Advances in 3D-Printed Surface-Modified Ca-Si Bioceramic Structures and Their Potential for Bone Tumor Therapy
Abstract
:1. Introduction
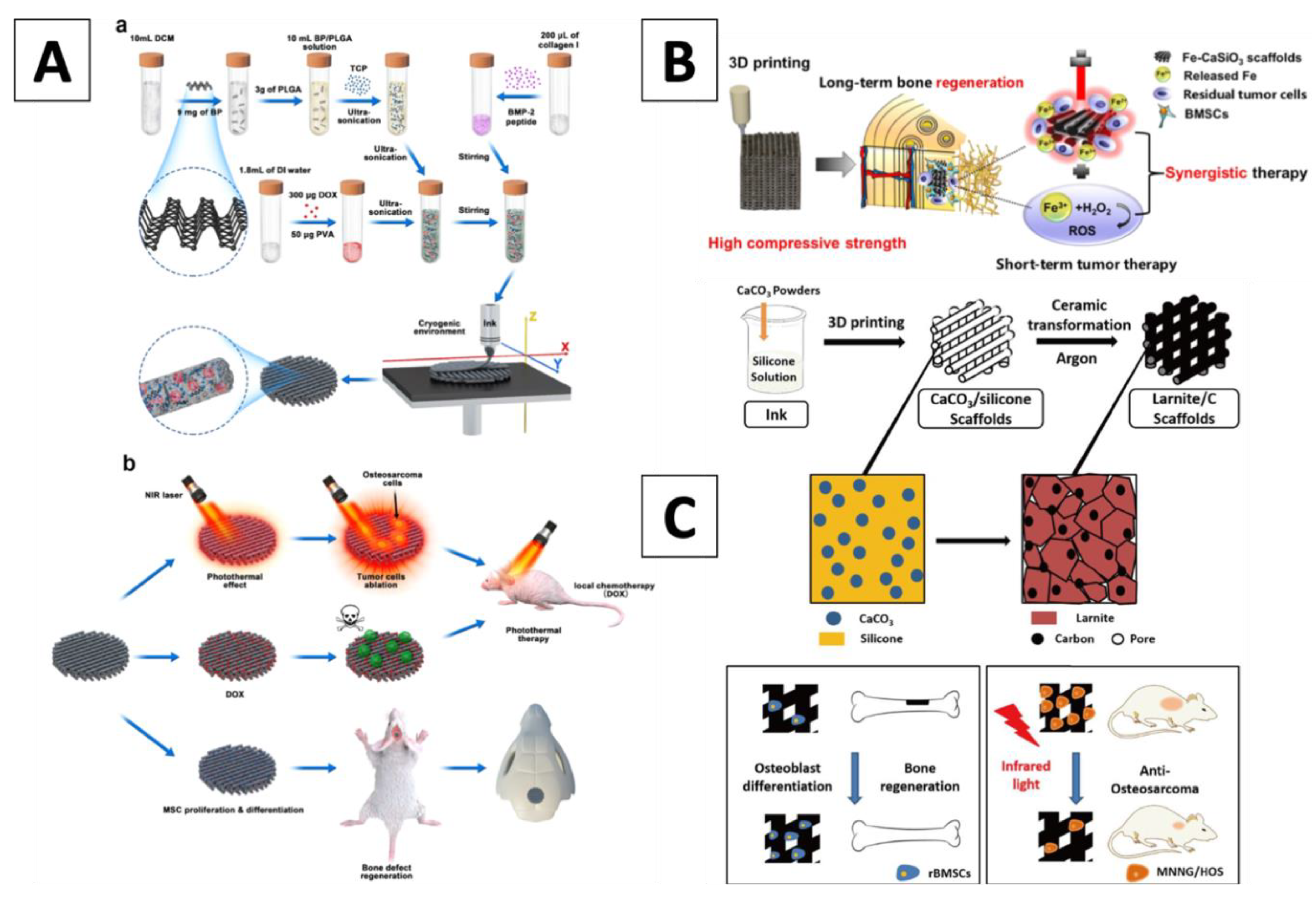
2. External Surface Functionalization of Scaffolds
3. Internal Dispersion Functionalization of Scaffolds
4. Challenges and Prospects
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. SICOT-J 2018, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef] [Green Version]
- Loh, A.H.P.; Navid, F.; Wang, C.; Bahrami, A.; Wu, J.; Neel, M.D.; Rao, B.N. Management of Local Recurrence of Pediatric Osteosarcoma Following Limb-Sparing Surgery. Ann. Surg. Oncol. 2014, 21, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.R.; Lazarides, A.L.; Visgauss, J.D.; Somarelli, J.A.; Blazer, D.G.; Brigman, B.E.; Eward, W.C. Limb salvage versus amputation in patients with osteosarcoma of the extremities: An update in the modern era using the National Cancer Database. BMC Cancer 2020, 20, 995. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Hamada, M.; Sugihara, S.; Hashizume, H.; Nagashima, H.; Inoue, H. Rotationplasty for patients with osteosarcoma around the knee joint. Acta Med. Okayama 1995, 49, 221–226. [Google Scholar] [PubMed]
- Demiralp, B.; Ege, T.; Kose, O.; Yurttas, Y.; Basbozkurt, M. Reconstruction of intercalary bone defects following bone tumor resection with segmental bone transport using an Ilizarov circular external fixator. J. Orthop. Sci. 2014, 19, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.S.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [Green Version]
- Bielack, S.S.; Hecker-Nolting, S.; Blattmann, C.; Kager, L. Advances in the management of osteosarcoma. F1000Research 2016, 5, 2767. [Google Scholar] [CrossRef]
- Beck, J.C.; Wara, W.M.; Bovill, E.G.; Phillips, T.L. The Role of Radiation Therapy in the Treatment of Osteosarcoma. Radiology 1976, 120, 163–165. [Google Scholar] [CrossRef]
- Schwarz, R.; Bruland, Ø.; Cassoni, A.; Schomberg, P.; Bielack, S. The Role of Radiotherapy in Oseosarcoma. Cancer Treat. Res. 2009, 152, 147–164. [Google Scholar] [CrossRef]
- Melamed, J.; Edelstein, R.S.; Day, E. Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Yang, X.-X.; Liu, R.-Q.; Zhao, D.; Guo, C.-N.; Zhu, A.-C.; Wen, M.-N.; Liu, Z.; Qu, G.-F.; Meng, H.-X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xie, X.; Shao, X.; Gao, F.; Jin, H.; Zhou, J.; Du, L.; Zhang, Y.; Ouyang, W.; Wang, X.; et al. Effect of hyperthermia on invasion ability and TGF-?1 expression of breast carcinoma MCF-7 cells. Oncol. Rep. 2011, 25, 1573–1579. [Google Scholar] [CrossRef]
- Doughty, A.C.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Cruz, D.; Mostafavi, E.; Vernet-Crua, A.; Cheng, J.; Shah, V.; Cholula-Diaz, J.L.; Guisbiers, G.; Tao, J.; García-Martín, J.M.; Webster, T.J. Green nanotechnology-based drug delivery systems for osteogenic disorders. Expert Opin. Drug Deliv. 2020, 17, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.S.; Jadhav, G.R.; Gathani, K.M.; Kotadia, P. Bioceramics in endodontics—A review. J. Istanb. Univ. Fac. Dent. 2017, 51, S128–S137. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Zhang, F.; Chang, J.; Lu, J.; Lin, K.; Ning, C. Bioinspired structure of bioceramics for bone regeneration in load-bearing sites. Acta Biomater. 2007, 3, 896–904. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From Bone Regeneration to Cancer Nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Andrei, M.; Vacaru, R.; Coricovac, A.; Ilinca, R.; Didilescu, A.; Demetrescu, I. The Effect of Calcium-Silicate Cements on Reparative Dentinogenesis Following Direct Pulp Capping on Animal Models. Molecules 2021, 26, 2725. [Google Scholar] [CrossRef]
- Panseri, S.; Montesi, M.; Hautcoeur, D.; Dozio, S.M.; Chamary, S.; De Barra, E.; Tampieri, A.; Leriche, A. Bone-like ceramic scaffolds designed with bioinspired porosity induce a different stem cell response. J. Mater. Sci. Mater. Med. 2021, 32, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef]
- Srinath, P.; Azeem, P.A.; Reddy, K.V. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020, 17, 2450–2464. [Google Scholar] [CrossRef]
- Du, Z.; Leng, H.; Guo, L.; Huang, Y.; Zheng, T.; Zhao, Z.; Liu, X.; Zhang, X.; Cai, Q.; Yang, X. Calcium silicate scaffolds promoting bone regeneration via the doping of Mg2+ or Mn2+ ion. Compos. Part B Eng. 2020, 190, 107937. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. A Novel Akermanite Bioceramic: Preparation and Characteristics. J. Biomater. Appl. 2006, 21, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhai, W.; Ni, S.; Chang, J.; Zeng, Y.; Qian, W. Study of the mechanical property and in vitro biocompatibility of CaSiO3 ceramics. Ceram. Int. 2005, 31, 323–326. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Saghati, S.; Akbarzadeh, A.; Del Bakhshayesh, A.R.; Sheervalilou, R.; Mostafavi, E. Chapter 6. Electrospinning and 3D Printing: Prospects for Market Opportunity. In Soft Matter Series; Royal Society of Chemistry (RSC): London, UK, 2018; pp. 136–155. [Google Scholar]
- Chang, C.-H.; Lin, C.-Y.; Liu, F.-H.; Chen, M.H.-C.; Lin, C.-P.; Ho, H.-N.; Liao, Y.-S. 3D Printing Bioceramic Porous Scaffolds with Good Mechanical Property and Cell Affinity. PLoS ONE 2015, 10, e0143713. [Google Scholar] [CrossRef]
- Roohani, I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lin, R.; Wang, X.; Xue, J.; Deng, C.; Feng, C.; Zhuang, H.; Ma, J.; Qin, C.; Wan, L.; et al. 3D printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 2020, 6, eaaz6725. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, E.; Soltantabar, P.; Webster, T.J. Nanotechnology and picotechnology. In Biomaterials in Translational Medicine; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 191–212. [Google Scholar]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2012, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Perikamana, S.K.M.; Lee, J.; Bin Lee, Y.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, W.; Lee, J.H.; Kondyurin, A.; Lord, M.S.; Jang, J.-H.; Kim, H.-W.; Bilek, M.M.M. Nano-Bio-Chemical Braille for Cells: The Regulation of Stem Cell Responses using Bi-Functional Surfaces. Adv. Funct. Mater. 2015, 25, 193–205. [Google Scholar] [CrossRef]
- Ma, H.; Luo, J.; Sun, Z.; Xia, L.; Shi, M.; Liu, M.; Chang, J.; Wu, C. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 2016, 111, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, A.; Abudula, T.; Russell, C.S.; Mostafavi, E.; Williams, T.J.; Salah, N.; Alshahrie, A.; Harris, S.; Basri, S.M.M.; Mishra, Y.K.; et al. In situ printing of scaffolds for reconstruction of bone defects. Acta Biomater. 2021, 127, 313–326. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Uskoković, V.; Batarni, S.S.; Schweicher, J.; King, A.; Desai, T.A. Effect of Calcium Phosphate Particle Shape and Size on Their Antibacterial and Osteogenic Activity in the Delivery of Antibiotics in Vitro. ACS Appl. Mater. Interfaces 2013, 5, 2422–2431. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Ma, B.; Li, B.; Huan, Z.; Ma, N.; Zhu, H.; Chang, J.; Xiao, Y.; Wu, C. 3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication 2020, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Lattanzi, W.; Perini, G.; Augello, A.; Papi, M.; De Spirito, M. 3D-printed graphene for bone reconstruction. 2D Mater. 2020, 7, 022004. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verron, E.; Schmid-Antomarchi, H.; Pascal-Mousselard, H.; Schmid-Alliana, A.; Scimeca, J.-C.; Bouler, J.-M. Therapeutic strategies for treating osteolytic bone metastases. Drug Discov. Today 2014, 19, 1419–1426. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, C.; Zhai, D.; Luo, Y.; Chen, Y.; Lv, F.; Yi, Z.; Deng, Y.; Wang, J.; Chang, J.; et al. A Bifunctional Biomaterial with Photothermal Effect for Tumor Therapy and Bone Regeneration. Adv. Funct. Mater. 2016, 26, 1197–1208. [Google Scholar] [CrossRef]
- Chhetri, M.; Maitra, S.; Chakraborty, H.; Waghmare, U.V.; Rao, C.N.R. Superior performance of borocarbonitrides, BxCyNz, as stable, low-cost metal-free electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2016, 9, 95–101. [Google Scholar] [CrossRef]
- Vrouwenvelder, W.; Groot, C.; de Groot, K. Better histology and biochemistry for osteoblasts cultured on titanium-doped bioactive glass: Bioglass 45S5 compared with iron-, titanium-, fluorine- and boron-containing bioactive glasses. Biomaterials 1994, 15, 97–106. [Google Scholar] [CrossRef]
- Xia, L.; Yin, Z.; Mao, L.; Wang, X.; Liu, J.; Jiang, X.; Zhang, Z.; Lin, K.; Chang, J.; Fang, B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci. Rep. 2016, 6, 22005. [Google Scholar] [CrossRef]
- Gencoglu, M.F.; Spurri, A.; Franko, M.; Chen, J.; Hensley, D.K.; Heldt, C.L.; Saha, D. Biocompatibility of Soft-Templated Mesoporous Carbons. ACS Appl. Mater. Interfaces 2014, 6, 15068–15077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Liu, J.; Wang, H.; Sun, W.; Lin, K.; Wang, X.; Shen, S.G. Amorphous carbon modification on implant surface: A general strategy to enhance osteogenic differentiation for diverse biomaterials via FAK/ERK1/2 signaling pathways. J. Mater. Chem. B 2019, 7, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, K.; Xie, Y.; Qi, S.; Shen, Q.; Yu, J.; Huang, L.; Zheng, X. Improved osteogenesis of boron incorporated calcium silicate coatings via immunomodulatory effects. J. Biomed. Mater. Res. Part A 2019, 107, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Zhaoa, C.; Shenab, A.; Zhangc, L.; Lina, K.; Wanga, X. Borocarbonitrides nanosheets engineered 3D-printed scaffolds for integrated strategy of osteosarcoma therapy and bone regeneration. Chem. Eng. J. 2020, 401, 125989. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, Z.; Kohler, N.; Kim, J.; Chung, M.A.; Sun, S. FePt Nanoparticles as an Fe Reservoir for Controlled Fe Release and Tumor Inhibition. J. Am. Chem. Soc. 2009, 131, 15346–15351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, X.; Abboud, J.; Zhang, Z. Plasmonics Resonance Enhanced Active Photothermal Effects of Aluminum and Iron Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Lamy, P.-J.; Durigova, A.; Jacot, W. Iron homeostasis and anemia markers in early breast cancer. Clin. Chim. Acta 2014, 434, 34–40. [Google Scholar] [CrossRef]
- Li, H.; Xue, K.; Kong, N.; Liu, K.; Chang, J. Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells. Biomaterials 2014, 35, 3803–3818. [Google Scholar] [CrossRef]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef]
- Valerio, P.; Pereira, M.M.; Goes, A.M.; Leite, M. The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials 2004, 25, 2941–2948. [Google Scholar] [CrossRef]
- Ma, H.; Li, T.; Huan, Z.; Zhang, M.; Yang, Z.; Wang, J.; Chang, J.; Wu, C. 3D printing of high-strength bioscaffolds for the synergistic treatment of bone cancer. NPG Asia Mater. 2018, 10, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chang, J.; Ni, S.; Wang, J. In vitro bioactivity of akermanite ceramics. J. Biomed. Mater. Res. Part A 2006, 76, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yan, T.; Xue, Y.; Guo, L.; Zhang, L.; Han, Y. Intrinsically ferromagnetic Fe-doped TiO2 coatings on titanium for accelerating osteoblast response in vitro. J. Mater. Chem. B 2018, 6, 5756–5767. [Google Scholar] [CrossRef]
- Zhuang, H.; Lin, R.; Liu, Y.; Zhang, M.; Zhai, D.; Huan, Z.; Wu, C. Three-Dimensional-Printed Bioceramic Scaffolds with Osteogenic Activity for Simultaneous Photo/Magnetothermal Therapy of Bone Tumors. ACS Biomater. Sci. Eng. 2019, 5, 6725–6734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shan, Y.; Chen, K. A facile approach to fabricate of photothermal functional Fe3O4@CuS microspheres. Mater. Chem. Phys. 2017, 193, 82–88. [Google Scholar] [CrossRef]
- Luther, J.; Jain, P.K.; Ewers, T.; Alivisatos, P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat. Mater. 2011, 10, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Torres, P.; Vieira, S.; Cerqueira, A.; Pina, S.; Silva, O.D.C.; Abrantes, J.; Ferreira, J. Effects of Mn-doping on the structure and biological properties of β-tricalcium phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, D.; Xu, M.; Yao, Q.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds with a Fe3O4/graphene oxide nanocomposite interface for hyperthermia therapy of bone tumor cells. J. Mater. Chem. B 2016, 4, 2874–2886. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Ma, H.; Zhai, D.; Deng, C.; Wang, J.; Zhuo, S.; Chang, J.; Wu, C. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018, 73, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Ye, X.; Zhao, Y.; Bai, L.; He, Z.; Tong, Q.; Xie, X.; Zhu, H.; Cai, D.; Zhou, Y.; et al. Cryogenic 3D printing of porous scaffolds for in situ delivery of 2D black phosphorus nanosheets, doxorubicin hydrochloride and osteogenic peptide for treating tumor resection-induced bone defects. Biofabrication 2020, 12, 035004. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Chang, J.; Zhai, W. Preparation and characterization of novel bioactive dicalcium silicate ceramics. J. Eur. Ceram. Soc. 2005, 25, 1507–1514. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Lu, M.; Xu, D.; Zheng, X.; Pan, Y.; Song, Y.; Xu, J.; Li, M.; Zhang, M.; Li, J.; et al. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2017, 5, 1820–1827. [Google Scholar] [CrossRef]
- Fu, S.; Hu, H.; Chen, J.; Zhu, Y.; Zhao, S. Silicone resin derived larnite/C scaffolds via 3D printing for potential tumor therapy and bone regeneration. Chem. Eng. J. 2020, 382, 122928. [Google Scholar] [CrossRef]
- Mehranfar, S.; Rad, I.A.; Mostafavi, E.; Akbarzadeh, A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: An overview of clinical trials. Artif. Cells Nanomed. Biotechnol. 2019, 47, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef] [Green Version]
- Thrivikraman, G.; Madras, G.; Basu, B. In vitro/In vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahani, R.D.; Dubé, M.; Therriault, D. Three-Dimensional Printing of Multifunctional Nanocomposites: Manufacturing Techniques and Applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xue, J.; Yu, X.; Zhai, D.; Lin, R.; Zhang, M.; Xia, L.; Wang, X.; Yao, Q.; Chang, J.; et al. Co-inspired hydroxyapatite-based scaffolds for vascularized bone regeneration. Acta Biomater. 2021, 119, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-containing bioceramics inhibit osteoclastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, Y.; Liu, Y.; Ye, R.; Chen, L.; Bai, G.; Xu, S. Erbium-doped tungsten selenide nanosheets with near-infrared II emission and photothermal conversion. Chem. Eng. J. 2021, 411, 128610. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, e1904209. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Lui, Y.S.; Sow, W.T.; Tan, L.P.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef]
- Miri, A.K.; Mostafavi, E.; Khorsandi, D.; Hu, S.-K.; Malpica, M.; Khademhosseini, A. Bioprinters for organs-on-chips. Biofabrication 2019, 11, 042002. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Yarholar, L.M. Plastic Surgery Innovation with 3D Printing for Craniomaxillofacial Operations. Mo. Med 2020, 117, 136–142. [Google Scholar]
- Wang, H.; Peng, R.; Li, X.; Wang, Y.; Jiang, Y.; Ji, Z.; Guo, F.; Tian, S.; Sun, H.; Fan, J.; et al. The dosimetry evaluation of 3D printing non-coplanar template-assisted CT-guided 125I seed stereotactic ablation brachytherapy for pelvic recurrent rectal cancer after external beam radiotherapy. J. Radiat. Res. 2021, 62, 473–482. [Google Scholar] [CrossRef]
- Rooney, M.; Rosenberg, D.M.; Braunstein, S.; Cunha, A.; Damato, A.L.; Ehler, E.; Pawlicki, T.; Robar, J.; Tatebe, K.; Golden, D.W. Three-dimensional printing in radiation oncology: A systematic review of the literature. J. Appl. Clin. Med Phys. 2020, 21, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, R.V.; Castilho, M.; De Grauw, J.; Cokelaere, S.; Plomp, S.; Groll, J.; Van Weeren, P.R.; Gbureck, U.; Malda, J. Long-Term in Vivo Performance of Low-Temperature 3D-Printed Bioceramics in an Equine Model. ACS Biomater. Sci. Eng. 2020, 6, 1681–1689. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.K.; Kang, S.S.; Han, S.J.; Lee, C.-K.; Chang, B.-S. A Long-Term Follow-up, Multicenter, Comparative Study of the Radiologic, and Clinical Results Between a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramics (BGS-7) Intervertebral Spacer and Titanium Cage in 1-Level Posterior Lumbar Interbody Fusion. Clin. Spine Surg. 2020, 33, E322–E329. [Google Scholar] [CrossRef]
- Lee, U.-L.; Lim, J.-Y.; Park, S.-N.; Choi, B.-H.; Kang, H.; Choi, W.-C. A Clinical Trial to Evaluate the Efficacy and Safety of 3D Printed Bioceramic Implants for the Reconstruction of Zygomatic Bone Defects. Materials 2020, 13, 4515. [Google Scholar] [CrossRef]
- He, Y.; Laugesen, K.; Kamp, D.; Sultan, S.A.; Oddershede, L.B.; Jauffred, L. Effects and side effects of plasmonic photothermal therapy in brain tissue. Cancer Nanotechnol. 2019, 10, 8. [Google Scholar] [CrossRef]

| Functionalization | BC Composition | Functionalizing Agents | The Temperature Achieved during NIR Radiation | In-Vitro Antitumor (% Cancer Cell Viability) during NIR (W/cm2) | Ref. |
|---|---|---|---|---|---|
| External Surface | Nagel (Ca7Si2P2O16) | Ca-P/polydopamine | 50 °C at 0.34 W/cm2 | 0.8–1%/0.38 | (Ma and Luo, et al, 2016) |
| Bioglass (Ca-Si-P) | Black Phosphorus | 70 °C at 1 W/cm2 | <5%/1 | (Yang 2018) | |
| Beta-tricalcium phosphate | Copper-tetrakis (4-carboxyphenyl) porphyrin (Cu-TCPP) | 55 °C at 0.9 W/cm2 | <10%/1 | (Dang 2020) | |
| Beta-tricalcium phosphate | Graphene Oxide | 40–90 °C at 0.36 W/cm2 | 14%/0.36 | (Ma and Jiang, et al, 2016) | |
| Akermanite (Ca2MgSi2O7) | Borocarbonitrides | 55 °C at 0.35 W/cm2 | 11%/0.3 | (Zhaoa 2020) | |
| Internal Dispersion | Wollastonite (CaSiO3) | Iron (Fe) | 50 °C at 0.6 W/cm2 | 8.6%/0.6 | (Wu 2006) |
| Akermanite (Ca2MgSi2O7) | Iron (Fe) | 45 °C at 0.7 W/cm2 | 40%/0.7; 2%/0.7 in combination with 896.8 A/m magnetic field | (Zhang 2017) | |
| Glass ceramic (Ca0.25-0XP0.05Si0.25) | Copper, Iron, Cobalt, Manganese | 40–55 °C at 0.36 W/cm2 | 0–10%/0.54 | (Qiu 2018) | |
| Beta-tricalcium phosphate | Black phosphorus—doxorubicin hydrochloride | 50 °C at 1.5 W/cm2 | No in vitro data, but tumor volume decrease is recorded In vivo | (Gou 2005) | |
| Larnite (Ca2SiO4) | Free Carbon from CaCO3 | 50 °C at 0.75 W/cm2 | 50%/0.75 | (Mehranfar 2019) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, L.B.; Medina Cruz, D.; Mostafavi, E.; O’Connell, C.P.; Webster, T.J. Advances in 3D-Printed Surface-Modified Ca-Si Bioceramic Structures and Their Potential for Bone Tumor Therapy. Materials 2021, 14, 3844. https://doi.org/10.3390/ma14143844
Truong LB, Medina Cruz D, Mostafavi E, O’Connell CP, Webster TJ. Advances in 3D-Printed Surface-Modified Ca-Si Bioceramic Structures and Their Potential for Bone Tumor Therapy. Materials. 2021; 14(14):3844. https://doi.org/10.3390/ma14143844
Chicago/Turabian StyleTruong, Linh B., David Medina Cruz, Ebrahim Mostafavi, Catherine P. O’Connell, and Thomas J. Webster. 2021. "Advances in 3D-Printed Surface-Modified Ca-Si Bioceramic Structures and Their Potential for Bone Tumor Therapy" Materials 14, no. 14: 3844. https://doi.org/10.3390/ma14143844
APA StyleTruong, L. B., Medina Cruz, D., Mostafavi, E., O’Connell, C. P., & Webster, T. J. (2021). Advances in 3D-Printed Surface-Modified Ca-Si Bioceramic Structures and Their Potential for Bone Tumor Therapy. Materials, 14(14), 3844. https://doi.org/10.3390/ma14143844








