Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Microstructural and Hardness Analysis
2.3. Surface Topography Analysis
2.4. Phase Composition Analysis
2.5. Corrosion Measurements
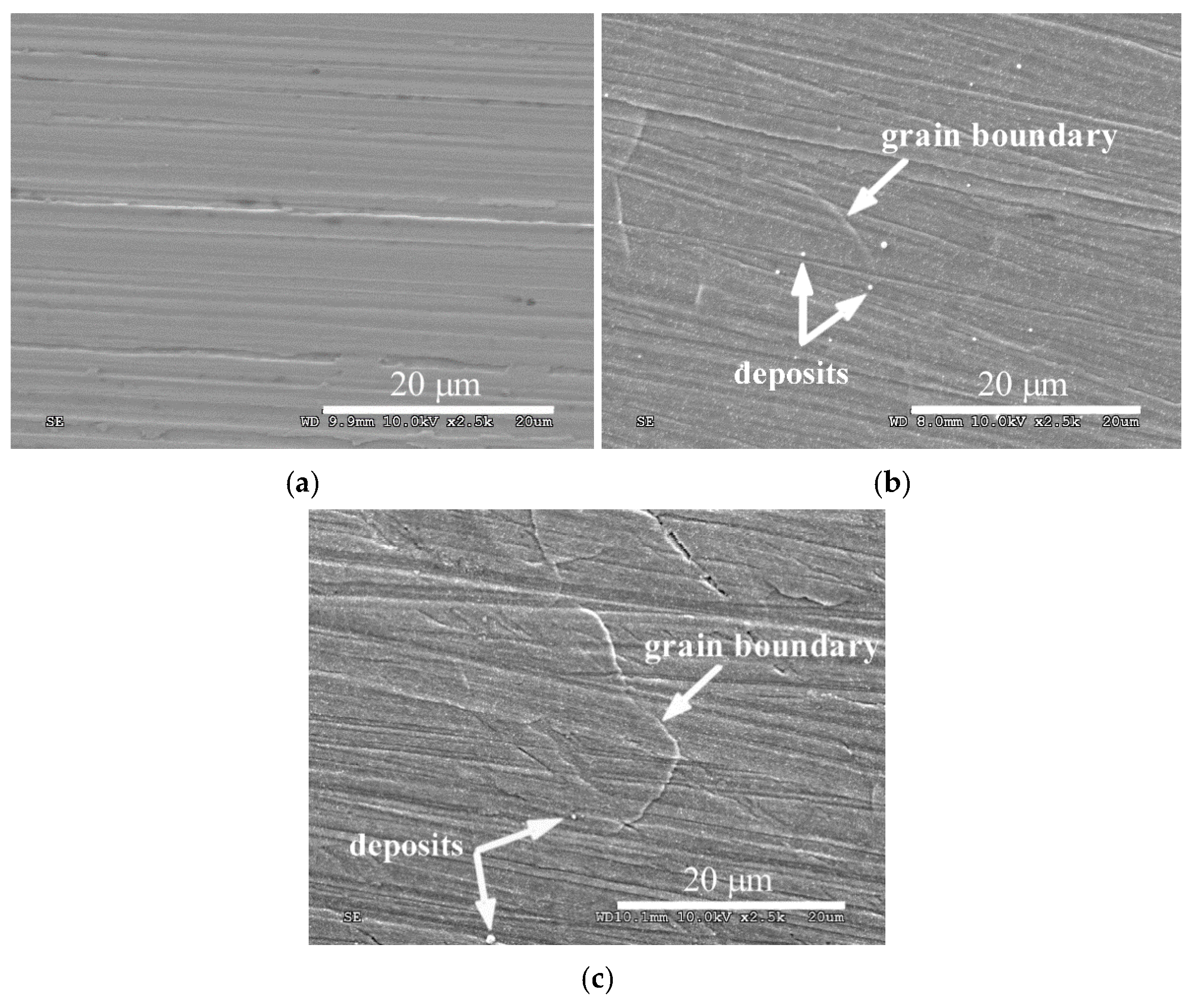
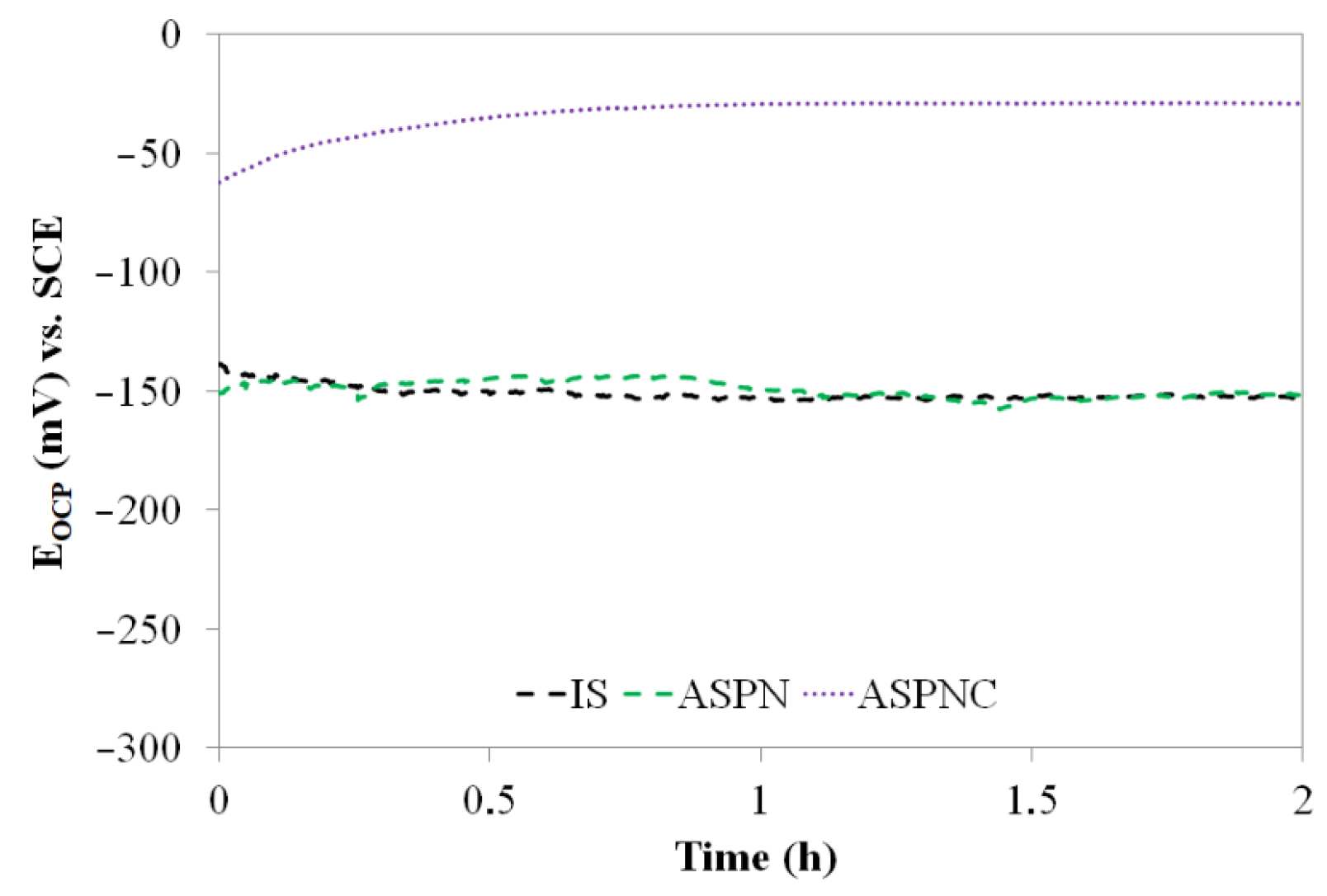
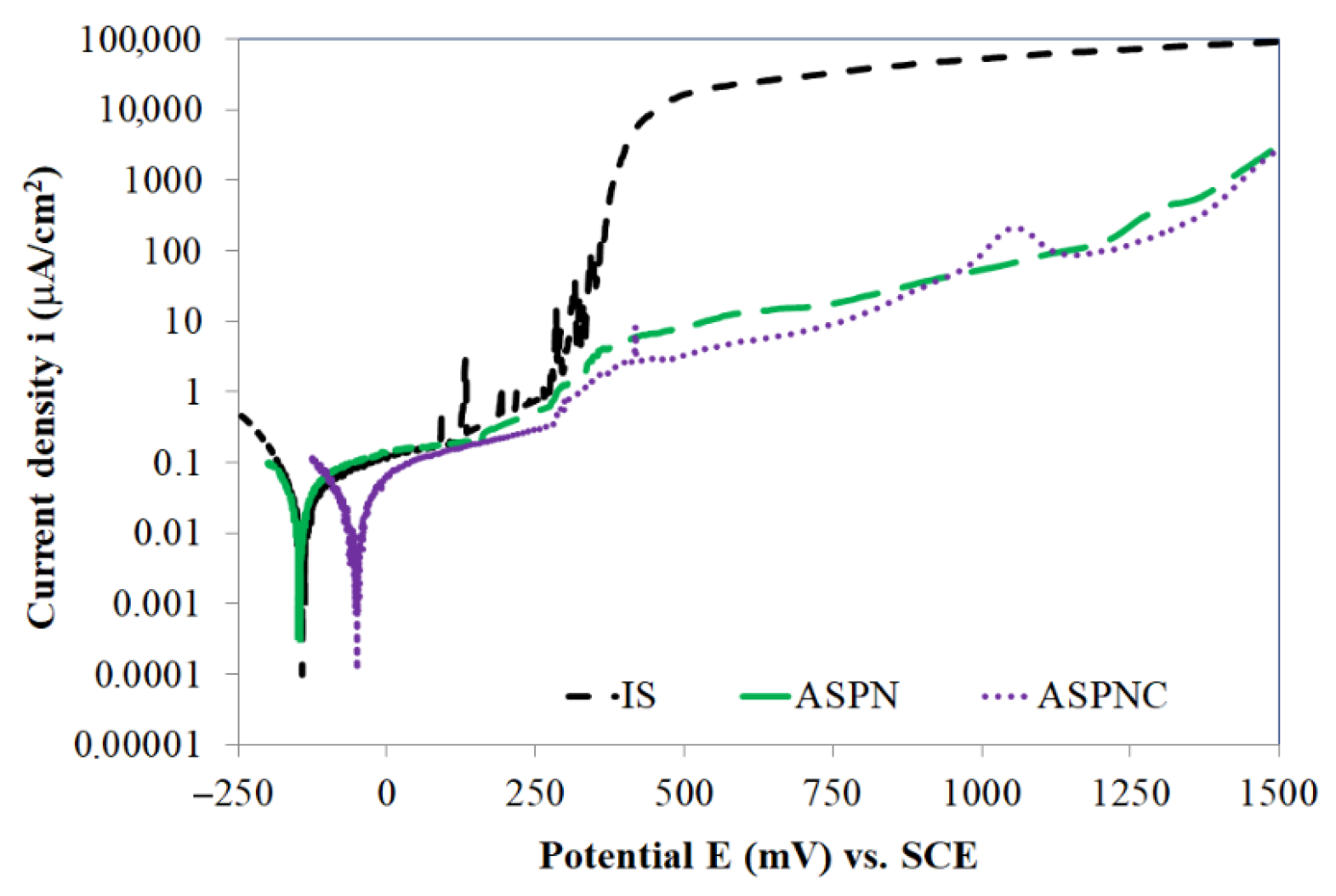
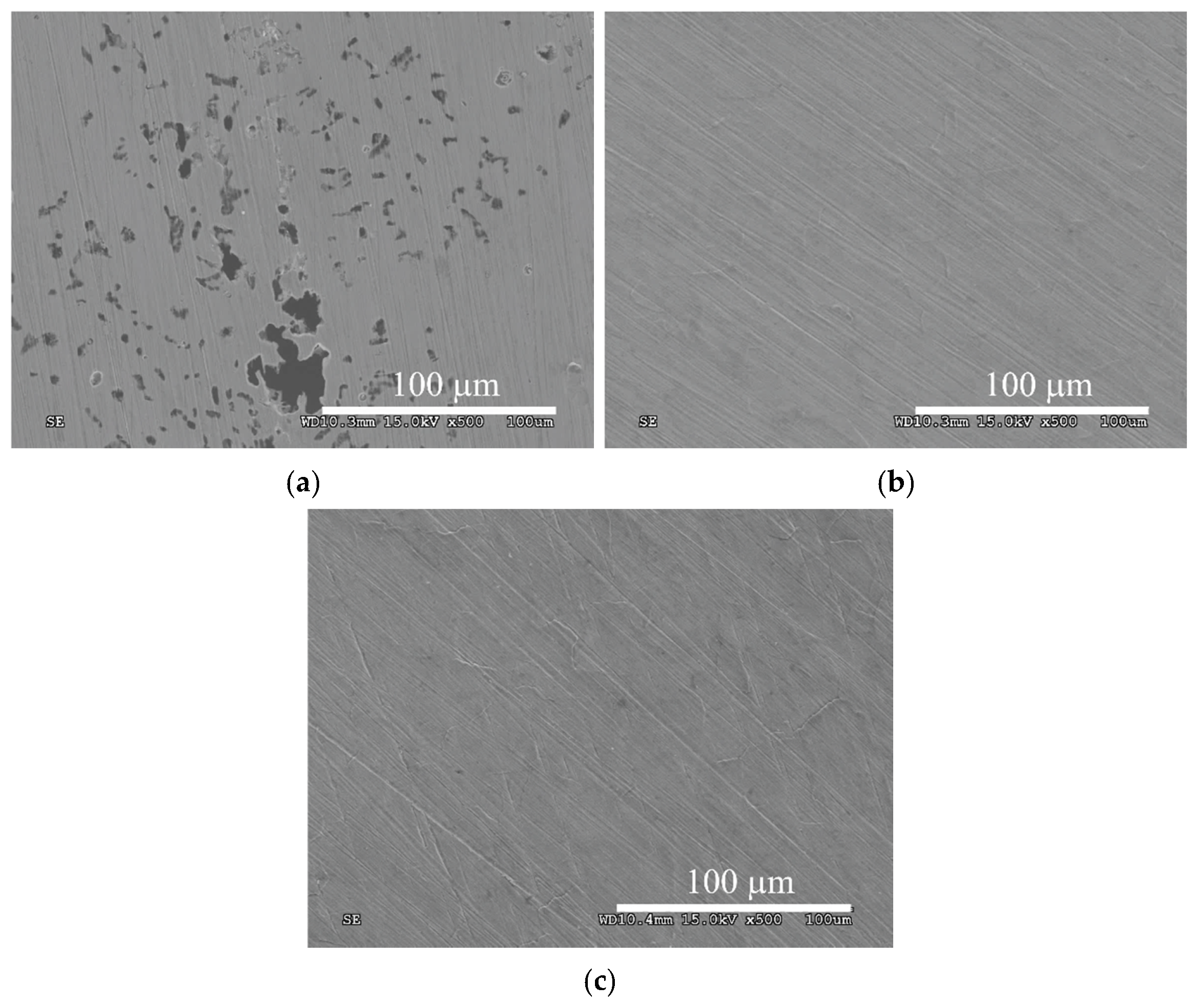
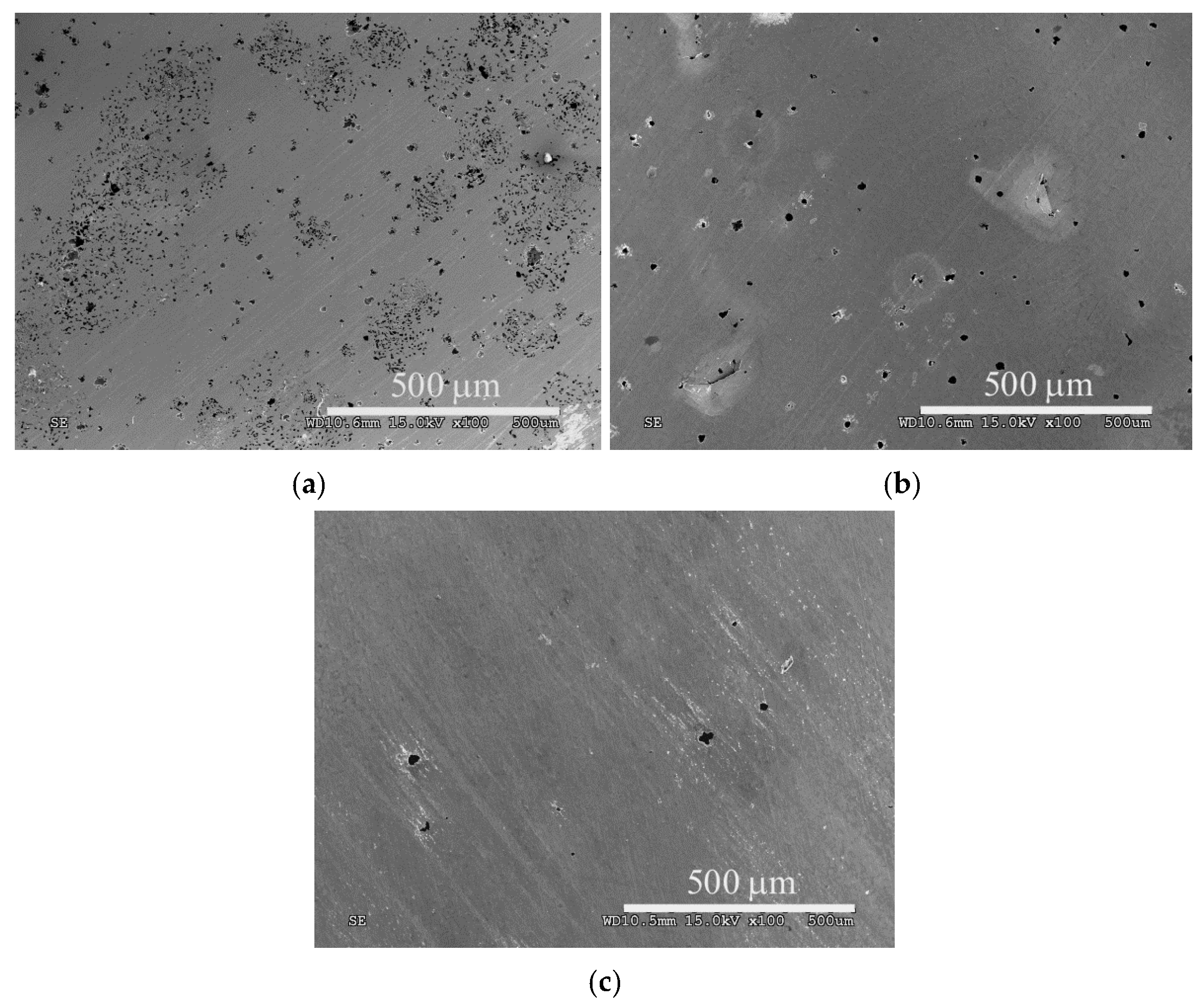
3. Results and Discussion
4. Conclusions
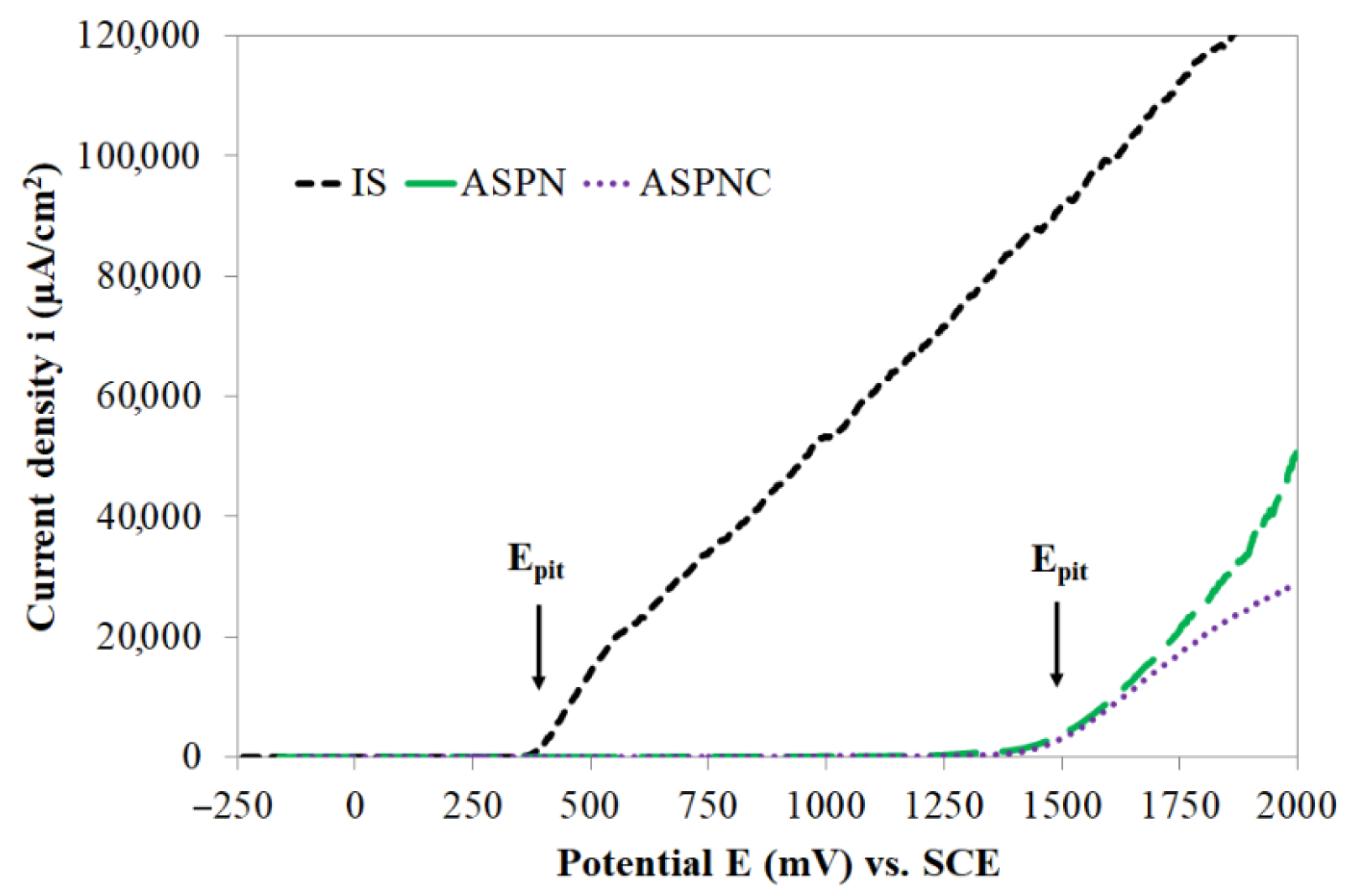
- Active screen plasma nitriding or nitrocarburising are technologies that guarantee a very large increase in the corrosion resistance of austenitic steels in an aggressive environment containing Cl− ions. The use of active screen plasma processes makes it possible to produce an austenitic steel surface layer characterised by a significant increase in the durability of the passive layer over a broad range of potentials.
- The pitting potentials for ASPN and ASPNC layers are similar, i.e., 1500 mV, however, the kinetics of pit formations are different in both cases. In the case of nitro-carbon expanded austenite, pits are formed much slower than in the case of the nitrogen austenite layer, which is indicative of the significant role that carbon, in combination with nitrogen, plays in inhibiting pitting corrosion processes.
- The changes in properties that carbon contributed to also included increased layer thickness, a change in the microstructure (a double-layer structure consisting of γNC and γC) and in the degree of hardness.
- Nitrocarburising carried out using the proposed technology and parameters may help extend the scope of application of cheaper, conventional austenitic steels. Steels subjected to this type of treatment can be utilised in various sophisticated solutions, which typically involve the use of superaustenitic steels. These can be found, among others, in the medical, petrochemical and power industries, where high corrosion resistance of some structural elements is often crucial.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Blawert, C.; Kalvelage, H.; Mordike, B.; Collins, G.; Short, K.; Jirásková, Y.; Schneeweiss, O. Nitrogen and carbon expanded austenite produced by PI3. Surf. Coat. Technol. 2001, 136, 181–187. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Casteletti, L.C.; Gallego, J. Microstructure of nitrided and nitrocarburized layers produced on a superaustenitic stainless steel. J. Mater. Res. Technol. 2013, 2, 158–164. [Google Scholar] [CrossRef]
- Boillot, P.; Peultier, J. Use of Stainless Steels in the Industry: Recent and Future Developments. Procedia Eng. 2014, 83, 309–321. [Google Scholar] [CrossRef]
- Nagarajan, S.; Rajendran, N. Crevice corrosion behaviour of superaustenitic stainless steels: Dynamic electrochemical impedance spectroscopy and atomic force microscopy studies. Corros. Sci. 2009, 51, 217–224. [Google Scholar] [CrossRef]
- Hänninen, H.; Romu, J.; Ilola, R.; Tervo, J.; Laitinen, A. Effects of processing and manufacturing of high nitrogen-containing stainless steels on their mechanical, corrosion and wear properties. J. Mater. Process. Technol. 2001, 117. [Google Scholar] [CrossRef]
- Orellana, L.M.; Pérez, F.J.; Gómez, C. The effect of nitrogen ion implantation on the corrosion behaviour of stainless steels in chloride media. Surf. Coat. Technol. 2005, 200. [Google Scholar] [CrossRef]
- Baranowska, J.; Arnold, B. Corrosion resistance of nitrided layers on austenitic steel. Surf. Coat. Technol. 2006, 200. [Google Scholar] [CrossRef]
- Celik, O.; Baydogan, M.; Atar, E.; Kayali, E.S.; Cimenoglu, H. Fatigue performance of low temperature nitrided AISI 321 grade austenitic stainless steel. Mater. Sci. Eng. A 2013, 565. [Google Scholar] [CrossRef]
- Biehler, J.; Hoche, H.; Oechsner, M. Corrosion properties of polished and shot-peened austenitic stainless steel 304L and 316L with and without plasma nitriding. Surf. Coat. Technol. 2017, 313. [Google Scholar] [CrossRef]
- Rajendran, P.; Devaraju, A. Experimental Evaluation of Mechanical and Tribological Behaviours of Gas Nitride treated AISI 316LN Austenitic Stainless Steel. Mater. Today Proc. 2018, 5. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, X.; Wang, S.; Li, J.; Wei, W.; Hu, J. Performance enhancement by plasma nitriding at low gas pressure for 304 austenitic stainless steel. Vacuum 2017, 145. [Google Scholar] [CrossRef]
- Sun, Y. Corrosion behaviour of low temperature plasma carburised 316L stainless steel in chloride containing solutions. Corros. Sci. 2010, 52. [Google Scholar] [CrossRef]
- Hummelshøj, T.S.; Christiansen, T.L.; Somers, M.A.J. Lattice expansion of carbon-stabilized expanded austenite. Scr. Mater. 2010, 63. [Google Scholar] [CrossRef]
- Souza, R.M.; Ignat, M.; Pinedo, C.E.; Tschiptschin, A.P. Structure and properties of low temperature plasma carburized austenitic stainless steels. Surf. Coat. Technol. 2009, 204. [Google Scholar] [CrossRef]
- Blawert, C.; Mordike, B.L.; Collins, G.A.; Short, K.T.; Jirásková, Y.; Schneeweiss, O.; Perina, V. Characterisation of duplex layer structures produced by simultaneous implantation of nitrogen and carbon into austenitic stainless steel X5CrNi189. Surf. Coat. Technol. 2000, 128–129. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Somers, M.A.J. Low-temperature gaseous surface hardening of stainless steel: The current status. Int. J. Mater. Res. 2009, 100. [Google Scholar] [CrossRef]
- Kajzer, A.; Ceglarska, M.; Sura, N.; Kajzer, W.; Borowski, T.; Tarnowski, M.; Pilecki, Z. Effect of Nitrided and Nitrocarburised Austenite on Pitting and Crevice Corrosion Resistance of 316 LVM Steel Implants. Materials 2020, 13, 5484. [Google Scholar] [CrossRef]
- Cisquini, P.; Ramos, S.V.; Viana, P.R.P.; de Freitas Cunha Lins, V.; Franco, A.R., Jr.; Vieira, E.A. Effect of the roughness produced by plasma nitrocarburizing on corrosion resistance of AISI 304 austenitic stainless steel. J. Mater. Res. Technol. 2019, 8. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. On the crystallographic structure of S-phase. Scr. Mater. 2004, 50. [Google Scholar] [CrossRef]
- Mingolo, N.; Tschiptschin, A.P.; Pinedo, C.E. On the formation of expanded austenite during plasma nitriding of an AISI 316L austenitic stainless steel. Surf. Coat. Technol. 2006, 201. [Google Scholar] [CrossRef]
- Fewell, M.; Mitchell, D.R.; Priest, J.; Short, K.; Collins, G. The nature of expanded austenite. Surf. Coat. Technol. 2000, 131. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Li, X.; Dong, H. Dry Sliding Wear of Active Screen Plasma Carburised Austenitic Stainless Steel. Tribol. Lett. 2012, 45. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Chen, Y.; Sun, J.; He, Q.; Liu, Q.; Liu, G.; Xie, K. Abrasive resistance and corrosion properties of AISI 316 sieve via low-temperature gaseous nitriding. Surf. Coat. Technol. 2019, 361. [Google Scholar] [CrossRef]
- Ou, K.-L.; Chou, H.-H.; Liu, C.-M.; Peng, P.-W. Surface modification of austenitic stainless steel with plasma nitriding for biomedical applications. Surf. Coat. Technol. 2011, 206. [Google Scholar] [CrossRef]
- Mändl, S.; Manova, D.; Neumann, H.; Pham, M.T.; Richter, E.; Rauschenbach, B. Correlation between PIII nitriding parameters and corrosion behaviour of austenitic stainless steels. Surf. Coat. Technol. 2005, 200. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Heck, S.C.; Pereira, R.G.; Picon, C.A.; Nascente, P.A.P.; Casteletti, L.C. Ion nitriding of a superaustenitic stainless steel: Wear and corrosion characterization. Surf. Coat. Technol. 2010, 204. [Google Scholar] [CrossRef]
- Li, C.; Bell, T. Corrosion properties of active screen plasma nitrided 316 austenitic stainless steel. Corros. Sci. 2004, 46. [Google Scholar] [CrossRef]
- De Sousa, R.R.M.; de Araújo, F.O.; Gontijo, L.C.; da Costa, J.A.P.; Alves, C. Cathodic cage plasma nitriding (CCPN) of austenitic stainless steel (AISI 316): Influence of the different ratios of the (N2/H2) on the nitrided layers properties. Vacuum 2012, 86. [Google Scholar] [CrossRef]
- De Sousa, R.R.M.; de Araújo, F.O.; Barbosa, J.C.P.; Ribeiro, K.J.B.; da Costa, J.A.P.; Alves, C. Nitriding using cathodic cage technique of austenitic stainless steel AISI 316 with addition of CH4. Mater. Sci. Eng. A 2008, 487. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Yoshida, D.; Yamauchi, N.; Ueda, N.; Sone, T.; Tanaka, S. Surface material design of 316 stainless steel by combination of low temperature carburizing and nitriding. Surf. Coat. Technol. 2005, 200. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, C.X.; Dong, H.; Bell, T. Low temperature plasma nitrocarburising of AISI 316 austenitic stainless steel. Surf. Coat. Technol. 2005, 191. [Google Scholar] [CrossRef]
- Borowski, T.; Adamczyk-Cieślak, B.; Brojanowska, A.; Kulikowski, K.; Wierzchoń, T. Surface modification of austenitic steel by various glow-discharge nitriding methods. Medziagotyra 2015, 21. [Google Scholar] [CrossRef]
- Czerwiec, T.; He, H.; Marcos, G.; Thiriet, T.; Weber, S.; Michel, H. Fundamental and Innovations in Plasma Assisted Diffusion of Nitrogen and Carbon in Austenitic Stainless Steels and Related Alloys. Plasma Process. Polym. 2009, 6. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Yamauchi, N.; Ueda, N.; Sone, T.; Hirose, Y. Behavior of carbon in low temperature plasma nitriding layer of austenitic stainless steel. Surf. Coat. Technol. 2005, 193. [Google Scholar] [CrossRef]
- Pillaca, E.J.D.M.; Ueda, M.; Oliveira, R.M.; Pichon, L. Study of the effects of E × B fields as mechanism to carbon–nitrogen plasma immersion ion implantation on stainless steel samples. Appl. Surf. Sci. 2014, 310. [Google Scholar] [CrossRef]
- Borowski, T.; Jeleńkowski, J.; Psoda, M.; Wierzchoń, T. Modifying the structure of glow discharge nitrided layers produced on high-nickel chromium-less steel with the participation of an athermal martensitic transformation. Surf. Coat. Technol. 2010, 204. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Wang, L.; Qiang, J.; Hei, Z. Structural characteristics of low-temperature plasma-nitrided layers on AISI 304 stainless steel with an α′-martensite layer. Surf. Coat. Technol. 2002, 153. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Dong, H. New insights into the mechanism of low-temperature active-screen plasma nitriding of austenitic stainless steel. Scr. Mater. 2012, 67. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Dong, H.; Guo, P.; Gu, D. Nitrogen mass transfer and surface layer formation during the active screen plasma nitriding of austenitic stainless steels. Vacuum 2018, 148. [Google Scholar] [CrossRef]
- Nishimoto, A.; Fukube, T.; Tanaka, T. Effect of Surface Deposits on Nitriding Layer Formation of Active Screen Plasma Nitriding. Mater. Trans. 2016, 57. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Luqman, M.; Ragab, S.A.; Almotairy, S.M. Influence of Chloride Ions on Electrochemical Corrosion Behavior of Dual-Phase Steel over Conventional Rebar in Pore Solution. Appl. Sci. 2020, 10, 4568. [Google Scholar] [CrossRef]
- Lei, M.; Zhu, X.M. In vitro corrosion resistance of plasma source ion nitrided austenitic stainless steels. Biomaterials 2001, 22, 641–647. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion behaviour of low temperature nitrided nickel-free, AISI 200 and AISI 300 series austenitic stainless steels in NaCl solution. Corros. Sci. 2018, 136. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, L. Surface properties of nitrided layer on AISI 316L austenitic stainless steel produced by high temperature plasma nitriding in short time. Appl. Surf. Sci. 2014, 298. [Google Scholar] [CrossRef]
- Grabke, H.J. High Nitrogen Steels. The Role of Nitrogen in the Corrosion of Iron and Steels. ISIJ Int. 1996, 36. [Google Scholar] [CrossRef]


| Material | a, nm | ε, % |
|---|---|---|
| IS | 0.360 | - |
| ASPN | 0.391 | 8.6 |
| ASPNC | 0.393 | 9.2 |
| Material | HV0.05 | Ra, nm |
|---|---|---|
| IS | 264 ± 5 | 62 ± 3 |
| ASPN | 1207 ± 38 | 158 ± 3 |
| ASPNC | 972 ± 16 | 169 ± 1 |
| Material | Ecorr, mV | icorr, μA·cm−2 | Rpol, kΩ·cm2 | ipas, μA·cm−2 (at 750 mV) | Epit, mV |
|---|---|---|---|---|---|
| IS | −142 | 0.008 | 516 | - | 355 |
| ASPN | −148 | 0.007 | 502 | 18 | ≥1500 |
| ASPNC | −51 | 0.003 | 766 | 9 | ≥1500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowski, T. Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising. Materials 2021, 14, 3320. https://doi.org/10.3390/ma14123320
Borowski T. Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising. Materials. 2021; 14(12):3320. https://doi.org/10.3390/ma14123320
Chicago/Turabian StyleBorowski, Tomasz. 2021. "Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising" Materials 14, no. 12: 3320. https://doi.org/10.3390/ma14123320
APA StyleBorowski, T. (2021). Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising. Materials, 14(12), 3320. https://doi.org/10.3390/ma14123320






