Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal
Abstract
1. Introduction
2. Ceramic Powder Waste [CPW]
3. Ceramic Waste as Aggregate
4. Ceramic Industry Sludge
5. Characteristics of Ceramic Waste Incorporating Geopolymer Composites
5.1. Flow/Slump
5.2. Setting Time
5.3. Compressive Strength
5.4. Split Tensile Strength
5.5. Flexural Strength
5.6. Modulus of Elasticity
5.7. Water Absorption
5.8. Carbonation Resistance
5.9. Acid Resistance
5.10. Sulphate Attack
5.11. Freeze–Thaw Cycles
5.12. Wet–Dry Cycles
6. Thermal and Elevated Temperature Study
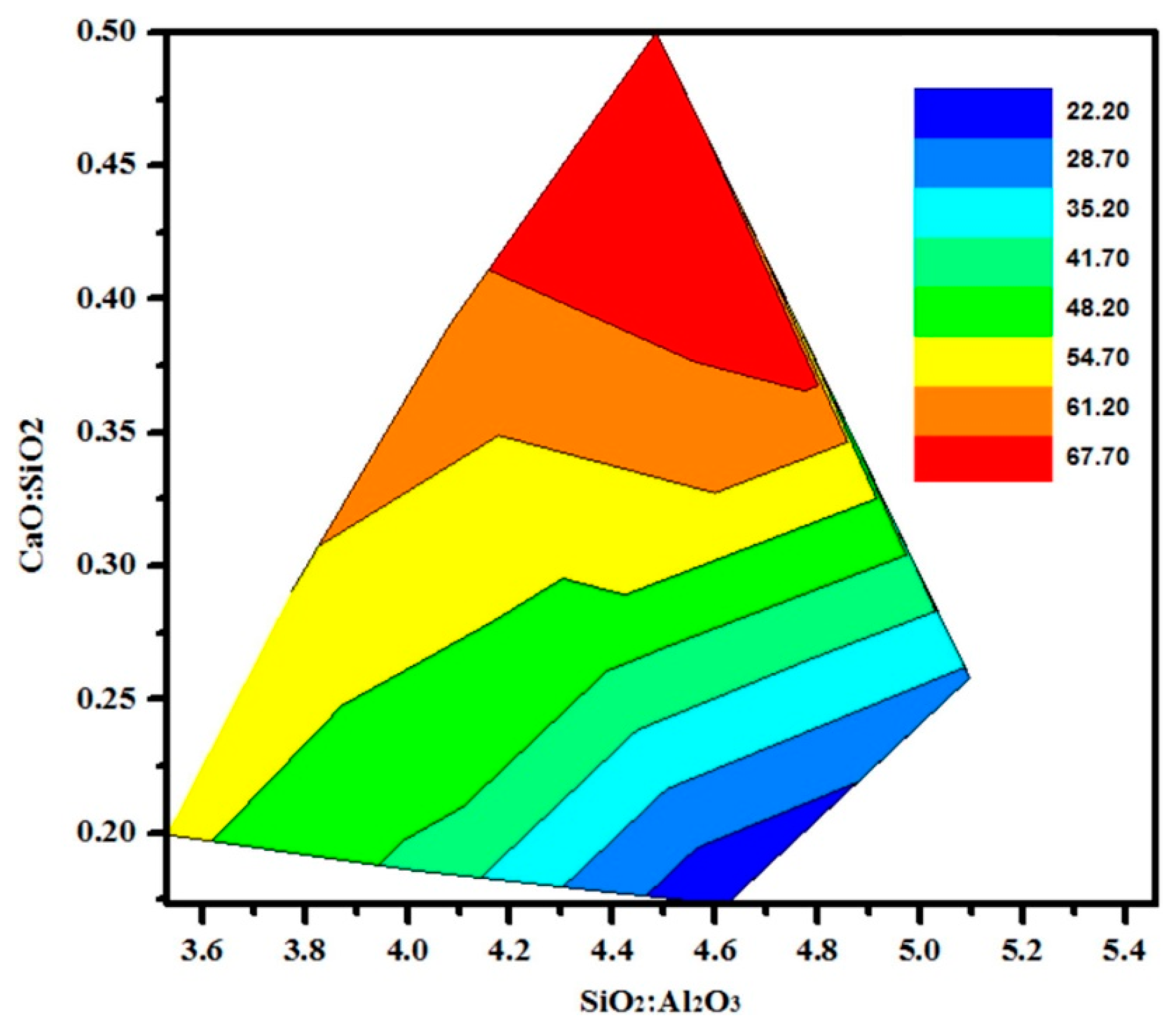
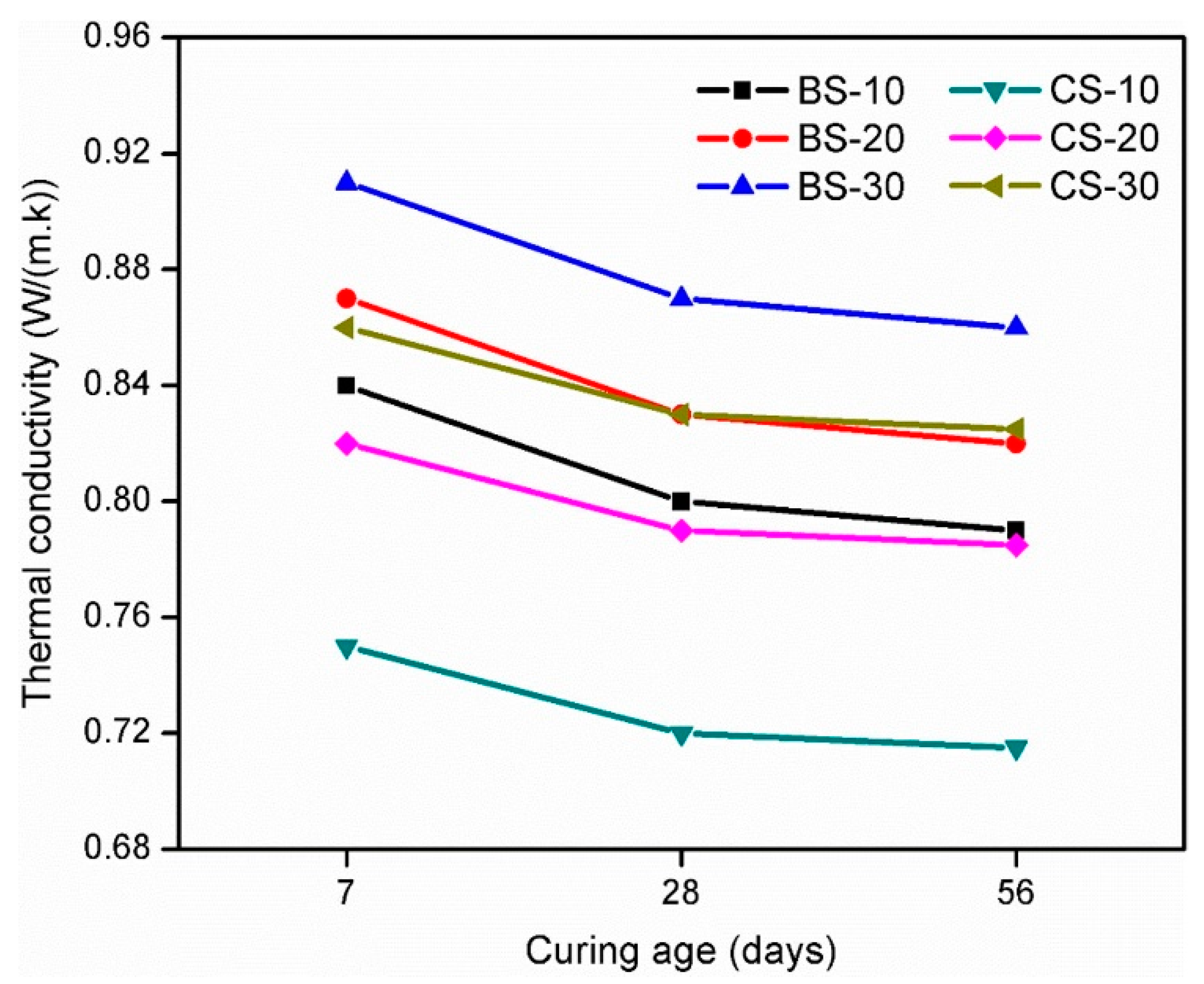
6.1. Thermal Conductivity
6.2. Residual Mechanical Strength after Elevated Temperature Exposure
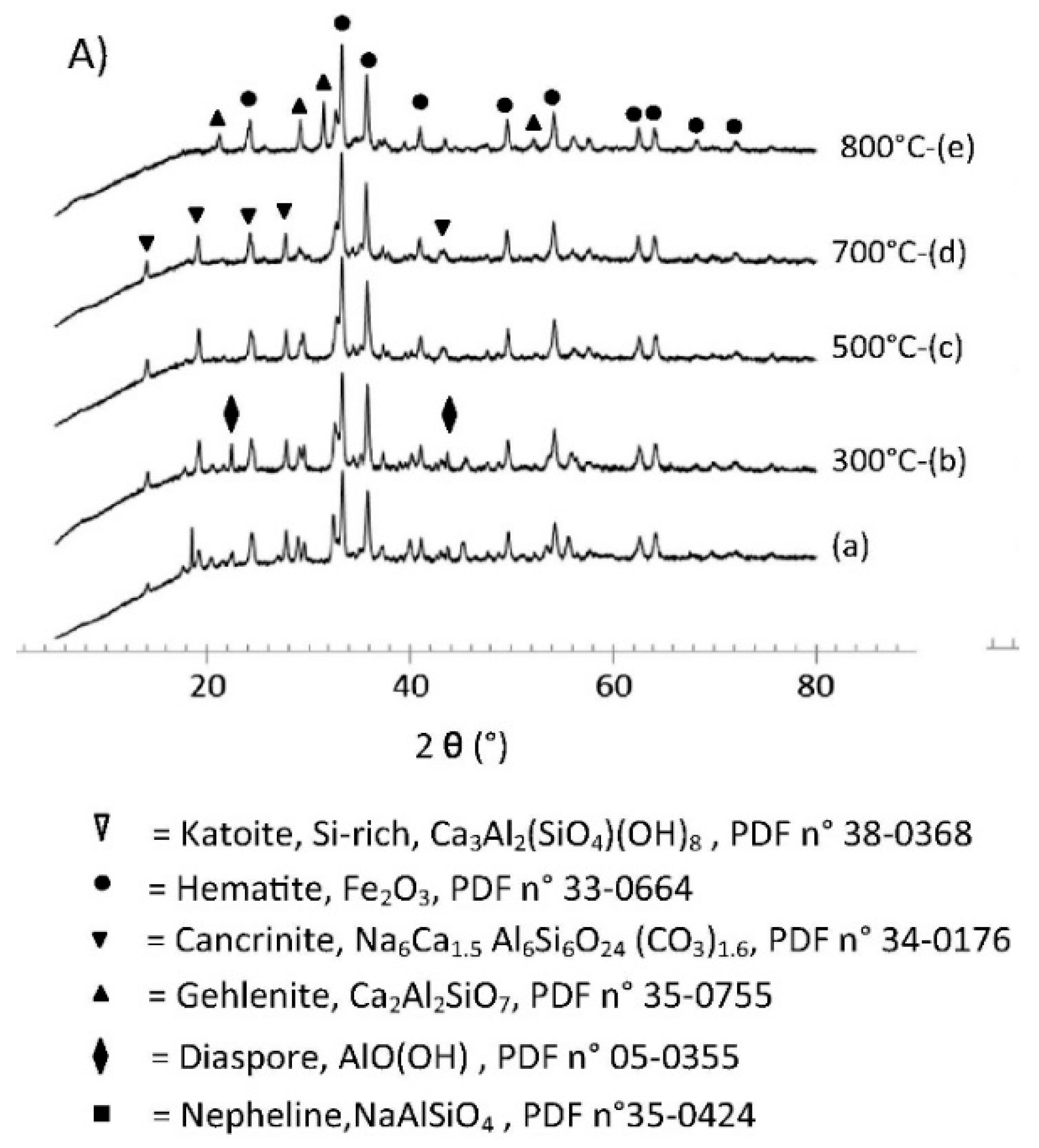
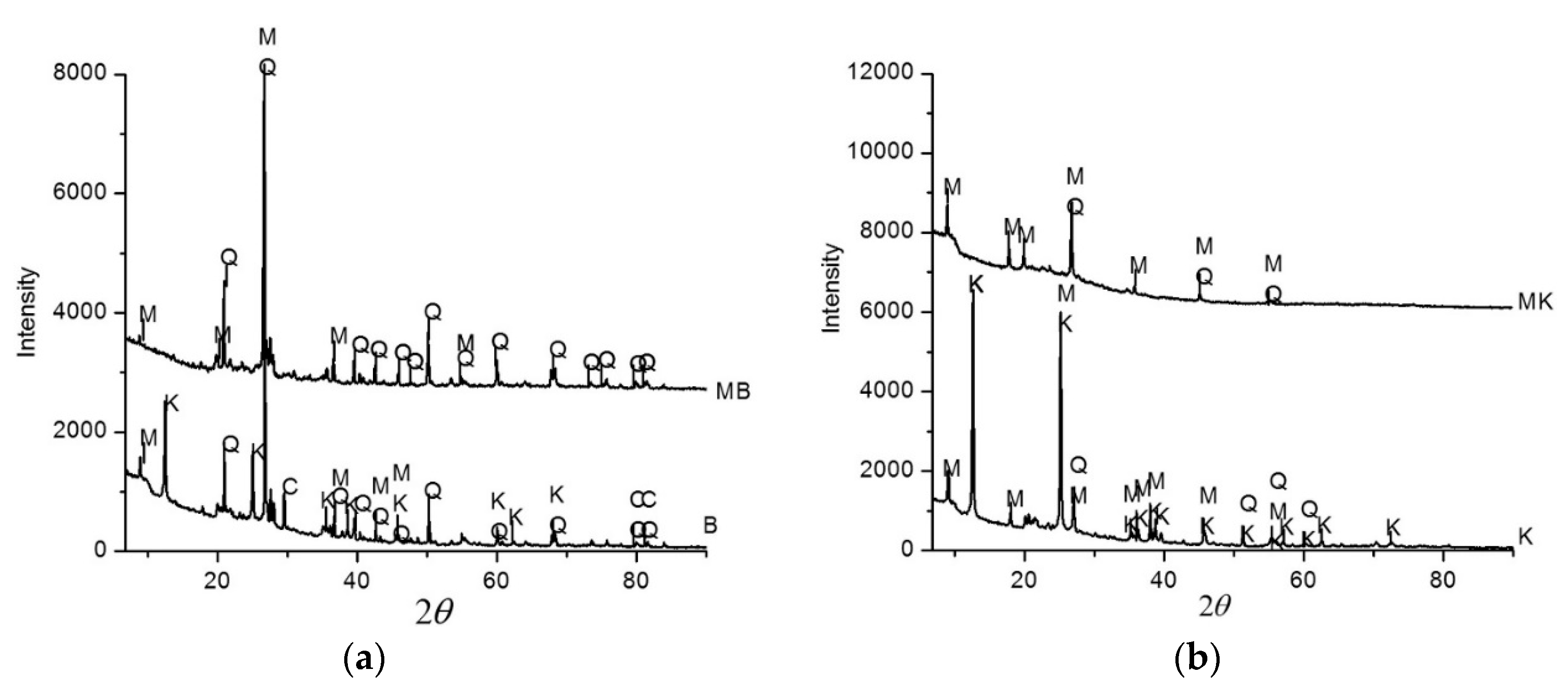
6.3. X-Ray Diffraction (XRD)
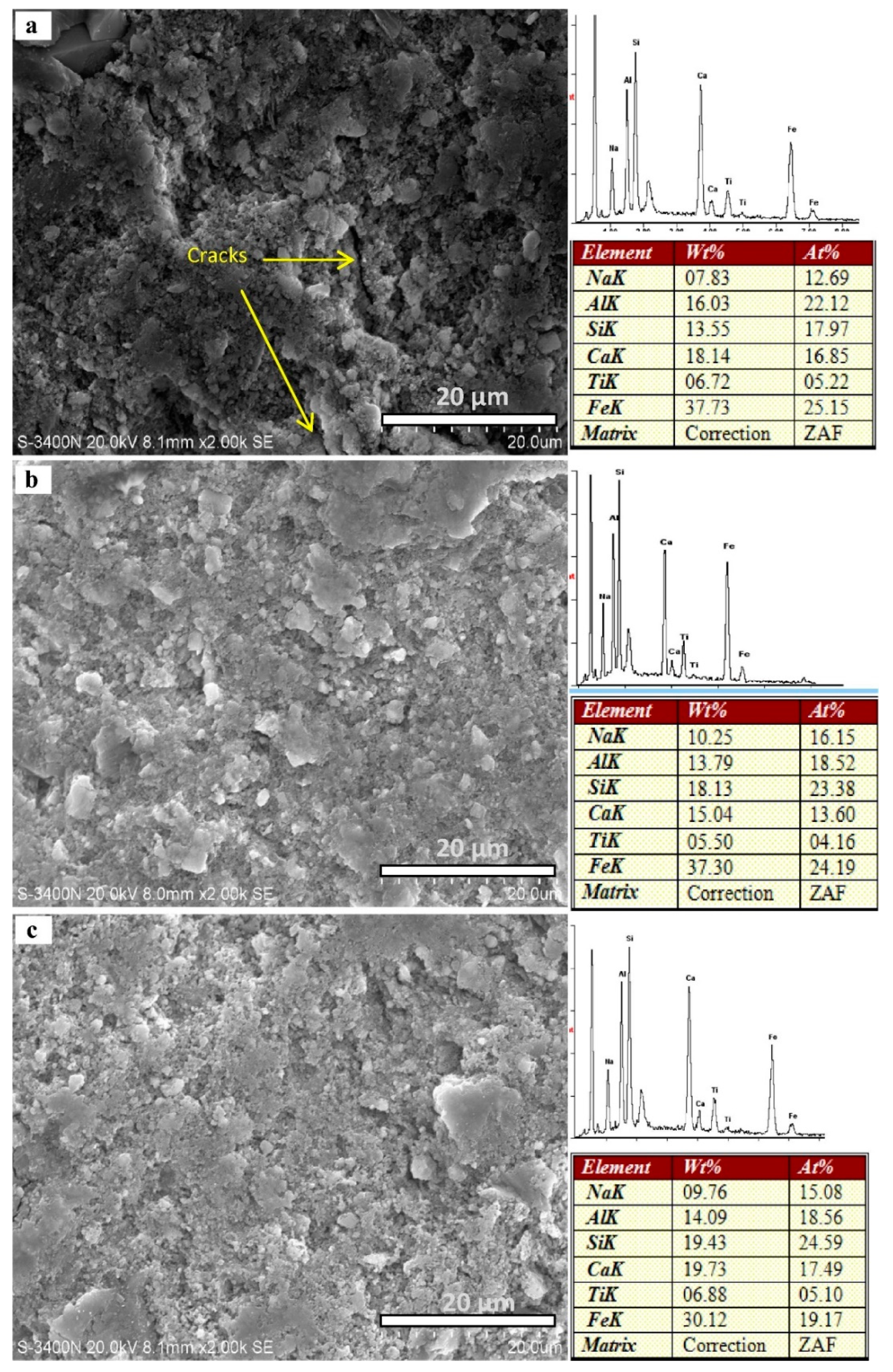
6.4. Scanning Electron Microscopy
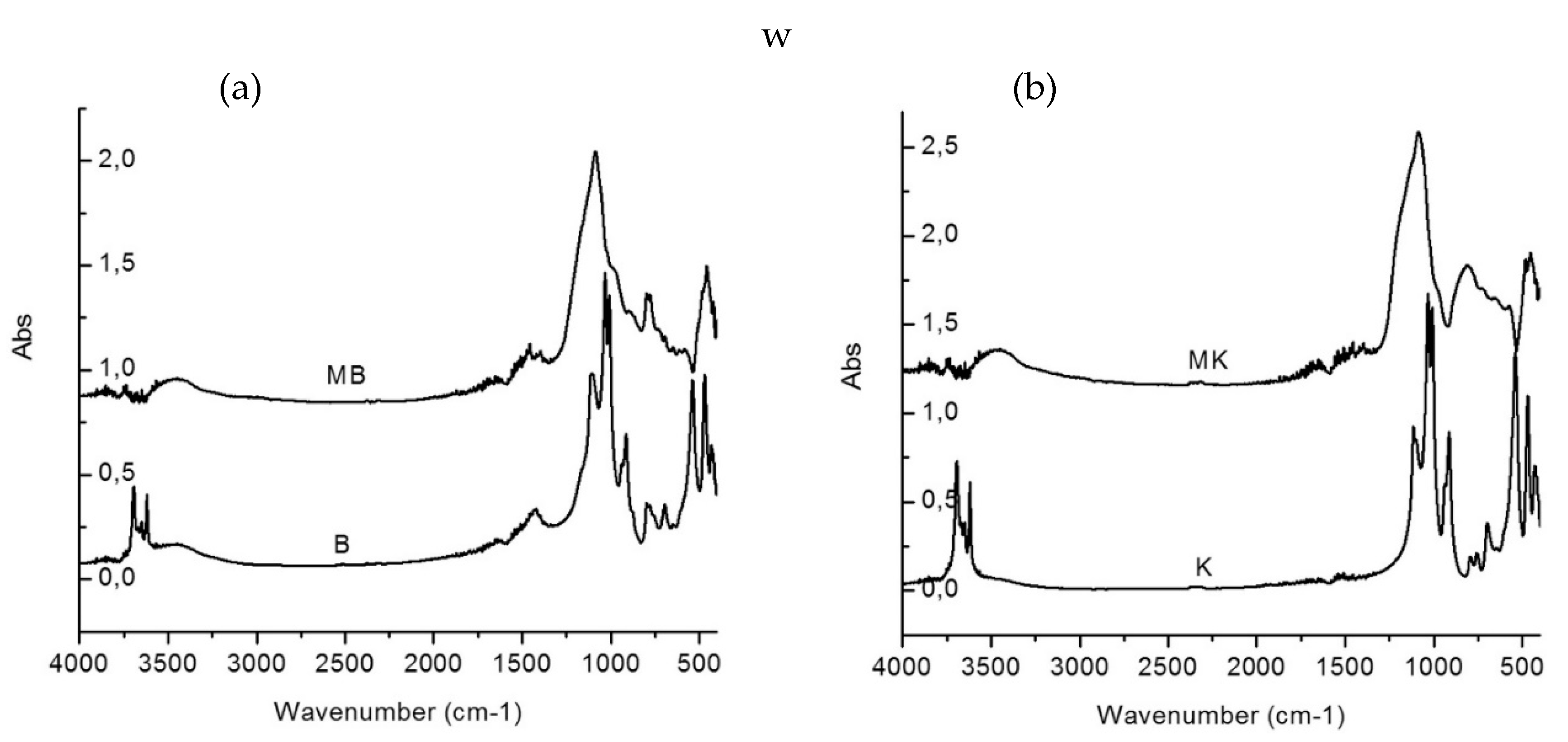
6.5. Fourier Transform Infrared Spectroscopy (FTIR)
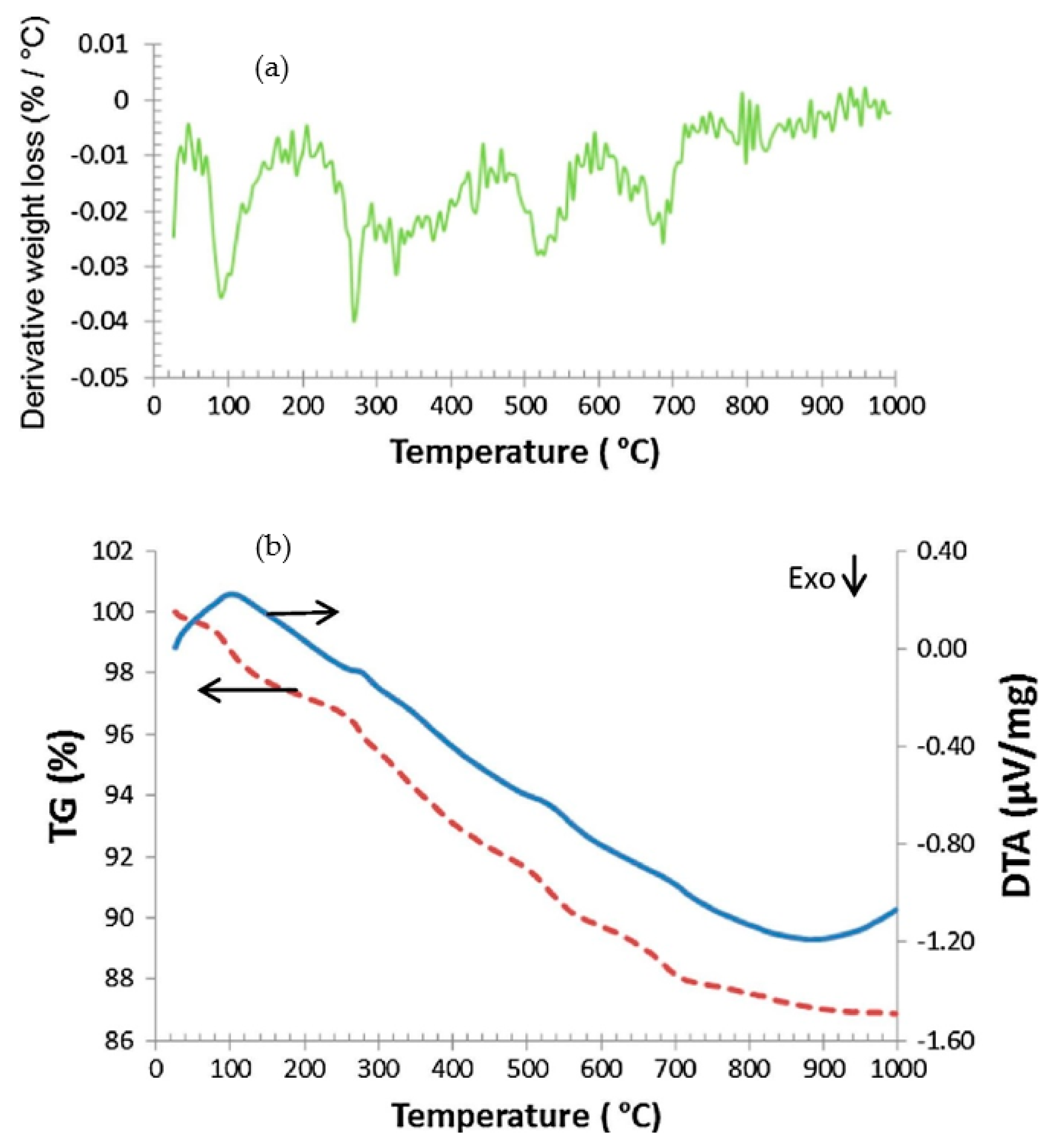
6.6. Thermogravimetric and Differential Thermal Analyser (TGA)
7. Conclusions
- The application of ceramic aggregate as a substitution of natural sand has an optimistic result by fabricating higher compressive strength, flexural strength, and splitting tensile strength;
- The workability and setting time properties of alkali-activated mortars heightened with the upsurge in WCP content;
- Resistance against the elevated temperature of alkali-activated mortars was improved through the rise in WCP content;
- The substitution of GGBS by FA in the ternary assortments led to the lessening of deterioration up to 900 °C;
- The mortars incorporated with tile ceramic waste could provide a probable reapplication of waste material;
- Alkali-activated mortar incorporated a higher amount of FA and promoted the upgrade of the presentation of sulphate as well as acid resistance;
- The XRD examination reveals that the ceramic sludge contains kaolinite;
- From the TGA analysis of ceramic sludge and kaolin, it could be concluded that kaolinite is an inimitable phase able to be dehydroxylated;
- FTIR research shows that a structural modification of the kaolinite found in ceramic sludge is caused by a dihydroxylation process;
- The combination of geopolymers with ceramic waste is promising for lowering the energy and cost of materials in the construction sector.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teng, Y.; Li, K.; Pan, W.; Ng, T. Reducing building life cycle carbon emissions through prefabrication: Evidence from and gaps in empirical studies. Build. Environ. 2018, 132, 125–136. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Luhar, I. Incorporation of natural waste from agricultural and aquacultural farming as supplementary materials with green concrete: A review. Compos. Part B: Eng. 2019, 175. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass wastes for the development of geopolymer composites—Durability, thermal and microstructural properties: A review. Constr. Build. Mater. 2019, 222, 673–687. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Luhar, S.; Arel, H. Şahan; Luhar, I. Performance evaluation of Ultrahigh performance fibre reinforced concrete—A review. Constr. Build. Mater. 2020, 232. [Google Scholar] [CrossRef]
- Chong, B.; Othman, R.; Jaya, R.P.; Hasan, M.M.; Sandu, A.; Nabiałek, M.; Jeż, B.; Pietrusiewicz, P.; Kwiatkowski, D.; Postawa, P.; et al. Design of Experiment on Concrete Mechanical Properties Prediction: A Critical Review. Materials 2021, 14, 1866. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass waste for development of Geopolymer composites—Mechanical properties and rheological characteristics: A review. Constr. Build. Mater. 2019, 220, 547–564. [Google Scholar] [CrossRef]
- Rehan, R.; Nehdi, M. Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environ. Sci. Policy 2005, 8, 105–114. [Google Scholar] [CrossRef]
- Stern, N.; Stern, N.H. The Economics of Climate Change: The Stern Review; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Institut Geopolymere: Saint-Quentin, France, 2015. [Google Scholar]
- Pacheco-Torgal, F. Introduction to Handbook of Alkali-activated Cements, Mortars and Concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–16. [Google Scholar]
- Xing, R.; Hanaoka, T.; Kanamori, Y.; Masui, T. Estimating energy service demand and CO2 emissions in the Chinese service sector at provincial level up to 2030. Resour. Conserv. Recycl. 2018, 134, 347–360. [Google Scholar] [CrossRef]
- Asaad, M.A.; Sarbini, N.N.; Sulaiman, A.; Ismail, M.; Huseien, G.F.; Majid, Z.A.; Raja, P.B. Improved corrosion resistance of mild steel against acid activation: Impact of novel Elaeis guineensis and silver nanoparticles. J. Ind. Eng. Chem. 2018, 63, 139–148. [Google Scholar] [CrossRef]
- Rashad, A.M. Properties of alkali-activated fly ash concrete blended with slag. Iran. J. Mater. Sci. Eng. 2013, 10, 57–64. [Google Scholar]
- Asaad, M.A.; Ismail, M.; Tahir, M.M.; Huseien, G.F.; Raja, P.B.; Asmara, Y.P. Enhanced corrosion resistance of reinforced concrete: Role of emerging eco-friendly Elaeis guineensis/silver nanoparticles inhibitor. Constr. Build. Mater. 2018, 188, 555–568. [Google Scholar] [CrossRef]
- Daniyal, M.; Ahmad, S. Application of waste ceramic tile aggregates in concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 12808–12815. [Google Scholar]
- Senthamarai, R.; Manoharan, P.D. Concrete with ceramic waste aggregate. Cem. Concr. Compos. 2005, 27, 910–913. [Google Scholar] [CrossRef]
- Senthamarai, R.; Manoharan, P.D.; Gobinath, D. Concrete made from ceramic industry waste: Durability properties. Constr. Build. Mater. 2011, 25, 2413–2419. [Google Scholar] [CrossRef]
- Huang, B.; Dong, Q.; Burdette, E.G. Laboratory evaluation of incorporating waste ceramic materials into Portland cement and asphaltic concrete. Constr. Build. Mater. 2009, 23, 3451–3456. [Google Scholar] [CrossRef]
- Hussein, A.A.; Jaya, R.P.; Hassan, N.A.; Yaacob, H.; Huseien, G.F.; Ibrahim, M.H.W. Performance of nanoceramic powder on the chemical and physical properties of bitumen. Constr. Build. Mater. 2017, 156, 496–505. [Google Scholar] [CrossRef]
- Samadi, M.; Hussin, M.W.; Lee, H.S.; Sam, A.R.M.; Ismail, M.; Lim, N.H.A.S.; Ariffin, N.F.; Khalid, N.H.A. Poperties of mortar containing ceramic powder waste as cement replacement. J. Teknol. 2015, 77. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Reusing ceramic wastes in concrete. Constr. Build. Mater. 2010, 24, 832–838. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Hossain, M.; Karim, M.; Islam, M.; Zain, M. Durability of mortar and concrete containing alkali-activated binder with pozzolans: A review. Constr. Build. Mater. 2015, 93, 95–109. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Hussin, M.W.; Ariffin, M.A.M. Potential use coconut milk as alternative to alkali solution for geopolymer production. J. Teknol. 2016, 78. [Google Scholar] [CrossRef][Green Version]
- Zulkifly, K.; Cheng-Yong, H.; Yun-Ming, L.; Bayuaji, R.; Abdullah, M.M.A.B.; Ahmad, S.B.; Stachowiak, T.; Szmidla, J.; Gondro, J.; Jeż, B.; et al. Elevated-Temperature Performance, Combustibility and Fire Propagation Index of Fly Ash-Metakaolin Blend Ge-opolymers with Addition of Monoaluminium Phosphate (MAP) and Aluminum Dihydrogen Triphosphate (ATP). Materials 2021, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, M.; Abdullah, M.; Rahim, S.A.; Gondro, J.; Pietrusiewicz, P.; Garus, S.; Stachowiak, T.; Sandu, A.; Tahir, M.M.; Korkmaz, M.; et al. Tool Wear and Surface Evaluation in Drilling Fly Ash Geopolymer Using HSS, HSS-Co, and HSS-TiN Cutting Tools. Materials 2021, 14, 1628. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Abdullah, M.M.A.B.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K. Utilization of Fly Ash Waste as Construction Material. Int. J. Conserv. Sci. 2016, 7, 161–166. [Google Scholar]
- Oikonomou, N. Recycled concrete aggregates. Cem. Concr. Compos. 2005, 27, 315–318. [Google Scholar] [CrossRef]
- Fatta, D.; Papadopoulos, A.; Avramikos, E.; Sgourou, E.; Moustakas, K.; Kourmoussis, F.; Mentzis, A.; Loizidou, M. Generation and management of construction and demolition waste in Greece—an existing challenge. Resour. Conserv. Recycl. 2003, 40, 81–91. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.; Monzó, J.; Cheeseman, C.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- El-Gamal, S.; El-Hosiny, F.; Amin, M.; Sayed, D. Ceramic waste as an efficient material for enhancing the fire resistance and mechanical properties of hardened Portland cement pastes. Constr. Build. Mater. 2017, 154, 1062–1078. [Google Scholar] [CrossRef]
- Amin, M.; Hashem, F. Hydration characteristics of hydrothermal treated cement kiln dust–sludge–silica fume pastes. Constr. Build. Mater. 2011, 25, 1870–1876. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.; Hashem, F.; Amin, M.; Sayed, D. Physicochemical characteristics of cementitious building materials de-rived from industrial solid wastes. Constr. Build. Mater. 2016, 126, 983–990. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.; Heikal, M.; Amin, M.; Negm, H. Reactivity of dealuminated kaolin and burnt kaolin using cement kiln dust or hydrated lime as activators. Constr. Build. Mater. 2013, 47, 1451–1460. [Google Scholar] [CrossRef]
- Sun, Z.; Cui, H.; An, H.; Tao, D.; Xu, Y.; Zhai, J.; Li, Q. Synthesis and thermal behavior of geopolymer-type material from waste ceramic. Constr. Build. Mater. 2013, 49, 281–287. [Google Scholar] [CrossRef]
- Rambaldi, E.; Esposito, L.; Tucci, A.; Timellini, G. Recycling of polishing porcelain stoneware residues in ceramic tiles. J. Eur. Ceram. Soc. 2007, 27, 3509–3515. [Google Scholar] [CrossRef]
- Wattanasiriwech, D.; Saiton, A. Paving blocks from ceramic tile production waste. J. Clean. Prod. 2009, 17, 1663–1668. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Bignozzi, M.C.; Sandrolini, F. New Blended Cement from Polishing and Glazing Ceramic Sludge. Int. J. Appl. Ceram. Technol. 2009, 7, 546–555. [Google Scholar] [CrossRef]
- Pelisser, F.; Steiner, L.R.; Bernardin, A.M. Recycling of porcelain tile polishing residue in Portland cement: Hydration efficiency. Environ. Sci. Technol. 2012, 46, 2368–2374. [Google Scholar] [CrossRef]
- Steiner, L.R.; Bernardin, A.M.; Pelisser, F. Effectiveness of ceramic tile polishing residues as supplementary cementitious materials for cement mortars. Sustain. Mater. Technol. 2015, 4, 30–35. [Google Scholar] [CrossRef]
- Baraldi, L. World sanitaryware production and exports. Ceram. World Rev. 2015, 114, 56–65. [Google Scholar]
- Medina, C.; Frías, M.; de Rojas, M.S. Microstructure and properties of recycled concretes using ceramic sanitary ware industry waste as coarse aggregate. Constr. Build. Mater. 2012, 31, 112–118. [Google Scholar] [CrossRef]
- Huseien, G.F.; Al-Fasih, M.Y.; Hamzah, H. Performance of self-compacting concrete with different sizes of recycled ceramic aggregates. Int. J. Innov. Res. Create. Technol. 2015, 1, 264–269. [Google Scholar]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Behera, M.; Bhattacharyya, S.; Minocha, A.; Deoliya, R.; Maiti, S. Recycled aggregate from C&D waste & its use in concrete—A breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar] [CrossRef]
- Zhan, L. Production of bricks from waste materials. Constr Build Mater. 2014, 47, 643–655. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; Van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Puertas, F.; García-Díaz, I.; Barba, A.; Gazulla, M.; Palacios, M.; Gómez, M.; Martínez-Ramírez, S. Ceramic wastes as alternative raw materials for Portland cement clinker production. Cem. Concr. Compos. 2008, 30, 798–805. [Google Scholar] [CrossRef]
- Torgal, F.P.; Jalali, S. Binders and Concretes. In Eco-efficient Construction and Building Materials; Springer: London, UK, 2011; pp. 75–129. [Google Scholar]
- Rakhimova, N.R.; Rakhimov, R.Z. Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste. Mater. Des. 2015, 85, 324–331. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Silva, R.; de Brito, J.; Dhir, R. Tensile strength behaviour of recycled aggregate concrete. Constr. Build. Mater. 2015, 83, 108–118. [Google Scholar] [CrossRef]
- Elchalakani, M.; Elgaali, E. Sustainable Concrete made of Construction and Demolition Wastes using Recycled Wastewater in the UAE. J. Adv. Concr. Technol. 2012, 10, 110–125. [Google Scholar] [CrossRef]
- Medina, C.; Juan, A.; Frías, M.; De Rojas, M.S.; Moran, J.M.; Guerra, M. Characterization of concrete made with recycled aggregate from ceramic sanitary ware. Mater. Construcc. 2011, 61, 533–546. [Google Scholar] [CrossRef]
- Alves, A.; Vieira, T.; De Brito, J.; Correia, J. Mechanical properties of structural concrete with fine recycled ceramic aggregates. Constr. Build. Mater. 2014, 64, 103–113. [Google Scholar] [CrossRef]
- Stock, D. World production and consumption of ceramic tiles. Tile Today 2014, 85, 30–37. [Google Scholar]
- Reig, L.; Soriano, L.; Borrachero, M.; Monzó, J.; Payá, J. Influence of the activator concentration and calcium hydroxide addition on the properties of alkali-activated porcelain stoneware. Constr. Build. Mater. 2014, 63, 214–222. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Arbi, K.; Palomo, A.; Fernández-Jiménez, A. Alkali-activated blends of calcium aluminate cement and slag/diatomite. Ceram. Int. 2013, 39, 9237–9245. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.; Soriano, L.; Borrachero, M.; Monzó, J.; Payá, J. Alkaline activation of ceramic waste materials. Waste Biomass Valorization. 2013, 4, 729–736. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.; Monzó, J.; Payá, J. Influence of calcium aluminate cement (CAC) on alkaline activation of red clay brick waste (RCBW). Cem. Concr. Compos. 2016, 65, 177–185. [Google Scholar] [CrossRef]
- Habert, G.; de Lacaillerie, J.D.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.; Postawa, P.; AbdRahim, S.Z.; Zainol, M.R.R.M.A.; Jaya, R.; Śliwa, A.; Omar, M.; Wysłocki, J.; Błoch, K.; et al. Effect of Aluminium Powder on Kaolin-Based Geopolymer Characteristic and Removal of Cu2+. Materials 2021, 14, 814. [Google Scholar] [CrossRef]
- Shvarzman, A.; Kovler, K.; Grader, G.; Shter, G. The effect of dehydroxylation/amorphization degree on pozzolanic activity of kaolinite. Cem. Concr. Res. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Constr. Build. Mater. 2012, 28, 276–281. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.; Irassar, E.; Scian, A. Thermal Treatment of Kaolin: Effect on the Pozzolanic Activity. Procedia Mater. Sci. 2012, 1, 343–350. [Google Scholar] [CrossRef]
- Ilic, B.; Mitrovic, A.; Milicic, L. Thermal treatment of kaolin clay to obtain metakaolin. Chem. Ind. 2010, 64, 351–356. [Google Scholar] [CrossRef]
- Vizcayno, C.; De Gutiérrez, R.; Castello, R.; Rodriguez, E.; Guerrero, C. Pozzolan obtained by mechanochemical and thermal treatments of kaolin. Appl. Clay Sci. 2010, 49, 405–413. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Havlica, J.; Brandštetr, J. The kinetic analysis of the thermal decomposition of kaolinite by DTG technique. Powder Technol. 2011, 208, 20–25. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Nosková, M.; Havlica, J.; Brandštetr, J. The non-isothermal kinetics analysis of the thermal decomposition of kaolinite by Effluent Gas Analysis technique. Powder Technol. 2010, 203, 272–276. [Google Scholar] [CrossRef]
- Hounsi, A.D.; Lecomte, G.L.; Djétéli, G.; Blanchart, P.; Alowanou, D.; Kpelou, P.; Napo, K.; Tchangbédji, G.; Praisler, M. How does Na, K alkali metal concentration change the early age structural characteristic of kaolin-based geopolymers. Ceram. Int. 2014, 40, 8953–8962. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Tahir, M.M.; Mirza, J. Properties of ceramic tile waste based alkali-activated mortars incorporating GBFS and fly ash. Constr. Build. Mater. 2019, 214, 355–368. [Google Scholar] [CrossRef]
- Jamil, N.; Abdullah, M.; Pa, F.C.; Hasmaliza, M.; Ibrahim, W.W.; Aziz, I.A.; Jeż, B.; Nabiałek, M. Phase Transformation of Kaolin-Ground Granulated Blast Furnace Slag from Geopolymerization to Sintering Process. Magnetochemistry 2021, 7, 32. [Google Scholar] [CrossRef]
- Bouaissi, A.; Li, L.-Y.; Abdullah, M.M.A.B.; Bui, Q.-B. Mechanical properties and microstructure analysis of FA-GGBS-HMNS based geopolymer concrete. Constr. Build. Mater. 2019, 210, 198–209. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Li, L.-y.; Jaya, N.A.; Abdullah, M.M.A.B.; Tan, S.J.; Hussin, K. Formation of one-part-mixing geo-polymers and geopolymer ceramics from geopolymer powder. Constr. Build. Mater. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Keppert, M.; Vejmelková, E.; Bezdička, P.; Doleželová, M.; Čáchová, M.; Scheinherrová, L.; Pokorný, J.; Vyšvařil, M.; Rov-naníková, P.; Černý, R. Red-clay ceramic powders as geopolymer precursors: Consideration of amorphous portion and CaO content. Appl. Clay Sci. 2018, 161, 82–89. [Google Scholar] [CrossRef]
- Shahedan, N.; Abdullah, M.; Mahmed, N.; Kusbiantoro, A.; Tammas-Williams, S.; Li, L.-Y.; Aziz, I.; Vizureanu, P.; Wysłocki, J.; Błoch, K.; et al. Properties of a New Insulation Material Glass Bubble in Geo-Polymer Concrete. Materials 2021, 14, 809. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Yehualaw, M.D.; Vo, D.-H.; Huynh, T.-P. Development of high-strength alkali-activated pastes containing high volumes of waste brick and ceramic powders. Constr. Build. Mater. 2019, 218, 519–529. [Google Scholar] [CrossRef]
- Kuenzel, C.; Grover, L.; Vandeperre, L.; Boccaccini, A.; Cheeseman, C. Production of nepheline/quartz ceramics from geo-polymer mortars. J. Eur. Ceram. Soc. 2013, 33, 251–258. [Google Scholar] [CrossRef]
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.-H. Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram. Int. 2017, 43, 11433–11441. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Tahir, M.M.; Mirza, J.; Khalid, N.H.A.; Asaad, M.A.; Husein, A.A.; Sarbini, N.N. Synergism be-tween palm oil fuel ash and slag: Production of environmental-friendly alkali activated mortars with enhanced properties. Constr. Build. Mater. 2018, 170, 235–244. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Yong, H.C.; Ming, L.Y.; Panias, D.; Sakkas, K. Correlation of the Processing Parameters in the Formation of Granulated Ground Blast Furnace Slag Geopolymer. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Innovative Research—ICIR Euroinvent, Iasi, Romania, 25–26 May 2017; IOP Publishing: Bristol, UK, 2017; Volume 209, p. 209. [Google Scholar]
- Huseien, G.F.; Mirza, J.; Ismail, M. Effects of high volume ceramic binders on flexural strength of self-compacting geo-polymer concrete. Adv. Sci. Lett. 2018, 24, 4097–4101. [Google Scholar] [CrossRef]
- Reig, L.; Sanz, M.; Borrachero, M.; Monzó, J.; Soriano, J.; Payá, J. Compressive strength and microstructure of alkali-activated mortars with high ceramic waste content. Ceram. Int. 2017, 43, 13622–13634. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high vol-ume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.; De Gutiérrez, R.M. Synthesis of ceramic materials from ecofriendly geopolymer precursors. Mater. Lett. 2018, 230, 300–304. [Google Scholar] [CrossRef]
- Badanoiu, A.I.; Al Saadi, T.H.A.; Stoleriu, S.; Voicu, G. Preparation and characterization of foamed geopolymers from waste glass and red mud. Constr. Build. Mater. 2015, 84, 284–293. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium additions on the compressive strength and microstructure of alkali-activated ceramic sanitary-ware. J. Am. Ceram. Soc. 2018, 101, 3094–3104. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Vázquez, T.; Vallepu, R.; Terai, T.; Ikeda, K. Alkaline Activation of Blends of Metakaolin and Calcium Aluminate. J. Am. Ceram. Soc. 2008, 91, 1231–1236. [Google Scholar] [CrossRef]
- Pastor, C.; Fernández-Jiménez, A.; Vázquez, T.; Palomo, A. Calcium aluminate cement hydration in a high alkalinity envi-ronment. Mater. Construcc. 2009, 59, 21–34. [Google Scholar]
- Ariffin, M.A.; Hussin, M.W.; Samadi, M.; Lim, N.H.A.S.; Mirza, J.; Awalluddin, D.; Othman, N. Effect of ceramic aggregate on high strength multi blended ash geopolymer mortar. J. Teknol. 2015, 77. [Google Scholar] [CrossRef]
- Ramos, G.A.; Pelisser, F.; Gleize, P.; Bernardin, A.M.; Michel, M.D. Effect of porcelain tile polishing residue on geopolymer cement. J. Clean. Prod. 2018, 191, 297–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Wang, H.; Bullen, F.; Reid, A. Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin. Thermochim. Acta 2013, 565, 163–171. [Google Scholar] [CrossRef]
- Abdullah, A.; Hussin, K.; Abdullah, M.; Yahya, Z.; Sochacki, W.; Razak, R.; Błoch, K.; Fansuri, H. The Effects of Various Concentrations of NaOH on the Inter-Particle Gelation of a Fly Ash Geopolymer Aggregate. Materials 2021, 14, 1111. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.; Abdullah, M.; Muniandy, R.; Abu Hashim, M.; Błoch, K.; Jeż, B.; Garus, S.; Palutkiewicz, P.; Mortar, N.M.; Ghazali, M. Comparison of Hook and Straight Steel Fibers Addition on Malaysian Fly Ash-Based Geopolymer Concrete on the Slump, Density, Water Absorption and Mechanical Properties. Materials 2021, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Mohd, M.A.B.A.; Jamaludin, L.; Hussin, K.; Binhussain, M.; Ghazali, C.M.R.; Izzat, A.M.; Abdullah, M.M.A.B.; Kamarudin, H.; Mohd, A.M.A.B. Study on Fly Ash Based Geopolymer for Coating Applications. Adv. Mater. Res. 2013, 686, 227–233. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Yong, H.C.; Ming, L.Y.; Hussin, K.; Surleva, A.; Azimi, E.A. Manufacturing parameters in-fluencing fire resistance of geopolymers: A review. Proc. Inst. Mech. Eng. Part. L: J. Mater. Des. Appl. 2016, 233, 721–733. [Google Scholar] [CrossRef]
- Huseien, G.; Ismail, M.; Tahir, M.; Mirza, J.; Hussein, A.; Khalid, N.; Sarbini, N. Performance of sustainable alkali activated mortars containing solid waste ceramic powder. Chem. Eng Trans. 2018, 63, 673–678. [Google Scholar]
- Deb, P.S.; Nath, P.; Sarker, P. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Ismail, M.; Ismail, M.E.; Muhammad, B. Influence of elevated temperatures on physical and compressive strength properties of concrete containing palm oil fuel ash. Constr. Build. Mater. 2011, 25, 2358–2364. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.M.; Yoriya, S.; Chaiprapa, J.; Rojviriya, C.; Li, L.Y. Microstructure and porosity evolution of alkali activated slag at various heating temperatures. J. Mater. Res. Technol. 2020, 9, 15894–15907. [Google Scholar] [CrossRef]
- Noruzman, A.H.; Ismail, M.; Bhutta, M.A.R.; Yusuf, T.O.; Shehu, I.A.; Hassan, I.O. Strength and Durability Characteristics of Polymer Modified Concrete Incorporating Vinyl Acetate Effluent. Adv. Mater. Res 2013, 690–693, 1053–1056. [Google Scholar] [CrossRef]
- Bamaga, S.O.; Ismail, M.; Majid, Z.A.; Ismail, M.; Hussin, M.W. Evaluation of Sulfate Resistance of Mortar Containing Palm Oil Fuel Ash from Different Sources. Arab. J. Sci. Eng. 2013, 38, 2293–2301. [Google Scholar] [CrossRef]
- Budiea, A.; Hussin, M.; Muthusamy, K.; Ismail, M. Performance of high strength POFA concrete in acidic environment. Concr. Res. Lett. 2010, 1, 14–18. [Google Scholar]
- Cai, L.; Wang, H.; Fu, Y. Freeze–thaw resistance of alkali–slag concrete based on response surface methodology. Constr. Build. Mater. 2013, 49, 70–76. [Google Scholar] [CrossRef]
- Chang, H.; Mu, S.; Xie, D.; Wang, P. Influence of pore structure and moisture distribution on chloride “maximum phenome-non” in surface layer of specimens exposed to cyclic drying-wetting condition. Constr. Build. Mater. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Krishnamoorthy, S. Permeable Porosity and Thermal Conductivity of Construction Materials. J. Mater. Civ. Eng. 2004, 16, 322–330. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Thermal behaviour of inorganic geopolymers and composites derived from sodium pol-ysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Ferraro, R.M.; Nanni, A. Effect of off-white rice husk ash on strength, porosity, conductivity and corrosion resistance of white concrete. Constr. Build. Mater. 2012, 31, 220–225. [Google Scholar] [CrossRef]
- Shin, A.H.-C.; Kodide, U. Thermal conductivity of ternary mixtures for concrete pavements. Cem. Concr. Compos. 2012, 34, 575–582. [Google Scholar] [CrossRef]
- Bessenouci, M.; Bibi-Triki, N.; Bendimerad, S.; Nakoul, Z.; Khelladi, S.; Hakem, A. Influence of Humidity on the Apparent Thermal Conductivity of Concrete Pozzolan. Phys. Procedia 2014, 55, 150–156. [Google Scholar] [CrossRef]
- Li, O.H.; Yun-Ming, L.; Cheng-Yong, H.; Bayuaji, R.; Abdullah, M.M.A.B.; Loong, F.K.; Jin, T.S.; Teng, N.H.; Nabiałek, M.; Jeż, B.; et al. Evaluation of the Effect of Silica Fume on Amorphous Fly Ash Geopolymers Exposed to Elevated Temperature. Magnetochemistry 2021, 7, 9. [Google Scholar] [CrossRef]
- Uysal, M.; Yilmaz, K. Effect of mineral admixtures on properties of self-compacting concrete. Cem. Concr. Compos. 2011, 33, 771–776. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Huynh, T.-P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and resid-ual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Uysal, H.; Demirboğa, R.; Şahin, R.; Gül, R. The effects of different cement dosages, slumps, and pumice aggregate ratios on the thermal conductivity and density of concrete. Cem. Concr. Res. 2004, 34, 845–848. [Google Scholar] [CrossRef]
- Asadi, I.; Shafigh, P.; Hassan, Z.F.B.A.; Mahyuddin, N.B. Thermal conductivity of concrete–A review. J. Build. Eng. 2018, 20, 81–93. [Google Scholar] [CrossRef]
- Kovářík, T.; Rieger, D.; Kadlec, J.; Křenek, T.; Kullová, L.; Pola, M.; Bělský, P.; Franče, P.; Říha, J. Thermomechanical proper-ties of particle-reinforced geopolymer composite with various aggregate gradation of fine ceramic filler. Constr. Build. Mater. 2017, 143, 599–606. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Tarallo, O. Fire resistant melamine based organic-geopolymer hybrid com-posites. Cem. Concr. Compos. 2015, 59, 89–99. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, Y.; Yan, F. Preparation of macroporous ceramic from metakaolinite-based geopolymer by calcina-tion. Ceram. Int. 2015, 41, 11177–11183. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surfaces A: Physi-cochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Cui, X.-M. Synthesis and characterization of low temperature (<800 C) ceramics from red mud geopolymer precursor. Constr. Build. Mater. 2017, 131, 564–573. [Google Scholar]
- Mladenovič, A.; Mirtič, B.; Meden, A.; Serjun, V.Z. Calcium aluminate rich secondary stainless steel slag as a supplementary cementitious material. Constr. Build. Mater. 2016, 116, 216–225. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Le Saout, G.; Kulik, D.; Scrivener, K. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Campostrini, R.; Maurina, S.; Carturan, G.; Monagheddu, M.; Budroni, G.; Cocco, G. Bauxite ‘red mud’in the ceramic industry. Part 1: Thermal behaviour. J. Eur. Ceram. Soc. 2000, 20, 235–244. [Google Scholar]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Hajjaji, W.; Andrejkovičová, S.; Zanelli, C.; Alshaaer, M.; Dondi, M.; Labrincha, J.; Rocha, F. Composition and technological properties of geopolymers based on metakaolin and red mud. Mater. Des. 2013, 52, 648–654. [Google Scholar] [CrossRef]
- Kaya, K.; Soyer-Uzun, S. Evolution of structural characteristics and compressive strength in red mud–metakaolin based geo-polymer systems. Ceram. Int. 2016, 42, 7406–7413. [Google Scholar] [CrossRef]
- Belmokhtar, N.; El Ayadi, H.; Ammari, M.; Ben Allal, L. Effect of structural and textural properties of a ceramic industrial sludge and kaolin on the hardened geopolymer properties. Appl. Clay Sci. 2018, 162, 1–9. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Wang, M.; Zhou, Y. Thermal evolution and crystallization kinetics of potassium-based geopolymer. Ceram. Int. 2011, 37, 59–63. [Google Scholar] [CrossRef]
- Musil, S.S.; Kriven, W.M. In Situ Mechanical Properties of Chamotte Particulate Reinforced, Potassium Geopolymer. J. Am. Ceram. Soc. 2014, 97, 907–915. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Xiao, B.; Huang, Q. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Hairi, S.N.M.; Jameson, G.N.L.; Rogers, J.J.; MacKenzie, K.J.D. Synthesis and properties of inorganic polymers (geopolymers) derived from Bayer process residue (red mud) and bauxite. J. Mater. Sci. 2015, 50, 7713–7724. [Google Scholar] [CrossRef]
- Novoselova, L. Hematite nanopowder obtained from waste: Iron-removal sludge. Powder Technol. 2016, 287, 364–372. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Pronier, S.; Sobrados, I.; Sanz, J.; Rossignol, S. Role of metakaolin dehydroxylation in geopolymer synthesis. Powder Technol. 2013, 250, 33–39. [Google Scholar] [CrossRef]
- Janković, B.; Smiciklas, I.; Stajić-Trošić, J.; Antonović, D. Thermal characterization and kinetic analysis of non-isothermal decomposition process of Bauxite red mud. Estimation of density distribution function of the apparent activation energy. Int. J. Miner. Process. 2013, 123, 46–59. [Google Scholar] [CrossRef]
- Alp, A.; Goral, M.S. The influence of soda additive on the thermal properties of red mud. J. Therm. Anal. Calorim. 2003, 73, 201–207. [Google Scholar] [CrossRef]
- Grassmann, O.; Müller, G.; Löbmann, P. Organic−Inorganic Hybrid Structure of Calcite Crystalline Assemblies Grown in a Gelatin Hydrogel Matrix: Relevance to Biomineralization. Chem. Mater. 2002, 14, 4530–4535. [Google Scholar] [CrossRef]
- Harabi, A.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Guechi, A.; Foughali, L. A new and economic approach to fabricate resistant porous membrane supports using kaolin and CaCO3. J. Eur. Ceram. Soc. 2014, 34, 1329–1340. [Google Scholar] [CrossRef]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of ceramics from metakaolin-based geopolymers. Part II: K-based geopol-ymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar]
- He, P.; Jia, D.; Wang, M.; Zhou, Y. Effect of cesium substitution on the thermal evolution and ceramics formation of potassi-um-based geopolymer. Ceram. Int. 2010, 36, 2395–2400. [Google Scholar] [CrossRef]
- Wang, L.-J.; Liu, H.; Zhao, S.-Y. Aggregate gradation design of asphalt mixture with stone-to-stone contact based on fuller’s model. J. Shanghai Univ. (Engl. Ed.) 2010, 14, 387–390. [Google Scholar] [CrossRef]
- Salahuddin, M.M.; Norkhairunnisa, M.; Mustapha, F. A review on thermophysical evaluation of alkali-activated geo-polymers. Ceram. Int. 2015, 41, 4273–4281. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Nabiałek, M.; Sandu, A.V.; Szmidla, J.; Jurczyńska, A.; Razak, R.A.; Aziz, I.H.A.; Jamil, N.H.; et al. Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal. Materials 2021, 14, 3279. https://doi.org/10.3390/ma14123279
Luhar I, Luhar S, Abdullah MMAB, Nabiałek M, Sandu AV, Szmidla J, Jurczyńska A, Razak RA, Aziz IHA, Jamil NH, et al. Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal. Materials. 2021; 14(12):3279. https://doi.org/10.3390/ma14123279
Chicago/Turabian StyleLuhar, Ismail, Salmabanu Luhar, Mohd Mustafa Al Bakri Abdullah, Marcin Nabiałek, Andrei Victor Sandu, Janusz Szmidla, Anna Jurczyńska, Rafiza Abdul Razak, Ikmal Hakem A Aziz, Noorina Hidayu Jamil, and et al. 2021. "Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal" Materials 14, no. 12: 3279. https://doi.org/10.3390/ma14123279
APA StyleLuhar, I., Luhar, S., Abdullah, M. M. A. B., Nabiałek, M., Sandu, A. V., Szmidla, J., Jurczyńska, A., Razak, R. A., Aziz, I. H. A., Jamil, N. H., & Deraman, L. M. (2021). Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal. Materials, 14(12), 3279. https://doi.org/10.3390/ma14123279









