Biomaterials for Periodontal and Peri-Implant Regeneration
Abstract
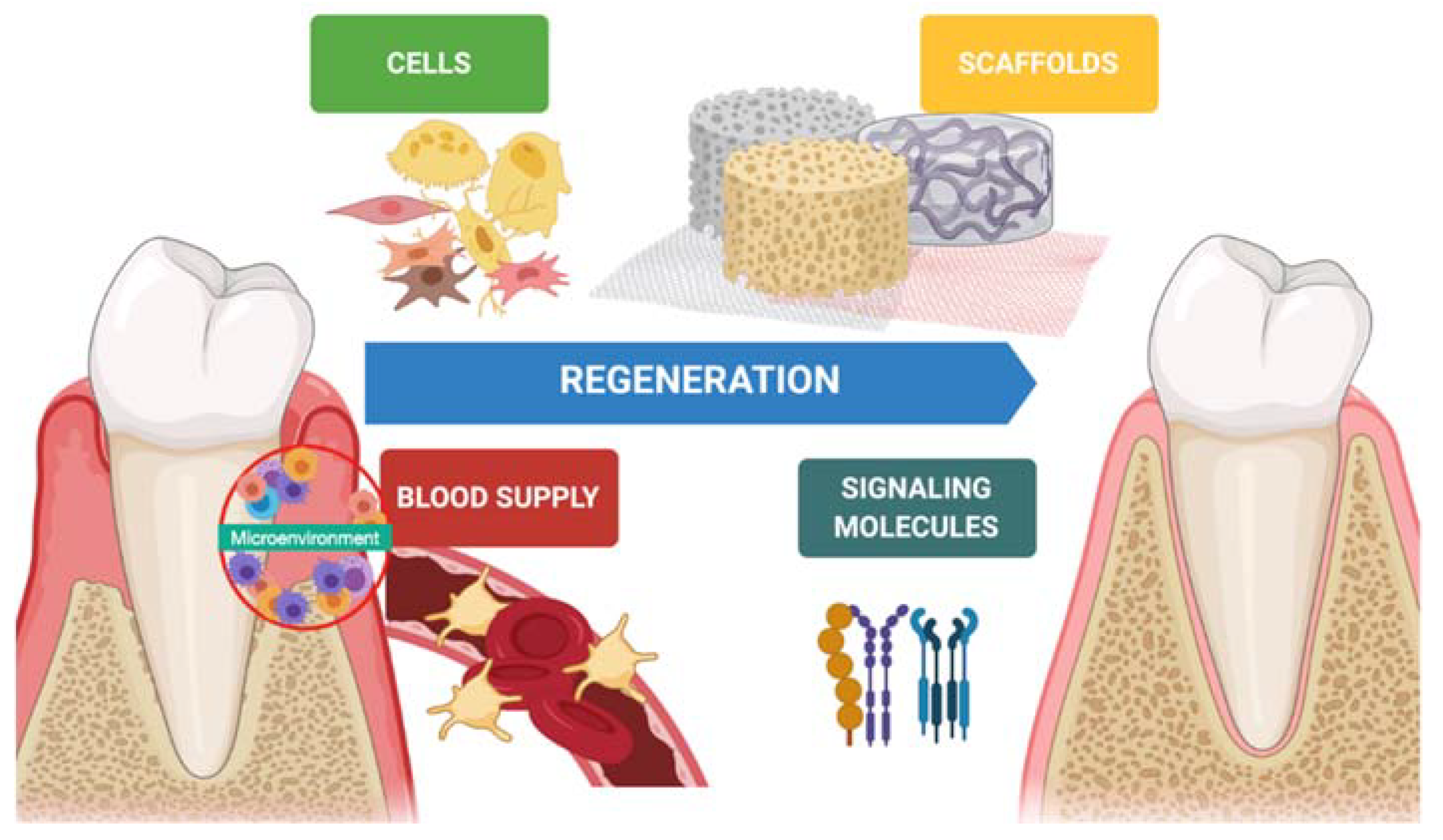
1. Introduction
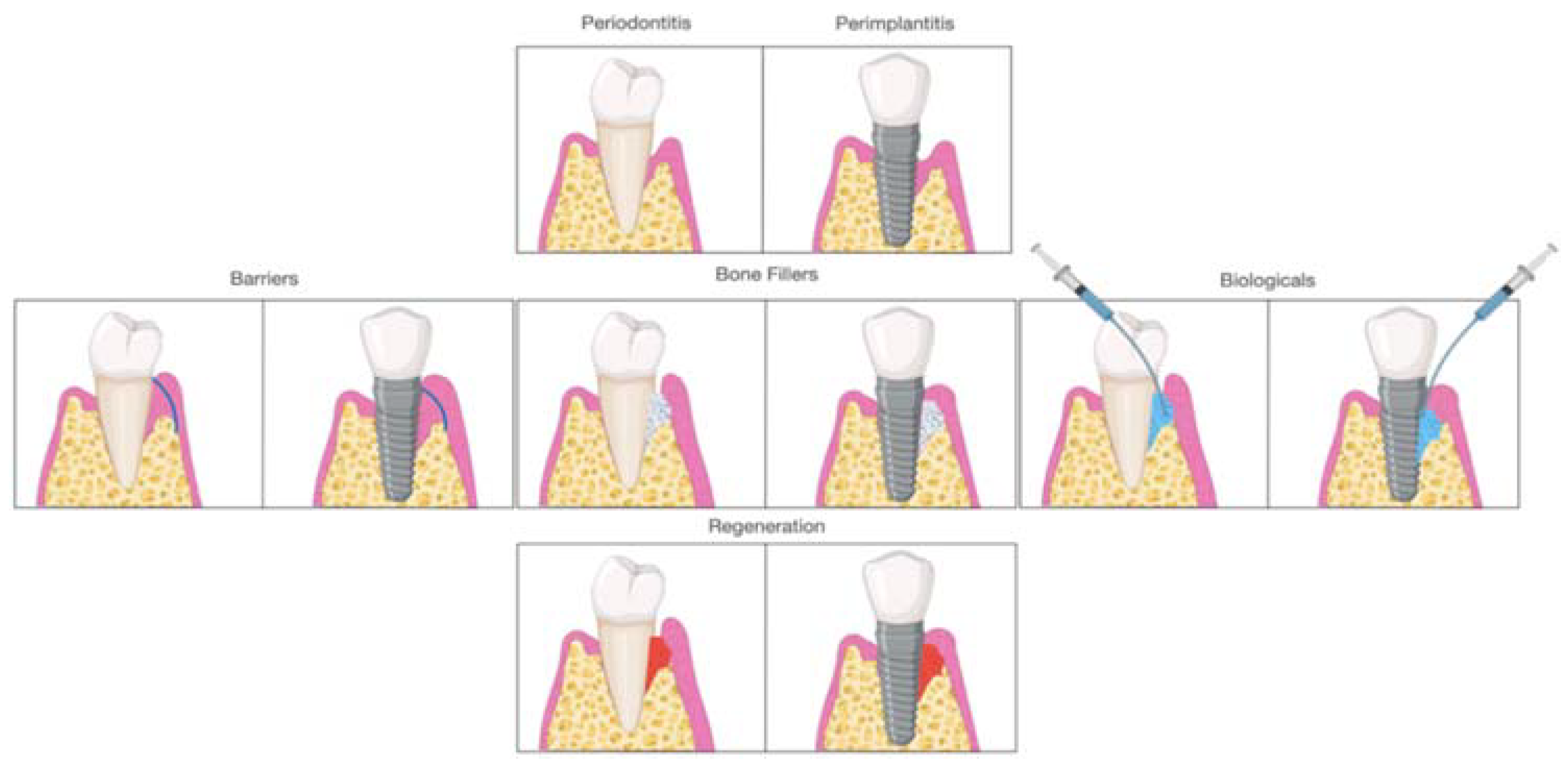
2. Materials
2.1. Bone Fillers
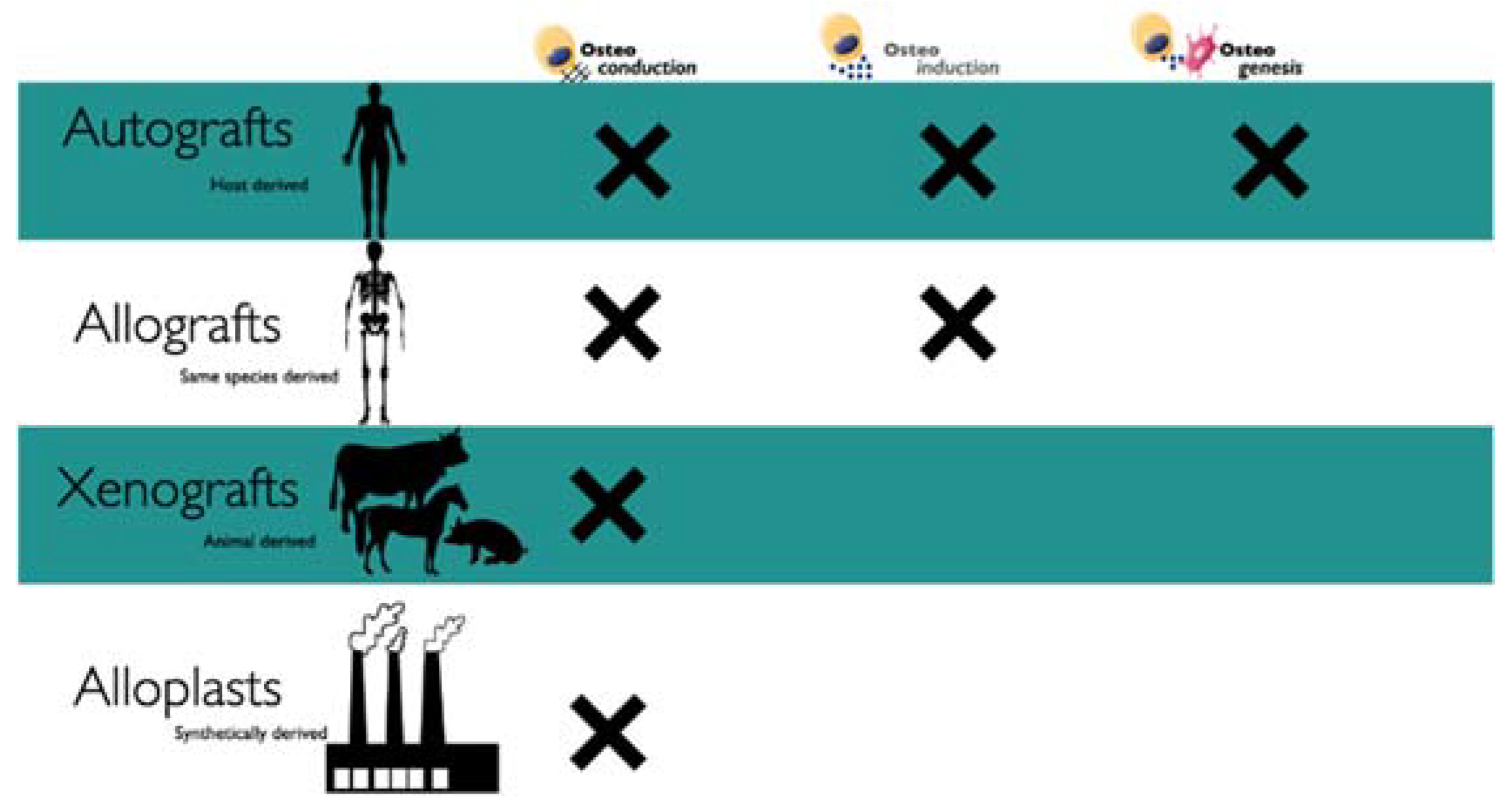
- Autografts are the gold standard due to the osteogenic, osteoconductive, and osteoinductive potential and the absence of foreign body reactions (FBR) [23]. Depending on the size of the defect, the autograft is usually harvested intraorally from the extraction socket, edentulous ridge, symphysis, tuberosity, or buccal plate (Figure 4).
- Allografts are biological materials harvested from the same species. The advantage of allografts is the elimination of a second surgical site and tissue availability. Tissue banks are involved in the extraction process from which tissue is extracted, and depending on the treatment, it is possible to obtain freeze-dried bone (FDBAs) or decalcified freeze-dried bone (DFDBAs). The disadvantage in using these types of biomaterials is the possible FBR and disease transmission; although, in the last years, severe and rigid procedures have been developed to reduce the risk [34,35]. Nevertheless, researchers and clinicians identified allografts as reliable sources for the regenerative procedure since they can serve as osteoconductive or osteoinductive biomaterials preserved of proteins in their matrix [36]. The allograft’s decalcification leads to an exposure of bone morphogenic proteins (BMPs) that are effective molecules in bone regeneration [36]. In the case of allograft, the exposure of BMPs showed an increase in bone resorption during the follow-up period. Nevertheless, a disadvantage in using allografts is the high cost compared to xenografts and autografts [37]. Moreover, it is not available in several counties for ethical and legal reasons.
- Xenografts are bone substitutes obtained from other species, such as bovine or porcine grafts, and transplanted into humans. The main disadvantage of xenografts is the antigenicity; indeed, these tissues need to be carefully treated to remove the organic components [18]. Several commercial products have been proposed based on this protocol (Table 1), such as Geistlich Bio-Oss® particles (Geistlich Pharma, Wolhusen, Switzerland), which are harvested bovine and is considered a global reference product in oral regeneration (Figure 5). Despite positive results from several studies, the disadvantage is in the unpredictable grade of regeneration and resorption. The advantages are a single surgical procedure, availability, and reduced patient morbidity. According to Stavropoulos et al. (2005, 2010), the use of deproteinized bovine bone (DBB) in adjunct to GTR renders the defect more stable on a long-term follow-up [38,39,40].
2.2. Barriers
2.2.1. Resorbable Barriers
2.2.2. Non-Resorbable Barriers
2.3. Biologics
- PDGF is primarily involved in wound healing; several studies showed its function and ability to enhance the proliferation and migration of PDL cells [71,72]. Moreover, the chemotactic effect leads to a promotion of collagen synthesis and can stimulate gingival fibroblasts to the hyaluronate synthesis [73,74,75,76]. This growth factor might be effective alone or in combination with other growth factors, such as the insulin-like growth factor-1 (IGF-1). Indeed, several in vivo studies showed the efficacy of PDGF in periodontal regeneration alone or combined, and it always demonstrated the new formation of cementum and the production of collagen [77,78,79]. Thanks to molecular cloning, it is now possible to reproduce a recombinant human PDGF [77]. Nevertheless, this type of recombinant product is not sold in several nations, such as Italy, for ethical problems. The most used and analyzed product is GEM 21S®, (Osteohealth, Shirley, NY, USA) with in vivo and in vitro studies [80].
- BMPs are factors that belong to the superfamily of transforming growth factor-beta (TGF-ß), are abundant in bone tissue, and are produced by several cells including osteoclasts and osteoblasts. Two types (BMP-4 and BMP-7) are commonly enclosed in allografts, demonstrating osteoinductivity and influencing cells’ behavior in bone regeneration [81,82,83]. Moreover, BMPs act as a chemoattractant for osteoblast precursors and undifferentiated stem cells (MSCs) through the activation of genes related to bone formation, such as osteocalcin [84,85]. A disadvantage in the extraction of BMPs is the synthetic production, which is very expensive, and there is a limitation for the encapsulation in synthetic biomaterials [85].
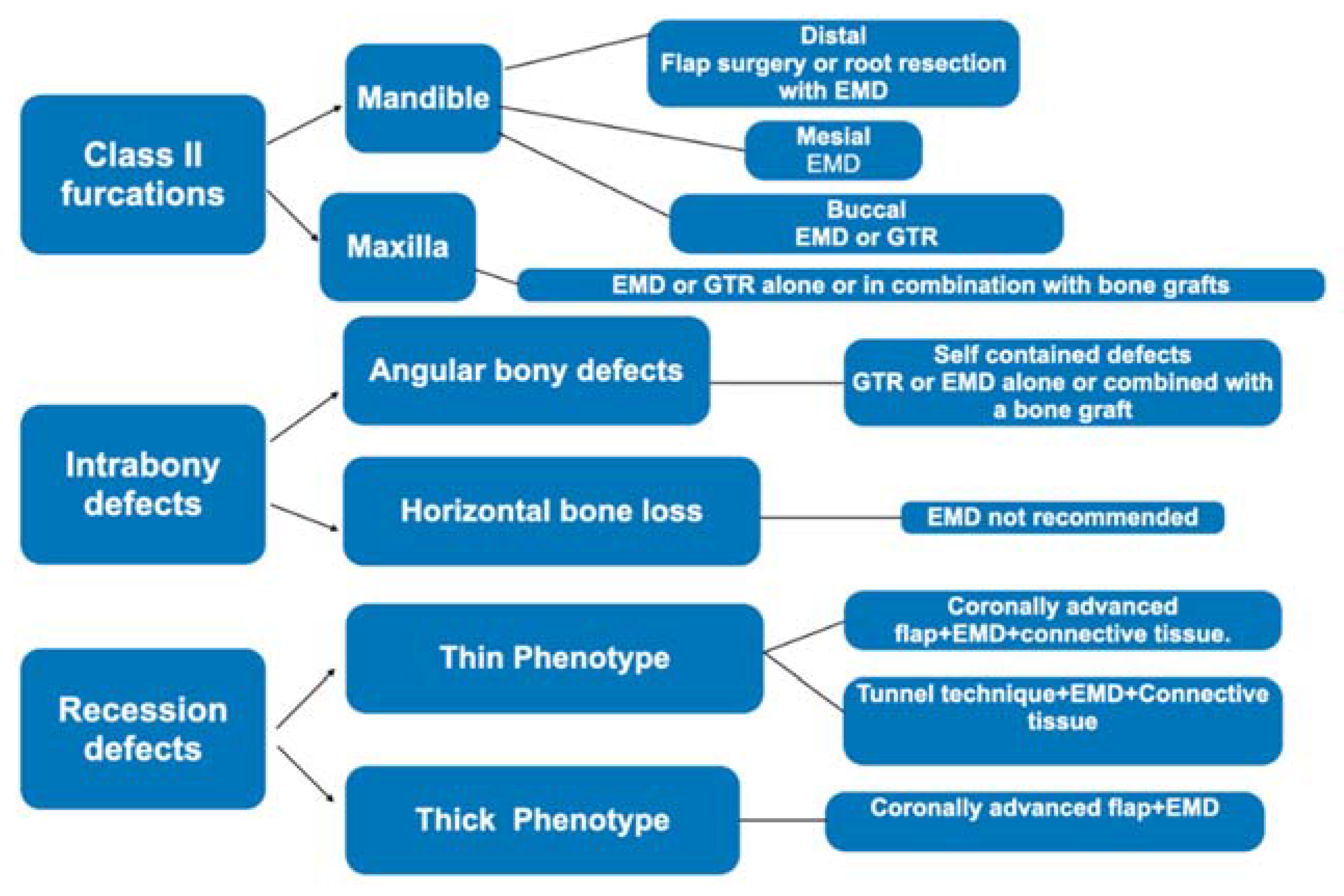
- EMD is released by Hertwig’s cells during the formation of teeth and periodontal tissue, and these proteins are situated on the root surface, influencing the initial steps of cementum, alveolar bone, and periodontal ligament formation [86,87]. In origin (1996), a Swedish factory (Biora, Malmö, Sweden) released the actual and unique EMD derivatives extracted from porcine enamel in the form of purified acid. Later, Straumann AS acquired the title and Emdogain® (Straumann AG, Basel, Switzerland) is the name of the unique enamel derivates on the market. It is composed mainly of amelogenins, which are specific proteins fundamental in the enamel mineralization process. In physiological conditions, the amelogenins are nano formed, and during the enzymatic degradation by metalloproteinases (MMP), they release bioactive peptides for weeks [88]. In this process, there are advantages, such as the stimulation of new bone and wound healing conditioning. On the other hand, this process might create root resorption due to the presence of MMP and an inflammatory pattern during the regenerative phase [89]. The advantage of using EMD is the mimic action, which can recruit cementoblasts to form new root cementum and consequently facilitate the formation of a new periodontal ligament [89]. This product has been on the market since 1997, and several articles underlined the ease of handling, an interesting result in periodontal regeneration [90,91,92,93,94]. Miron et al. in (2016) collected all the data regarding EMD in periodontal regeneration, and in this study, the use of EMD was relevant in adjunct to non-surgical therapy and regenerative procedures, according to the defect size and shape (Figure 7) [95]. According to the literature, EMD, after 25 years from its introduction, seems to be unique in demonstrating a histological periodontal regeneration with new cementum and periodontal ligament and the presence of Sharpey’s fibers in the periodontal structure [95]. Regarding the use of EMD around implants, data collected from a randomized clinical trial, according to Isehed et al. (2016), revealed that EMD delivered promising but insufficient regeneration associated with an alteration of the Gram-negative flora [96].
- Hyaluronic acid (HA) is a natural glycosaminoglycan contained in several tissues, such as connective tissue. It is an excellent scaffold for periodontal regeneration. Moreover, it seems to have an antimicrobial and anti-inflammatory effect [97,98]. The principal factor that makes this a promising biomaterial is the viscoelastic property and the capacity for absorbing a considerable amount of water. This renders hyaluronic acid a periodontal filler, and, in several situations, it has a protective function as a barrier for bacteria and viruses. Pilloni et al. (2019) suggested the use of HA with a collagen membrane in periodontal defects [99,100]. A systematic review from Eliezer et al. suggested that the addition of HA to non-surgical and surgical periodontal therapy may have additional clinical effects on the clinical attachment level (CAL, 0.73 mm; 95% CI, 0.28 to 1.17 mm; p < 0.0001), periodontal depth (PD, 0.36 mm; 95% CI, −0.54 to −0.19 mm; p < 0.0001), and bleeding on probing (BoP, 5%; 95% CI, −22 to −8%; p < 0.001) [101]. Regarding the use of HA in peri-implant defects, several studies suggest the benefit in microflora diversity, and at the same time, HA acts as a protective shield against bacteria colonization [102,103]. Interesting data from an animal study suggested the inhibition of the downgrowth of connective tissue inside the peri-implant defect, facilitating bone regeneration and implant stability [104].
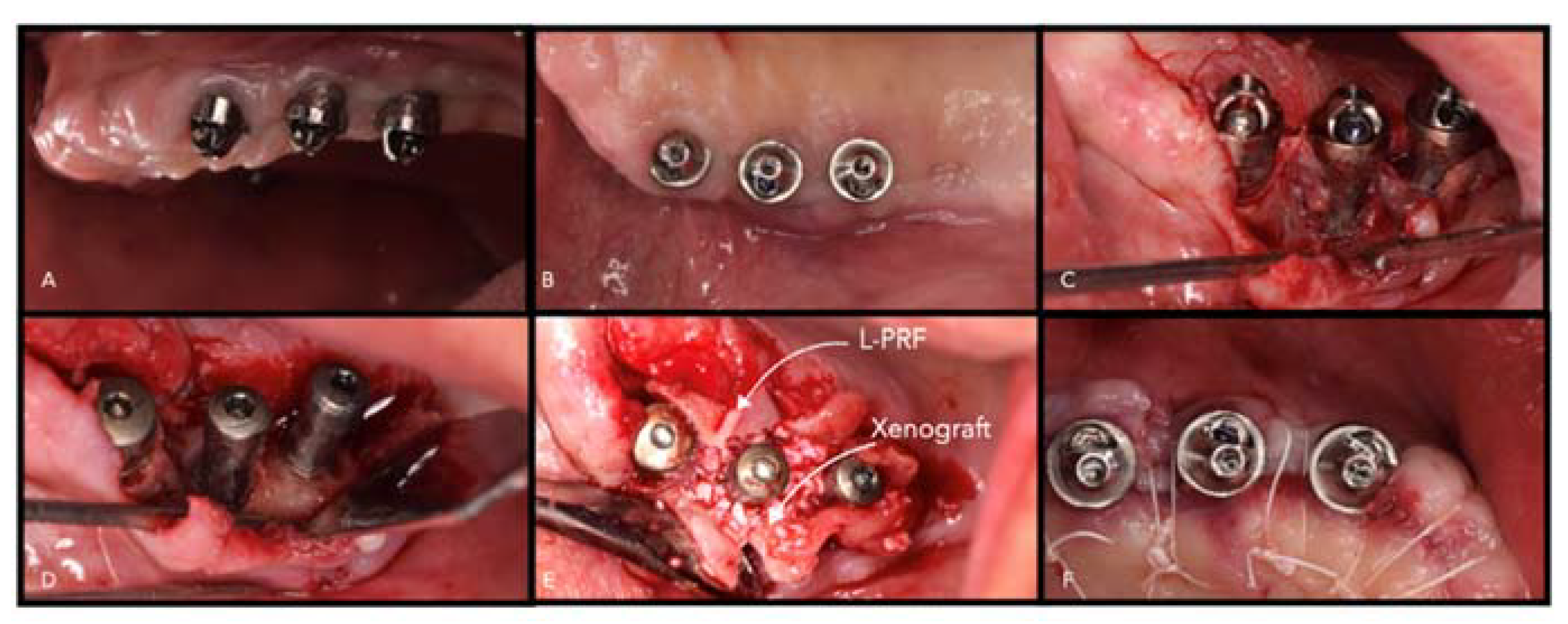
- Autologous platelet concentrates (APG) are promising biomaterials in periodontal and peri-implant regeneration. There are several protocols published (platelet-rich fibrin, PRF/A-PRF/L-PRF; platelet-rich plasma (PRP) platelet-rich growth factors, (PRGF) in the literature, and the main composition is based on platelet fibrin and growth factors, such as PDGF, vascular endothelial growth factors (VEGF), and transforming growth factors beta (TGF- b) [75,76,105]. They are defined as natural living cell scaffolds and according to several systematic reviews are valid biomaterials in periodontal and peri-implant regeneration [106,107,108] (Figure 8). The advantages are autologous origin and the fast and chip protocol. On the other hand, the handling and the production process differs among the types (PRF, A-PRF, PRP, PRGF). Another disadvantage is the fast resorption pattern that was estimated to be among 14 and 20 days [105]. Nevertheless, due to the fibrin scaffold and the presence of growth factors, they are promising biomaterial. Future studies are investigating PRF as a drug delivery system in periodontal defects [109].
3. Emerging Technologies
3.1. Stem Cell Therapies
3.2. Three-Dimensional Printing
- Inkjet model: consists of using inkjet printing with powder and liquid solutions to select and dispose of cells, create an extracellular matrix, and allows the use of a customized scaffold [116]. Park et al. (2012, 2014) published the use of a 3D fiber scaffold for guiding PDL cells and facilitating the mineralization of tissue [117,118]. Goh et al. (2015) analyzed the use of a 3D scaffold in socket preservation with normal bone healing and better-preserved volume [109].
- Fusion model: allows building personalized scaffolds but without the inclusion of cells, growth factors, and proteins [116]. The polymer used is lactic-co-glycolic acid with good characteristics of resorption and mechanical strength.
- 3D plotting allows the production of a soft scaffold composed of hydrogel with easy incorporation of cells. A limitation is the possible inhibition of cell-to-cell communication, influencing the signaling and proliferation process [119,120]. On the other hand, the use of living cells in the scaffold has great results for tissue formation.
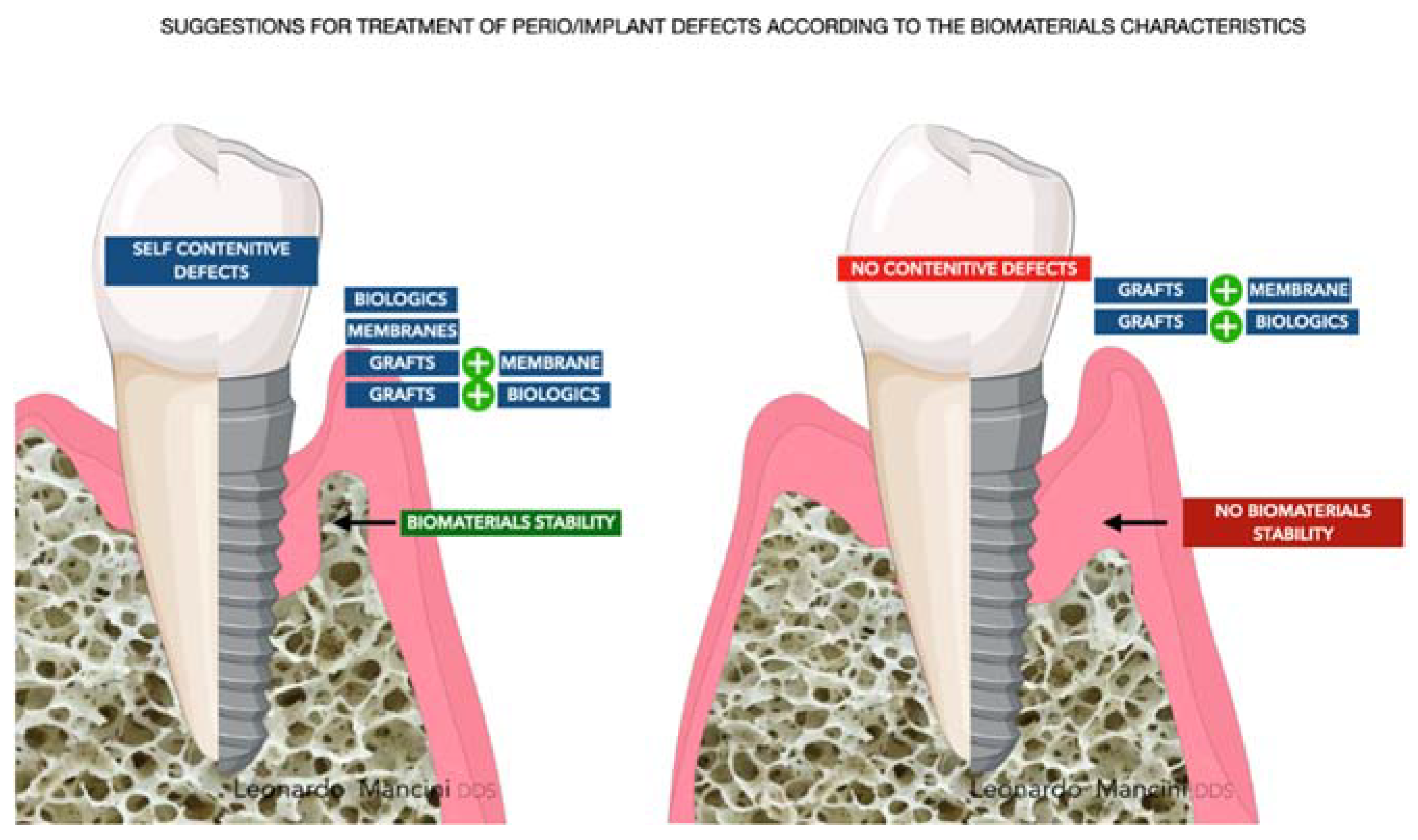
4. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, M.H. Changes in regenerative capacity through lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [PubMed]
- Sallum, E.A.; Ribeiro, F.V.; Ruiz, K.S.; Sallum, A.W. Experimental and clinical studies on regenerative periodontal therapy. Periodontology 2000 2019, 79, 22–55. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000 2017, 73, 73–83. [Google Scholar] [CrossRef]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Romandini, M.; Gioco, G.; Perfetti, G.; Deli, G.; Staderini, E.; Lafori, A. The association between periodontitis and sleep duration. J. Clin. Periodontol. 2017, 44, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Shin, H.S.; Romandini, P.; Laforí, A.; Cordaro, M. Hormone-related events and periodontitis in women. J. Clin. Periodontol. 2020, 47, 429–441. [Google Scholar] [CrossRef]
- Schwarz, F.; Giannobile, W.V.; Jung, R.E.; Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 2—Effects of hard tissue augmentation procedures on the maintenance of peri-implant tissues. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 11–13. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Schwarz, F.; Jung, R.; Sanz, M.; Thoma, D. Effects of lateral bone augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 18–31. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Biologics-based regenerative technologies for periodontal soft tissue engineering. J. Periodontal. 2020, 91, 147–154. [Google Scholar] [CrossRef]
- Romandini, M.; Pedrinaci, I.; Lima, C.; Soldini, M.C.; Araoz, A.; Sanz, M. Prevalence and risk/protective indicators of buccal soft tissue dehiscence around dental implants. J. Clin. Periodontol. 2021, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Tavelli, L.; McGuire, M.K.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J. Periodontal. 2020, 91, 9–16. [Google Scholar] [CrossRef]
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New attachment formation in the human periodontium by guided tissue regeneration: Case reports. J. Clin. Periodontol. 1986, 13, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjo, U.M.E. Wound healing following surgical and regenerative periodontal therapy. Periodontology 2000 2015, 68, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2000 2009, 51, 208–219. [Google Scholar] [CrossRef]
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigon, A.; et al. Regeneration of alveolar ridge defects—Consensus report of Group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 82–91. [Google Scholar] [CrossRef]
- Cosyn, J.; Thoma, D.S.; Hämmerle, C.H.; De Bruyn, H. Esthetic assessments in implant dentistry: Objective and subjective criteria for clinicians and patients. Periodontology 2000 2017, 73, 193–202. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef]
- Bernardi, S.; Macchiarelli, G.; Bianchi, S. Autologous Materials in Regenerative Dentistry: Harvested Bone, Platelet Concentrates and Dentin Derivates. Molecules 2020, 25, 5330. [Google Scholar] [CrossRef] [PubMed]
- Torroni, A.; Marianetti, T.M.; Romandini, M.; Gasparini, G.; Cervelli, D. Mandibular reconstruction with different techniques. J. Craniofac. Surg. 2015, 26, 885–890. [Google Scholar] [CrossRef]
- Nabers, C.L.; O’Leary, T.J. Autogenous bone trasplant in the treatment of osseus defects. J. Periodontol. 1965, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cushing, M. Autogenous red marrow grafts: Potential induction of osteogenesis. J. Periodontol. 1969, 40, 492–497. [Google Scholar] [CrossRef]
- Sottosanti, J.S.; Bierly, J.A. The storage of marrow and its relation to periodontal grafting procedures. J. Periodontol. 1975, 46, 162–170. [Google Scholar] [CrossRef]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, P.; Duvina, M.; Barbato, L.; Biondi, E.; Nuti, N. Bone regeneration in dentistry. Clin. Cases Miner. Bone Metab. 2011, 8, 24. [Google Scholar]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef]
- Proussaefs, P.; Lozada, J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: A human study. Int. J. Periodontics Restor. Dent. 2005, 25, 351–363. [Google Scholar]
- Schallhorn, R.G. The use of autogenous hip marrow biopsy implants for bony crater defects. J. Periodontol. 1968, 39, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone: The influence of processing on safety and performance. Orthop. Clin. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Tomford, W.W. Transmission of disease through transplantation of musculoskeletal allografts. J. Bone Jt. Surg. Am. 1995, 77, 1742–1754. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Al Ghamdi, A.; Shibly, O.; Ciancio, S. Osseous grafting part II: Xenografts and alloplasts for periodontal regeneration—A literature review. J. Int. Acad. Periodontol. 2010, 12, 39–44. [Google Scholar]
- Stavropoulos, A.; Karring, T. Five-year results of guided tissue regeneration in combination with deproteinized bovine bone (Bio-Oss) in the treatment of intrabony periodontal defects: A case series report. Clin. Oral Investig. 2005, 9, 271–277. [Google Scholar] [CrossRef]
- Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A. Maxillary Sinus Augmentation with Decellularized Bovine Compact Particles: A Radiological, Clinical, and Histologic Report of 4 Cases. BioMed Res. Int. 2017, 2594670. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Wach, T. New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation. Materials 2020, 13, 2935. [Google Scholar] [CrossRef]
- Kollati, P.; Koneru, S.; Dwarakanath, C.D.; Gottumukkala, S. Effectiveness of naturally derived bovine hydroxyapatite (Cerabone™) combined with platelet-rich fibrin matrix in socket preservation: A randomized controlled clinical trial. J. Indian Soc. Periodontol. 2019, 23, 145–151. [Google Scholar] [PubMed]
- Kyyak, S.; Blatt, S.; Schiegnitz, E.; Heimes, D.; Staedt, H.; Thiem, D.; Sagheb, K.; Al-Nawas, B.; Kämmerer, P.W. Activation of Human Osteoblasts via Different Bovine Bone Substitute Materials with and without Injectable Platelet Rich Fibrin In Vitro. Front. Bioeng. Biotechnol. 2021, 9, 599224. [Google Scholar] [CrossRef] [PubMed]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: Preliminary study in a rabbit cranial bone model. Clin. Oral Implant. Res. 2012, 23, 698–705. [Google Scholar] [CrossRef]
- Ortiz-Puigpelat, O.; Simões, A.; Caballé-Serrano, J.; Hernández-Alfaro, F. Blood absorption capacity of different xenograft bone substitutes: An in vitro study. J. Clin. Exp. Dent. 2019, 11, e1018–e1024. [Google Scholar] [CrossRef]
- Rombouts, C.; Jeanneau, C.; Camilleri, J.; Laurent, P.; About, I. Characterization and angiogenic potential of xenogeneic bone grafting materials: Role of periodontal ligament cells. Dent. Mater. J. 2016, 35, 900–907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, V.J.; Michalek, J.E.; Liu, Q.; Mealey, B.L. Ridge preservation following tooth extraction using bovine xenograft compared with porcine xenograft: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 361–368. [Google Scholar] [CrossRef]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef]
- Stievano, D.; di Stefano, A.; Ludovichetti, M.; Pagnutti, S.; Gazzola, F.; Boato, C.; Stellini, E. Maxillary sinus lift through heterologous bone grafts and simultaneous acid-etched implants placement: Five-year follow-up. Minerva Chirurgica 2008, 63, 79–91. [Google Scholar] [PubMed]
- Stavropoulos, A.; Karring, T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss) in the treatment of intrabony periodontal defects—6-year results from a randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 200–210. [Google Scholar] [CrossRef]
- Caton, J.; de Furia, E.; Polson, A.; Nyman, S. Periodontal regeneration via selective cell repopulation. J. Periodontol. 1987, 58, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Gottlow, J.; Lindhe, J.; Karring, T.; Wennstrom, J. New attachment formation by guided tissue regeneration. J. Periodontal Res. 1987, 22, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration—Intrabony defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. S2), S77–S104. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Jiménez Garcia, J.; Berghezan, S.; Caramês, J.; Dard, M.M.; Marques, D. Effect of cross-linked vs non-cross-linked collagen membranes on bone: A systematic review. J. Periodontal Res. 2017, 52, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Aurer, A.; Jorgic-Srdjak, K. Membranes for periodontal regeneration. Acta Stomatol. Croat. 2005, 39, 107–112. [Google Scholar]
- Singh, A.K. GTR membranes: The barriers for periodontal regeneration. DHR Int. J. Med. Sci. 2013, 4, 31–38. [Google Scholar]
- Minabe, M. A critical review of the biologic rationale for guided tissue regeneration. J. Periodontol. 1991, 62, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef]
- Bartee, B.K.; Carr, J. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J. Oral Implantol. 1995, 21, 88–95. [Google Scholar]
- Marouf, H.A.; El-Guindi, H.M. Efficacy of high-density versus semipermeable PTFE membranes in an elderly experimental model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2000, 89, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Babo, P.S.; Pires, R.L.; Reis, R.L.; Gomes, M.E. Membranes for periodontal tissues regeneration. Ciência Tecnol. Mater. 2014, 26, 108–117. [Google Scholar]
- Monteiro, A.; Macedo, L.; Macedo, N.-L.; Balducci, I. Polyurethane and PTFE membranes for guided bone regeneration: Histopathological and ultrastructural evaluation. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e401–e406. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Pini Prato, G.; Tonetti, M.S. Periodontal regeneration of human intrabony defects with titanium reinforced membranes: A controlled clinical trial. J. Periodontol. 1995, 66, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G. Postoperative healing complications associated with Gore-Tex periodontal material, part I: Incidence and characterization. Int. J. Periodontics Restor. Dent. 1995, 15, 363–375. [Google Scholar]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The modified papilla preservation technique: A new surgical approach for interproximal regenerative procedures. J. Periodontol. 1995, 66, 261–266. [Google Scholar] [CrossRef]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The simplified papilla preservation flap: A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599. [Google Scholar]
- Cortellini, P.; Tonetti, M.S. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: A novel approach to limit morbidity. J. Clin. Periodontol. 2007, 34, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Franceschetti, G. Use of the single flap approach in periodontal reconstructive surgery. Dent. Cadmos 2007, 8, 15–25. [Google Scholar]
- Aimetti, M.; Fratini, A.; Manavella, V.; Giraudi, M.; Citterio, F.; Ferrarotti, F.; Mariani, G.M.; Cairo, F.; Baima, G.; Romano, F. Pocket resolution in regenerative treatment of intrabony defects with papilla preservation techniques: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2021, 48, 843–858. [Google Scholar] [CrossRef]
- Dennison, D.K.; Vallone, D.R.; Pinero, G.J.; Rittman, B.; Caffesse, R.G. Differential effect of TGF-β1 and PDGF on proliferation of periodontal ligament cells and gingival fibroblasts. J. Periodontol. 1994, 65, 641–648. [Google Scholar] [CrossRef]
- Oates, T.W.; Rouse, C.A.; Cochran, D.L. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J. Periodontol. 1993, 64, 142–148. [Google Scholar] [CrossRef]
- Giannobile, W.; Whitson, S.; Lynch, S. Non-coordinate control of bone formation displayed by growth factor combinations with IGF-I. J. Dent. Res. 1997, 76, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Stähli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 6–19. [Google Scholar] [CrossRef]
- Mancini, L.; Tarallo, F.; Quinzi, V.; Fratini, A.; Mummolo, S. Platelet-Rich Fibrin in Single and Multiple Coronally Advanced Flap for Type 1 Recession: An Updated Systematic Review and Meta-Analysis. Medicina 2021, 57, 144. [Google Scholar] [CrossRef]
- Tarallo, F.; Mancini, L.; Pitzurra, L.; Bizzarro, S.; Tepedino, M.; Marchetti, E. Use of Platelet-Rich Fibrin in the Treatment of Grade 2 Furcation Defects: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J. Periodontol. 2003, 74, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Hernandez, R.A.; Finkelman, R.D.; Ryarr, S.; Kiritsy, C.P. Comparative effects of plateletderived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J. Periodontal Res. 1996, 31, 301–312. [Google Scholar] [CrossRef]
- Lynch, S.E.; Buser, D.; Hernandez, R.A.; Weber, H.; Stich, H.; Fox, C.H.; Williams, R.C. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants: Results of a pilot study in beagle dogs. J. Periodontol. 1991, 62, 710–716. [Google Scholar] [CrossRef]
- Izumi, Y.; Aoki, A.; Yamada, Y.; Kobayashi, H.; Iwata, T.; Akizuki, T.; Suda, T.; Nakamura, S.; Wara-Aswapati, N.; Ueda, M.; et al. Current and future periodontal tissue engineering. Periodontology 2000 2011, 56, 166–187. [Google Scholar] [CrossRef]
- Thoma, D.S.; Payer, M.; Jakse, N.; Bienz, S.P.; Husler, J.; Schmidlin, P.R.; Jung, U.-W.; Hammerle, C.H.F.; Jung, R.E. Randomized, controlled clinical two-centre study using xenogeneic block grafts loaded with recombinant human bone morphogenetic protein-2 or autogenous bone blocks for lateral ridge augmentation. J. Clin. Periodontol. 2018, 45, 265–276. [Google Scholar] [CrossRef]
- Giannobile, W. Periodontal tissue engineering by growth factors. Bone 1996, 19, S23–S37. [Google Scholar] [CrossRef]
- Chiu, H.C.; Chiang, C.Y.; Tu, H.P.; Wikesjo, U.M.; Susin, C. Effects of bone morphogenetic protein-6 on periodontal wound healing/regeneration in supraalveolar periodontal defects in dogs. J. Clin. Periodontol. 2013, 40, 624–630. [Google Scholar] [CrossRef]
- Sigurdsson, T.J.; Lee, M.B.; Kubota, K.; Turek, T.J.; Wozney, J.M. Periodontal repair in dogs: Recombinant human bone morphogenetic protein-2 significantly enhances periodontal regeneration. J. Periodontol. 1995, 66, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.; Guglielmoni, P.; Promsudthi, A.; Cho, K.S.; Trombelli, L.; Selvig, K.A.; Jin, L.; Wozney, J.M. Periodontal repair in dogs: Effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J. Clin. Periodontol. 1999, 26, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Ito, Y.; Diekwisch, T.G. Evolution and development of Hertwig’s epithelial root sheath. Dev. Dyn. 2006, 235, 1167–1180. [Google Scholar] [CrossRef]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.S.; Arzate, H.; Bringas, P., Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. 2003, 228, 651–663. [Google Scholar] [CrossRef]
- Giannobile, W.V.; Somerman, M.J. Growth and amelogenin-like factors in periodontal wound healing—A systematic review. Ann. Periodontol. 2003, 8, 193–204. [Google Scholar] [CrossRef]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Purschwitz, R. A clinical study evaluating the treatment of supra-alveolar-type defects with access flap surgery with and without an enamel matrix protein derivative: A pilot study. J. Clin. Periodontol. 2008, 35, 713–718. [Google Scholar] [CrossRef]
- Jepsen, S.; Heinz, B.; Jepsen, K.; Arjomand, M.; Hoffmann, T.; Richter, S.; Reich, E.; Sculean, A.; Gonzales, J.R.; Bodeker, R.H.; et al. A randomized clinical trial comparing enamel matrix derivative and membrane treatment of buccal Class II furcation involvement in mandibular molars, Part I: Study design and results for primary outcomes. J. Periodontal. 2004, 75, 1150–1160. [Google Scholar] [CrossRef]
- McGuire, M.K.; Nunn, M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue, Part 1: Comparison of clinical parameters. J. Periodontal. 2003, 74, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Tonetti, M.S.; Zabalegui, I.; Sicilia, A.; Blanco, J.; Rebelo, H.; Rasperini, G.; Merli, M.; Cortellini, P.; Suvan, J.E. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: Results from a multicenter practice-based clinical trial. J. Periodontol. 2004, 75, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Calatrava, J.; Nobili, A.; Calzavara, D.; Sanz, M. Treatment of severe intra-bony lesions with emd application and different soft-tissue management techniques: A case report. Dent. Cadmos 2021, 89, 314–318. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Isehed, C.; Holmlund, A.; Renvert, S.; Svenson, B.; Johansson, I.; Lundberg, P. Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerative treatment of peri-implantitis: A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 863–873. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Müller, H.D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef]
- Pilloni, A.; Nardo, F.; Rojas, M.A. Surgical treatment of a cemental tear-associated bony defect using hyaluronic acid and a resorbable collagen membrane: A 2-year follow-up. Clin. Adv. Periodontics 2019, 9, 64–69. [Google Scholar] [CrossRef]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef]
- Eliezer, M.; Imber, J.C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Lerma, A.; Magan-Fernandez, A.; Gijon, J.; Sanchez-Fernandez, E.; Soriano, M.; Garcia-Salcedo, J.A.; Mesa, F. Short-term effects of hyaluronic acid on the subgingival microbiome in peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 734–745. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Nobre, M.; Cintra, N.; Maló, P. Peri-implant maintenance of immediate function implants: A pilot study comparing hyaluronic acid and chlorhexidine. Int. J. Dent. Hyg. 2007, 5, 87–94. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Hilbig, U.; Hoffmann, C.; Unger, R.E.; Sader, R.A.; Peters, F.; Kirkpatrick, C.J. An injectable bone substitute composed of beta-tricalcium phosphate granules, methylcellulose and hyaluronic acid inhibits connective tissue influx into its implantation bed in vivo. Acta Biomater. 2011, 7, 4018–4028. [Google Scholar] [CrossRef]
- Marchetti, E.; Mancini, L.; Bernardi, S.; Bianchi, S.; Cristiano, L.; Torge, D.; Marzo, G.; Macchiarelli, G. Evaluation of Different Autologous Platelet Concentrate Biomaterials: Morphological and Biological Comparisons and Considerations. Materials 2020, 13, 2282. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Karanxha, L.; Panda, S.; Bucchi, C.; Nadathur Doraiswamy, J.; Sankari, M.; Ramamoorthi, S.; Varghese, S.; Taschieri, S. Autologous platelet concentrates for treating periodontal infrabony defects. Cochrane Database Syst. Rev. 2018, 11, CD011423. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Karanxha, L.; Goker, F.; Satpathy, A.; Taschieri, S.; Francetti, L.; Chandra Das, A.; Kumar, M.; Panda, S.; del Fabbro, M. Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 1347. [Google Scholar] [CrossRef]
- Panda, S.; Purkayastha, A.; Mohanty, R.; Nayak, R.; Satpathy, A.; Chandra Das, A.; Kumar, M.; Mohanty, G.; Panda, S.; Fabbro, M.D. Plasma rich in growth factors (PRGF) in non-surgical periodontal therapy: A randomized clinical trial. Braz. Oral Res. 2020, 34, e034. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Clemer-Shamai, N.; Shapira, L. Incorporating antibiotics into platelet-rich fibrin: A novel antibiotics slow-release biological device. J. Clin. Periodontol. 2019, 46, 241–247. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Trovato, L.; Naro, F.; D’Aiuto, F.; Moreno, F. Promoting tissue repair by micrograft stem cells delivery. Stem Cells Int. 2020, 2195318. [Google Scholar] [CrossRef] [PubMed]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of preclinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® autologous micrografts in oral regeneration: Clinical, histological, and radiographical evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Zheng, R.C.; Park, Y.K.; Cho, J.J.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H. Bone regeneration at dental implant sites with suspended stem cells. J. Dent. Res. 2014, 93, 1005–1013. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Taut, A.D.; Padial-Molina, M.; Flanagan, C.L.; Pilipchuk, S.P.; Hollister, S.J.; Giannobile, W.V. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng. Part C Methods 2014, 20, 533–542. [Google Scholar] [CrossRef]
- Goh, B.T.; Teh, L.Y.; Tan, D.B.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation—A pilot randomised controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 271–277. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J. Dent. Res. 2015, 94 (Suppl. S9), S143–S152. [Google Scholar] [CrossRef]
| Commercial Name | Sources | Heating Temperature |
|---|---|---|
| Bio-Oss® | Bovine | 300 °C [38] |
| Re-bone® | Bovine | −80 °C to 121 °C [41] |
| Endobon® | Bovine | 900 °C [42] |
| cerabone® | Bovine | 1250 °C [43] |
| creosTM | Bovine | 600 °C [44] |
| PepGen P-15® | Bovine | 1100 °C [45] |
| SmartBone® | Bovine + Porcine | 50 °C < [46] |
| Gen-Os® | Porcine | 130 °C [47] |
| Zcore® | Porcine | 500 °C to 620 °C [48] |
| THE GraftTM | Porcine | 400 °C [49] |
| Equimatrix® | Equine | N/A |
| Bio-Gen® | Equine | 130 °C [50] |
| Commercial Name | Sources | Main Components | Cross-Linking Agent | Resorption Rate |
|---|---|---|---|---|
| Bio-Gide | Porcine | Type I and III collagen | None | 24 weeks |
| Biostite | Calfskin | 88% HA 9.5% type I collagen and 2.5% chondroitin sulfate | Diphenylphosphoryl azide | 4–8 weeks |
| BioMend | Bovine | 100% type I collagen | Formaldehyde | 6–8 weeks |
| BioBar | Bovine | 100% type I collagen | N/A | 6–8 months |
| BioMend-Extend | Bovine | 100% type I collagen | Formaldehyde | 18 weeks |
| Periogen | Bovine | Type I and III collagen | Glutaraldehyde | 4–8 weeks |
| Paroguide | Calfskin | 96% type I collagen and 4% chondroitin sulfate | Diphenylphosphoryl azide | 4–8 weeks |
| OsteoBiol | Equine | 100% equine collagen | None | 8 weeks |
| Tissue Guide | Bovine dermis + tendon | Atelocollagen + tendon collagen | Hexamethylene diisocyanate | 4–8 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.M.; Panda, S.; Marchetti, E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials 2021, 14, 3319. https://doi.org/10.3390/ma14123319
Mancini L, Romandini M, Fratini A, Americo LM, Panda S, Marchetti E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials. 2021; 14(12):3319. https://doi.org/10.3390/ma14123319
Chicago/Turabian StyleMancini, Leonardo, Mario Romandini, Adriano Fratini, Lorenzo Maria Americo, Saurav Panda, and Enrico Marchetti. 2021. "Biomaterials for Periodontal and Peri-Implant Regeneration" Materials 14, no. 12: 3319. https://doi.org/10.3390/ma14123319
APA StyleMancini, L., Romandini, M., Fratini, A., Americo, L. M., Panda, S., & Marchetti, E. (2021). Biomaterials for Periodontal and Peri-Implant Regeneration. Materials, 14(12), 3319. https://doi.org/10.3390/ma14123319