Assessment of the Bone Healing Process Mediated by Periosteum-Derived Mesenchymal Stem Cells’ Secretome and a Xenogenic Bioceramic—An In Vivo Study in the Rabbit Critical Size Calvarial Defect Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Periosteum MSC Isolation and Characterization
2.3. Preparation of Secretomes
2.4. Sample Preparation for Proteomic Analysis
2.5. LC–MS-Based Protein Identification
2.6. In Vivo Evaluation of Secretomes’ Biological Response on the Bone Healing Process
2.7. Characterization of the Bone Healing Process
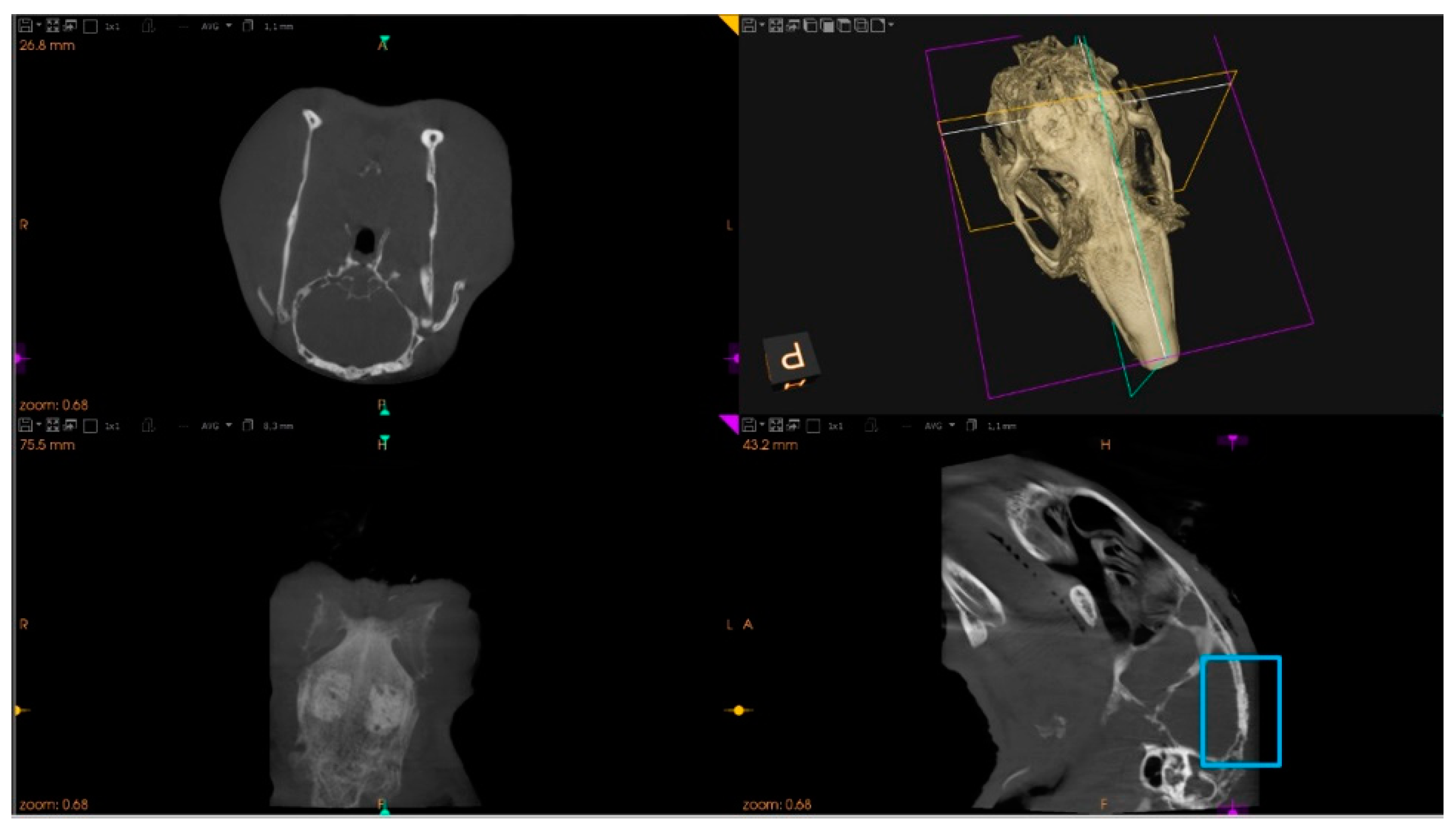
2.7.1. Microtomographic Evaluation
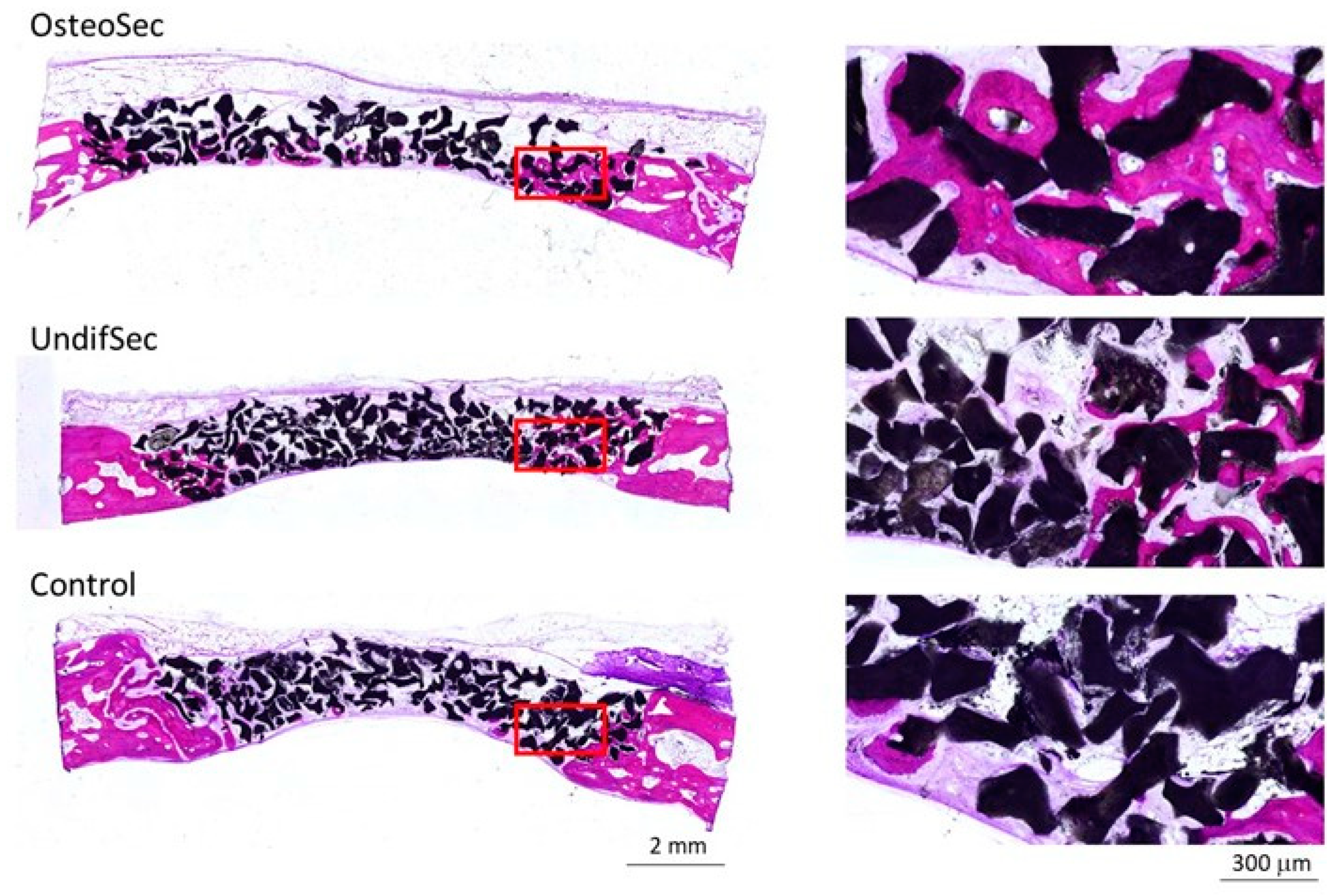
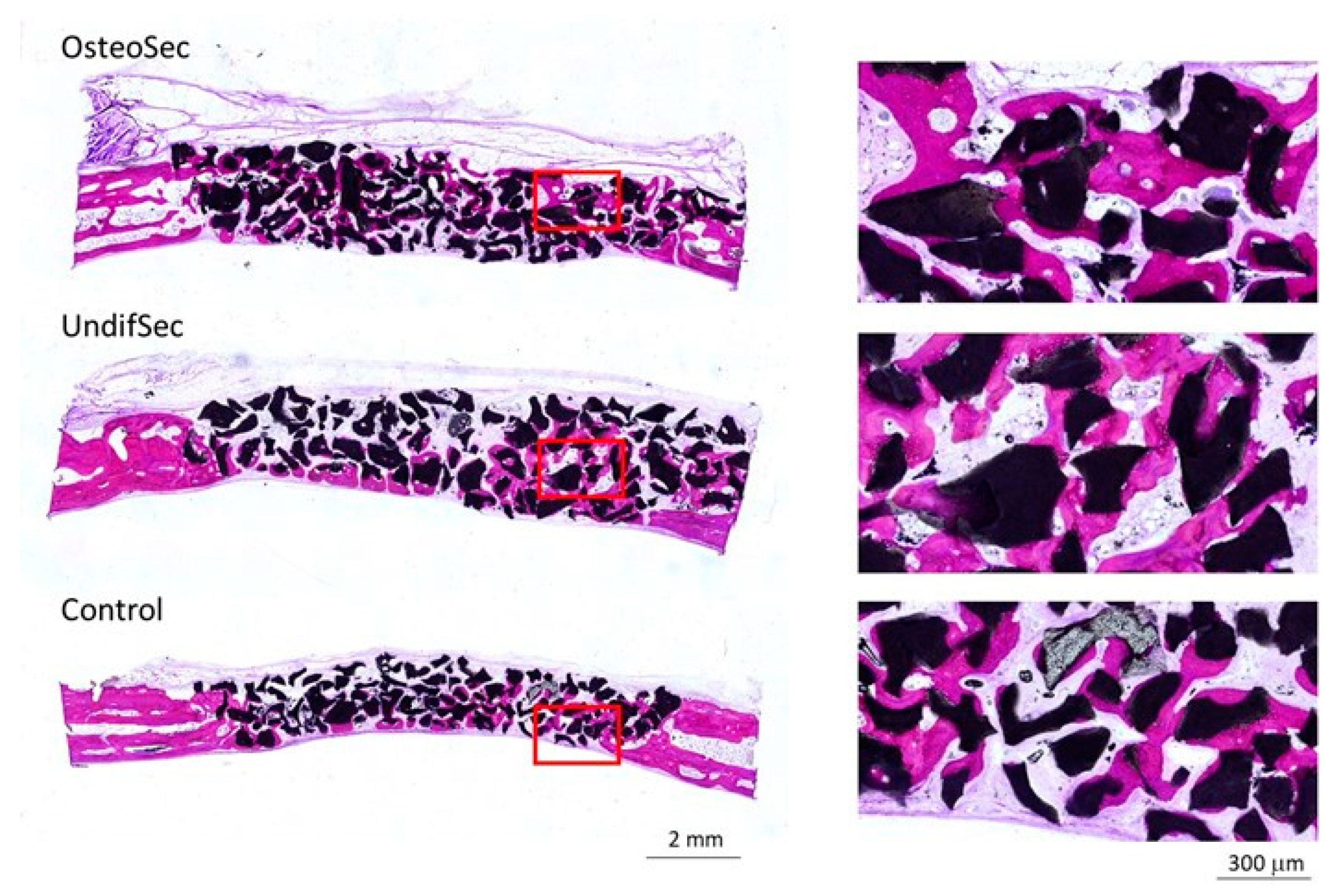
2.7.2. Histological and Histomorphometric Evaluation
2.8. Statistical Analysis
3. Results and Discussion
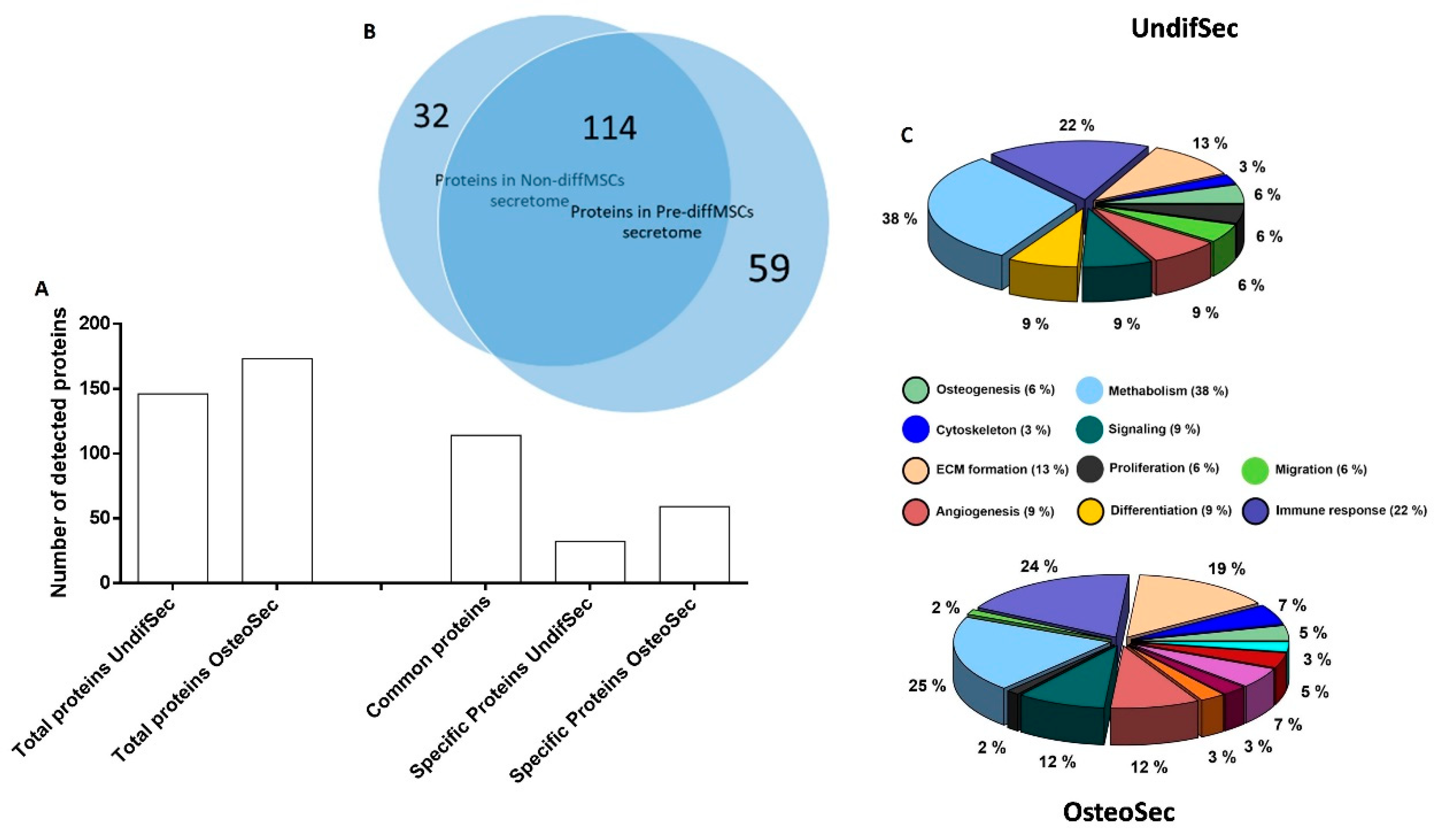
3.1. Secretome Characterization
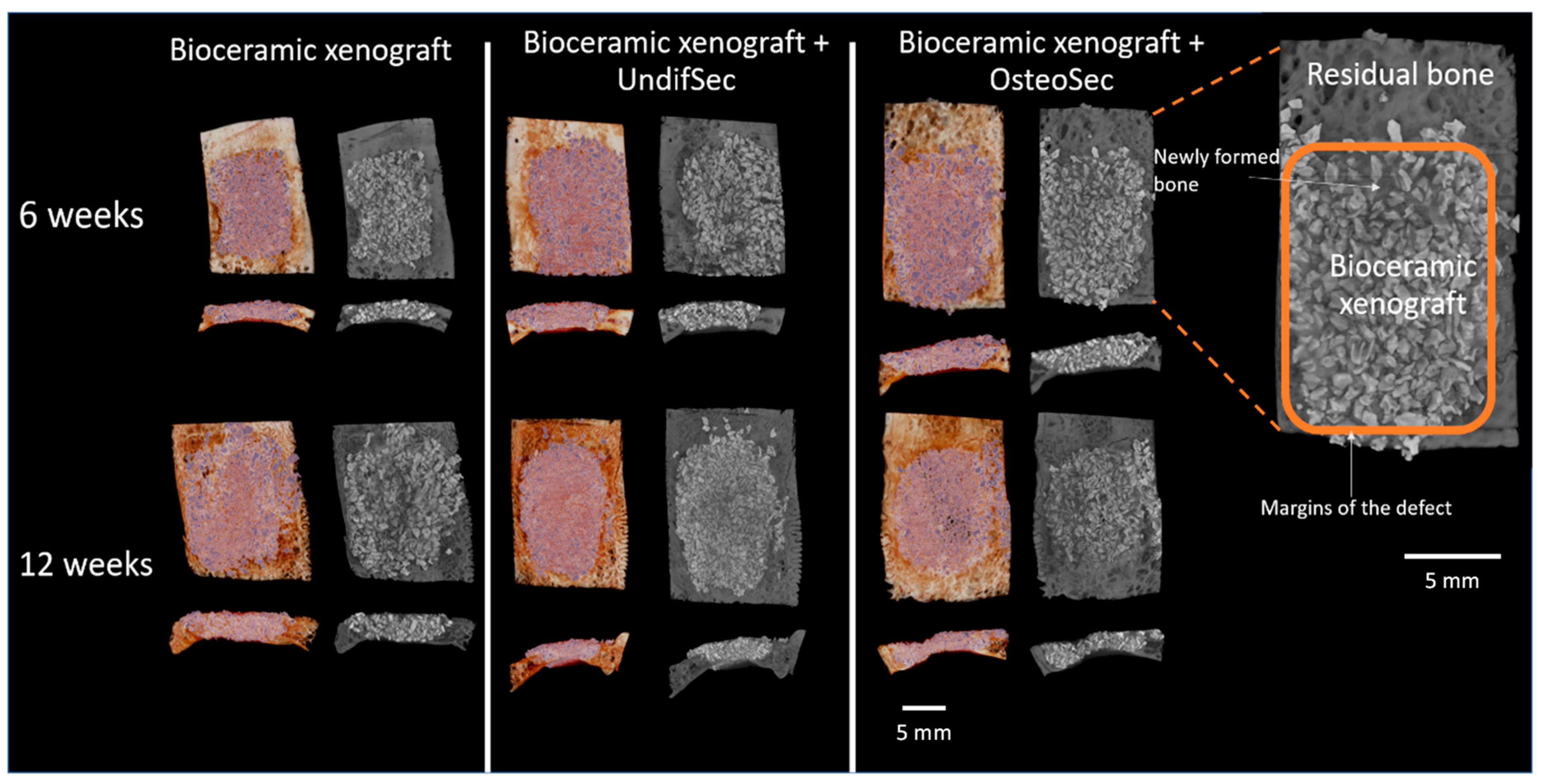
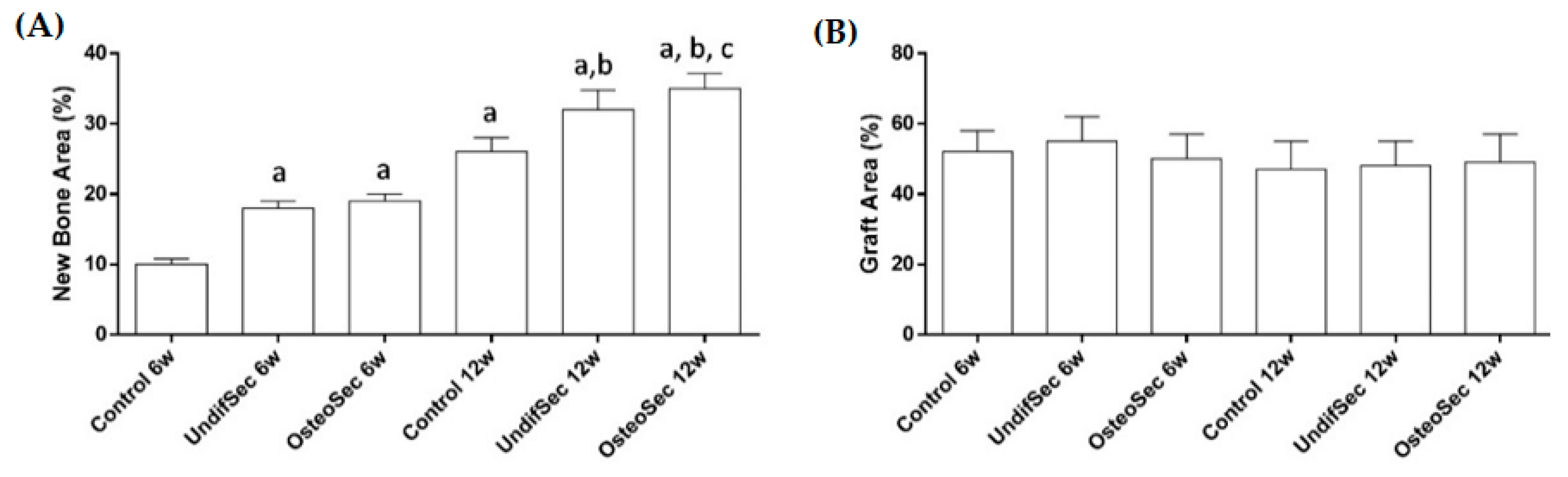
3.2. The Bone Healing in Rabbit Calvarial Critical Size Defects
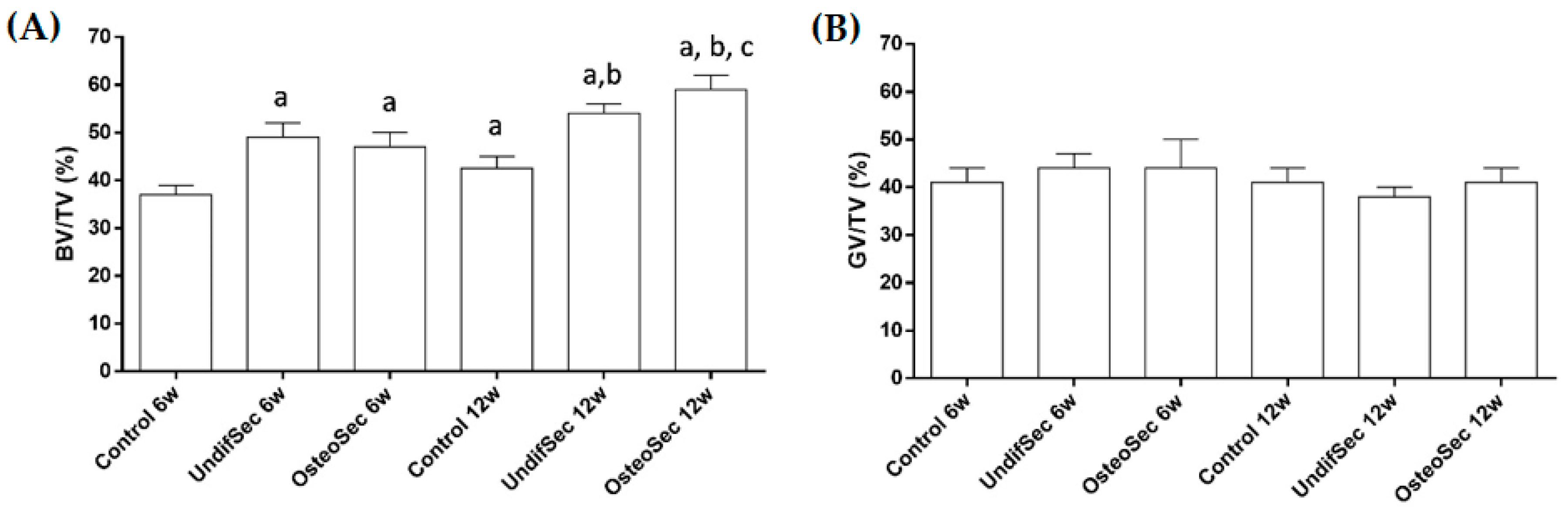
3.3. Secretome Modulation of the Bone Healing Process
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fierabracci, A.; del Fattore, A.; Luciano, R.; Muraca, M.; Teti, A.; Muraca, M. Recent Advances in Mesenchymal Stem Cell Immunomodulation: The Role of Microvesicles. Cell Transplant. 2015, 24, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Pacini, S. Deterministic and Stochastic Approaches in the Clinical Application of Mesenchymal Stromal Cells (MSCs). Front. Cell Dev. Biol. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Yong, K.W.; Choi, J.R.; Dolbashid, A.S.; Wan Safwani, W.K.Z. Biosafety and Bioefficacy Assessment of Human Mesenchymal Stem Cells: What Do We Know so Far? Regen. Med. 2018, 13, 219–232. [Google Scholar] [CrossRef]
- Ismail, H.D.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Kusnadi, Y.; Merlina, M.; Yulisa, N.D. Mesenchymal Stem Cell Implantation in Atrophic Nonunion of the Long Bones: A Translational Study. Bone Jt. Res. 2016, 5, 287–293. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone Marrow Mesenchymal Stem Cells: Aging and Tissue Engineering Applications to Enhance Bone Healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Kotobuki, N.; Katsube, Y.; Katou, Y.; Tadokoro, M.; Hirose, M.; Ohgushi, H. In Vivo Survival and Osteogenic Differentiation of Allogeneic Rat Bone Marrow Mesenchymal Stem Cells (MSCs). Cell Transplant. 2008, 17, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A Review of Therapeutic Effects of Mesenchymal Stem Cell Secretions and Induction of Secretory Modification by Different Culture Methods. J. Transl. Med. 2014, 12, 1–14. [Google Scholar] [CrossRef]
- Wee Yeh Yeo, R.; Chai Lai, R.; Hian Tan, K.; Kiang Lim, S. Exosome: A Novel and Safer Therapeutic Refinement of Mesenchymal Stem Cell. J. Circ. Biomark. Exosome 2013, 1, 7. [Google Scholar]
- Zullo, J.; Matsumoto, K.; Xavier, S.; Ratliff, B.; Goligorsky, M.S. The Cell Secretome, a Mediator of Cell-to-Cell Communication. Prostaglandins Other Lipid Mediat. 2015, 120, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Damaser, M.S. Stem Cells as Drug Delivery Methods: Application of Stem Cell Secretome for Regeneration. Adv. Drug Deliv. Rev. 2015, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Hsiao, S.T.F.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells Derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Pires, A.O.; Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Ribeiro-Samy, S.; Gomes, E.D.; Serra, S.C.; Silva, N.A.; Manadas, B.; Sousa, N.; et al. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: A proteomic analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.S.; Jeong, J.H.; Choi, S.J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef]
- Ayaz-Guner, S.; Alessio, N.; Acar, M.B.; Aprile, D.; Özcan, S.; di Bernardo, G.; Peluso, G.; Galderisi, U. A Comparative Study on Normal and Obese Mice Indicates That the Secretome of Mesenchymal Stromal Cells Is Influenced by Tissue Environment and Physiopathological Conditions. Cell Commun. Signal. 2020, 18, 118. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.C.; Fernández, V.; Cifuentes, M.; Becerra, J. Comparative Analysis and Characterization of Soluble Factors and Exosomes from Cultured Adipose Tissue and Bone Marrow Mesenchymal Stem Cells in Canine Species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.R.; Bhonde, R.; Pal, R. Secretome Studies of Mesenchymal Stromal Cells (MSCs) Isolated from Three Tissue Sources Reveal Subtle Differences in Potency. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 689–700. [Google Scholar] [CrossRef]
- Dwek, J.R. The Periosteum: What Is It, Where Is It, and What Mimics It in Its Absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M. Periosteal Cells in Bone Tissue Engineering. Tissue Eng. 2003, 9. [Google Scholar] [CrossRef]
- Ferretti, C.; Borsari, V.; Falconi, M.; Gigante, A.; Lazzarini, R.; Fini, M.; di Primio, R.; Mattioli-Belmonte, M. Human Periosteum-Derived Stem Cells for Tissue Engineering Applications: The Role of VEGF. Stem Cell Rev. Rep. 2012, 8, 882–890. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Silva, J.C.; Colaco, B.; Gama, A.; Duarte-Araújo, M.; Fernandes, M.H.; Bettencourt, A.; Gomes, P. In Vivo Tissue Response and Antibacterial Efficacy of Minocycline Delivery System Based on Polymethylmethacrylate Bone Cement. J. Biomater. Appl. 2018, 33, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Sousa Gomes, P. Novel Cellulose/Hydroxyapatite Scaffolds for Bone Tissue Regeneration: In Vitro and in Vivo Study. J. Tissue Eng. Regen. Med. 2018, 12. [Google Scholar] [CrossRef]
- Coelho, M.J.; Fernandes, M.H. Human Bone Cell Cultures in Biocompatibility Testing. Part II: Effect of Ascorbic Acid, β-Glycerophosphate and Dexamethasone on Osteoblastic Differentiation. Biomaterials 2000, 21, 1095–1102. [Google Scholar] [CrossRef]
- Choi, Y.A.; Lim, J.; Kim, K.M.; Acharya, B.; Cho, J.Y.; Bae, Y.C.; Shin, H.I.; Kim, S.Y.; Park, E.K. Secretome Analysis of Human BMSCs and Identification of SMOC1 as an Important ECM Protein in Osteoblast Differentiation. J. Proteome Res. 2010, 9, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, J.; Kim, Y.H.; Kim, K.T.; Ryu, S.H.; Lee, T.G.; Suh, P.G. Comparative Secretome Analysis of Human Bone Marrow-Derived Mesenchymal Stem Cells during Osteogenesis. J. Cell. Physiol. 2013, 228, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation in Vivo and in Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Kim, S.W.; Muise, A.M.; Lyons, P.J.; Ro, H.S. Regulation of Adipogenesis by a Transcriptional Repressor That Modulates MAPK Activation. J. Biol. Chem. 2001, 276, 10199–10206. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. P38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ohno, I.; Hashimoto, J.; Shimizu, K.; Takaoka, K.; Ochi, T.; Matsubara, K.; Okubo, K. A CDNA Cloning of Human AEBP1 from Primary Cultured Osteoblasts and Its Expression in a Differentiating Osteoblastic Cell Line. Biochem. Biophys. Res. Commun. 1996, 228, 411–414. [Google Scholar] [CrossRef]
- Yoshiko, Y.; Aubin, J.E. Stanniocalcin 1 as a Pleiotropic Factor in Mammals. Peptides 2004, 25, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, Y.; Maeda, N.; Aubin, J.E. Stanniocalcin 1 Stimulates Osteoblast Differentiation in Rat Calvaria Cell Cultures. Endocrinology 2003, 144, 4134–4143. [Google Scholar] [CrossRef]
- Smaldone, S.; Ramirez, F. Fibrillin Microfibrils in Bone Physiology. Matrix Biol. 2016, 52–54, 191–197. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Norris, S.A.; Pettifor, J.M.; Gray, D.A.; Buffenstein, R. Calcium Metabolism and Bone Mass in Female Rabbits during Skeletal Maturation: Effects of Dietary Calcium Intake. Bone 2001, 29, 62–69. [Google Scholar] [CrossRef]
- Djasim, U.M.; Wolvius, E.B.; van Neck, J.W.; Weinans, H.; van der Wal, K.G.H. Recommendations for Optimal Distraction Protocols for Various Animal Models on the Basis of a Systematic Review of the Literature. Int. J. Oral Maxillofac. Surg. 2007, 36, 877–883. [Google Scholar] [CrossRef]
- Frame, J.W. A Convenient Animal Model for Testing Bone Substitute Materials. J. Oral Surg. 1980, 38, 176–180. [Google Scholar] [PubMed]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the Critical Size in Calvarial Bone Defects: Revisiting the Concept of a Critical-Size Defect. Plast. Reconstr. Surg. 2008, 125, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Rodent Models in Bone-Related Research: The Relevance of Calvarial Defects in the Assessment of Bone Regeneration Strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Critical Size Defects for Bone Regeneration Experiments in Rabbit Calvariae: Systematic Review and Quality Evaluation Using ARRIVE Guidelines. Clin. Oral Implant. Res. 2015, 26, 915–930. [Google Scholar] [CrossRef]
- Bosch, C.; Melsen, B.; Gibbons, R.; Vargervik, K. Human Recombinant Transforming Growth Factor-Beta 1 in Healing of Calvarial Bone Defects. J. Craniofacial Surg. 1996, 7, 300–310. [Google Scholar] [CrossRef]
- Haddad, A.J.; Peel, S.A.F.; Clokie, C.M.L.; Sándor, G.K.B. Closure of Rabbit Calvarial Critical-Sized Defects Using Protective Composite Allogeneic and Alloplastic Bone Substitutes. J. Craniofacial Surg. 2006, 17, 926–934. [Google Scholar] [CrossRef]
- Seidel, P.; Dingeldein, E. Cerabone®—Eine Spongiosa-Keramik Bovinen Ursprungs. Mater. Werkst. 2004, 35, 208–212. [Google Scholar] [CrossRef]
- Murugan, R.; Rao, K.P.; Sampath Kumar, T.S. Heat-Deproteinated Xenogeneic Bone from Slaughterhouse Waste: Physico-Chemical Properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef]
- Mahesh, L.; Mascarenhas, G.; Bhasin, M.T.; Guirado, C.; Juneja, S. Histological Evaluation of Two Different Anorganic Bovine Bone Matrixes in Lateral Wall Sinus Elevation Procedure: A Retrospective Study. Natl. J. Maxillofac. Surg. 2020, 11, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Sandgren, R.; Pippenger, B.; Fricain, J.; Herber, V.; el Chaar, E. A Novel Xenograft Bon e Substitute Supports Stable Bone Formation in Circumferential Defects Around Dental Implants in Minipigs. Int. J. Oral Maxillofac. Implant. 2020, 35, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Kapogianni, E.; Barbeck, M.; Jung, O.; Arslan, A.; Kuhnel, L.; Xiong, X.; Krastev, R.; Friedrich, R.; Schnettler, R.; Fienitz, T.; et al. Comparison of Material-Mediated Bone Regeneration Capacities of Sintered and Non-Sintered Xenogeneic Bone Substitutes via 2D and 3D Data. In Vivo 2019, 33, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Ravindran, S.; Huang, C.C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Burdette, A.J.; Guda, T.; Thompson, M.E.; Banas, R.; Sheppard, F. A Novel Secretome Biotherapeutic Influences Regeneration in Critical Size Bone Defects. J. Craniofacial Surg. 2018, 29, 116–123. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. Stem Cells and Cell Therapies in Lung Biology and Diseases: Conference Report. Ann. Am. Thorac. Soc. 2010, 12, 181–204. [Google Scholar] [CrossRef]
- Chang, W.; Kim, R.; Park, S.I.; Jung, Y.J.; Ham, O.; Lee, J.; Kim, J.H.; Oh, S.; Lee, M.Y.; Kim, J.; et al. Enhanced Healing of Rat Calvarial Bone Defects with Hypoxic Conditioned Medium from Mesenchymal Stem Cells through Increased Endogenous Stem Cell Migration via Regulation of ICAM-1 Targeted-MicroRNA-221. Mol. Cells 2015, 38, 643–650. [Google Scholar] [CrossRef]
- Katagiri, W.; Osugi, M.; Kawai, T.; Ueda, M. Novel Cell-Free Regeneration of Bone Using Stem Cell–Derived Growth Factors. Int. J. Oral Maxillofac. Implant. 2013, 28, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem Cell-Conditioned Medium Accelerates Distraction Osteogenesis through Multiple Regenerative Mechanisms. Bone 2014, 61, 82–90. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Hara, K.; Ikeno, M.; Okamoto, Y.; Hibi, H.; Ueda, M. Rat Bone Marrow Stromal Cell–Conditioned Medium Promotes Early Osseointegration of Titanium Implants. Int. J. Oral Maxillofac. Implant. 2013, 28, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Osugi, M.; Kawai, T.; Wakayama, Y.; Sakaguchi, K.; Nakamura, S.; Katagiri, W. Secretomes of Mesenchymal Stem Cells Induce Early Bone Regeneration by Accelerating Migration of Stem Cells. J. Oral Maxillofac. Surgery, Med. Pathol. 2018, 30, 445–451. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.P.; Chen, L.; Overbeck Nielsen, M.; Qanie, D.W.; Kratchmarova, I.; Kassem, M.; Andersen, J.S. Temporal Profiling and Pulsed SILAC Labeling Identify Novel Secreted Proteins During. Mol. Cell. Proteom. 2012, 11, 989–1007. [Google Scholar] [CrossRef] [PubMed]

| Osteogenesis-Related Proteins Identified on the Periosteum-Derived MSCs Secretomes | ||
|---|---|---|
| Common to UndifSec and OsteoSec | Specific to UndifSec | Specific to OsteoSec |
| Actinin alpha 4 Annexin A2 Collagen alpha-2(I) chain Peroxiredoxin-1 Collagen alpha-1(XII) chain Chitinase-3-like protein 1 Fibrillin-1 Macrophage colony-stimulating factor 1 receptor Pro-low-density lipoprotein receptor-related protein 1 | Fibrillin-2 Cathepsin K | Collagen alpha-1(I) chain AE binding protein 1 Stanniocalcin-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranskunas, M.; Šimoliūnas, E.; Alksne, M.; Martin, V.; Gomes, P.S.; Puisys, A.; Kaupinis, A.; Juodzbalys, G. Assessment of the Bone Healing Process Mediated by Periosteum-Derived Mesenchymal Stem Cells’ Secretome and a Xenogenic Bioceramic—An In Vivo Study in the Rabbit Critical Size Calvarial Defect Model. Materials 2021, 14, 3512. https://doi.org/10.3390/ma14133512
Pranskunas M, Šimoliūnas E, Alksne M, Martin V, Gomes PS, Puisys A, Kaupinis A, Juodzbalys G. Assessment of the Bone Healing Process Mediated by Periosteum-Derived Mesenchymal Stem Cells’ Secretome and a Xenogenic Bioceramic—An In Vivo Study in the Rabbit Critical Size Calvarial Defect Model. Materials. 2021; 14(13):3512. https://doi.org/10.3390/ma14133512
Chicago/Turabian StylePranskunas, Mindaugas, Egidijus Šimoliūnas, Milda Alksne, Victor Martin, Pedro Sousa Gomes, Algirdas Puisys, Algirdas Kaupinis, and Gintaras Juodzbalys. 2021. "Assessment of the Bone Healing Process Mediated by Periosteum-Derived Mesenchymal Stem Cells’ Secretome and a Xenogenic Bioceramic—An In Vivo Study in the Rabbit Critical Size Calvarial Defect Model" Materials 14, no. 13: 3512. https://doi.org/10.3390/ma14133512
APA StylePranskunas, M., Šimoliūnas, E., Alksne, M., Martin, V., Gomes, P. S., Puisys, A., Kaupinis, A., & Juodzbalys, G. (2021). Assessment of the Bone Healing Process Mediated by Periosteum-Derived Mesenchymal Stem Cells’ Secretome and a Xenogenic Bioceramic—An In Vivo Study in the Rabbit Critical Size Calvarial Defect Model. Materials, 14(13), 3512. https://doi.org/10.3390/ma14133512








