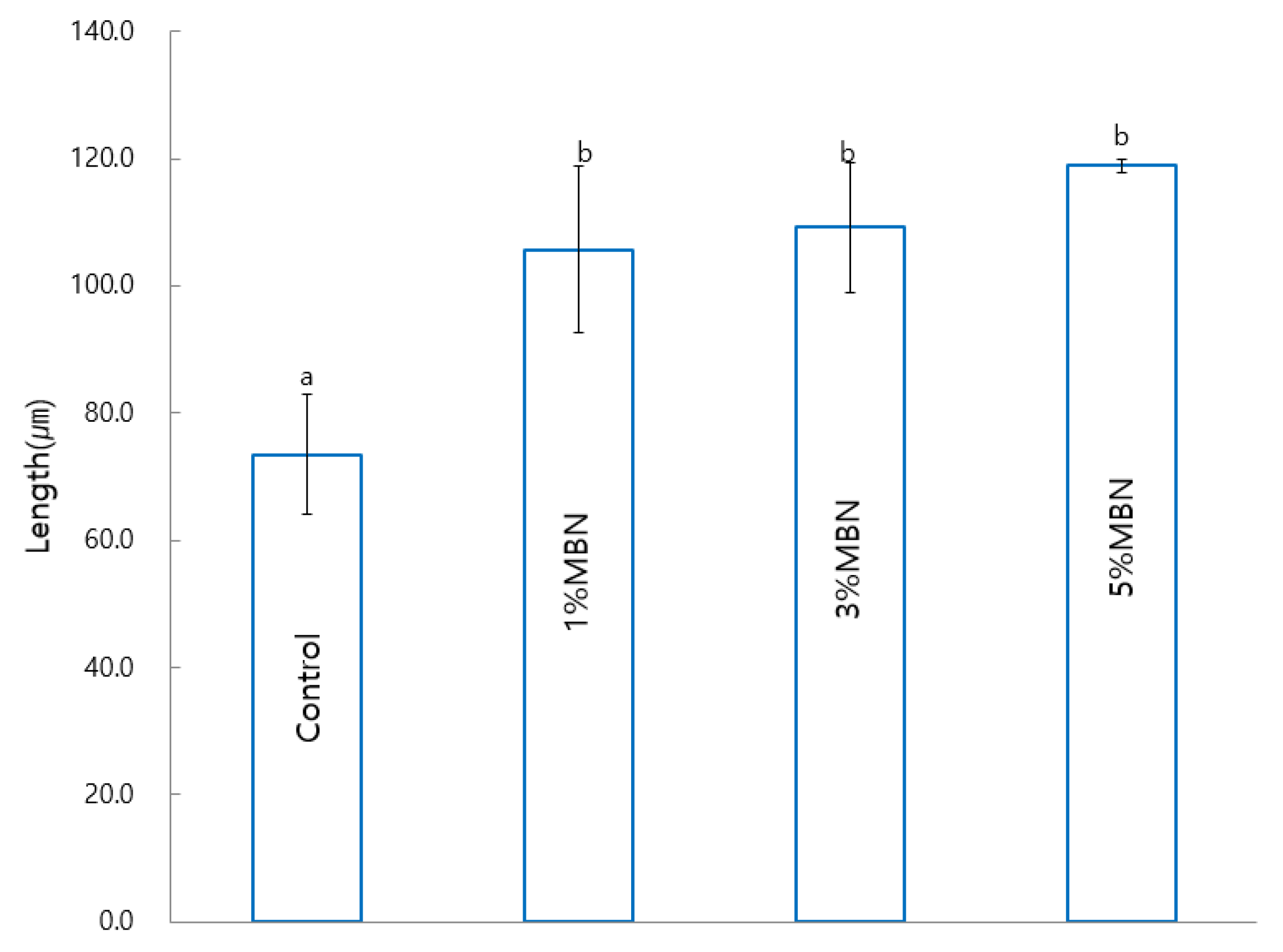
2.7. Anti-Demineralization Test
2.8. Statistical Analysis
3. Results
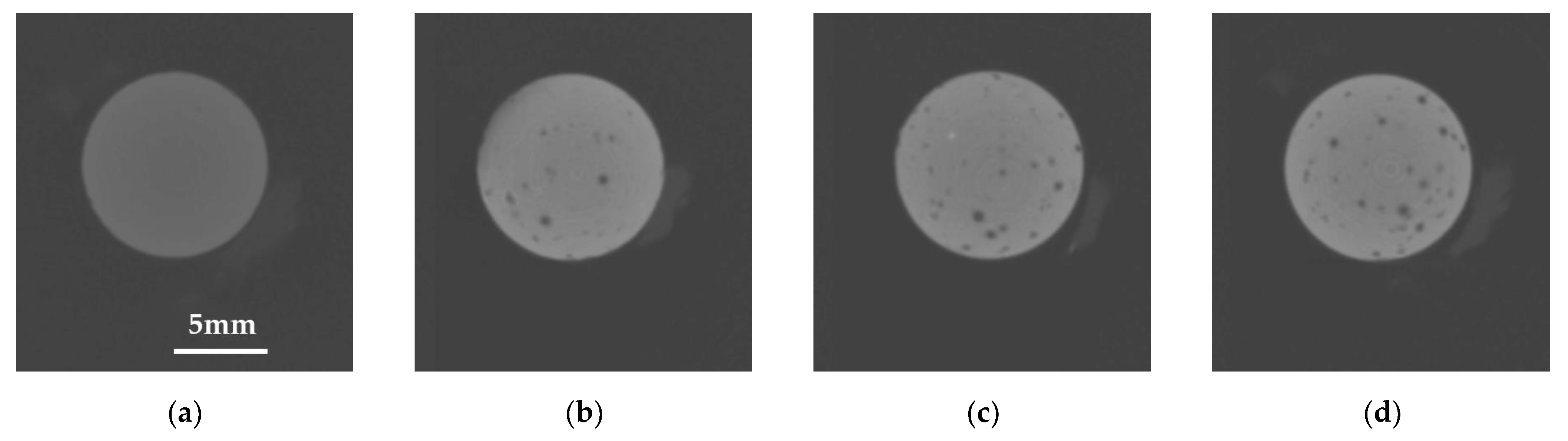
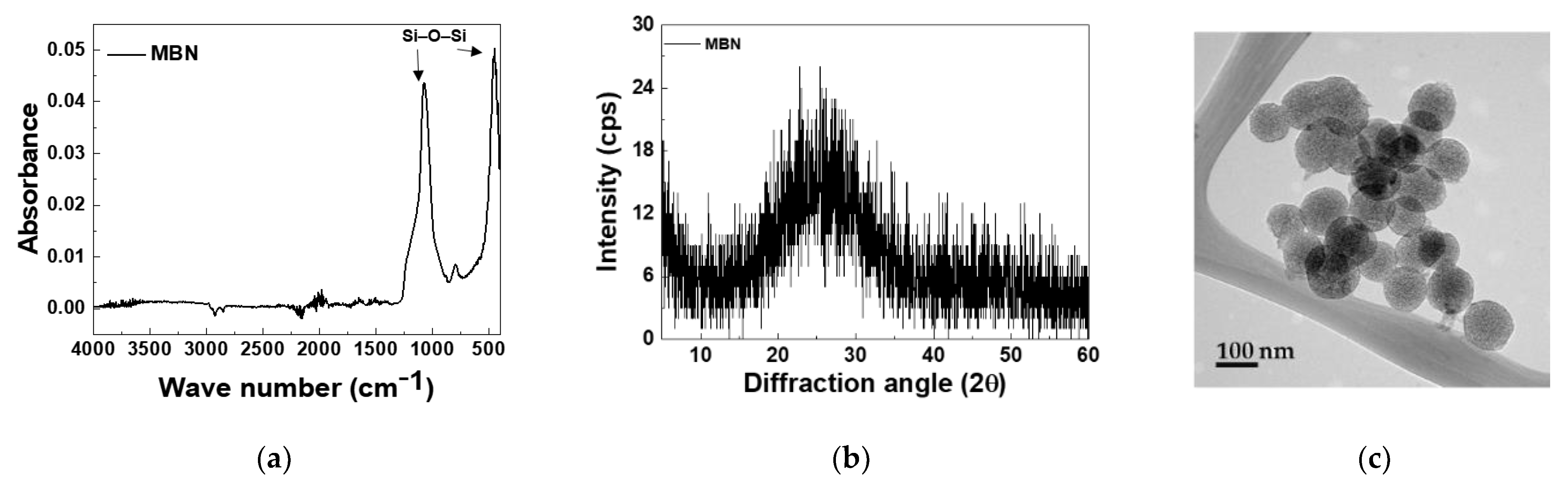
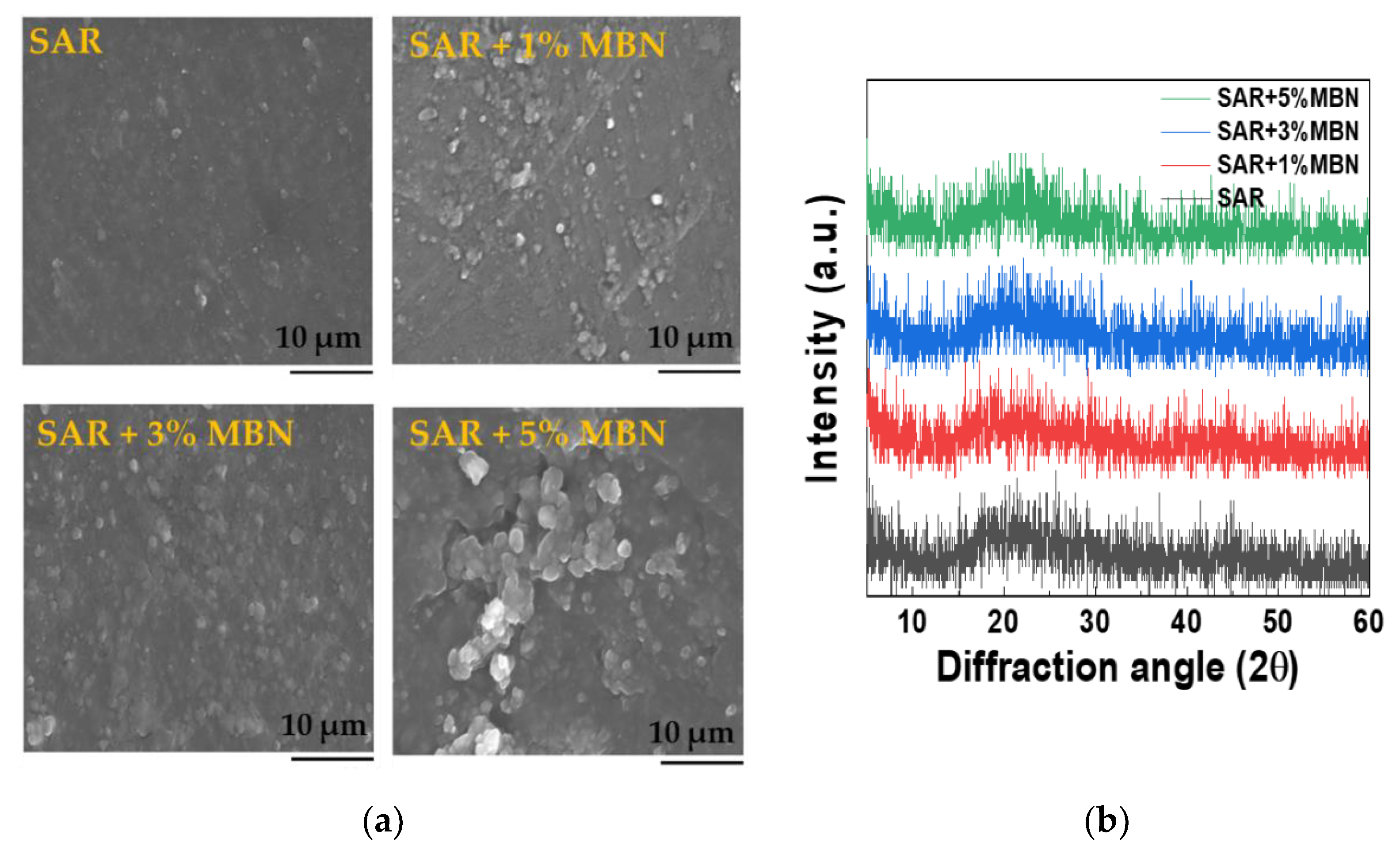
3.1. Characterization of MBNs and Fabricated Resin Discs
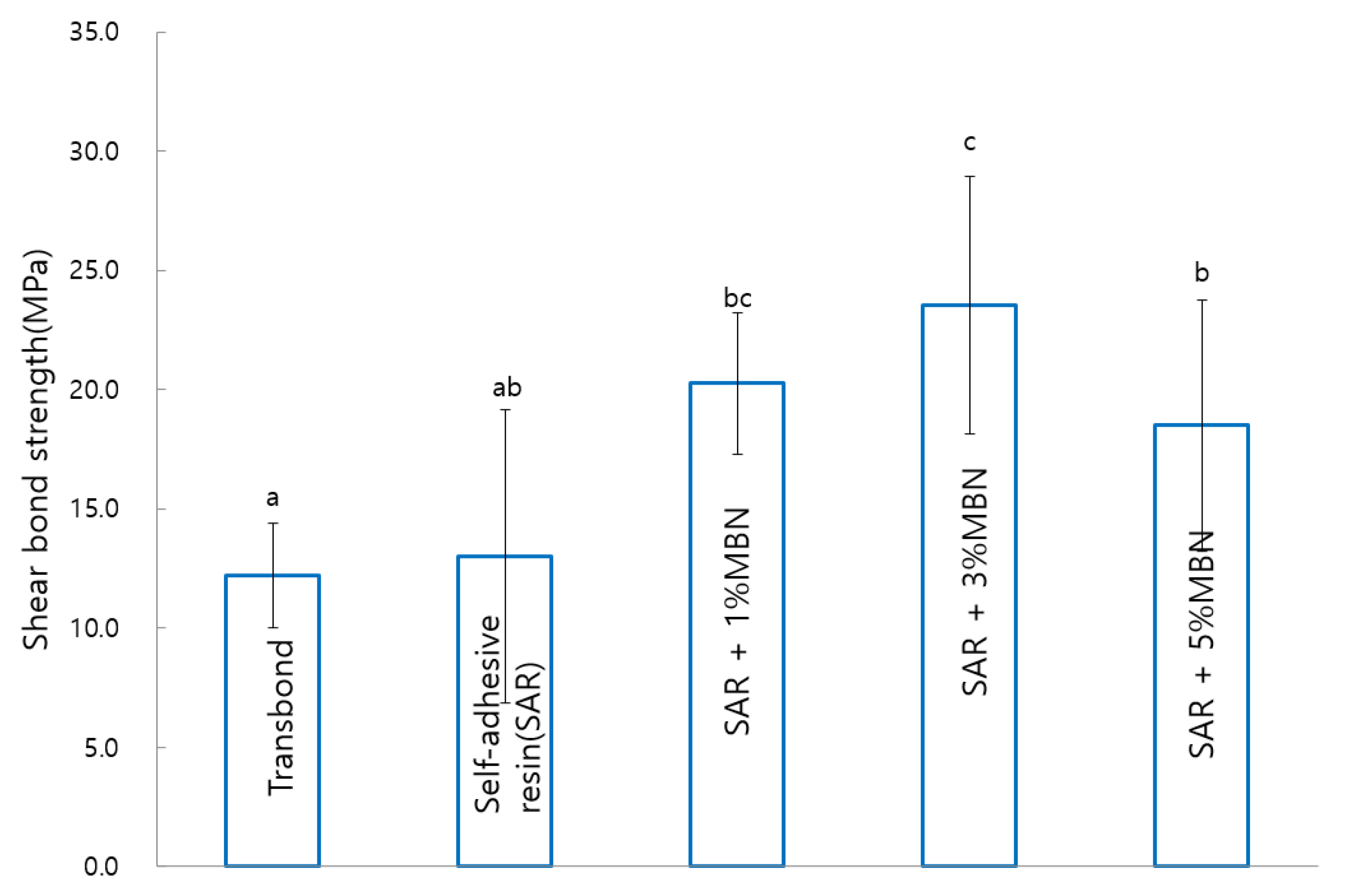
3.2. Shear Bond Strength (SBS)
3.3. ARI Score
3.4. Microhardness
3.5. Anti-Bacterial Test
3.6. Degree of Conversion
3.7. Anti-Demineralization Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latta, M.A.; Radniecki, S.M. Bond Strength of Self-Adhesive Restorative Materials Affected by Smear Layer Thickness but not Dentin Desiccation. J. Adhes. Dent. 2020, 22, 79–84. [Google Scholar]
- Chantchaimongkol, S.; Sirivimol Srisawasdi, D. Microtensile Bond. Strength of Self-Adhesive Resin Composite to Dentin; Chulalongkorn University: Bangkok, Thailand, 2013. [Google Scholar]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Poitevin, A.; De Munck, J.; Van Ende, A.; Suyama, Y.; Mine, A.; Peumans, M.; Van Meerbeek, B. Bonding effectiveness of self-adhesive composites to dentin and enamel. Dent. Mater. 2013, 29, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.S.; Buciumeanu, M.; Martinelli, A.E.; Nascimento, R.M.D.; Henriques, B.; Silva, F.; Souza, J.C.M. Mechanical Strength and Wear of Dental Glass-Ionomer and Resin Composites Affected by Porosity and Chemical Composition. J. Bio- Tribo-Corros. 2015, 1, 24. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Wu, W.; Xu, T.; Fong, H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing halloysite nanotubes. Dent. Mater. 2012, 28, 1071–1079. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munk, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Latta, M.A.; Tsujimoto, A.; Takamizawa, T.; Barkmeier, W.W. In Vitro Wear Resistance of Self-Adhesive Restorative Materials. J. Adhes. Dent. 2020, 22, 59–64. [Google Scholar] [PubMed]
- Hosseinipour, Z.S.; Heidari, A.; Shahrabi, M.; Poorzandpoush, K. Microleakage of a Self-Adhesive Flowable Composite, a Self-Adhesive Fissure Sealant and a Conventional Fissure Sealant in Permanent Teeth with/without Saliva Contamination. Front. Dent. 2019, 16, 239. [Google Scholar]
- El-Fiqi, A.; Lee, J.H.; Lee, E.-J.; Kim, H.-W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013, 9, 9508–9521. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, M.G.; Ferracane, J.L.; Davis, H.; Bae, H.E.; Choi, D.; Kim, D.-S. Effect of bioactive glass-containing resin composite on dentin remineralization. J. Dent. 2018, 75, 58–64. [Google Scholar] [CrossRef]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part. H J. Eng. Med. 1998, 212, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Vidor, M.M.; Felix, R.; Marchioro, E.M.; Hahn, L. Enamel surface evaluation after bracket debonding and different resin removal methods. Dent. Press J. Orthod. 2015, 20, 61–67. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkali-mediated sol–gel method. Mater. Lett. 2007, 61, 3251–3253. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoo, K.-H.; Yoon, S.-Y.; Son, W.-S.; Kim, Y.-I. Synergetic Effect of 2-Methacryloyloxyethyl Phosphorylcholine and Mesoporous Bioactive Glass Nanoparticles on Antibacterial and Anti-Demineralisation Properties in Orthodontic Bonding Agents. Nanomaterials 2020, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.B.; Samuel, S.M.W. Discrepancies in degree of conversion measurements by FTIR. Braz. Oral Res. 2014, 28, 9–15. [Google Scholar]
- Cui, T.; Luo, W.; Xu, L.; Yang, B.; Zhao, W.; Cang, H. Progress of Antimicrobial Discovery Against the Major Cariogenic Pathogen Streptococcus mutans. Curr. Issues Mol. Biol. 2019, 32, 601–644. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Bishara, S.E.; Ajlouni, R.; Laffoon, J.F.; Warren, J.J. Comparison of shear bond strength of two self-etch primer/adhesive sys-tems. Angle Orthod. 2006, 76, 123–126. [Google Scholar]
- Stookey, G.K.; Featherstone, J.D.B.; Rapozo-Hilo, M.; Schemehorn, B.R.; Williams, R.A.; Baker, R.A.; Barker, M.L.; Kaminski, M.A.; McQueen, C.M.; Amburgey, J.S.; et al. The Featherstone laboratory pH cycling model: A prospective, multi-site validation exercise. Am. J. Dent. 2011, 24, 322. [Google Scholar]
- Bae, J.; Son, W.S.; Yoo, K.H.; Yoon, S.Y.; Bae, M.K.; Lee, D.J.; Ko, C.-C.; Choi, Y.K.; Kim, Y.I. Effects of Poly (Am-idoamine) Dendrimer-Coated Mesoporous Bioactive Glass Nanoparticles on Dentin Remineralization. Nanomaterials 2019, 9, 591. [Google Scholar] [CrossRef]
- Son, S.-A.; Kim, D.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.-I. Mesoporous Bioactive Glass Combined with Graphene Oxide Quantum Dot as a New Material for a New Treatment Option for Dentin Hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef]
- Antony, G.J.M.; Raja, S.; Aruna, S.T.; Jarali, C.S. Effect of the addition of diurethane dimethacrylate on the chemical and mechanical properties of tBA-PEGDMA acrylate based shape memory polymer network. J. Mech. Behav. Biomed. Mater. 2020, 110, 103951. [Google Scholar] [CrossRef]
- Shapinko, Y.; Eleftheriadi, I.; Brosh, T.; Adler-Abramovich, L.; Davidovitch, M.; Sella-Tunis, T.; Sarig, R.; Shpack, N. Evaluation of an Orthodontic Adhesive with Combined Primer and Composite. Open J. Stomatol. 2018, 08, 205–216. [Google Scholar] [CrossRef][Green Version]
- Khvostenko, D.; Mitchell, J.; Hilton, T.; Ferracane, J.; Kruzic, J. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent. Mater. 2013, 29, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Tauböck, T.T.; Attin, T.; Tarle, Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: The effect of post-cure heating. Sci. Rep. 2019, 9, 17245. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2009, 93, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef]
- Martins, C.H.G.; Carvalho, T.C.; Souza, M.G.M.; Ravagnani, C.; Peitl, O.; Zanotto, E.D.; Panzeri, H.; Casemiro, L.A. As-sessment of antimicrobial effect of Biosilicate® against anaerobic, microaerophilic and facultative anaerobic microorganisms. J. Mater. Sci. Mater. Med. 2011, 22, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Jibi Paul, M.; Basappa, N. Comparative evaluation of the remineralizing effects and surface micro hardness of glass ionomer cements containing bioactive glass (S53P4): An in vitro study. Int. J. Clin. Pediatr. Dent. 2010, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, O.E.; El-Brolossy, T. An Investigation about the Reminerlization Potential of Bio-active glass on Artificially Carious Enamel and Dentin using Raman Spectroscopy. Egypt. Dent. J. 2003, 49, 381–392. [Google Scholar]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Ko, C.-C.; Son, S.-A.; Kim, Y.-I. Enamel Anti-Demineralization Effect of Orthodontic Adhesive Containing Bioactive Glass and Graphene Oxide: An In-Vitro Study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-K.; Yoo, K.-H.; Yoon, S.-Y.; Na, H.S.; Chung, J.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. In Vitro Effect of Gallium-Doped Bioactive Glass on Enamel Anti-Demineralization and Bond Strength of Orthodontic Resins. Appl. Sci. 2019, 9, 4918. [Google Scholar] [CrossRef]

| Composition | Content % |
|---|---|
| Bisphenol A ethoxylate dimethacrylate | 34% |
| Barium monoxide | 5% |
| Diurethane dimethacrylate, mixture of isomers | 36% |
| α-Alumina | 2% |
| Diboron trioxide | 2% |
| 2-Propenoic acid | 2% |
| Benzoic acid, 4-(dimethylamino) | 1% |
| Phenol | 0.5% |
| Silane amine | 6% |
| Phosphine oxide | 0.5% |
| Silica | 11% |
| Score | Definition |
|---|---|
| 1 | All adhesive remained on the tooth |
| 2 | Over 90% of the adhesive remained on the tooth |
| 3 | 10–90% of the adhesive remained on the tooth |
| 4 | Below 10% of the adhesive remained on the tooth |
| 5 | No adhesive remained on the tooth |
| Groups | No. of Samples | ARI Scores | Statistics Kruskal-Wallis | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Commercial control (Transbond XT) | 20 | 0 | 0 | 20 | 0 | 0 | p = 0.567 |
| Self-adhesive resin (SAR) | 20 | 0 | 0 | 19 | 0 | 1 | |
| SAR + MBN 1% | 20 | 0 | 0 | 20 | 0 | 0 | |
| SAR + MBN 3% | 20 | 0 | 0 | 19 | 1 | 0 | |
| SAR + MBN 5% | 20 | 0 | 0 | 20 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, A.; Yoo, K.-H.; Yoon, S.-Y.; Park, B.-S.; Kim, I.-R.; Kim, Y.-I. Anti-Microbial and Remineralizing Properties of Self-Adhesive Orthodontic Resin Containing Mesoporous Bioactive Glass. Materials 2021, 14, 3550. https://doi.org/10.3390/ma14133550
Choi A, Yoo K-H, Yoon S-Y, Park B-S, Kim I-R, Kim Y-I. Anti-Microbial and Remineralizing Properties of Self-Adhesive Orthodontic Resin Containing Mesoporous Bioactive Glass. Materials. 2021; 14(13):3550. https://doi.org/10.3390/ma14133550
Chicago/Turabian StyleChoi, Aerin, Kyung-Hyeon Yoo, Seog-Young Yoon, Bong-Soo Park, In-Ryoung Kim, and Yong-Il Kim. 2021. "Anti-Microbial and Remineralizing Properties of Self-Adhesive Orthodontic Resin Containing Mesoporous Bioactive Glass" Materials 14, no. 13: 3550. https://doi.org/10.3390/ma14133550
APA StyleChoi, A., Yoo, K.-H., Yoon, S.-Y., Park, B.-S., Kim, I.-R., & Kim, Y.-I. (2021). Anti-Microbial and Remineralizing Properties of Self-Adhesive Orthodontic Resin Containing Mesoporous Bioactive Glass. Materials, 14(13), 3550. https://doi.org/10.3390/ma14133550






