Modelling the Leachability of Strontium and Barium from Stone Building Materials
Abstract
1. Introduction
1.1. The Geochemical and Toxicological Characteristics of Strontium (Sr) and Barium(Ba)
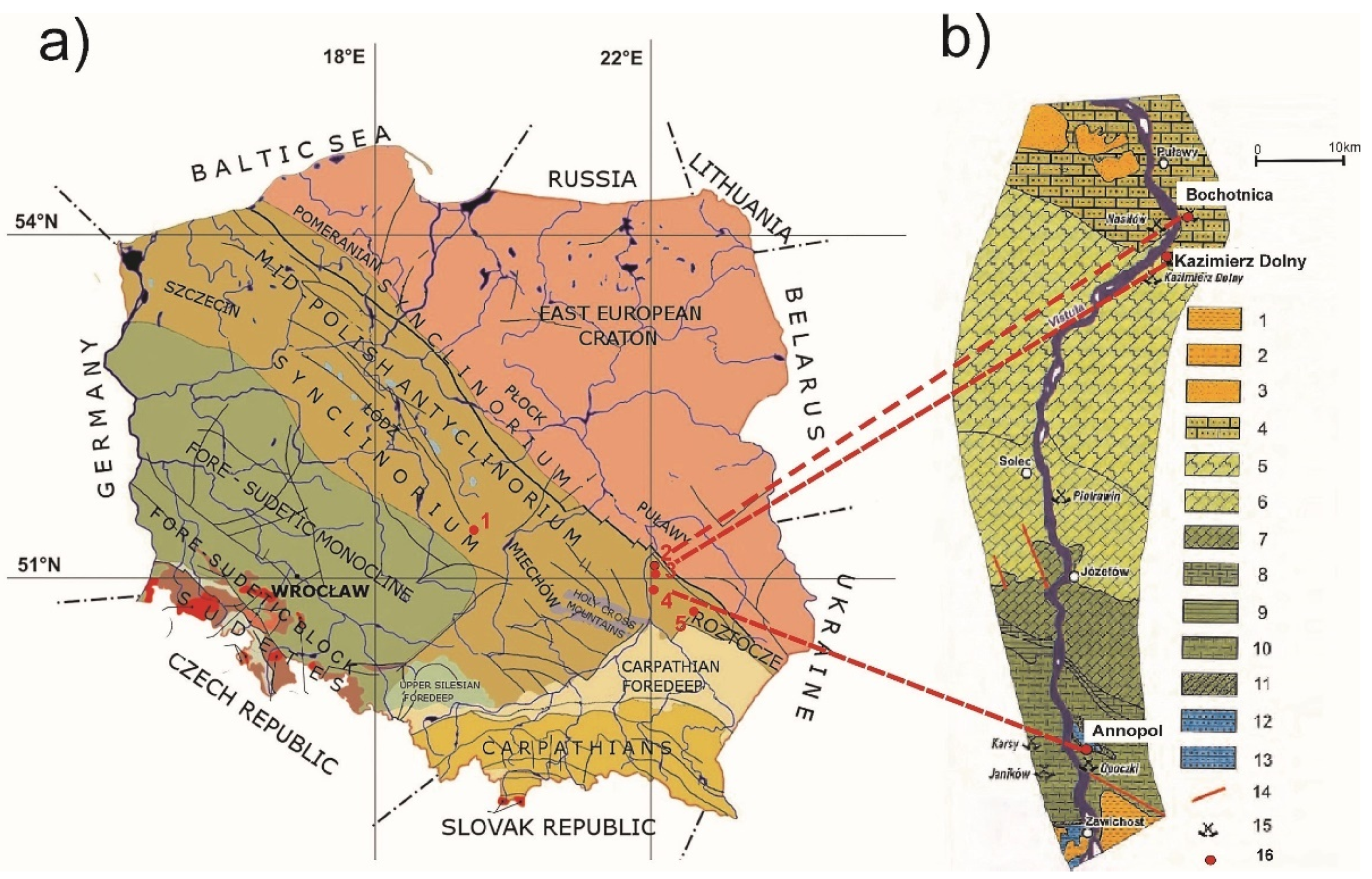
1.2. Geological Setting
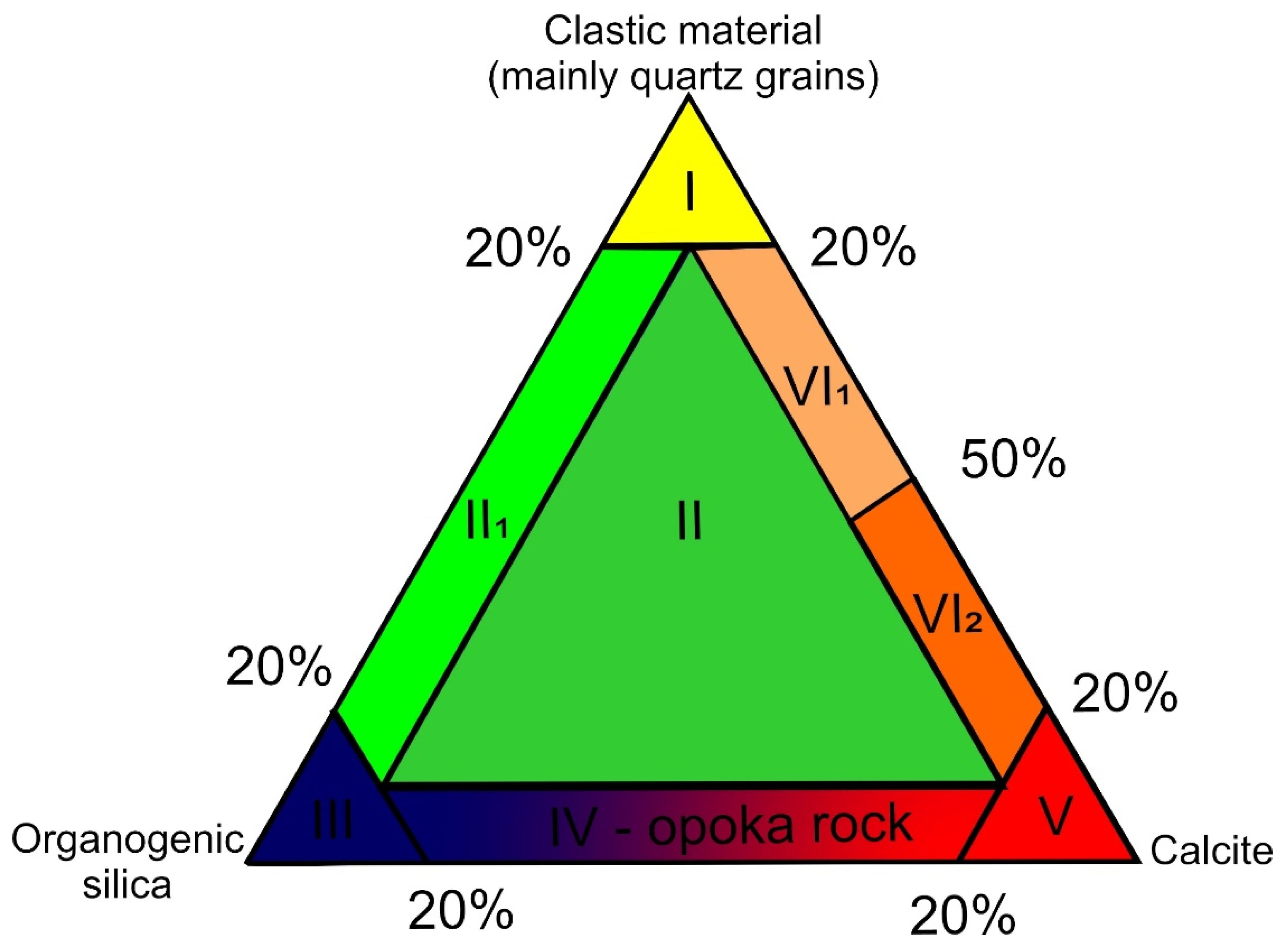
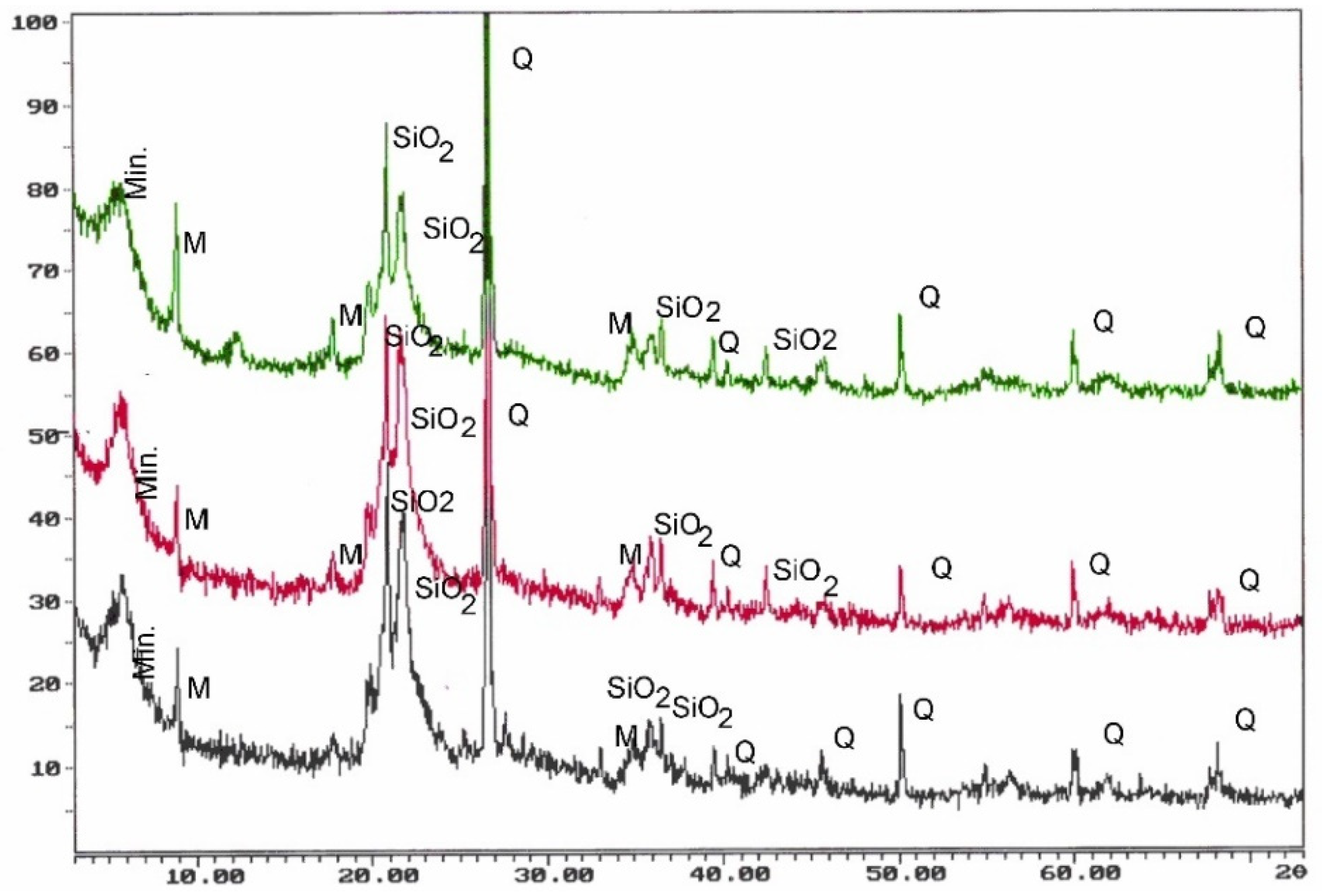
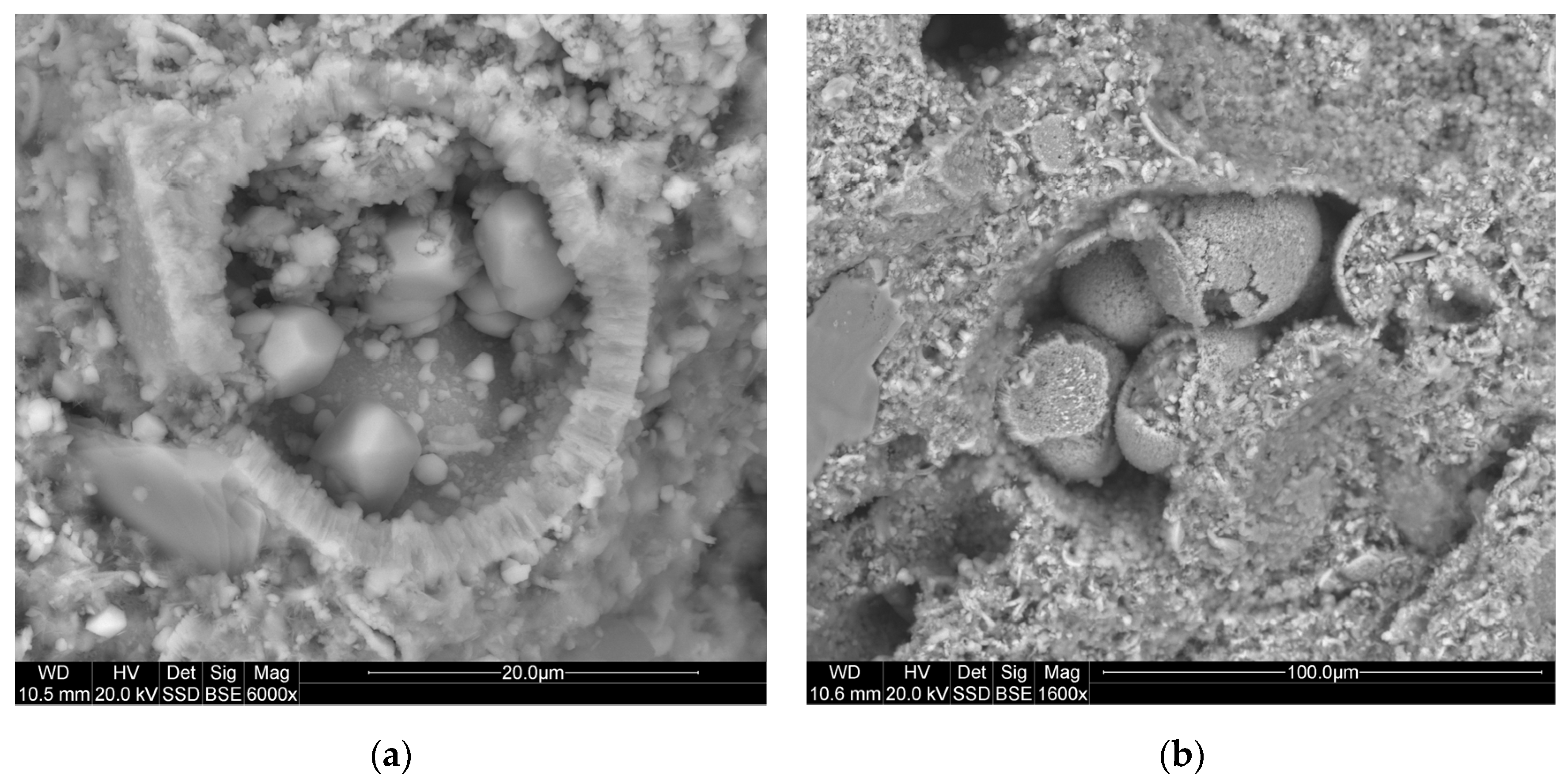
1.3. Materials
2. Methodology
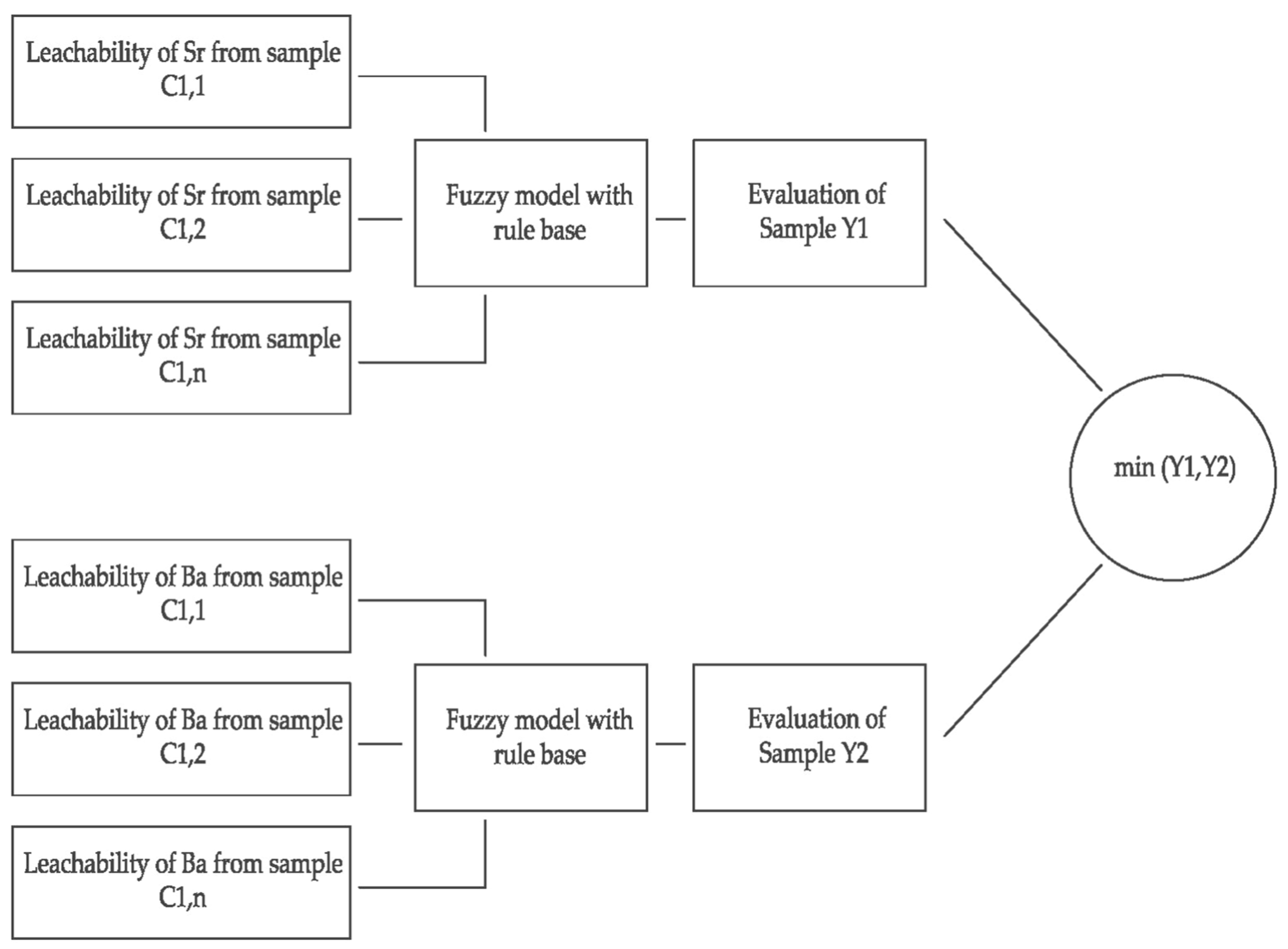
Calculation Method/Numerical Analysis for the Sr and Ba Leaching Model
- Definition of the membership function;
- Fuzzification;
- Knowledge representation;
- Inference;
- Defuzzification [65].
3. Results
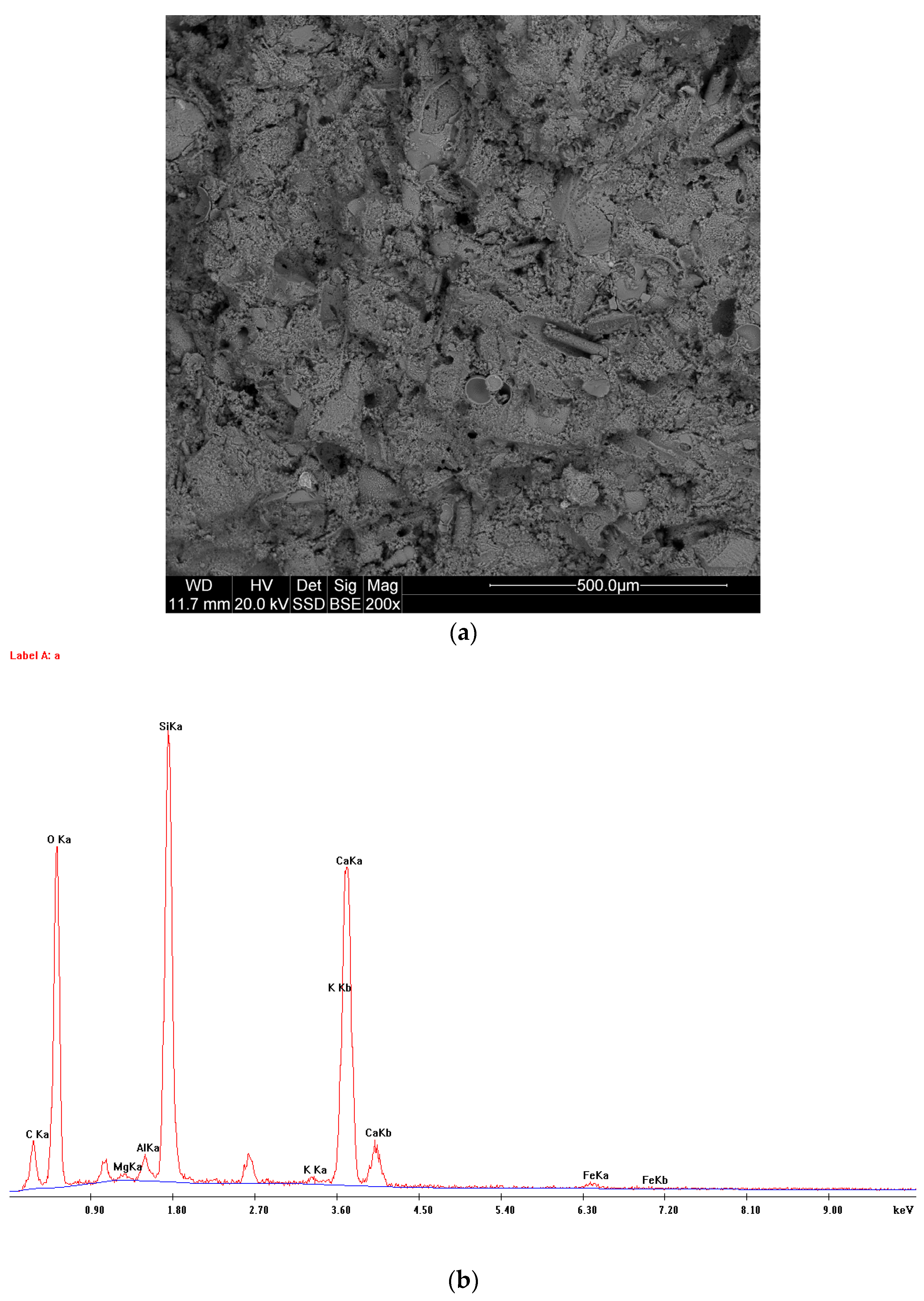
3.1. Empirical Resultes
3.2. The Model forSr and Ba Leachability
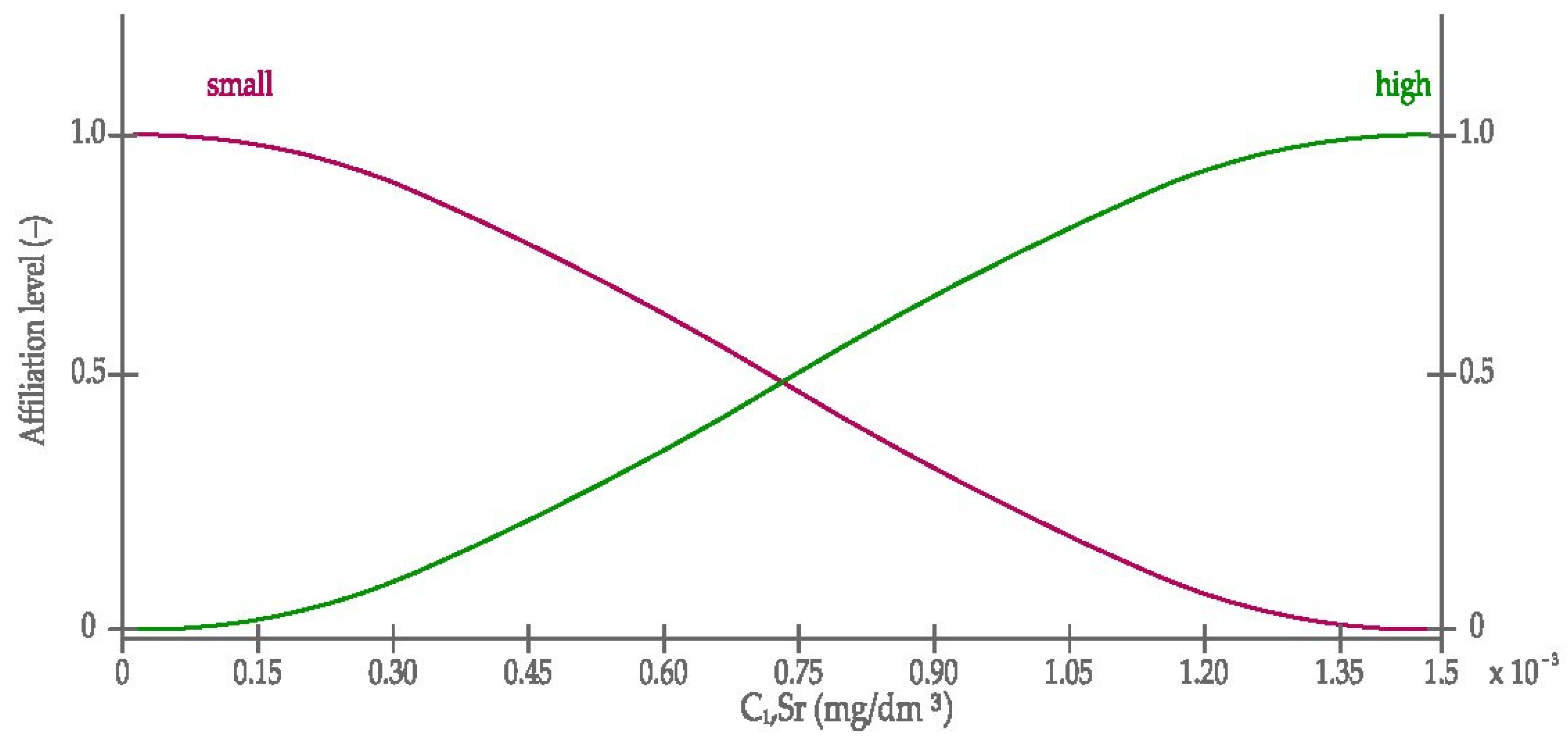
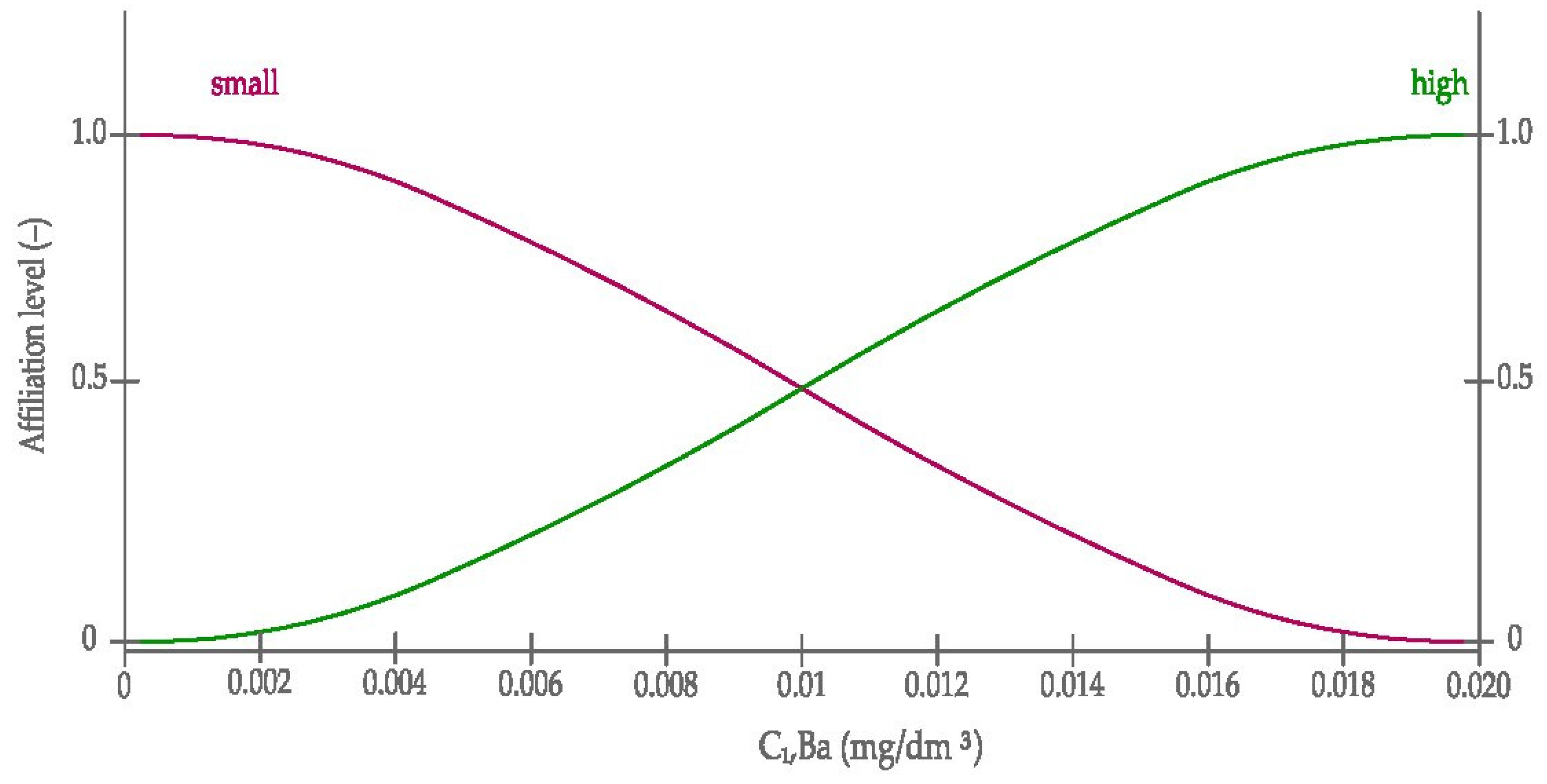
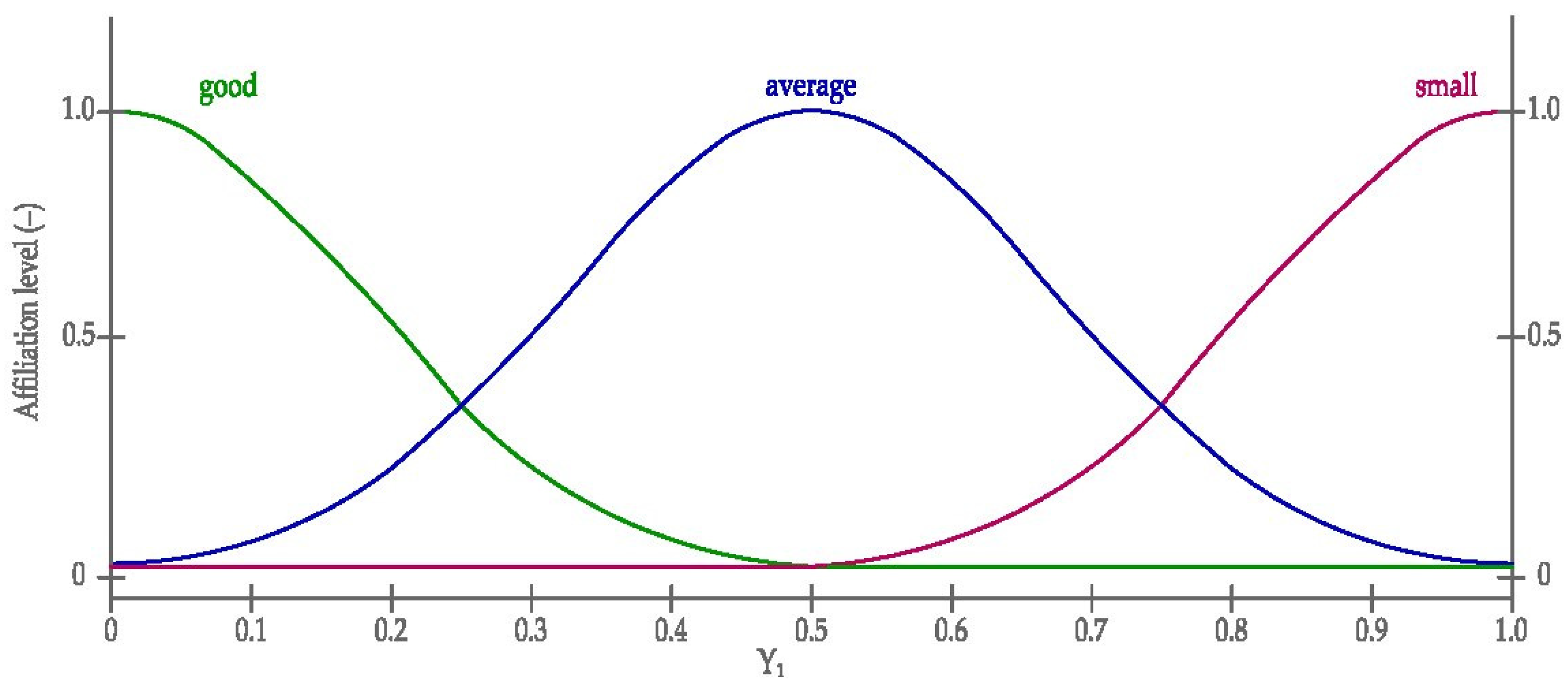
3.2.1. The Definition of Membership Functions Used in the Model
- The Gauss curve, Equation (1):where:
- σ—standard deviation,
- m—the expected value,
- t—the independent variable.
- The sigmoid curve, Equation (2):where:
- a—growth rate,
- c—the inflection point,
- t—the independent variable.
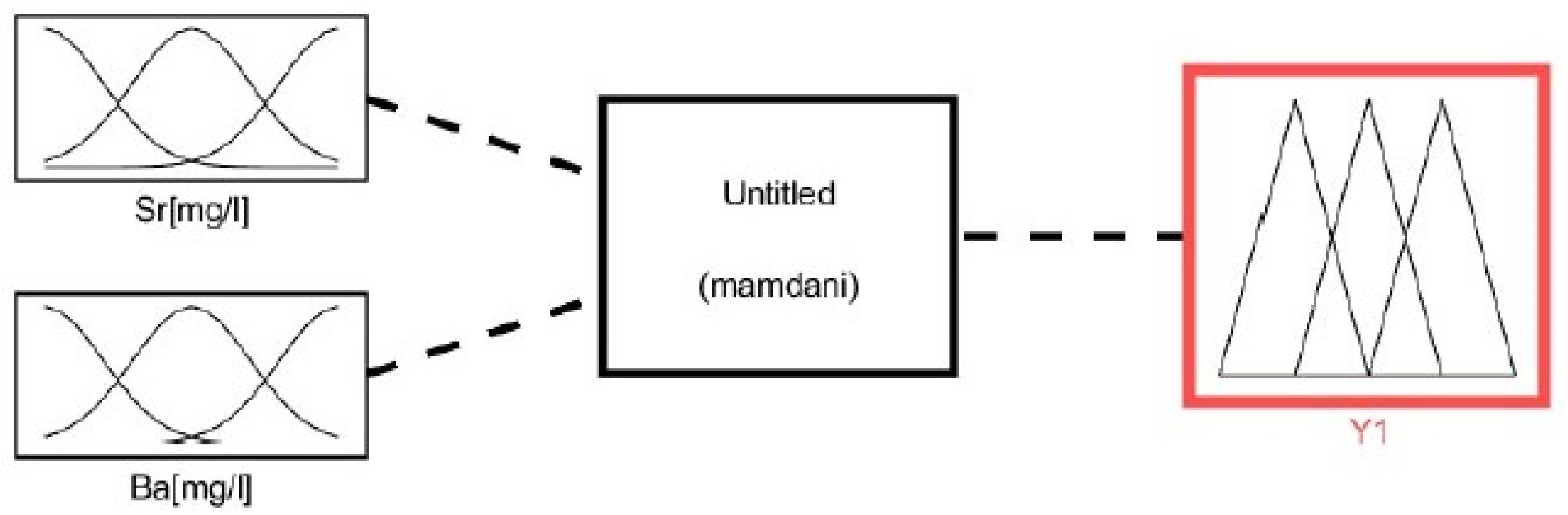
3.2.2. Fuzzification
- Two input variables for the leachability of Sr and Ba (predecessor linguistic variables);
- One output variable Y (the successor linguistic variables input variables).
3.2.3. Knowledge Representation
3.2.4. Inference
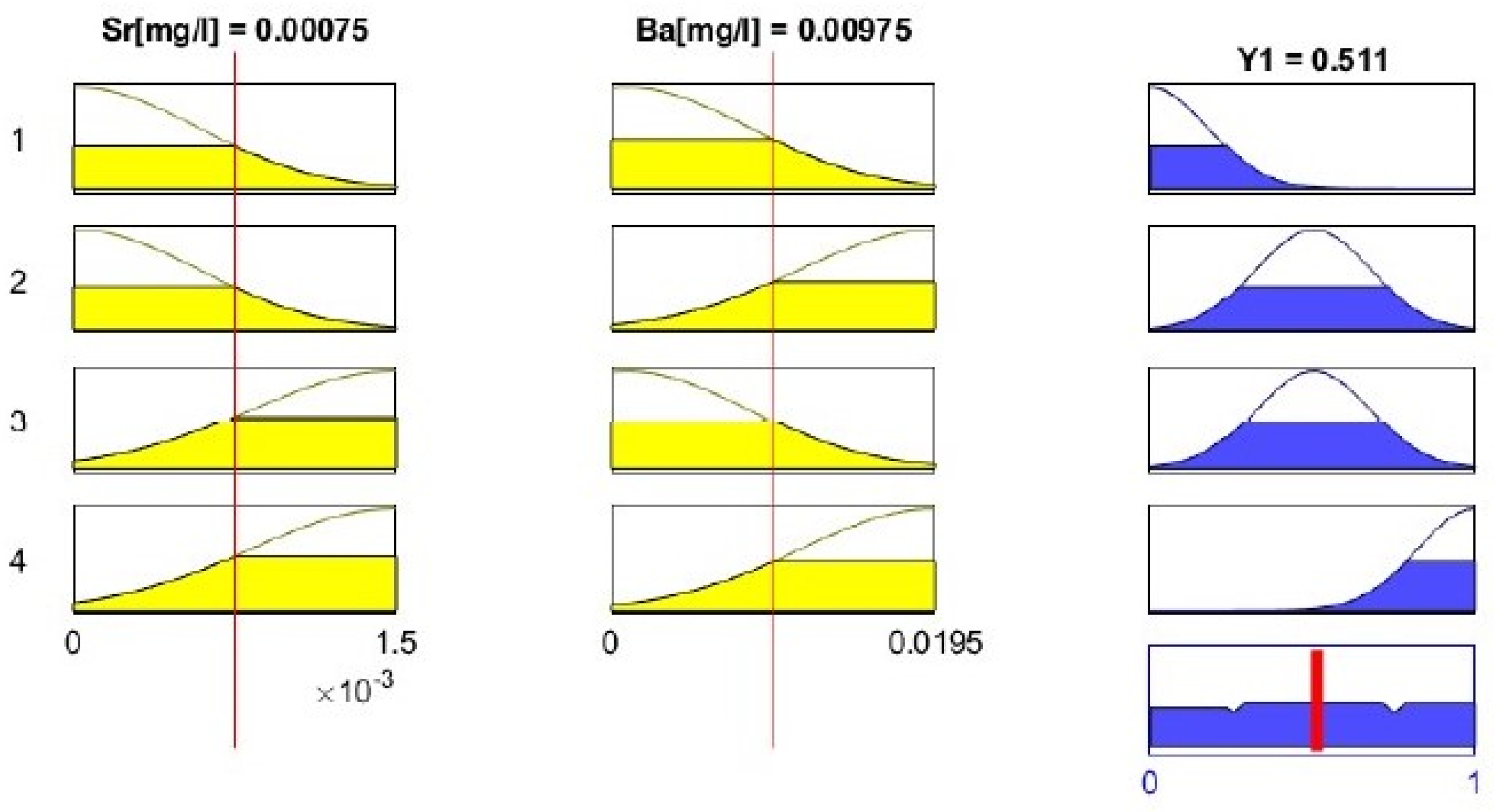
3.2.5. Defuzzification
- cl—fuzzy set center,
- μF(l)—function of membership of fuzzy sets F(l) corresponding to a given input variable.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vollprecht, D.; Berger, M.; Altenburger-Junker, I.; Neuhold, S.; Sedlazeck, K.P.; Aldrian, A.; Dijkstra, J.J.; Van Zomeren, A.; Raith, J.G.; Junker, A.; et al. Mineralogy and Leachability of Natural Rocks—A Comparison to Electric Arc Furnace Slags. Minerals 2019, 9, 501. [Google Scholar] [CrossRef]
- Tossavainen, M.; Forssberg, E. The potential leachability from natural road construction materials. Sci. Total. Environ. 1999, 239, 31–47. [Google Scholar] [CrossRef]
- Matschullat, J.; Ottenstein, N.R.; Reimann, C. Geochemical background—Can we calculate it? Environ. Geol. 2000, 39, 900–1000. [Google Scholar] [CrossRef]
- Söderström, T.; Stoica, P. System Identification; Prentice Hall: Englewood Cliffs, NJ, USA, 1989. [Google Scholar]
- PN-EN ISO 11885:1996. Water Quality—Determination of 33 Elements by Inductively Coupled Plasma Atomic Emission Y Spectroscopy; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Dijkstra, J.J.; Meeussen, J.C.L.; Comans, R.N.J. Leaching of Heavy Metals from Contaminated Soils: An Experimental and Modeling Study. Environ. Sci. Technol. 2004, 38, 16. [Google Scholar] [CrossRef]
- Dijkstra, J.J.; Van Zomeren, A.; Meeussen, J.C.L.; Comans, R.N.J. Effect of Accelerated Aging of MSWI Bottom Ash on the Leaching Mechanisms of Copper and Molybdenum. Environ. Sci. Technol. 2006, 40, 14. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.J.; Van Der Sloot, H.A.; Comansr, N.J. The leach-ing of major and trace elements from MSWI bottom ash as a function of pH and time. Appl. Geochem. 2006, 21, 335. [Google Scholar] [CrossRef]
- Geelhoed, J.S.; Meeussen, J.C.L.; Hillier, S.; Lumsdon, D.G.; Thomas, R.P.; Farmer, J.G.; Paterson, A.; Terson, E. Identification and geochemical modeling of processes controlling leaching of Cr (VI) and other major elements from chromite ore processing residue. Geochim. Cosmochim. Acta 2002, 66, 3927. [Google Scholar] [CrossRef]
- Geelhoed, J.S.; Meeussen, J.C.L.; Roe, M.J.; Hillier, S.; Thomas, R.P.; Farmier, J.G.; Paterson, E. Chromium Remediation or Re-lease? Effect of Iron (II) Sulfate Addition on Chromium (VI) Leaching from Columns of Chromite Ore Processing Residue. Environ. Sci. Technol. 2003, 37, 3206. [Google Scholar] [CrossRef]
- Hofmann, A.; Van Beinum, W.; Meeussen, J.C.L.; Kretzschmar, R. Sorption kinetics of strontium in porous hydrous ferric oxide aggregates II. Comparison of experimental results and model predictions. J. Colloid Interface Sci. 2005, 283, 2940. [Google Scholar] [CrossRef]
- Meeussen, J.C.L. Orchestra: An Object-Oriented Framework for Implementing Chemical Equilibrium Models. Environ. Sci. Technol. 2003, 37, 1175. [Google Scholar] [CrossRef]
- Van Der Sloot, H.A. Environmental Properties of Building Materials in Relation to the Construction Products Directive (CPD); ECN: Petten, The Netherlands, 2003. [Google Scholar]
- Van Beinum, W.; Hofmann, A.; Meeussen, J.C.L.; Kretzschmar, R. Sorption kinetics of strontium in porous hydrous ferric oxide aggregates: I. The Donnan diffusion model. J. Colloid Interface Sci. 2005, 283, 1828. [Google Scholar] [CrossRef] [PubMed]
- Van Zomerena, A.; Comansr, N.J. Contribution of Natural Organic Matter to Copper Leaching from Municipal Solid Waste Incinerator Bot-tom Ash. Environ. Sci. Technol. 2004, 38, 3927. [Google Scholar] [CrossRef] [PubMed]
- Moser, H.; Römbke, J. Ecotoxicological Characterization of Waste: Results and Experiences of an International Ring Test; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Harmonization of leaching test. Available online: www.leaching.org (accessed on 20 December 2020).
- Bardossy, A.; Duckstein, L. Fuzzy Rule-Based Modeling with Application to Geophysical, Biological and Engineering Systems; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Driankov, D.; Hellendoorn, H.; Reinfrank, M. An Introduction to Fuzzy Control; Springer-Verlag: Berlin, Germany, 1993. [Google Scholar]
- Kuncheva, L.I. Fuzzy Classifier Design; Physica-Verlag: Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Kacprzyk, J. Fuzzy Sets in System Analysis; Polish Scientific Publishers PWN: Warsaw, Poland, 1986. (In Polish) [Google Scholar]
- Piegat, A. Fuzzy Modeling and Control; Academic Publishing House EXIT: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- Rutkowski, L. Methods and Techniques of Artificial Intelligence, 2nd ed.; Polish Scientific Publishers PWN: Warsaw, Polish, 2002. (In Polish) [Google Scholar]
- Sugeno, M.; Yasukawa, T. A fuzzy-logic-based approach to qualitative modeling. IEEE Trans. Fuzzy Syst. 1993, 1, 7. [Google Scholar] [CrossRef]
- Pedrycz, W. (Ed.) Fuzzy Modelling, Paradigms and Practice; Kluwer Academic Press: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Akgun, A.; Sezer, E.A.; Nefeslioglu, H.A.; Gokceoglu, C.; Pradhan, B. Aneasy-to-use MATLAB program (MamLand) for the assessment of landslide susceptibility using a Mamdani fuzzy algorithm. Comput. Geosci. 2012, 38, 23. [Google Scholar] [CrossRef]
- Król, A. Release of Heavy Metals from Mineral Composites Taking into Account Environmental Impact. Studies and Monographs; Publishing House of the Opole University of Technology: Opole, Poland, 2012. [Google Scholar]
- Mamdani, E.H. Applications of fuzzy algorithms to approximate rea-soning using linguistic synthesis. Proc. IEEE Trans. Comput. 1977, 26, 1181. [Google Scholar]
- Report of the European Cement Association Cenbureau. Use of Slags and Non-Ferrous Metals as an Addition to Cement and Leaching in Drinking Water; CWB: Milton, ON, Canada, 1996. [Google Scholar]
- Tamas, F.D.; Tagnit-Hamou, J.; Tritthart, J. Trace Elements in Clinkier and the Iruse as “Fingerprints” to Facili Tatetheir Qualitativeidentification, Materials Science of Concrete; The Sidney Diamond Symposium: Honolulu, HI, USA; American Ceramic Society: Westerville, OH, USA, 1998. [Google Scholar]
- Kalarus, D. Chemical Identification of Portland Cements Produced in Poland Based on the Content of Trace Elements. Ph.D. Thesis, AGH University of Science and Technology, Kraków, Poland, 2007. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Grup: Milton Park, UK, 2011. [Google Scholar]
- Migaszewski, Z.M.; Gałuszka, A. Rudiments of Enviromental Geochemistry; Scientific and Technical: Warsaw, Poland, 2007. (In Polish) [Google Scholar]
- Polanski, A.; Smulikowski, K. Geochemistry; Geological Publishing House: Warsaw, Poland, 1969. (In Polish) [Google Scholar]
- Koljonen, T. The geochemical atlas of Finland. Geol. Surv. Finl. Espoo. I Environ. Qual. 1992, 32, 2230. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. Treatise on Geochemistry 2004. Available online: https://www.geol.umd.edu/~rudnick/PDF/Rudnick_Gao_Treatise.pdf (accessed on 20 December 2020).
- FOREGS: Geochemical Atlas. Available online: http://weppi.gtk.fi/publ/foregsatlas/index.php (accessed on 20 December 2020).
- FOREGS. Geochemical Atlas of Europe. Available online: http://www.integrated-assessment.eu/eu/indexaa6f.html?q=resource_centre/foregs_geochemical_atlas_europe (accessed on 20 December 2020).
- Wyrwicka, K. Stratigraphy, phation and tectology of the western Lublin mastrycht. Geol. Q. 1980, 24, 4. (In Polish) [Google Scholar]
- Walaszczyk, I. Turonian through Santonian deposits of the Central Polish Uplands; their facies development, inoceramid paleontology and stratigraphy. Acta. Geol. Pol. 1992, 42, 1–122. [Google Scholar]
- Włodek, M.; Gaździcka, E. Explanations to the Detailed Geological Map of Poland. Annopol Sheet; PIG-PIB: Warszawa, Poland, 2009. (In Polish) [Google Scholar]
- Bąk, B.; Radwanek-Bąk, B. Here is the opoka rock—And Kazimierz Dolny was built on it. Opencast Mining 2020, 1, 55–63. (In Polish) [Google Scholar]
- Vítek, J. Opukovýfenomén Na zvlněnýchokrajích České tabule. Vesmir 2011, 90, 704. (In Czech) [Google Scholar]
- Kotlík, J.; Šrámek, J.; Kaše, J. Monografie STOT: Opuka; Vyd. Praha.Stop: Czech Republic, 2000. (In Czech) [Google Scholar]
- Ginzburg, I.I. Principles of Geochemical Prospecting. In Techniques of Prospecting for Non-Ferrous Oras Rare Metals; Pergamon Press: Oxford, UK, 1960. [Google Scholar]
- Pękala, A. Research on Temporal Leachability of Trace Elements from Opoka-Rocks in The Aspect of Geochemical Environmental Indicators. IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 1755. [Google Scholar] [CrossRef]
- Znosko, J. Map of tectonic units of Poland. In Tectonic Atlas of Poland; Znosko, J., Ed.; Polish Geological Institute: Warsaw, Poland, 1998. [Google Scholar]
- Głodek, J.; Kęsik, A.; Kolago, C.; Mojski, E.; Starkel, L. Along the Vistula River; Wyd. Geol.: Warsaw, Poland, 1967. [Google Scholar]
- Pusch, G.G. Geognostische Beschreibung von Polen so wie der übrigen Nordkarpathen-Lander; Cotta'schen Buchhandlung: Stuttgard-Tübingen, Germany, 1836; p. 695. [Google Scholar]
- Sujkowski, Z. Petrography of chalk Polish. Chalk from deep drilling in Lublin compared to chalk in some other areas of Polish. Rep. State Geol. Inst. 1931, 6, 485–628. [Google Scholar]
- Pożaryski, W. An outline of stratigraphy and palaeogeography of the Cretaceous in the Polish Lowland (in Polish with English summary). State Geol. Inst. 1960, 30, 377–418. [Google Scholar]
- Pożaryska, K. The sedimentological problems of Upper Maastrichtian and Danian of the Pulawy Environment (Middle Vistula). Bull. State Geol. Inst. 1952, 81, 1–104. [Google Scholar]
- Kowalsk, W.C. The resistance to compression of the construction rocks of the Sleepy semi-groundbreaking section of the Central Vistula against the background of their lithology. Biul. Geol. U. W. 1961, 1, 2. [Google Scholar]
- Rutkowski, J. Senonian in the area of Miechow, southern Poland. Yearb. Pol. Geol. Soc. 1965, 35, 3–53. [Google Scholar]
- Harasimiuk, M. Relief evolution of the Chełm hills in the Tertiary and Quaternary. Geogr. Stud. 1975, 115, 7–105. [Google Scholar]
- Bolewski, A.; Parachoniak, W. Petrography; Geological Publishing: Warszawa, Poland, 1988. [Google Scholar]
- Kozłowski, S. Raw Materials Polish; Geological Publishing: Warszawa, Poland, 1986. [Google Scholar]
- Manecki, A.; Muszyński, M. A Guide to Petrography; AGH University of Science and Technology Press: Kraków, Poland, 2008. (In Polish) [Google Scholar]
- Trochonowicz, M. Analysis of the Efectiveness of Diaphragments Performer by Chemical Injection Methods in Opoka Rock Walls. Ph.D. Thesis, Lublin University of Technology, Lublin, Poland, 2011. [Google Scholar]
- Pękala, A.; Pytel, M. Evaluation of Temporal Leachability of Strontium from Building Materials to Environment. IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 012124. [Google Scholar] [CrossRef]
- EA NEN 7375:2004. Leaching Characteristics of Moulded or Monolithic Building and Waste Materials. Determination of Leaching of Inorganic Components with the Diffusion Test. Available online: https://www.alsenvironmental.co.uk/media-uk/method_statements/coventry/waste-sector/method-statement-wsm004.pdf (accessed on 20 December 2020).
- Pękala, A.; Hydzik-Wiśniewska, J. Analysis of temporal leachibility of trace elements to the environment of opoka-rocks used in historical building. E3S Web Conf. 2018, 49, 00081. [Google Scholar] [CrossRef]
- Musiał, M. Analysis of the impact of selected factors on the effectiveness of using PCM in mobile window insulation. E3S Web Conf. Solina 2018, 49, 00073. [Google Scholar] [CrossRef]
- Lichołai, L.; Musiał, M. Experimental analysis of the functioning of a window with a phase change heat accumulator. Materials 2020, 13, 3647. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, E.; De Baets, B. Monotone Mamdani-Assilian models under mean of maxima defuzzification. Fuzzy Sets Syst. 2008, 159, 2819. [Google Scholar] [CrossRef]
- Program Matlab, 2018b; The MathWorks Inc.: Natick, MA, USA, 2018.
- Pękala, A. Silification of the Mesozoic Rocks Accompanying the Bełchatów Lignite Deposit, Central Poland. Geosciences 2020, 10, 141. [Google Scholar] [CrossRef]
- Głowienka, E.; Michałowska, K.; Pękala, A. Spatio-Temporal Analysis of Soil Properties for the Eastern Border of the European Union; Taylor & Francis Group: Milton Park, UK, 2016. [Google Scholar] [CrossRef]
- Pękala, A. Mineralogical-geochemical study of the transitional rocks from the Mesozoic-Neogen contact zone in the “Bełchatów” lignite deposit. Min. Geol. 2012, 7, 187. (In Polish) [Google Scholar]






| Origin-Source | Median (mg·kg−1) | |||
|---|---|---|---|---|
| No. of Samples | Strontium (Sr) | Barium (Ba) | ||
| Crust [35] | upper continental | n.a. | 350 | 628 |
| Soil [36] | World | n.a. | 240 | 500 |
| Subsoil [37] | Europe | 788 | 95.0 | 385 |
| Topsoil [37] | Europe | 845 | 89.0 | 375 |
| Water [37] | World | n.a. | 0.50 (mg L−1) | 30 (μg L−1) |
| Water [37] | Europe | 807 | 0.11 (mg L−1) | 24.9 (μg L−1) |
| Carbonate rocks [38] | World | n.a. | 610 | 10 |
| Sandstones [38] | World | n.a. | 20 | 10 |
| Humus [37] | Europe | 367 | 17.4 | 60.6 |
| Time [h] | Average Leachability of Elements from Opoka Rock (mg/L) | |
|---|---|---|
| Sr | Ba | |
| 6 | 0.0015 | 0.0195 |
| 24 | 0.0587 | 0.0202 |
| 54 | 0.0785 | 0.0197 |
| 96 | 0.092 | 0.0197 |
| 216 | 0.1182 | 0.0197 |
| 384 | 0.1375 | 0.0198 |
| 864 | 0.1231 | 0.0197 |
| 1536 | 0.1225 | 0.0195 |
| Time [h] | Total Leachability of Elements from Opoka Rock (mg/L) | |
|---|---|---|
| Sr | Ba | |
| 6 | 0.0015 | 0.0195 |
| 24 | 0.0602 | 0.0397 |
| 54 | 0.1387 | 0.0594 |
| 96 | 0.2307 | 0.0791 |
| 216 | 0.3489 | 0.0988 |
| 384 | 0.4864 | 0.1186 |
| 864 | 0.6095 | 0.3156 |
| 1536 | 0.7320 | 0.3351 |
| C1-1,Sr (mg/L) | C1-1,Ba (mg/L) | Y |
|---|---|---|
| Small: sig {−0.00075; 0} | Small: sig {−0.00975; 0} | Good: sig {−10; 0.5} |
| High: sig {0; 0.00075} | High: sig {0.00075; 0} | Average: gaus {0.2; 0.5} |
| Bad: sig {10; 0.5} |
| No | The Rules of Fuzzy Inference |
|---|---|
| 1 | If (Sr (mg/dm3) is small) and (Ba (mg/dm3) is small) then (Y is good) |
| 2 | If (Sr (mg/dm3) is small) and (Ba (mg/dm3) is high) then (Y is average) |
| 3 | If (Sr (mg/dm3) is high) and (Ba (mg/dm3) is small) then (Y is average) |
| 4 | If (Sr (mg/dm3) is high) and (Ba (mg/dm3) is high) then (Y is bad) |
| Linguistic Operator | Operator Use Case |
|---|---|
| Conjunction | Min |
| Alternative | Max |
| Implication | Min |
| Aggregation | Max |
| Time | Cumulative Leachability of Metal (mg/dm3) | The Result of Defuzzification | |
|---|---|---|---|
| (h) | Ci,Sr | Ci,Ba | Yi |
| 6 | 0.0015 | 0.0195 | 0.511 |
| 24 | 0.0587 | 0.0202 | 0.500 |
| 54 | 0.0785 | 0.0197 | 0.500 |
| 96 | 0.092 | 0.0197 | 0.500 |
| 216 | 0.1182 | 0.0197 | 0.500 |
| 384 | 0.1375 | 0.0198 | 0.500 |
| 864 | 0.1231 | 0.0197 | 0.500 |
| 1536 | 0.1225 | 0.0195 | 0.500 |
| Time (h) | Quantity for the Variable Y Result | |
|---|---|---|
| Sr to Ba | Ba to Sr | |
| 6 | average | average |
| 24 | average | average |
| 54 | average | average |
| 96 | average | average |
| 216 | average | average |
| 384 | average | average |
| 864 | average | average |
| 1536 | average | average |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pękala, A.; Musiał, M. Modelling the Leachability of Strontium and Barium from Stone Building Materials. Materials 2021, 14, 3403. https://doi.org/10.3390/ma14123403
Pękala A, Musiał M. Modelling the Leachability of Strontium and Barium from Stone Building Materials. Materials. 2021; 14(12):3403. https://doi.org/10.3390/ma14123403
Chicago/Turabian StylePękala, Agnieszka, and Michał Musiał. 2021. "Modelling the Leachability of Strontium and Barium from Stone Building Materials" Materials 14, no. 12: 3403. https://doi.org/10.3390/ma14123403
APA StylePękala, A., & Musiał, M. (2021). Modelling the Leachability of Strontium and Barium from Stone Building Materials. Materials, 14(12), 3403. https://doi.org/10.3390/ma14123403






