In Vitro Ion Release of Wires in Removable Orthodontic Appliances
Abstract
1. Introduction
2. Materials and Methods
- Does the colour of the orthodontic resin influence the ion release?
- Does the presence of glitter particles influence the ion release?
- Does the use of cleaning tablets lower the ion release?
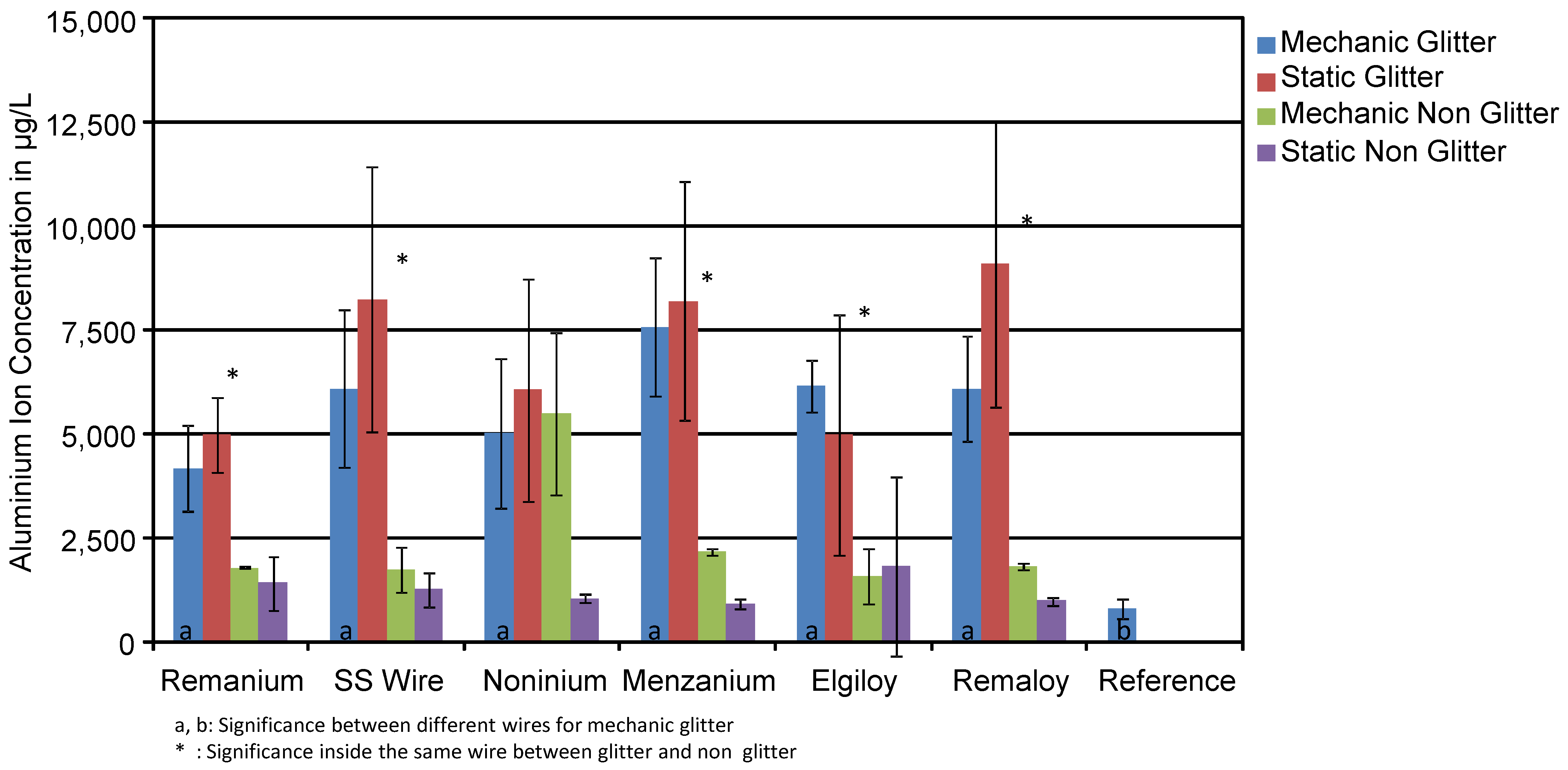
- Does the mechanical loading influence the ion release?
3. Results
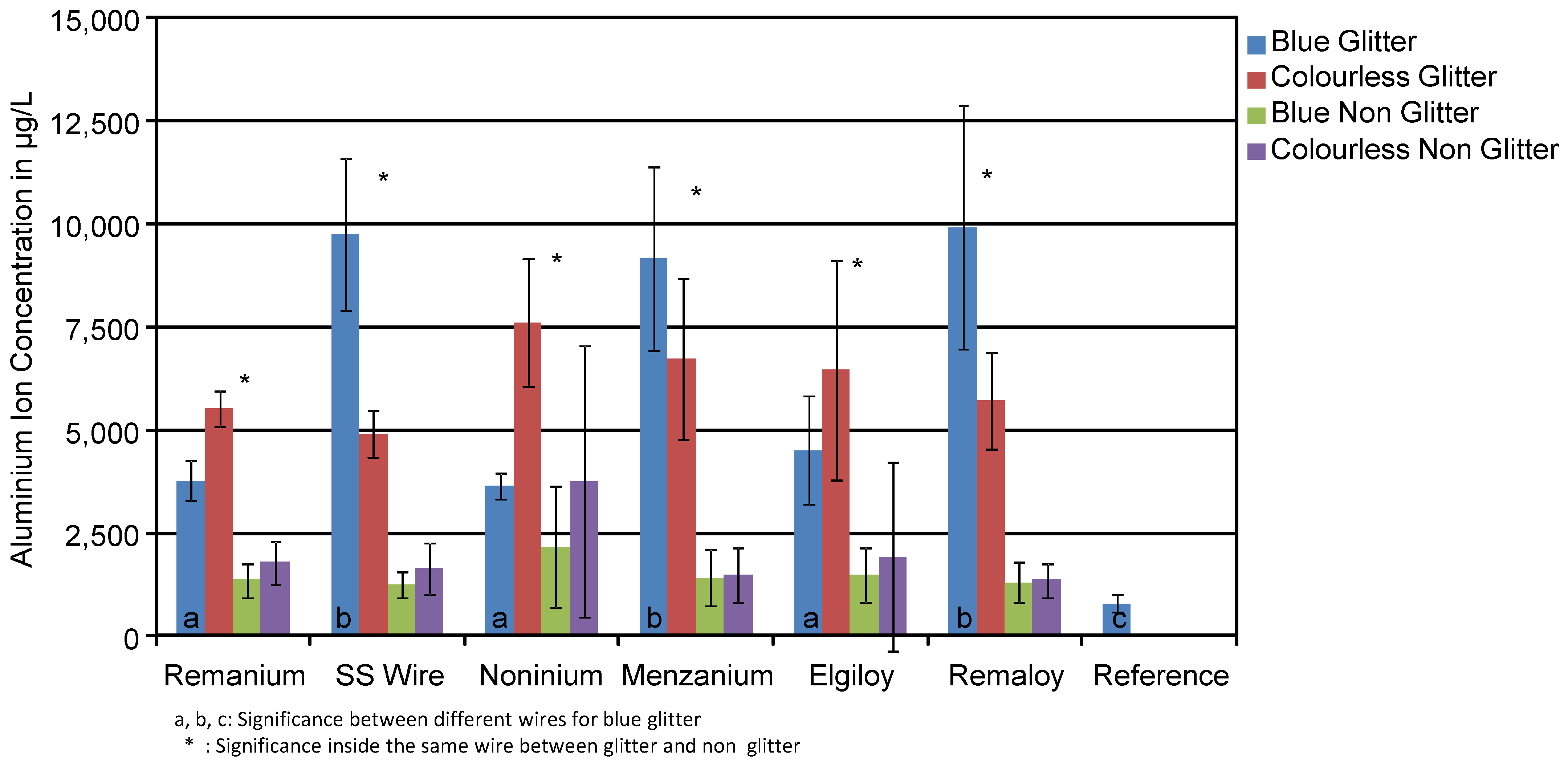
3.1. Aluminium
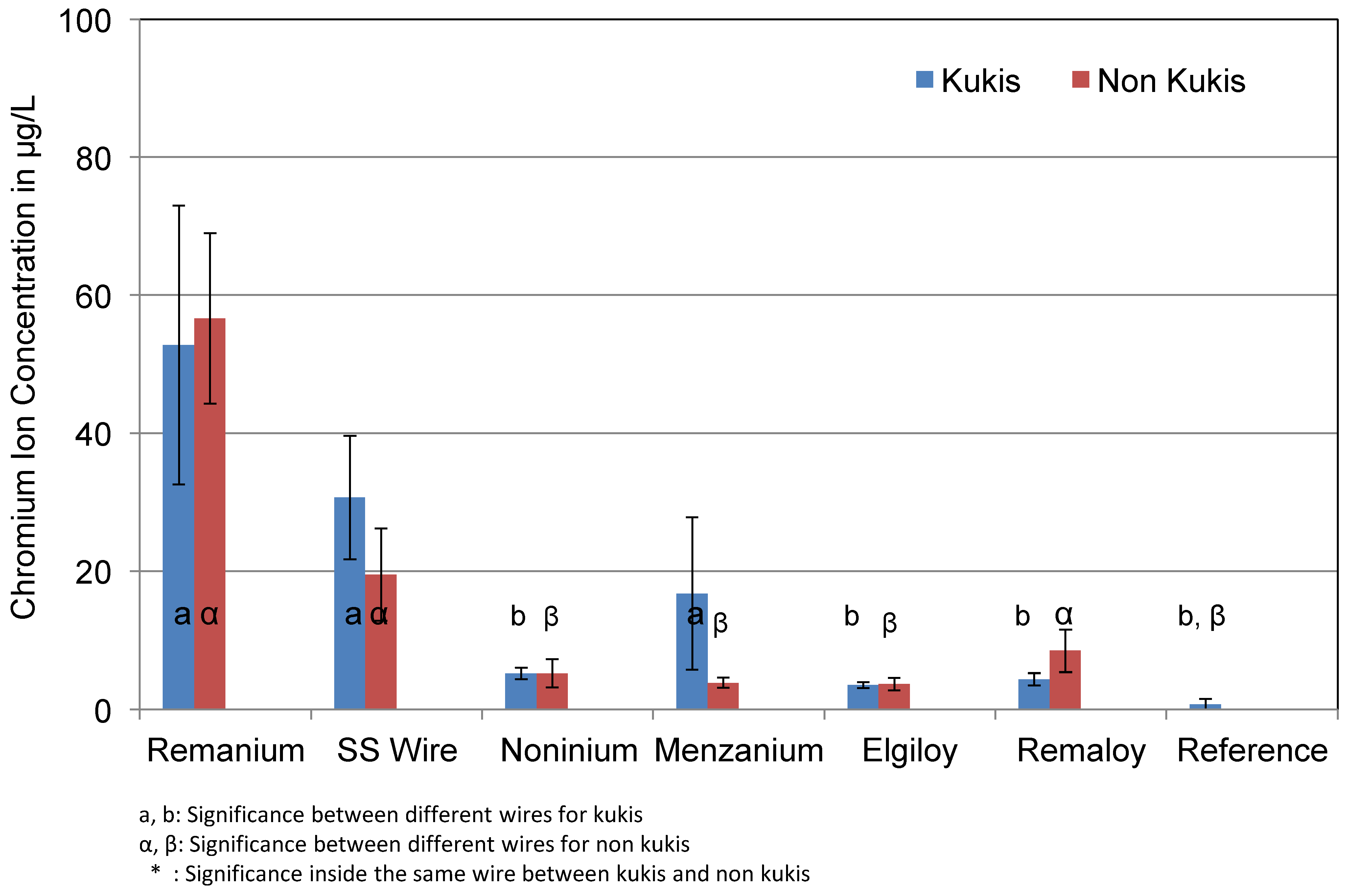
3.2. Chromium
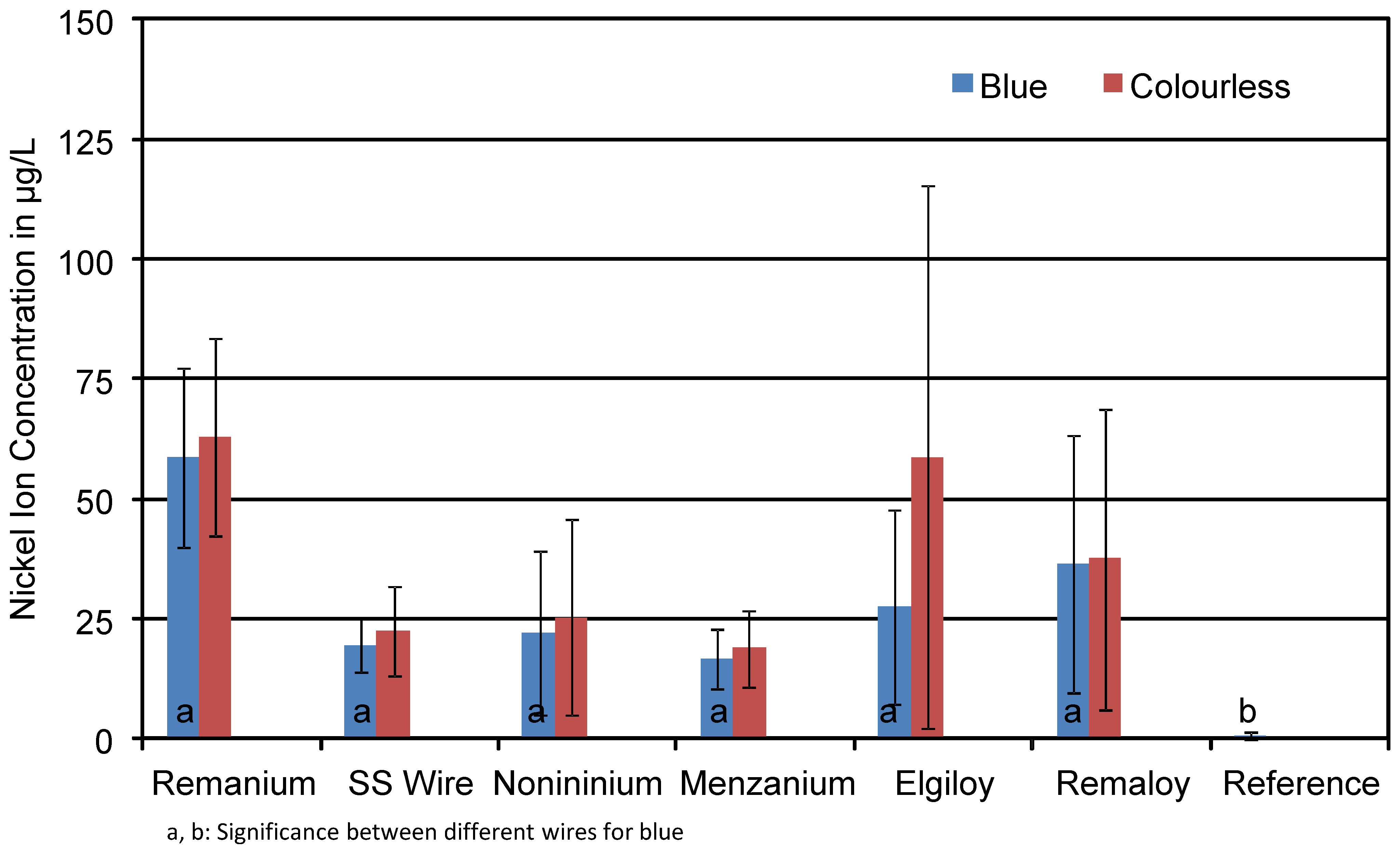
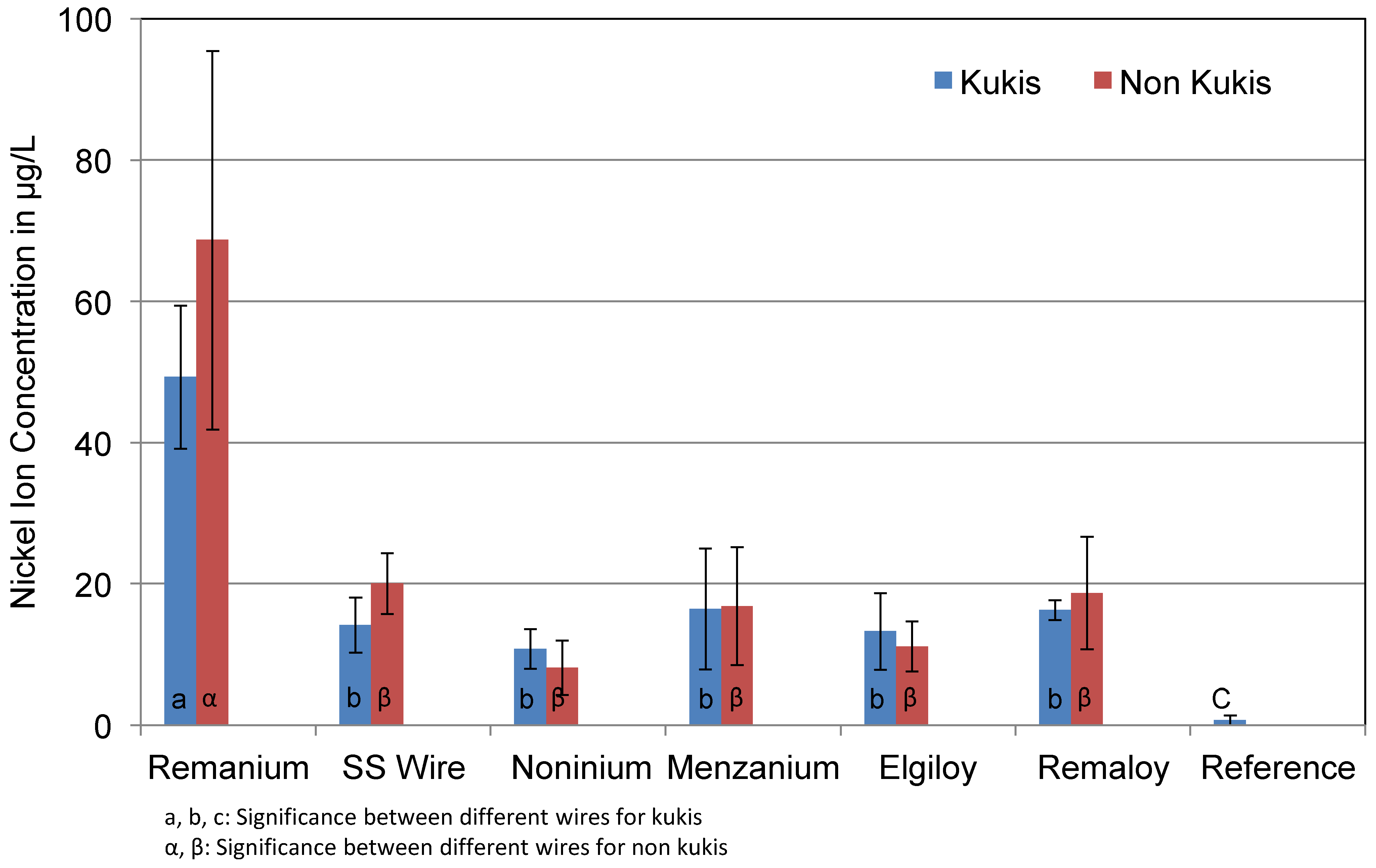
3.3. Nickel
4. Discussion
4.1. Materials and Methods
4.2. Aluminium
4.3. Chromium
4.4. Nickel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imani, M.M.; Mozaffari, H.R.; Ramezani, M.; Sadeghi, M. Effect of fixed orthodontic treatment on salivary nickel and chromium levels: A systematic review and meta-analysis of observational studies. Dent. J. 2019, 7, 21. [Google Scholar] [CrossRef]
- Mirhashemi, A.; Jahangiri, S.; Kharrazifard, M. Release of nickel and chromium ions from orthodontic wires following the use of teeth whitening mouthwashes. Prog. Orthod. 2018, 19, 4. [Google Scholar] [CrossRef]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.; Yang, H.; Su, K.; Hsu, C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Huang, H.H. Surface characterizations and corrosion resistance of nickel–titanium orthodontic archwires in artificial saliva of various degrees of acidity. J. Biomed. Mater. Res. A 2005, 74, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Lei, W.; Yajun, C.; Lei, L.; Yue, H.; Zhongtao, D. Corrosion behavior of titanium in artificial saliva by lactic acid. Materials 2014, 7, 5528–5542. [Google Scholar] [CrossRef]
- Koike, M.; Nakamura, S.; Fujii, H. In vitro assessment of release from titanium by immersion tests. J. Jpn. Prosthodont. Soc. 1997, 41, 675–679. [Google Scholar] [CrossRef]
- Bessho, K.; Hujimura, K.; Iizuka, T. Experimental long-term study of titanium ions eluted from pure titanium miniplates. J. Biomed. Mater. Res. 1995, 29, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, N.J. Biocompatibility of nickel and cobalt dental alloys. Gen. Dent. 2001, 49, 498–503. [Google Scholar]
- Gölz, L.; Bayer, S.; Keilig, L.; Jäger, A.; Stark, H.; Bourauel, C.; Götz, W.; Frede, S.; Winter, J.; Kraus, D. Possible implications of Ni (II) on oral IL-1β-induced inflammatory processes. Dent. Mater. 2014, 30, 1325–1335. [Google Scholar] [CrossRef]
- Bhasin, V.; Pustake, S.J.; Joshi, V.; Tiwari, A.; Bhasin, M.; Punia, R.S.; Patil, S. Assessment of Changes in Nickel and Chromium Levels in the Gingival Crevicular Fluid during Fixed Orthodontic Treatment. J. Contemp. Dent. Pr. 2017, 18, 675–678. [Google Scholar] [CrossRef]
- Downarowicz, P.; Mikulewicz, M. Trace metal ions release from fixed orthodontic appliances and DNA damage in oral mucosa cells by in vivo studies: A literature review. Adv. Clin. Exp. Med. 2017, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Quadras, D.D.; Nayak, U.S.K.; Kumari, N.S.; Priyadarshini, H.R.; Gowda, S.; Fernandes, B. In vivo study on the release of nickel, chromium, and zinc in saliva and serum from patients treated with fixed orthodontic appliances. Dent. Res. J. 2019, 16, 209–215. [Google Scholar] [CrossRef]
- Fitjer, L.C.; Jonas, I.E.; Kappert, H.F. Corrosion susceptibility of lingual wire extensions in removable appliances. An in vitro study. J. Orofac. Orthop. 2002, 63, 212–226. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, L. Quantification of metal ion induced DNA damage with single cell array based assay. Analyst 2013, 19, 5505–5840. [Google Scholar] [CrossRef] [PubMed]
- Maya, S.; Prakash, T.; Madhu, K.D.; Goli, D. Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Wendl, B.; Wiltsche, H.; Lankmayr, E.; Winsauer, H.; Walter, A.; Muchitsch, A.; Jakse, N.; Wendl, M.; Wendl, T. Metal release profiles of orthodontic bands, brackets, and wires: An in vitro study. J. Orofac. Orthop. 2017, 78, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, N. Korrosionsuntersuchungen und Metallionen analysen des Korrosionsmediums nach einem ISO-Normenentwurf für kieferorthopädische Brackets. Dr. med. dent. Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Deutschland, 2019. Online-Ausgabe in Bonndoc. Available online: https://nbn-resolving.org/urn:nbn:de:hbz:5n-55125 (accessed on 17 June 2021).
- Fusayama, T.; Katayori, T.; Nomoto, S. Corrosion of gold and amalgam placed in contact with each other. J. Dent. Res. 1963, 42, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Bumann, J.; Jonas, I.E.; Kappert, H.F. Contribution to the biological assessment of orthodontic acrylic materials. Measurement of their residual monomer output and cytotoxicity. J. Orofac. Orthop. 2000, 61, 246–257. [Google Scholar] [CrossRef]
- Segal, N.; Hell, J.; Berzins, D.W. Influence of stress and phase on corrosion of a super elastic nickel-titanium orthodontic wire. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 764–770. [Google Scholar] [CrossRef]
- Watt, B.E.; Proudfoot, A.T.; Vale, J.A. Hydrogen peroxide poisoning. Toxicol. Rev. 2004, 23, 51–57. [Google Scholar] [CrossRef]
- Backenstose, W.M.; Wells, J.G. Side effects of immersion-type cleansers on the metal components of dentures. J. Prosthet. Dent. 1977, 37, 615–621. [Google Scholar] [CrossRef]
- Pitner, P.; Rossiwall, B. Untersuchungen über die Korrosion von abnehmbaren kieferorthopädischen Apparaturen durch selbsttätige Reinigungsmittel. J. Orofac. Orthop. 1975, 36, 570–582. [Google Scholar] [CrossRef]
- Heidemann, J.; Witt, E.; Feeg, M.; Werz, R.; Pieger, K. Orthodontic soldering techniques: Aspects of quality assurance in the dental laboratory. J. Orofac. Orthop. 2002, 63, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cameán, A.; Jos, A.; Puerto, M.; Calleja, A.; Iglesias-Linares, A.; Solano, E.; Cameán, A.M. In vivo determination of aluminium, cobalt, chromium, copper, nickel, titanium and vanadium in oral mucosa cells from orthodontic patients with mini-implants by Inductively coupled plasma-mass spectrometry (ICP-MS). J. Trace Elem. Med. Biol. 2015, 32, 13–20. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, 6268. [Google Scholar] [CrossRef]
- Baričević, M.; Ratkaj, I.; Mladinić, M.; Zelježić, D.; Kraljević, S.P.; Lončar, B.; Stipetić, M.M. In vivo assessment of DNA damage induced in oral mucosa cells by fixed and removable metal prosthodontic appliances. Clin. Oral Investig. 2012, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tietz, T.; Lenzner, A.; Kolbaum, A.E.; Zellmer, S.; Riebeling, C.; Gürtler, R.; Jung, C.; Kappenstein, O.; Tentschert, J.; Giulbudagian, M.; et al. Aggregated aluminium exposure: Risk assessment for the general population. Arch. Toxicol. 2019, 93, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Berni Canani, R.; Fairweather-Tait, S. Scientific opinion on dietary reference values for chromium. EFSA J. 2014, 12, 3595. [Google Scholar] [CrossRef]
- Bącela, J.; Łabowska, M.B.; Detyna, J.; Zięty, A.; Michalak, I. Functional coatings for orthodontic arch wires—A Review. Materials 2020, 13, 3257. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Shariati, M.; Sobouti, F.; Rakhshan, V. Effects of fixed orthodontic treatment on nickel and chromium levels in gingival crevicular fluid as a novel systemic biomarker of trace elements: A longitudinal study. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 666–672. [Google Scholar] [CrossRef]
- Kuhta, M.; Pavlin, D.; Slaj, M.; Varga, S.; Lapter-Varga, M.; Slaj, M. Type of arch wire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009, 79, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Faccioni, F.; Franceschetti, P.; Cerpelloni, M.; Fracasso, M.E. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 687–693, discussion 693–694. [Google Scholar] [CrossRef] [PubMed]
- Mikulewicz, M.; Wołowiec, P.; Janeczek, M.; Gedrange, T.; Chojnacka, K. The release of metal ions from orthodontic appliances in animal tests. Angle Orthod. 2014, 84, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Karnam, S.K.; Reddy, A.N.; Manjith, C.M. Comparison of metal ion release from different bracket arch wire combinations: An in vitro study. J. Contemp. Dent. Pract. 2012, 13, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishnan, S.; Melath, A.; Ajith, V.V.; Mathews, N.B. A comparative study of bio degradation of various orthodontic arch wires: An in vitro study. J. Int. Oral Health 2015, 7, 12–17. [Google Scholar] [PubMed]





| Manufacturer | Alloy | Composition in% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Mo | Ni | P | S | Fe | Co | ||
| Dentaurum (Ispringen, Germany) | Remanium® | 0.05–0.15 | <2 | <2 | 16–19 | <0.8 | 6.0–9.5 | <1 | <1 | rest | - |
| American Orthodontics (Sheboygen, WI, USA) | SS Wire | 0.0–1.2 | 0–2 | 0–2 | 11.5–20 | 0.0–6.5 | 0–15 | / | / | rest | - |
| Dentaurum (Ispringen, Germany) | Noninium® | <0.1 | <1 | 16–20 | 16–20 | 1.8–2.5 | <0.2 | – | 0.05 | rest | - |
| Scheu Dental (Iserlohn) | Menzanium® | 0.1 | 1 | 16–20 | 16–20 | 1.6–2.5 | 0.2 | 0.05 | 0.05 | rest | - |
| Rocky Mountain Orthodontics® (Denver, CO, USA) | Elgiloy® | 0.15 | – | 1.5–2.5 | 19–21 | 7 | 14–16 | – | – | rest | 39–41 |
| Dentaurum (Ispringen, Germany) | Remaloy® | 0.03 | <0.5 | <0.1 | 18–22 | 3–5 | 19–23 | – | <0.1 | 4–6 | rest |
| Kukis® braces cleaner ingredients | Sodium Sulfate, Sodium Bicarbonate, Sodium Carbonate, Citric Acid, Malic Acid, Potassium Caroate, Sodium Carbonate Peroxide, PEG-150, Sulfamic Acid, Maltodextrin, TAED, PEG-90, Aroma, Sodium Chloride, Sodium Dodecylbenzensulfonate, Sodium Starch Ocetylsuccinate, Cellulose Gum, Methenamine, Cetylpyridinum Chloride, Aqua, CI 73015 |
| Wire Name | Analysed Element | Mean Concentration of Glitter-Containing Test Specimens in μg/L | Mean Concentration of Glitter-Free Test Specimens in μg/L |
|---|---|---|---|
| Remanium® | Aluminium | 4638 (1007) * | 1565 (504) |
| SS Wire | 7322 (2835) * | 1456 (514) | |
| Noninium® | 5613 (2322) * | 2957 (2593) | |
| Menzanium® | 7934 (2376) * | 1454 (646) | |
| Elgiloy® | 5484 (2250) * | 1706 (1630) | |
| Remaloy® | 7814 (3067) * | 129 (436) | |
| Reference | 770 (263) |
| Wire Name | Analysed Element | Mean Concentration of Glitter-Containing Test Specimens in μg/L | Mean Concentration of Glitter-Free Test Specimens in μg/L |
|---|---|---|---|
| Remanium® | Chromium | 65.3 (12.8) | 56.3 (24.0) |
| SS Wire | 19.9 (3.6) | 22.0 (10.2) | |
| Noninium® | 20.9 (13.9) | 26.4 (22.56) | |
| Menzanium® | 18.4 (7.2) | 16.9 (7.1) | |
| Elgiloy® | 40.4 (37.5) | 45.8 (52.1) | |
| Remaloy® | 35.0 (27.6) | 39.2 (30.4) | |
| Reference | 0.7 (0.7) |
| Wire Name | Analysed Element | Mean Concentration of Glitter-Containing Test Specimens in μg/L | Mean Concentration of Glitter-Free Test Specimens in μg/L |
|---|---|---|---|
| Remanium® | Nickel | 85.7 (45.2) | 68.5 (22.1) |
| SS Wire | 36.1 (10.2) | 29.5 (14.6) | |
| Noninium® | 20.3 (21.3) | 29.0 (28.3) | |
| Menzanium® | 24.4 (22.8) | 30.2 (38.6) | |
| Elgiloy® | 48.7 (58.2) | 52.2 (74.0) | |
| Remaloy® | 24.5 (24.6) | 27.7 (32.0) | |
| Reference | 0.8 (0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wepner, L.; Färber, H.A.; Jaensch, A.; Weber, A.; Heuser, F.; Keilig, L.; Singer, L.; Bourauel, C.P. In Vitro Ion Release of Wires in Removable Orthodontic Appliances. Materials 2021, 14, 3402. https://doi.org/10.3390/ma14123402
Wepner L, Färber HA, Jaensch A, Weber A, Heuser F, Keilig L, Singer L, Bourauel CP. In Vitro Ion Release of Wires in Removable Orthodontic Appliances. Materials. 2021; 14(12):3402. https://doi.org/10.3390/ma14123402
Chicago/Turabian StyleWepner, Lena, Harald Andreas Färber, Andreas Jaensch, Anna Weber, Florian Heuser, Ludger Keilig, Lamia Singer, and Christoph Peter Bourauel. 2021. "In Vitro Ion Release of Wires in Removable Orthodontic Appliances" Materials 14, no. 12: 3402. https://doi.org/10.3390/ma14123402
APA StyleWepner, L., Färber, H. A., Jaensch, A., Weber, A., Heuser, F., Keilig, L., Singer, L., & Bourauel, C. P. (2021). In Vitro Ion Release of Wires in Removable Orthodontic Appliances. Materials, 14(12), 3402. https://doi.org/10.3390/ma14123402






