Biomass Related Highly Porous Metal Free Carbon for Gas Storage and Electrocatalytic Applications
Abstract
:1. Introduction
2. Materials and Methods
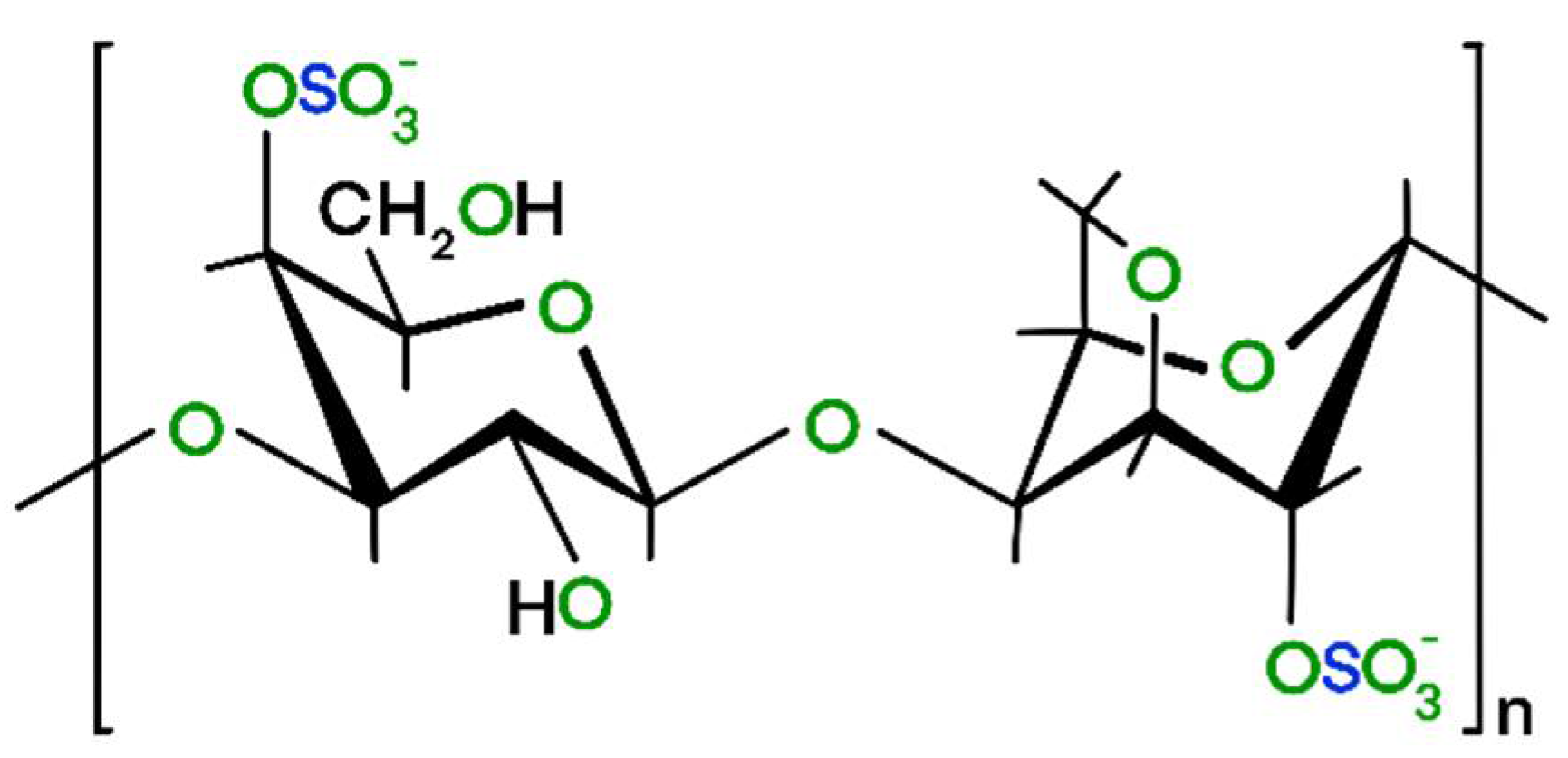
2.1. Synthesis
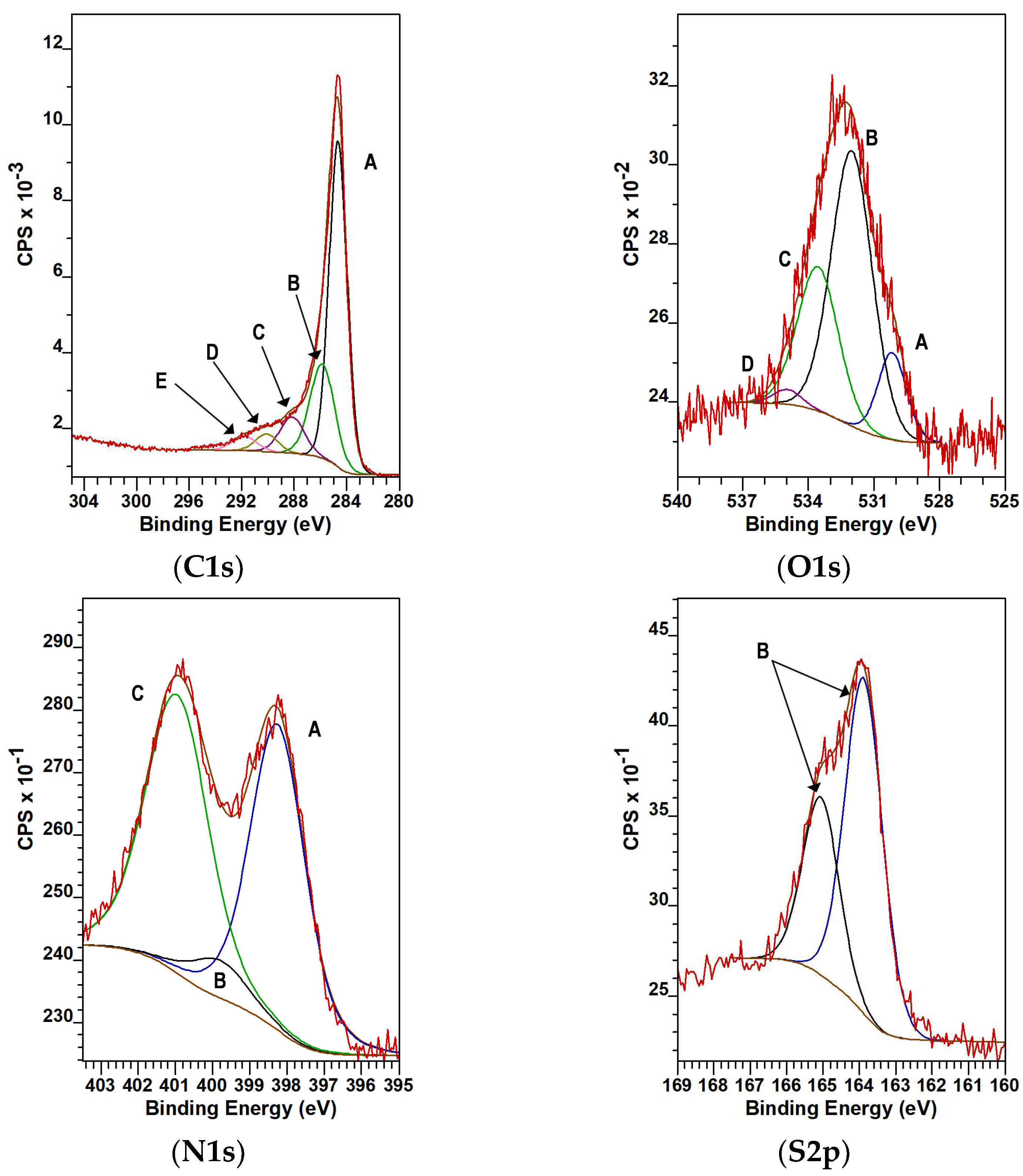
2.2. Characterization Methods
3. Results and Discussion
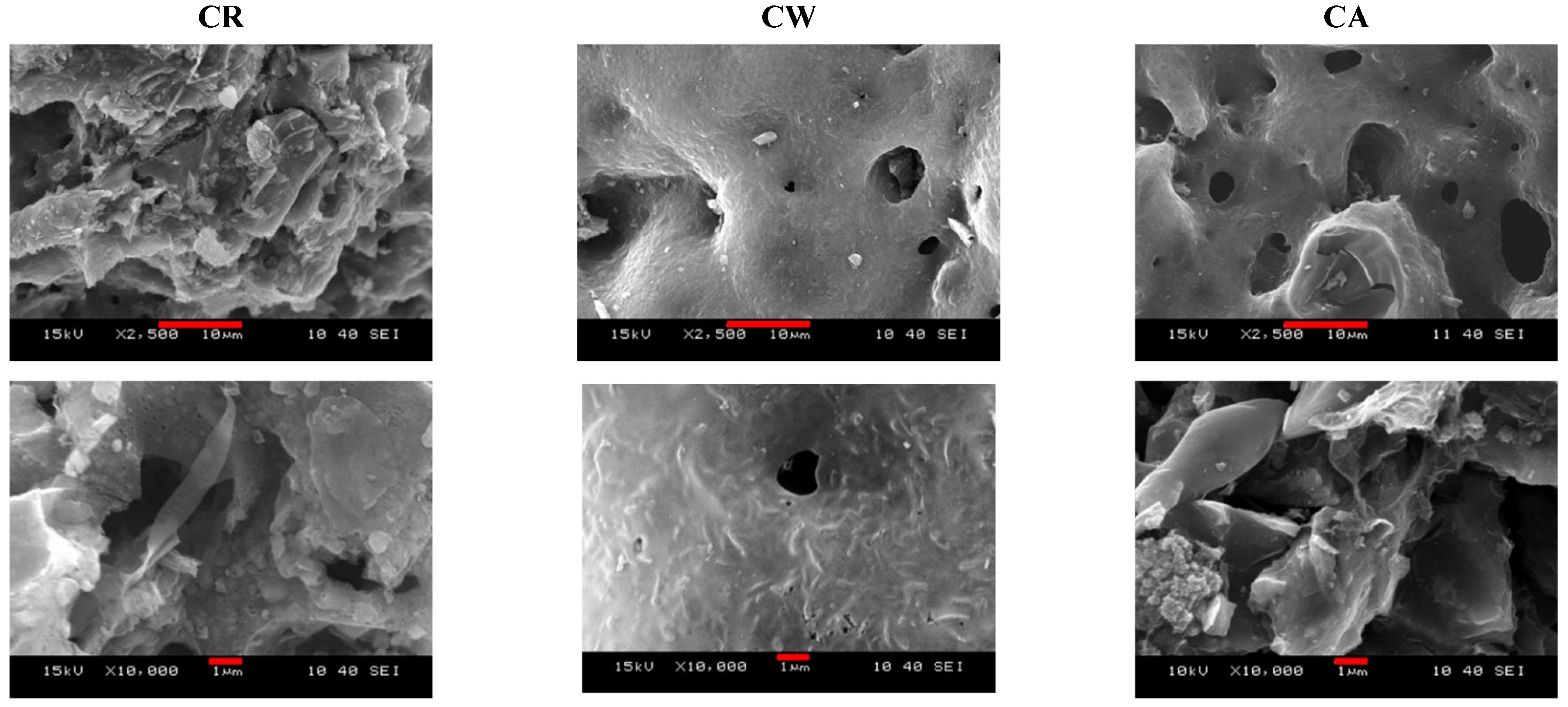
3.1. Development of the Morphology during the Synthesis
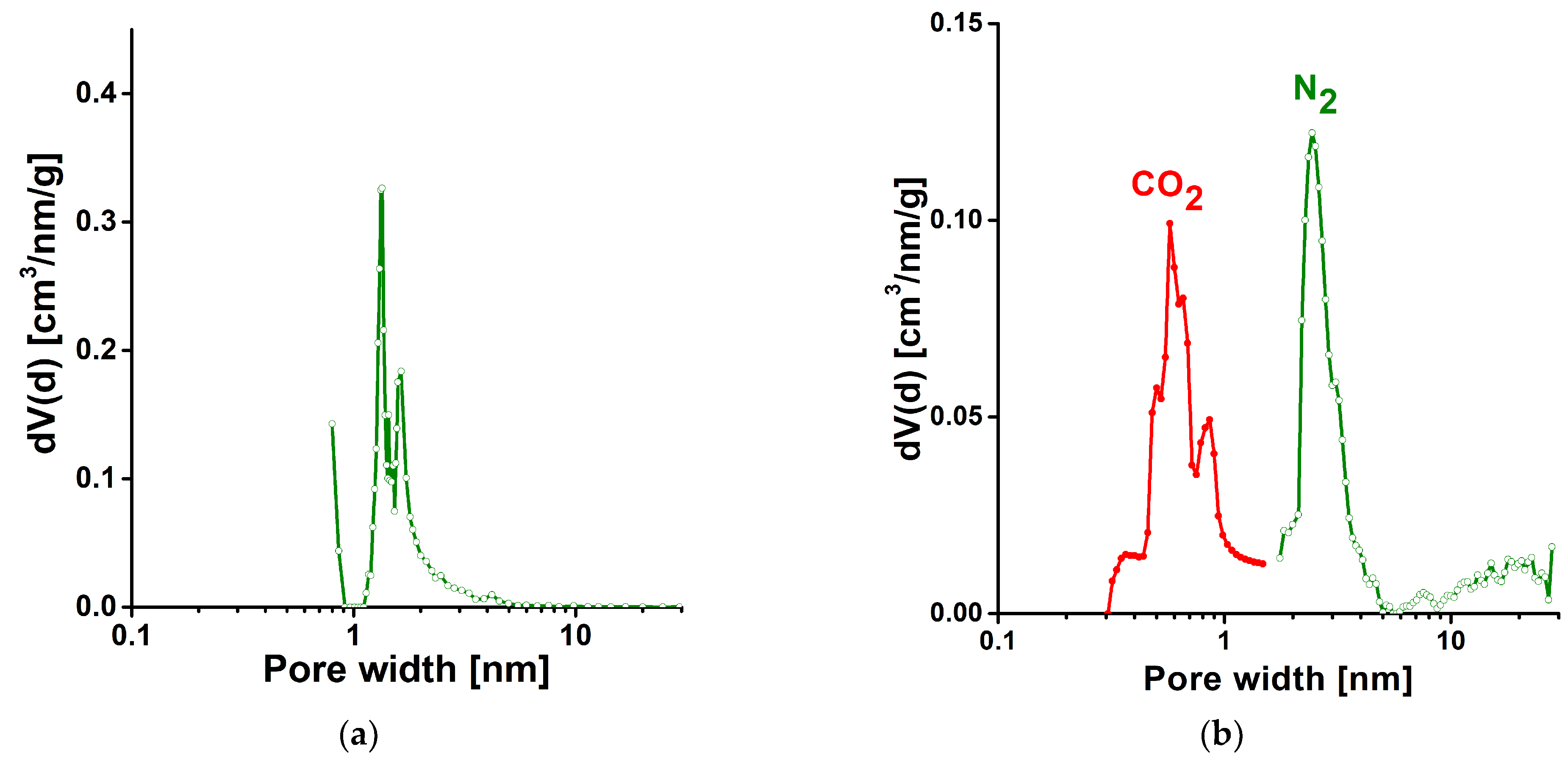
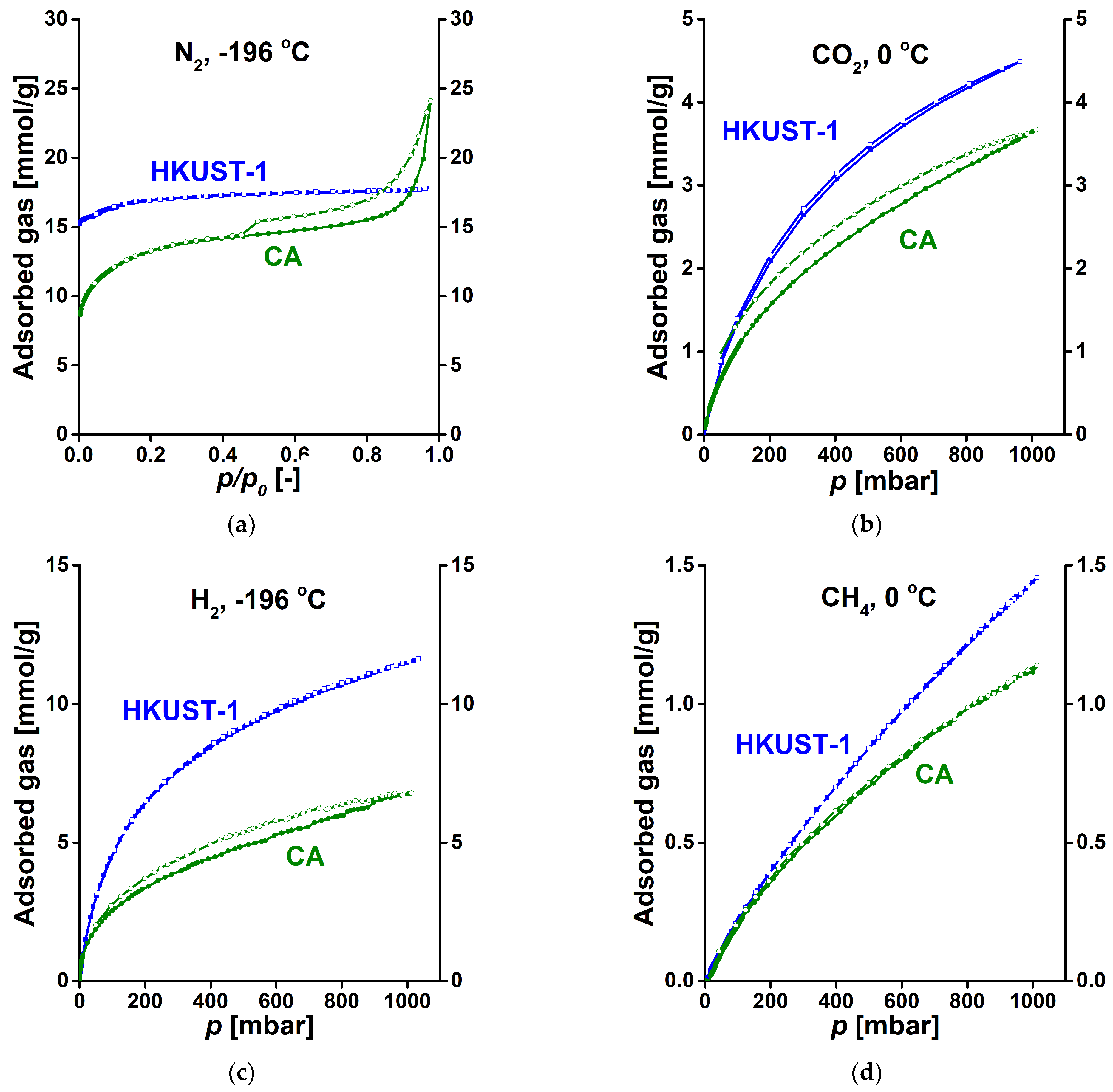
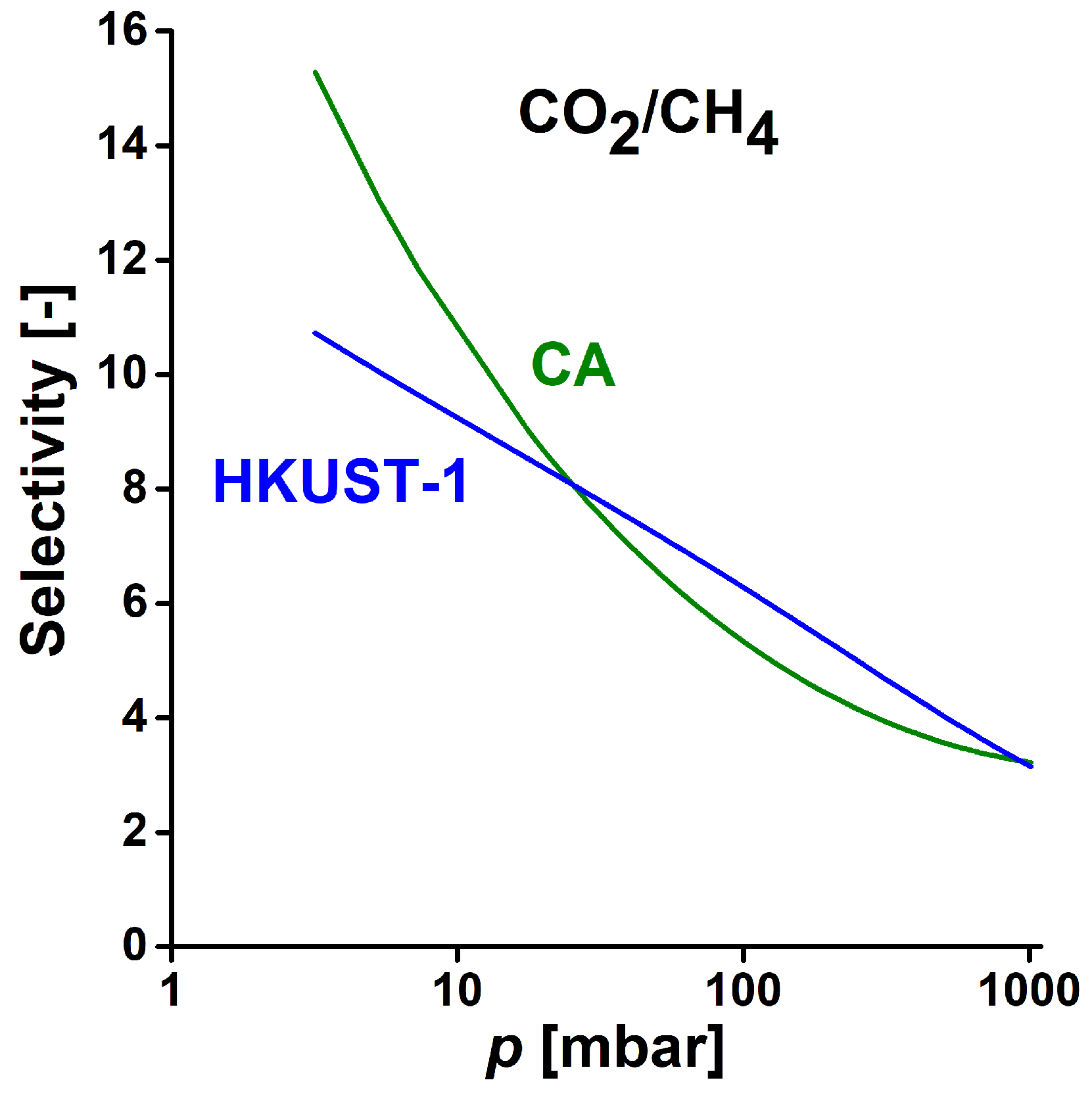
3.2. Gas Storage Application
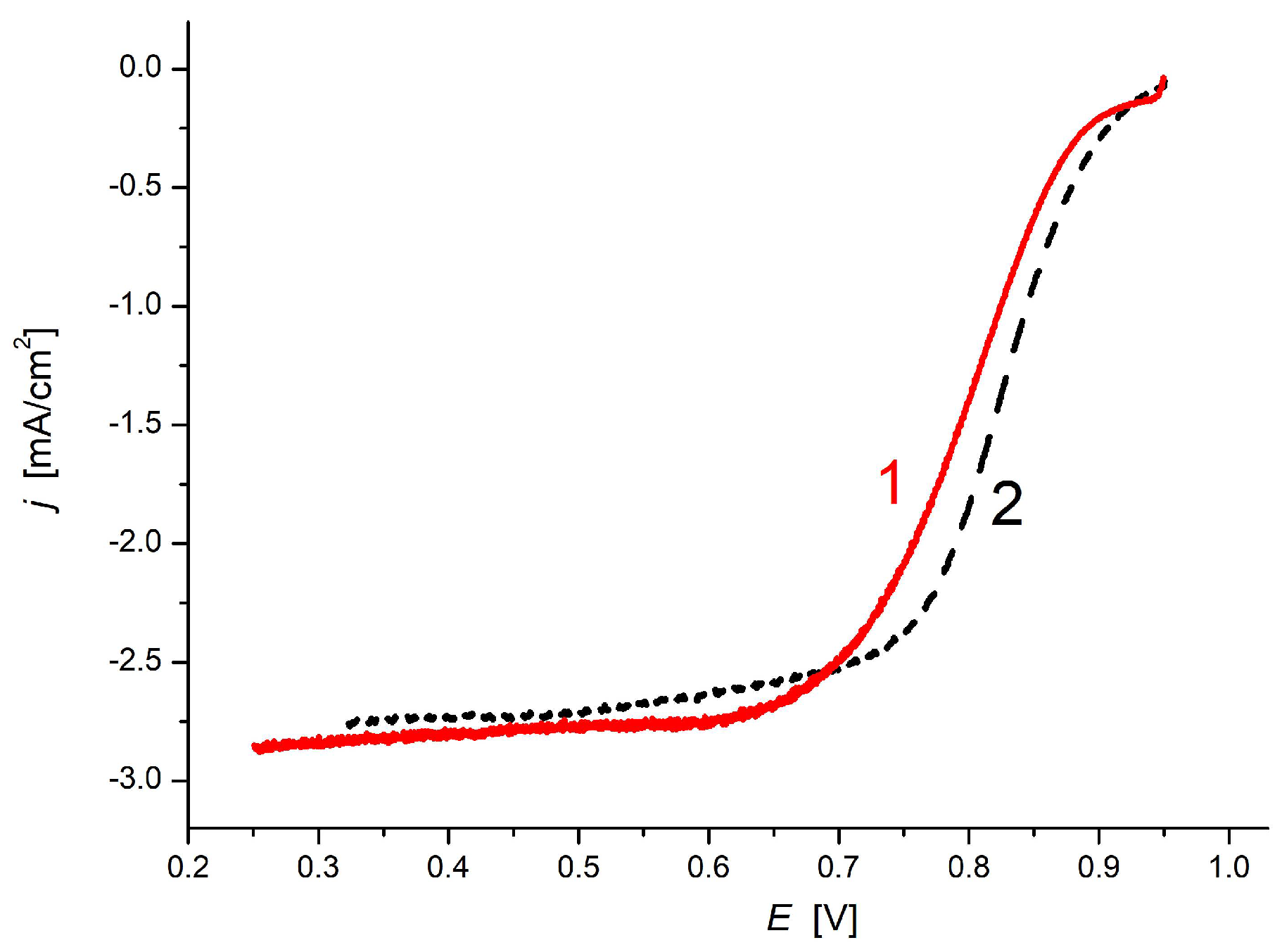
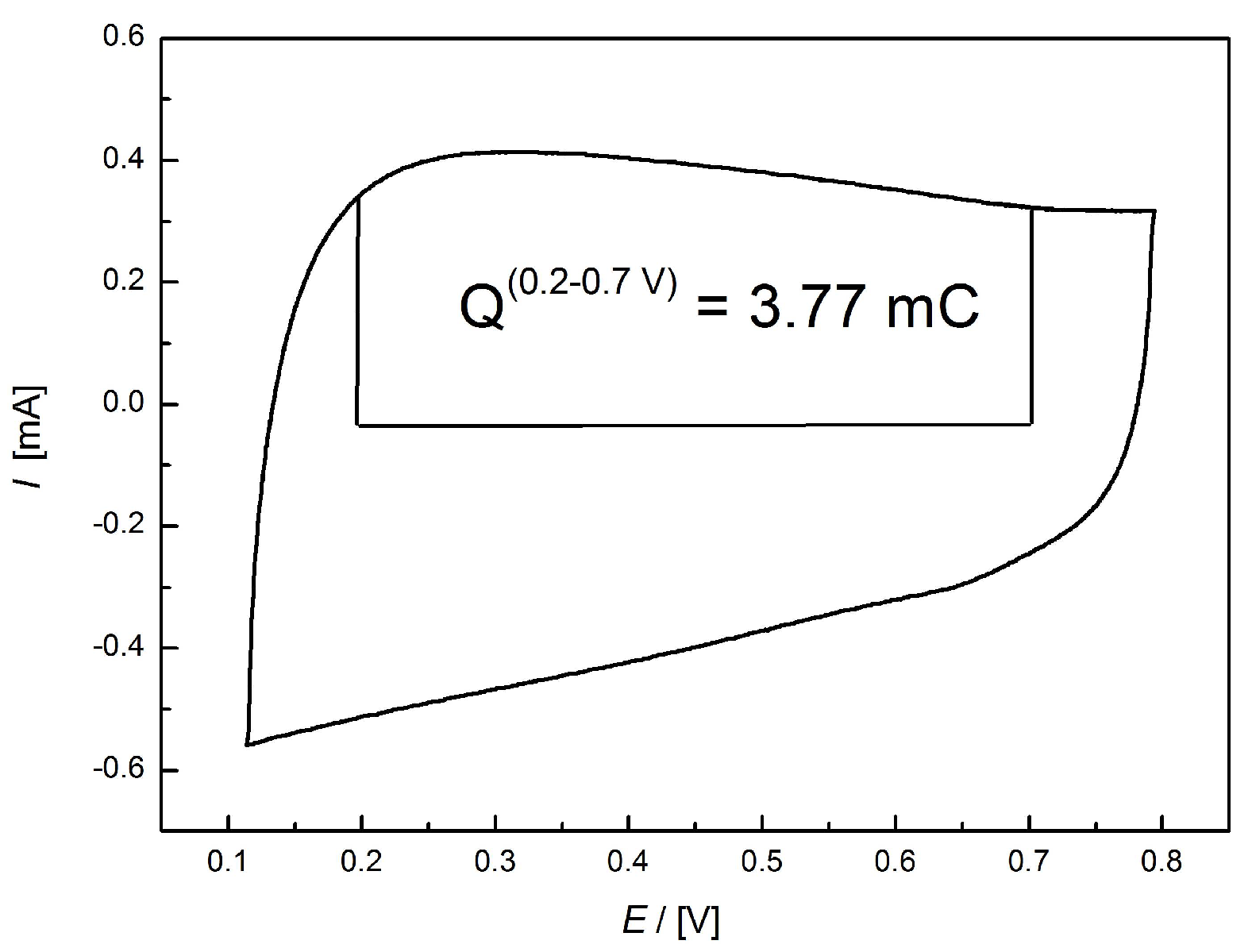
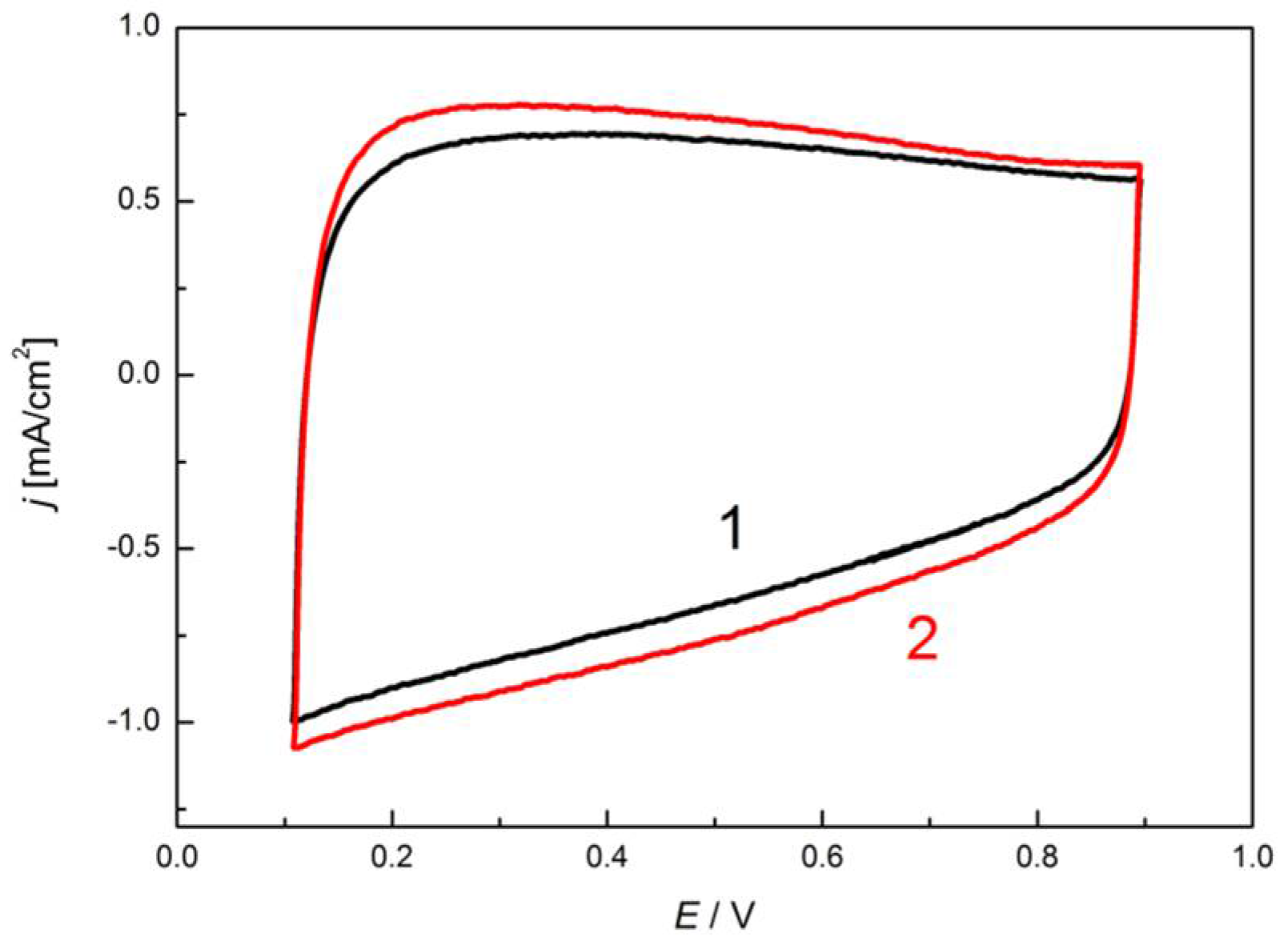
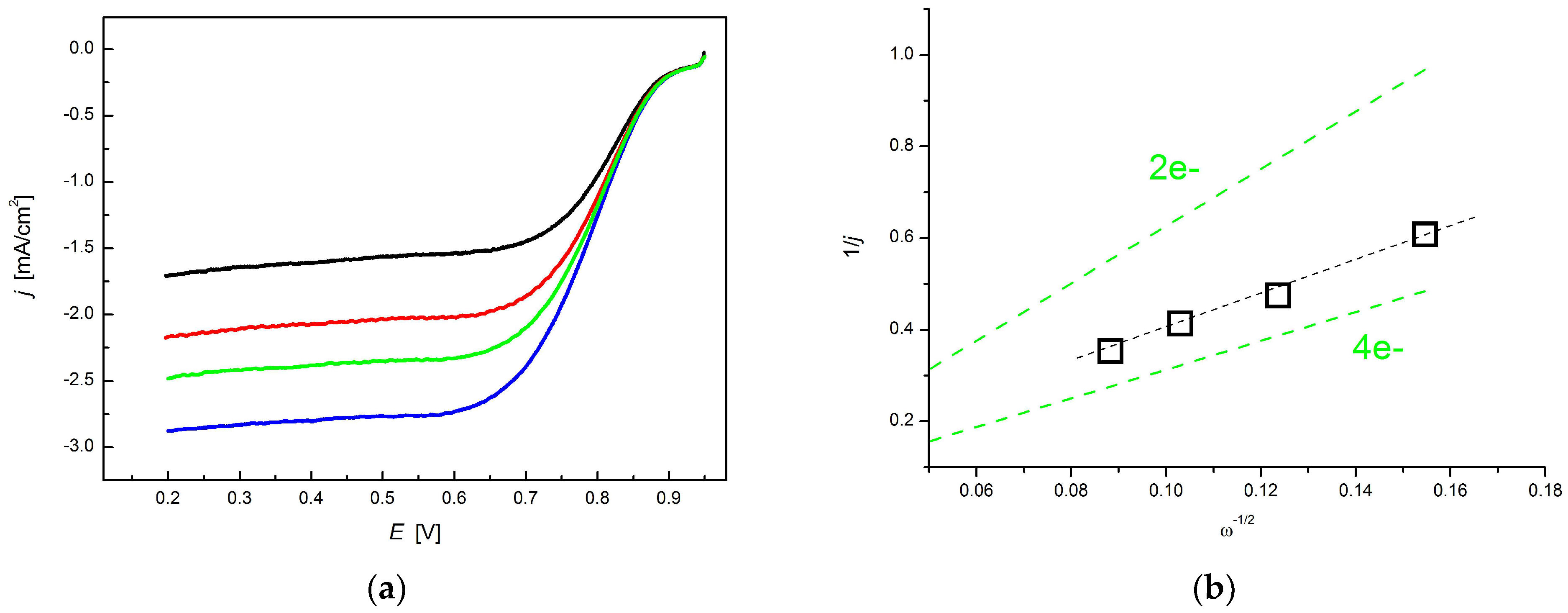
3.3. Electrocatalytic Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar-On, Y.M.; Philips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [Green Version]
- Gambles, R.; Zsuffa, L. The international energy agency–Cooperative research on biomass for energy. In Research in Thermochemical Biomass Conversion, 1st ed.; Bridgwater, A.V., Kuester, J.L., Eds.; Springer: Heidelberg, Germany, 1988. [Google Scholar]
- Research and Markets. Available online: https://www.businesswire.com/news/home/20161124005116/en/Global-Carrageenan-Market-Report-2015-2020 (accessed on 28 April 2021).
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Michel, A.-S.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties of carrageenan gels in presence of various cations. Int. J. Biol. Macromol. 1997, 21, 195–500. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Acetic acid-mediated cellulose-based carbons: Influence of activation conditions on textural features and carbon dioxide uptakes. J. Colloid Interface Sci. 2021, 594, 745–758. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Zhao, L.; Lu, J. Effects of organic and inorganic metal salts on thermogravimetric pyrolysis of biomass components. Korean J. Chem. Eng. 2017, 34, 3077–3084. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Tyc, M.; Kowalska, K.; Kamedulski, P.; Zielinski, W.; Lukaszewicz, J.P. Green algae and gelatine derived nitrogen rich carbon as an outstanding competitor to Pt loaded carbon catalysts. Sci. Rep. 2021, 11, 7084. [Google Scholar] [CrossRef] [PubMed]
- Ubaidullah., M.; Al-Enizi, A.M.; Ahamad, T.F.; Shaikh, S.F.; Al-Abdrabalnabi, M.A.; Samdani, M.S.; Kumar, D.; Alam, M.A.; Khan, M. N-doped mesoporous carbon using waste polyethylene terephthalate bottle-based MOF-5 for high performance supercapacitor. J. Energy Storage 2021, 33, 102125. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; He, F.; Chen, J.; Wang, D.; Shichun, M.; Yang, H.Y. Defect and doping co-engineered non-metal nanocarbon ORR electrocatalyst. Nano-Micro Lett. 2021, 13, 65. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niu, J.; Li, M.; Xia, Z. Catalytic mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells. J. Phys. Chem. C 2014, 118, 3545–3553. [Google Scholar] [CrossRef]
- Seredych, M.; László, K.; Bandosz, T. Sulfur-doped carbon aerogel as a metal-free oxygen reduction catalyst. ChemCatChem 2015, 7, 2924–2931. [Google Scholar] [CrossRef]
- Seredych, M.; Biggs, M.; Bandosz, T. Oxygen reduction on chemically heterogeneous iron-containing nanoporous carbon: The effects of specific surface functionalities. Micropor. Mesopor. Mater. 2016, 221, 137–149. [Google Scholar] [CrossRef]
- Voitko, K.; Tóth, A.; Demianenko, E.; Dobos, G.; Berke, B.; Bakalinska, O.; Grebenyuk, A.; Tombácz, E.; Kuts, V.; Tarasenko, Y.; et al. Catalytic performance of carbon nanotubes in H2O2 decomposition: Experimental and quantum chemical study. J. Colloid Interface Sci. 2015, 437, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Nagy, B.; Bakos, I.; Bertóti, I.; Domán, A.; Menyhárd, A.; Mohai, M.; László, K. Synergism of nitrogen and reduced graphene in the electrocatalytic behavior of resorcinol–Formaldehyde based carbon aerogels. Carbon 2018, 139, 872–879. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, M.; Song, P.; Fu, J.; Jin, Z.; Ma, L.; Ge, J.; Liu, C.; Chen, Z.; Xing, W. Highly polarized carbon nano-architecture as robust metal-free catalyst for oxygen reduction in polymer electrolyte membrane fuel cells. Nano Energy 2018, 49, 23–30. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-doped N-rich carbon nanosheets with expanded interlayer distance as anode materials for sodium-ion batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Luo, Z.; Jiang, J.; Zhu, J.; Du, Z.; Fan, Z.; Xie, L.; Zhang, H.; Huang, W.; Yu, T. Nitrogen and sulfur codoped graphene: Multifunctional electrode materials for high-performance Li-ion batteries and oxygen reduction reaction. Adv. Mater. 2014, 26, 6186–6192. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and Nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Weng, C.C.; Hu, Z.P.; Ge, L.; Yuan, Z.Y. CdS-polydopamine-derived N, S-codoped hierarchically porous carbons as highly active electrocatalyst for oxygen reduction. ACS Sustain. Chem. Eng. 2017, 5, 9914–9922. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, C.; Pan, Q.; Xin, Y.; Xu, F.; Qi, W.; Wei, H.; Yang, S.; Zhou, C.; Hu, N.; et al. High-voltage aqueous asymmetric pseudocapacitors based on methyl blue-doped polyaniline hydrogels and the derived N/S-codoped carbon aerogels. Chem. Eng. J. 2020, 383, 123153. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef]
- De Falco, G.; Florent, M.; Jagiello, J.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Bandosz, T.J. Alternative view of oxygen reduction on porous carbon electrocatalysts: The substance of complex oxygen-surface interactions. iScience 2021, 24, 102216. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, W.B.; Shi, J.S.; Xin, F.W. Influence of doping nitrogen, sulfur, and phosphorus on activated carbons for gas adsorption of H2, CH4 and CO2. RSC Adv. 2016, 6, 50138–50143. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Influence of porous texture and surface chemistry on the CO2 adsorption capacity of porous carbons: Acidic and basic site interactions. ACS Appl. Mater. Interfaces 2014, 6, 21237–21247. [Google Scholar] [CrossRef]
- Wang, D.; Shen, Y.; Chen, Y.; Liu, L.; Zhao, Y. Microwave-assistant preparation of N/S co-doped hierarchical porous carbons for hydrogen adsorption. Chem. Eng. J. 2019, 367, 260–268. [Google Scholar] [CrossRef]
- Li, D.; Jia, Y.; Chang, G.; Chen, J.; Liu, H.; Wang, J.; Hu, Y.; Xia, Y.; Yang, D.; Yao, X. A defect-driven metal-free electrocatalyst for oxygen reduction in acidic electrolyte. Chem 2018, 4, 2345–2356. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. 1947, 55, 327–329. [Google Scholar]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, K.; Rodríguez-Reinoso, F. Nanoporous Materials for Gas Storage, 1st ed.; Springer: Singapore, 2019; p. 403. [Google Scholar]
- Li, H.; Li, L.; Lin, R.B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Worrall, S.D.; Bissett, M.A.; Hill, P.I.; Rooney, A.P.; Haigh, S.J.; Attfield, M.P.; Dryfe, R.A.W. Metal-organic framework templated electrodeposition of functional gold nanostructures. Electrochim. Acta 2016, 222, 361–369. [Google Scholar] [CrossRef]
- Gotthardt, M.A.; Schoch, R.; Wolf, S.; Bauer, M.; Kleist, W. Synthesis and characterization of bimetallic metal-organic framework Cu-Ru-BTC with HKUST-1 structure. Dalton Trans. 2015, 44, 2052–2056. [Google Scholar] [CrossRef] [Green Version]
- Domán, A.; Madarász, J.; Sáfrán, G.; Wang, Y.; László, K. Copper benzene-1,3,5-tricarboxylate (HKUST-1)-graphene oxide pellets for methane adsorption. Microporous Mesoporous Mater. 2021, 316, 110948. [Google Scholar] [CrossRef]
- Barret, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. II. J. Chem. Phys. 1950, 18, 1024–1026. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036. [Google Scholar] [CrossRef]
- Kumari, S.; Verma, E.; Kumar, R.; Upreti, D.; Prakash, B.; Maruyama, T.; Bagchi, V. Micropores within N,S co-doped mesoporous 3D graphene-aerogel enhance the supercapacitive performance. New J. Chem. 2021, 45, 7523–7532. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ubaidullah, M.; Kumar, D. Carbon quantum dots (CQDs)/Ce doped NiO nanocomposite for high performance supercapacitor. Mater. Today Commun. 2021, 27, 10234. [Google Scholar]
- Panja, T.; Bhattacharjya, D.; Yu, J.S. Nitrogen and phosphorus co-doped cubic ordered mesoporous carbon as a supercapacitor electrode material with extraordinary cyclic stability. J. Mater. Chem. A 2015, 3, 18001–18009. [Google Scholar] [CrossRef]
- Xia, K.; Huang, Z.; Zheng, L.; Han, B.; Gao, Q.; Zhou, C.; Wang, H.; Wu, J. Facile and controllable synthesis of N/P co-doped graphene for high-performance supercapacitors. J. Power Sources 2017, 365, 380–388. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, L.; Cao, Y.; Tang, Y.; Li, Y. Gram-scale production of B, N co-doped graphene-like carbon for high performance supercapacitor electrodes. Appl. Surf. Sci. 2018, 435, 937–944. [Google Scholar] [CrossRef]
- Tian, P.; Zang, J.; Jia, S.; Zhang, Y.; Gao, H.; Zhou, S.; Wang, W.; Xu, H.; Wang, Y. Preparation of S/N co-doped graphene through a self-generated high gas pressure for high rate supercapacitor. Appl. Surf. Sci. 2018, 456, 781–788. [Google Scholar] [CrossRef]
- Shang, N.; Gao, S.; Zhang, X.; Zhang, S.; Feng, C.; Wang, Z.; Wang, C. N,P dual-doped hierarchically porous carbon derived from a polyelectrolyte complex as high-performance electrodes for supercapacitors. Energy Technol. 2019, 7, 1800641. [Google Scholar] [CrossRef]
- Akhter, T.; Islam, M.; Faisal, S.N.; Haque, E.; Minett, A.I.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Self-assembled N/S codoped flexible graphene paper for high performance energy storage and oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 2078–2087. [Google Scholar] [CrossRef]
- Miao, Y.; Ma, Y.; Wang, Q. Plasma-assisted simultaneous reduction and nitrogen/sulfur codoping of graphene oxide for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 7597–7608. [Google Scholar] [CrossRef]
- Kong, W.; Zhu, J.; Zhang, M.; Liu, Y.; Hu, J. Three-dimensional N- and S- codoped graphene hydrogel with in-plane pores for high performance supercapacitor. Microporous Mesoporous Mater. 2018, 268, 260–267. [Google Scholar] [CrossRef]
- Liu, M.; Huo, S.; Xu, M.; Wu, L.; Liu, M.; Xue, Y.; Yan, Y.M. Structural engineering of N/S co-doped carbon material as high-performance electrode for supercapacitors. Electrochim. Acta 2018, 274, 389–399. [Google Scholar] [CrossRef]
- Sun, L.; Yao, Y.; Zhou, Y.; Li, L.; Zhou, H.; Guo, M.; Liu, S.; Feng, C.; Qi, Z.; Gao, B. Solvent-free synthesis of N/S-codoped hierarchically porous carbon materials from protic ionic liquids for temperature-resistant, flexible supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 13494–13503. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Song, A.; Gao, L.; Su, L.; Shao, G. Supercapacitance of nitrogen-sulfur-oxygen co-doped 3D hierarchical porous carbon in aqueous and organic electrolyte. J. Power Sources 2017, 359, 556–567. [Google Scholar] [CrossRef]
- Wan, L.; Li, N.; Li, X.; Chen, J.; Zhang, Y.; Xie, M.; Du, C. One-step synthesis of N, S-codoped porous graphitic carbon derived from lotus leaves for high-performance supercapacitors. Ionics 2019, 25, 4891–4903. [Google Scholar] [CrossRef]
- Guo, D.; Ding, B.; Hu, X.; Wang, Y.; Han, F.; Wu, X. Synthesis of Boron and Nitrogen Codoped Porous Carbon Foam for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11441–11449. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Lv, S.; Zhou, Q.; Shen, X.; Mo, S.; Tong, H. Scalable one-step synthesis of N,S co-doped graphene-enhanced hierarchical porous carbon foam for high-performance solid-state supercapacitors. J. Mater. Chem. A 2019, 7, 7591–7603. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Xia, M.; Rehman, S.; Mu, S.; Kou, Z.; Zhang, Z.; Chen, Z.; Gao, F.; Hou, Y. N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted “cutting-thin” technique: A universal synthesis and multifunctional applications. Nano Energy 2016, 28, 346–355. [Google Scholar] [CrossRef]
- Lu, H.; Yang, C.; Chen, J.; Li, J.; Jin, H.; Wang, J.; Wang, S. Tailoring hierarchically porous nitrogen-, sulfur-, codoped carbon for high-performance supercapacitors and oxygen reduction. Small 2020, 16, 1906584. [Google Scholar] [CrossRef]
- Wadekar, P.H.; Khose, R.V.; Pethsangave, D.A.; Some, S. One-pot synthesis of sulfur and nitrogen co-functionalized graphene material using deep eutectic solvents for supercapacitors. ChemSusChem 2019, 12, 3326–3335. [Google Scholar] [CrossRef]
- Zhou, J.; Li, N.; Gao, F.; Zhao, Y.; Hou, L.; Xu, Z. Vertically-aligned BCN Nanotube Arrays with Superior Performance in Electrochemical capacitors. Sci. Rep. 2014, 4, 6083. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, L.X.; Wu, D.L.; Xia, W.; Jia, D.Z. Interaction between nitrogen and sulfur in co-doped graphene and synergetic effect in supercapacitor. Sci. Rep. 2015, 5, 9591. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-performance Platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 2018, 8, 3591. [Google Scholar] [CrossRef] [PubMed]
- Khotseng, L. Oxygen reduction reaction. In Electrocatalysts for Fuel Cells and Hydrogen Evolution–Theory to Design, 1st ed.; Ray, A., Mukhopadhyay, I., Pati, R.K., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef] [Green Version]

| Sample | SBET | V0.98 | Vmicro | |
|---|---|---|---|---|
| m2/g | cm3/g | % | ||
| CR | 272 | 0.23 | 0.10 | 42 |
| CW | 871 | 0.70 | 0.32 | 46 |
| CA | 1070 | 0.83 | 0.40 | 48 |
| Sample | C | O | N | S | K | Ca | Na |
|---|---|---|---|---|---|---|---|
| CR | 67.2 | 20.5 | 5.9 | 0.5 | 2.1 | 3.2 | 0.6 |
| CW | 81.9 | 6.9 | 9.3 | 2.0 | n.d. * | n.d. * | n.d. * |
| CA | 89.4 | 4.6 | 5.0 | 1.0 | n.d. * | n.d. * | n.d. * |
| Sample | Gas | Henry | Langmuir–Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| KH | R | nsat | K | m | R | |||
| CA | CO2 | 0.463 | 0.9974 | 10.5 | 12.5 | 0.00424 | 0.660 | 0.99987 |
| CH4 | 0.044 | 0.9963 | 2.75 | 0.00088 | 0.963 | 0.99953 | ||
| HKUST-1 | CO2 | 0.232 | 0.9998 | 5.7 | 8.30 | 0.00485 | 0.799 | 0.99999 |
| CH4 | 0.041 | 0.9984 | 7.01 | 0.00046 | 0.915 | 0.99997 | ||
| Electrode Material | Cg | Reference |
|---|---|---|
| F/g | ||
| P/N co-doped ordered mesoporous carbon | 210 | [47] |
| N, P co-doped graphene | 219 | [48] |
| B, N co-doped graphene | 225 | [49] |
| N, S co-doped graphene | 264.3 | [50] |
| N, P dual-doped hierarchically porous carbon | 289 | [51] |
| N, S co-doped flexible graphene paper | 305 | [52] |
| N, S co-doped graphene oxide | 307 | [53] |
| 3D N, S co-doped graphene hydrogel | 320 | [54] |
| N, S co-doped carbon | 322 | [55] |
| N, S co-doped porous carbon from ionic liquid precursor | 347 | [56] |
| N, S co-doped templated porous carbon | 367 | [57] |
| N, S co-doped biomass-based carbon aerogel (CA) | 377 | This work |
| N,S co-doped porous graphitic carbon from lotus leaves | 385 | [58] |
| B, N co-doped porous carbon foam | 402 | [59] |
| N, S co-doped graphene-enhanced hierarchical porous carbon foam | 405 | [60] |
| N, P co-doped high performance 3D graphene | 413 | [61] |
| N, S co-doped carbon (based on polymer from N and S containing aromatic precursors) | 461.5 | [62] |
| N, S co-doped graphene material | 503 | [63] |
| Vertically-aligned BC2N nanotube arrays | 547 | [64] |
| N, S co-doped graphene | 566 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, S.K.S.; Bakos, I.; Dobos, G.; Farkas, A.; Kiss, G.; Klébert, S.; Madarász, J.; László, K. Biomass Related Highly Porous Metal Free Carbon for Gas Storage and Electrocatalytic Applications. Materials 2021, 14, 3488. https://doi.org/10.3390/ma14133488
Andrade SKS, Bakos I, Dobos G, Farkas A, Kiss G, Klébert S, Madarász J, László K. Biomass Related Highly Porous Metal Free Carbon for Gas Storage and Electrocatalytic Applications. Materials. 2021; 14(13):3488. https://doi.org/10.3390/ma14133488
Chicago/Turabian StyleAndrade, Samantha K. Samaniego, István Bakos, Gábor Dobos, Attila Farkas, Gábor Kiss, Szilvia Klébert, János Madarász, and Krisztina László. 2021. "Biomass Related Highly Porous Metal Free Carbon for Gas Storage and Electrocatalytic Applications" Materials 14, no. 13: 3488. https://doi.org/10.3390/ma14133488
APA StyleAndrade, S. K. S., Bakos, I., Dobos, G., Farkas, A., Kiss, G., Klébert, S., Madarász, J., & László, K. (2021). Biomass Related Highly Porous Metal Free Carbon for Gas Storage and Electrocatalytic Applications. Materials, 14(13), 3488. https://doi.org/10.3390/ma14133488







