Zn–0.8Mg–0.2Sr (wt.%) Absorbable Screws—An In-Vivo Biocompatibility and Degradation Pilot Study on a Rabbit Model
Abstract
1. Introduction
- The In-Vivo Biological Behaviour of Zn- and Mg-Based Materials
2. Materials and Methods
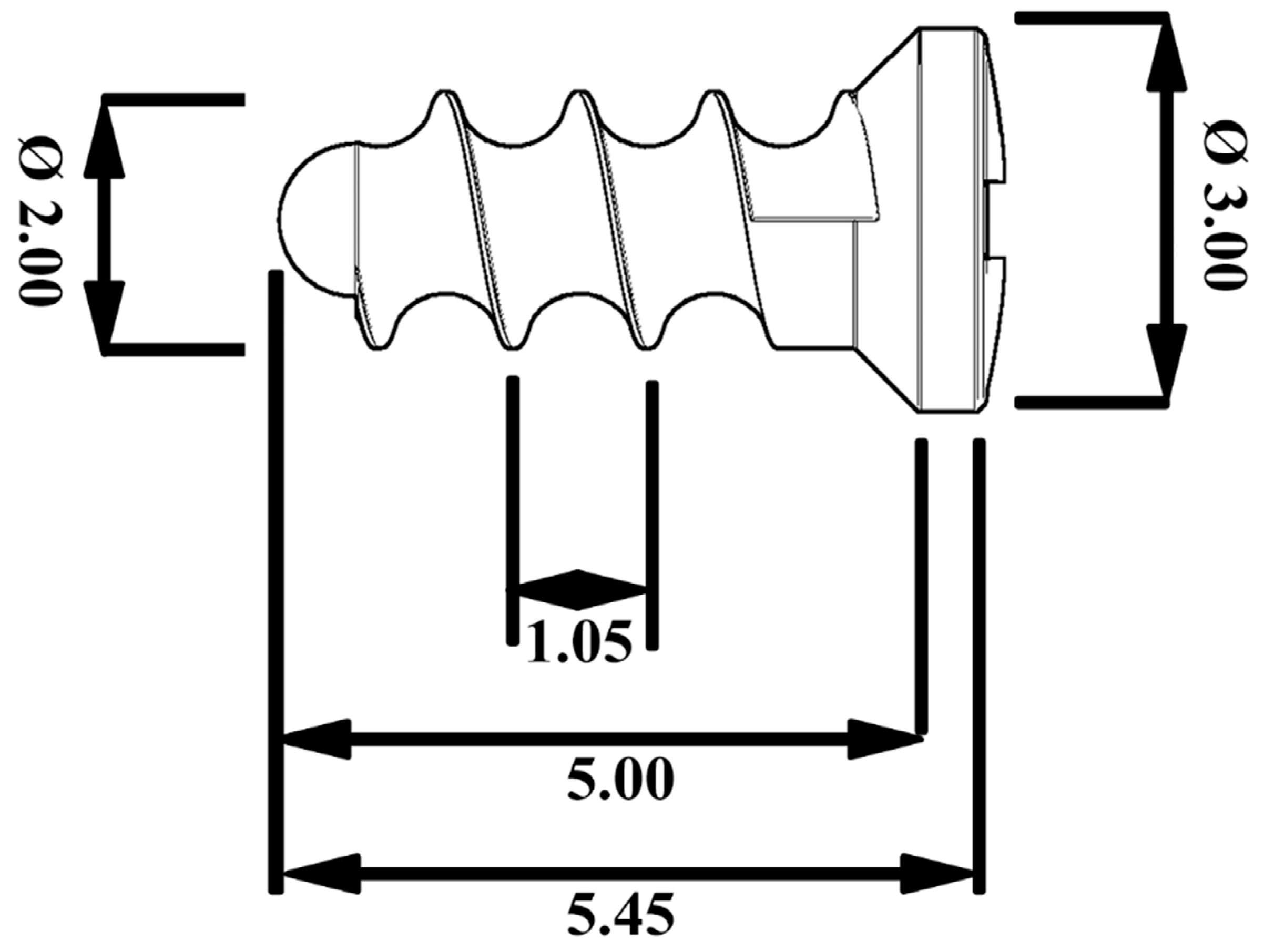
2.1. Implant Material
2.2. Animals
2.3. Experimental Group
2.4. Surgery
2.5. Euthanasia
2.6. Histological Methods for Analysis of the Bone Specimens
2.7. Histopathological Methods of Parenchymal Organ Processing
2.8. Methods of X-ray Examination
2.9. MicroCT Examination
2.10. Analysis of the Solid Corrosion Products
2.11. Analysis of Systemic Toxicity in the Vital Organs
2.12. Strontium and Zinc Analysis
2.13. Magnesium Analysis
3. Results
3.1. Mechanical Properties of the Alloy
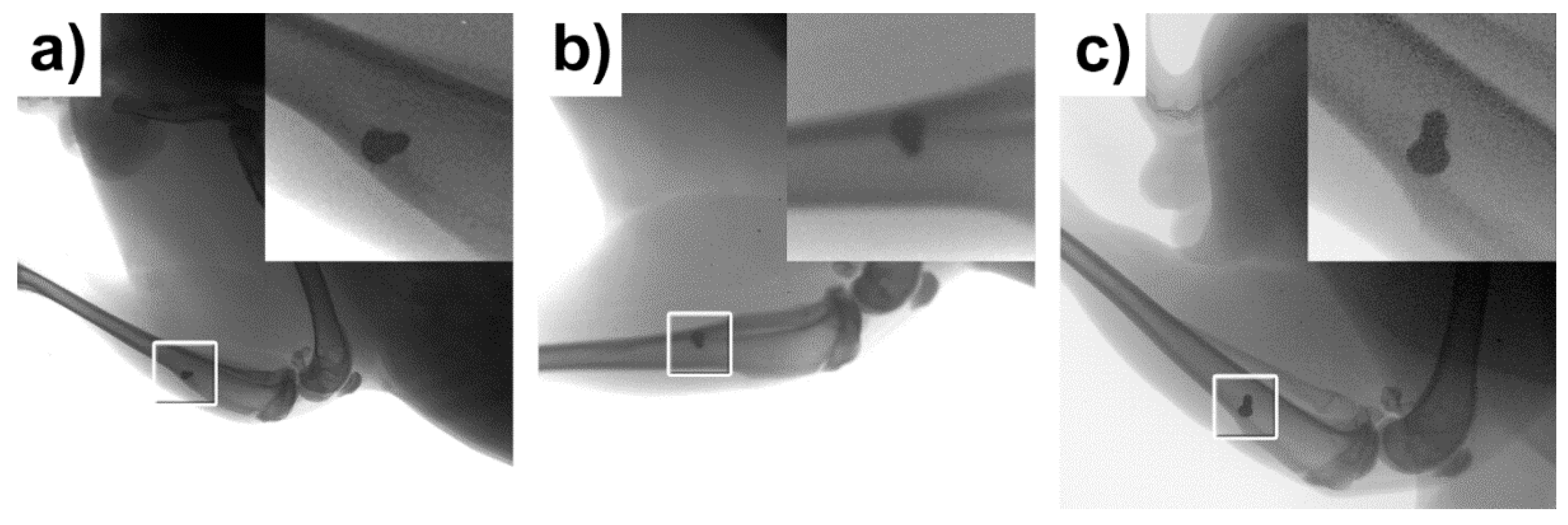
3.2. X-ray Examination
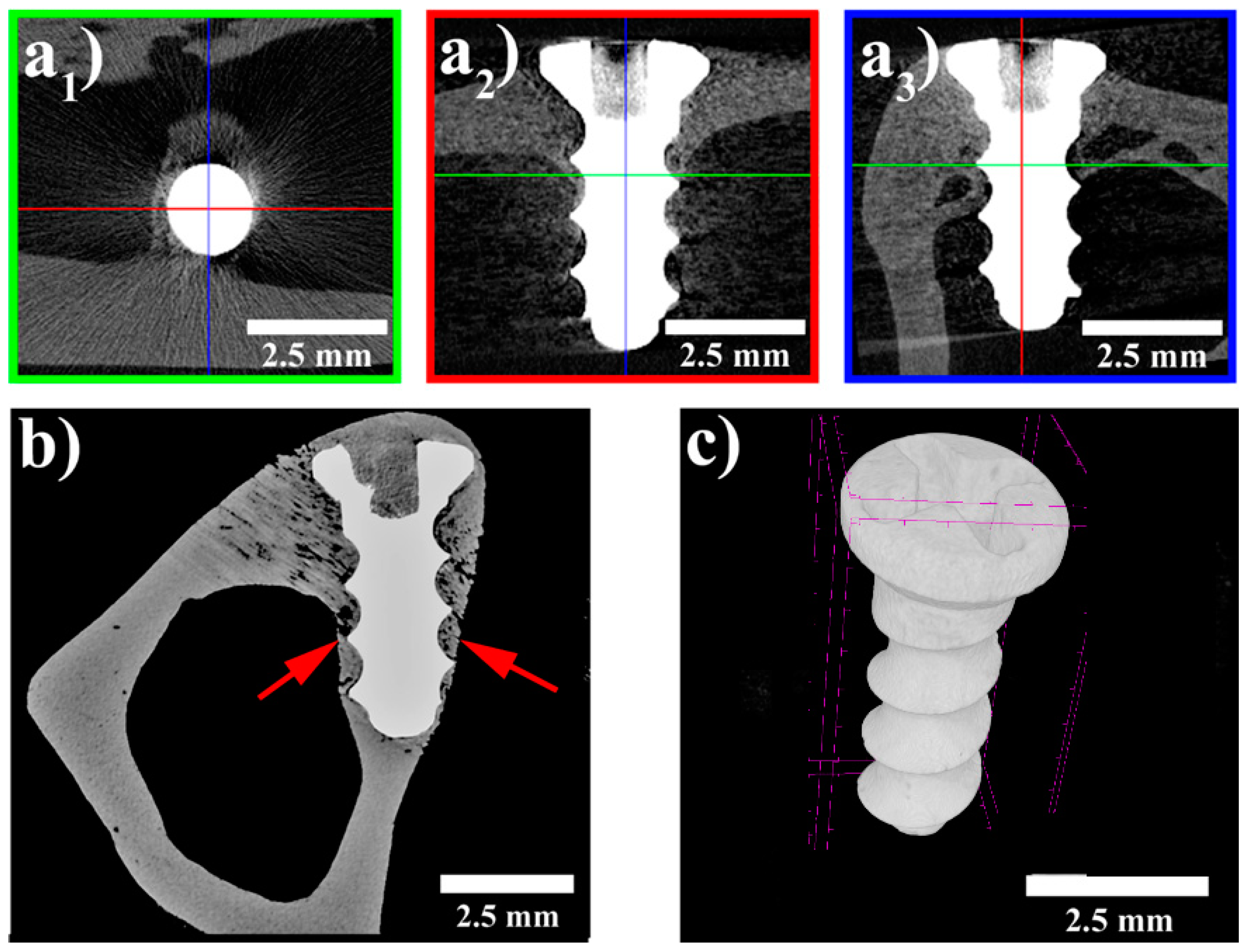
3.3. MicroCT Examination
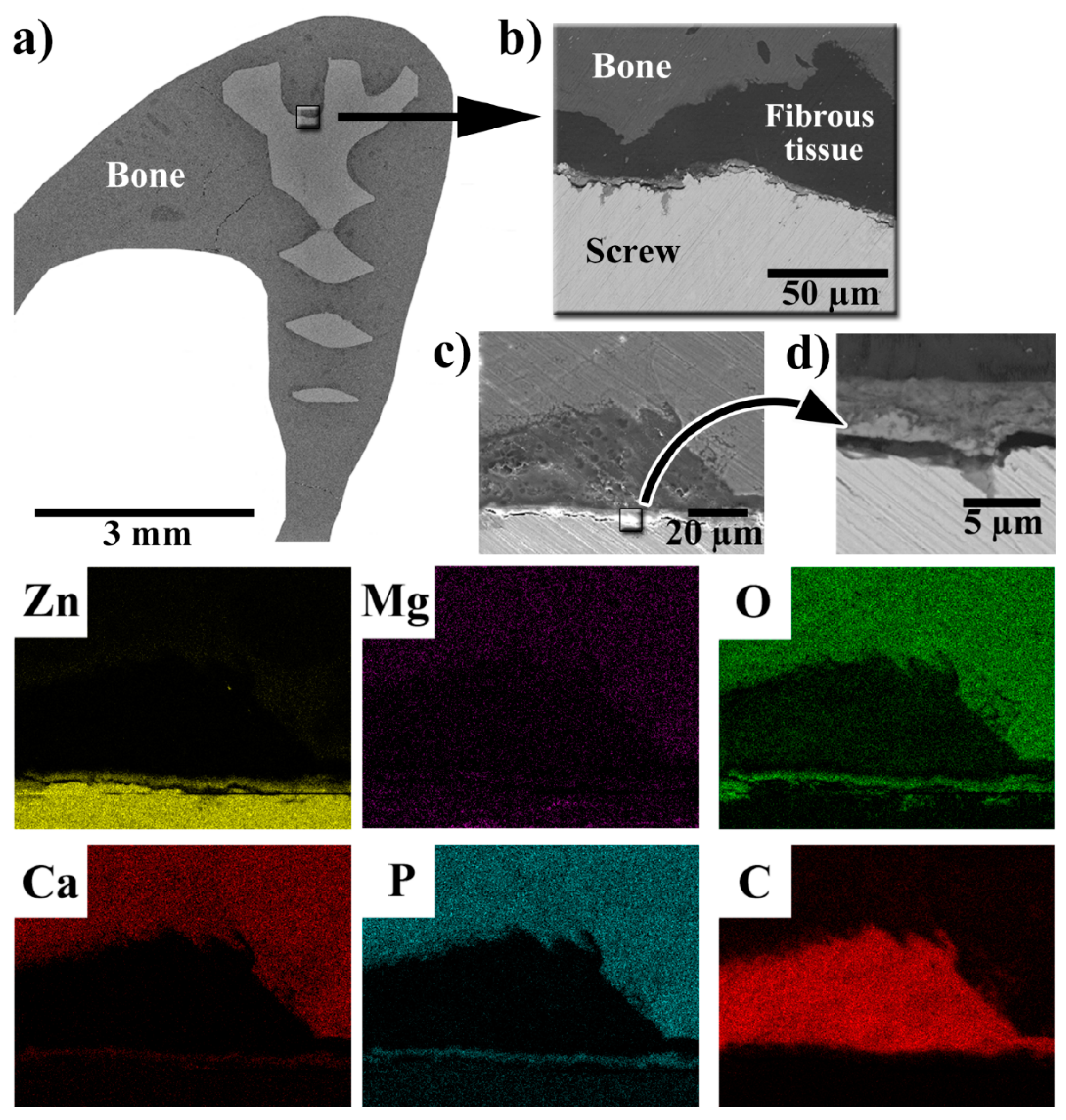
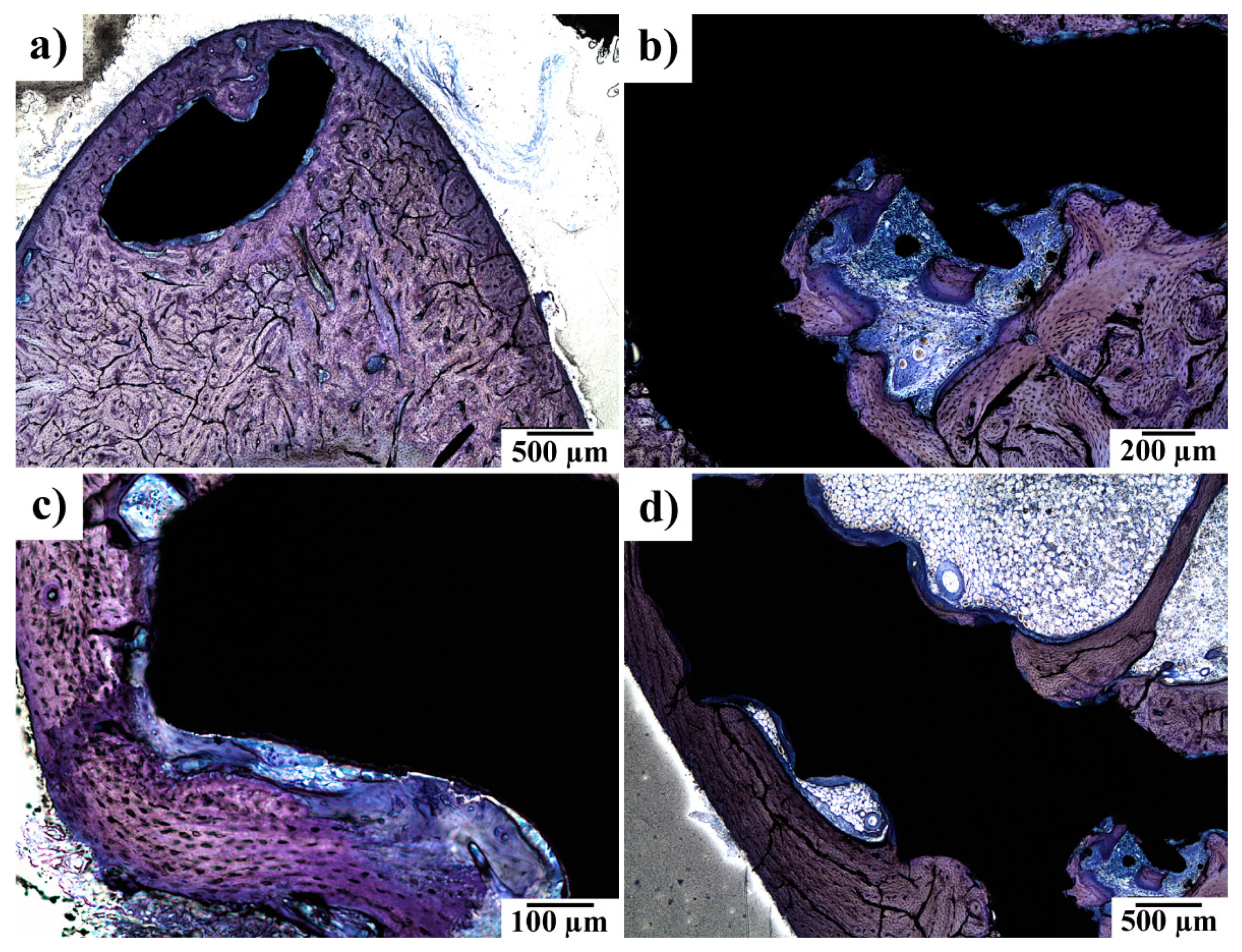
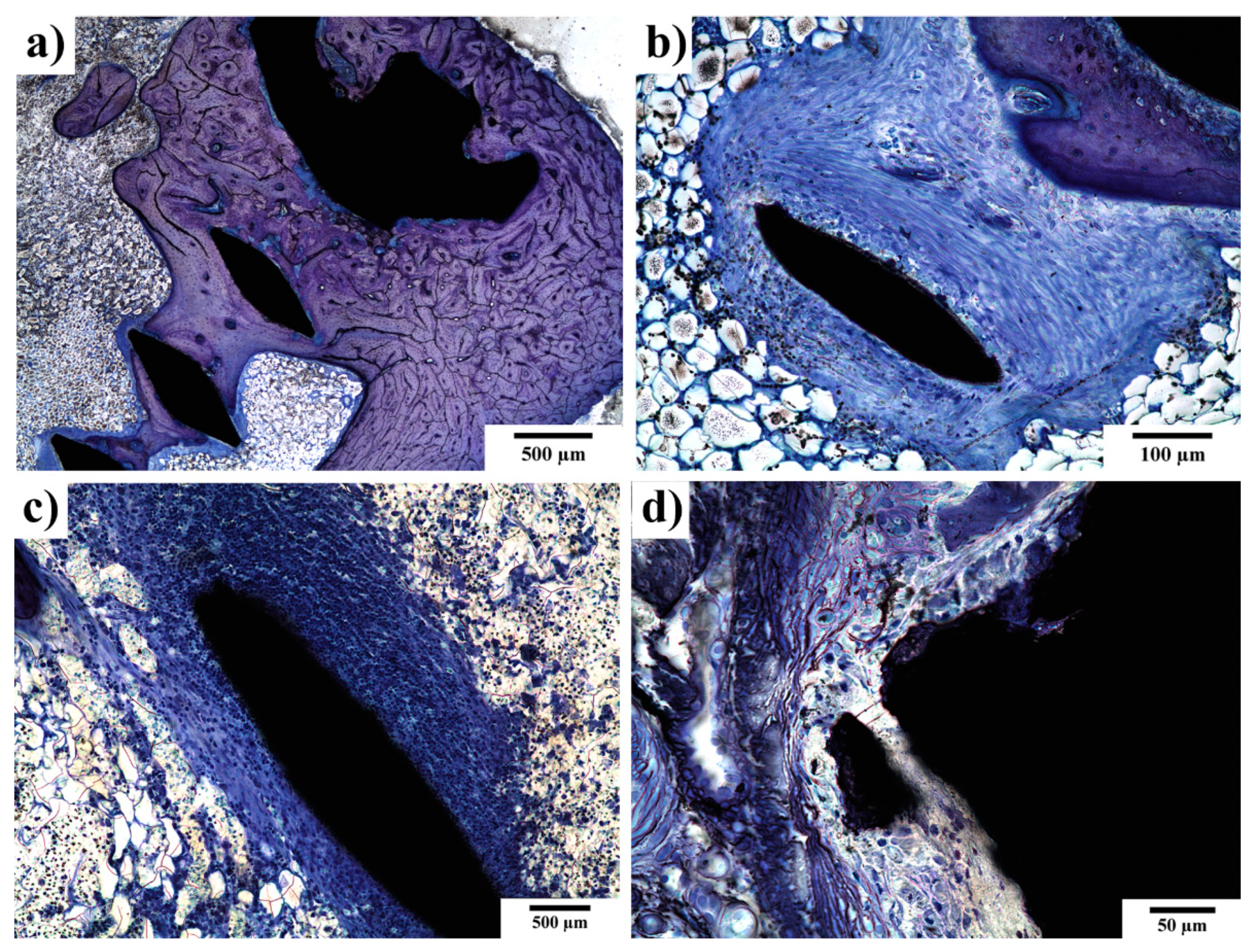
3.4. SEM-EDS Observations of the Implant–Bone Interface
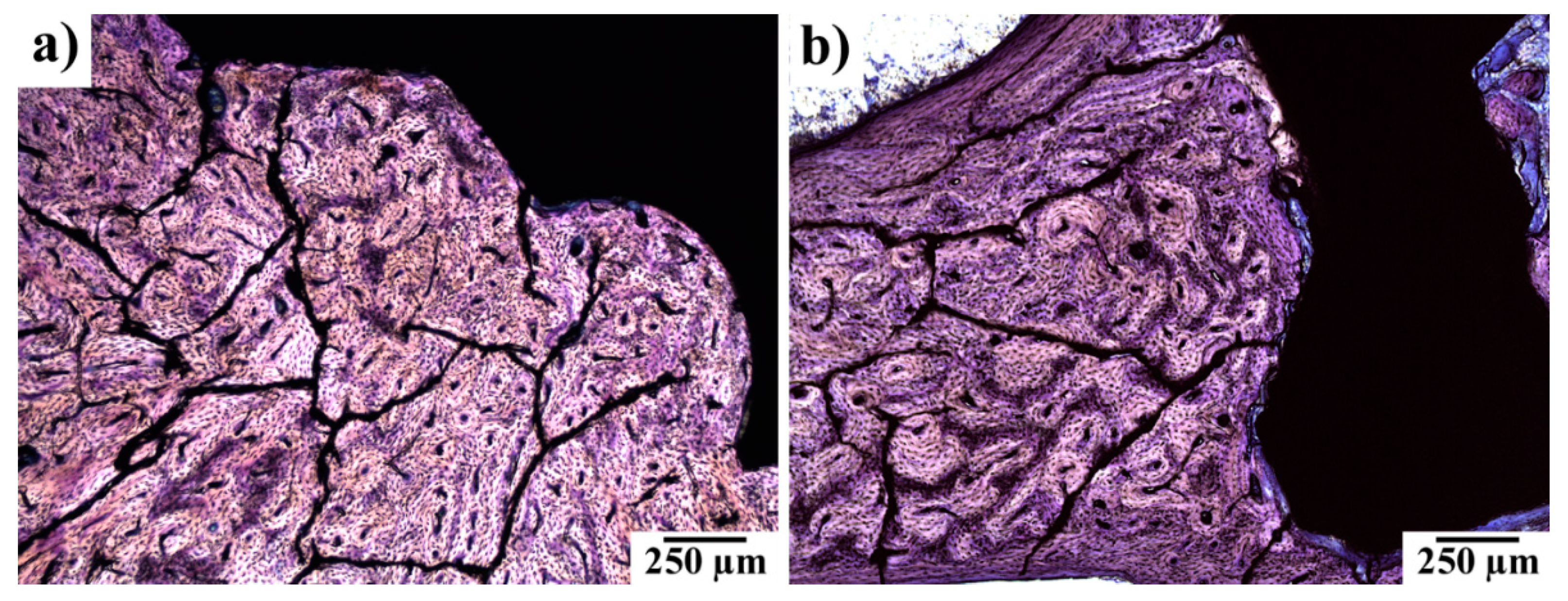
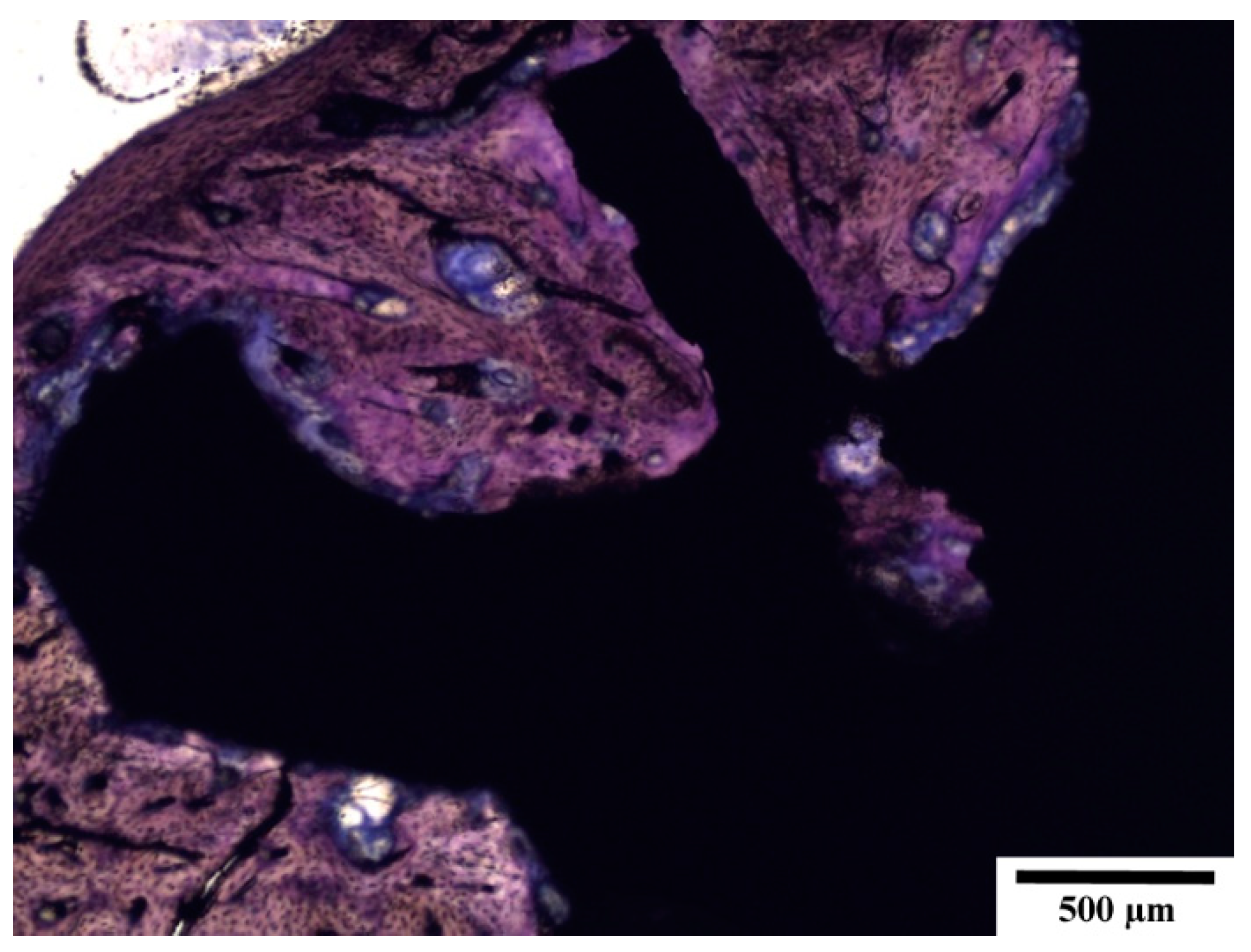
3.5. Histopathological Examination of Bone Specimens Containing Implants
3.6. Systemic Toxicity in the Liver, Kidneys and Brain Specimen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kose, O.; Turan, A.; Ünal, M.; Acar, B.; Guler, F. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: Short-term clinical and radiological outcomes in eleven patients. Arch. Orthop. Trauma Surg. 2018, 138, 1069–1075. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.-T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- ASTM F3160-21, Standard Guide for Metallurgical Characterization of Absorbable Metallic Materials for Medical Implants. Available online: https://www.astm.org/Standards/F3160.htm (accessed on 2 June 2021).
- ASTM F3268-18a, Standard Guide for In Vitro Degradation Testing of Absorbable Metals. Available online: https://www.astm.org/Standards/F3268.htm (accessed on 2 June 2021).
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Huse, E.C. A new ligature? Chic. Med. J. Exam. 1878, 171–172. [Google Scholar]
- Yue, Y.; Lei, L.; Ye, H.; Yang, S.; Wang, L.; Yang, N.; Huang, J.; Ren, L. Effectiveness of Biodegradable Magnesium Alloy Stents in Coronary Artery and Femoral Artery. J. Interv. Cardiol. 2015, 28, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Wu, Y.; Xiang, S.; Sun, C.; Wang, Y.; Yu, H.; Fu, Y.; Wang, X.; Yan, J.; Zhao, D.; et al. Corrosion Resistance and Biocompatibility Assessment of a Biodegradable Hydrothermal-Coated Mg–Zn–Ca Alloy: An in vitro and in vivo Study. ACS Omega 2020, 5, 4548–4557. [Google Scholar] [CrossRef]
- Angrisani, N.; Reifenrath, J.; Zimmermann, F.; Eifler, R.; Meyer-Lindenberg, A.; Herrera, K.V.; Vogt, C. Biocompatibility and degradation of LAE442-based magnesium alloys after implantation of up to 3.5years in a rabbit model. Acta Biomater. 2016, 44, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Lamaka, S.V.; Lu, X.; Zheludkevich, M.L. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals—A critical review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Venezuela, J.J.D.; Johnston, S.; Dargusch, M.S. The Prospects for Biodegradable Zinc in Wound Closure Applications. Adv. Health Mater. 2019, 8, e1900408. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- 21. Kubásek, J.; Dvorský, D.; Šedý, J.; Msallamová, Š.; Levorová, J.; Foltán, R.; Vojtěch, D. The Fundamental Comparison of Zn–2Mg and Mg–4Y–3RE Alloys as a Perspective Biodegradable Materials. Materials 2019, 12, 3745. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Zhang, Z.; Qu, X.; Jia, X.; Wu, Q.; Han, Y.; Zheng, Y.; Dai, K. Biodegradable Zn–Sr alloy for bone regeneration in rat femoral condyle defect model: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 1588–1604. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Dai, T.; Xu, F.; Zhou, J.G.; Qu, G.; Tian, L.; Liu, B.; Liu, Y. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system. Acta Biomater. 2019, 92, 351–361. [Google Scholar] [CrossRef]
- Tie, D.; Guan, R.; Liu, H.; Cipriano, A.; Liu, Y.; Wang, Q.; Huang, Y.; Hort, N. An in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg–1Sr alloy. Acta Biomater. 2016, 29, 455–467. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, B.; Zhang, X.; Lu, F.; Wang, Z.; Tian, S.; Li, D.; Yang, J.; Cao, F.; Cheng, L.; et al. High-purity weight-bearing magnesium screw: Translational application in the healing of femoral neck fracture. Biomaterials 2020, 238, 119829. [Google Scholar] [CrossRef]
- Diekmann, J.; Bauer, S.; Weizbauer, A.; Willbold, E.; Windhagen, H.; Helmecke, P.; Lucas, A.; Reifenrath, J.; Nolte, I.; Ezechieli, M. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: A pilot in vivo study in rabbits. Mater. Sci. Eng. C 2016, 59, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Cihova, M.; Martinelli, E.; Schmutz, P.; Myrissa, A.; Schäublin, R.; Weinberg, A.; Uggowitzer, P.; Löffler, J. The role of zinc in the biocorrosion behavior of resorbable Mg‒Zn‒Ca alloys. Acta Biomater. 2019, 100, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Hybasek, V.; Kubasek, J.; Capek, J.; Alferi, D.; Pinc, J.; Jiru, J.; Fojt, J. Influence of model environment complexity on corrosion mechanism of biodegradable zinc alloys. Corros. Sci. 2021, 187, 109520. [Google Scholar] [CrossRef]
- Čapek, J.; Kubásek, J.; Pinc, J.; Fojt, J.; Krajewski, S.; Rupp, F.; Li, P. Microstructural, mechanical, in vitro corrosion and biological characterization of an extruded Zn-0.8Mg-0.2Sr (wt%) as an absorbable material. Mater. Sci. Eng. C 2021, 122, 111924. [Google Scholar] [CrossRef]
- Luize, D.S.; Bosco, A.F.; Bonfante, S.; De Almeida, J.M. Influence of ovariectomy on healing of autogenous bone block grafts in the mandible: A histomorphometric study in an aged rat model. Int. J. Oral Maxillofac. Implant. 2008, 23, 207–214. [Google Scholar]
- Šedý, J.; Urdzíková, L.; Jendelová, P.; Syková, E. Methods for behavioral testing of spinal cord injured rats. Neurosci. Biobehav. Rev. 2008, 32, 550–580. [Google Scholar] [CrossRef] [PubMed]
- Reifenrath, J.; Bormann, D.; Meyer-Lindenberg, A. Magnesium Alloys as Promising Degradable Implant Materials in Orthopaedic Research. In Magnesium Alloys—Corrosion and Surface Treatments; IntechOpen: London, UK, 2011; p. 10577214143. [Google Scholar]
- Johansson, C.; Hansson, H.; Albrektsson, T. Qualitative interfacial study between bone and tantalum, niobium or commercially pure titanium. Biomaterials 1990, 11, 277–280. [Google Scholar] [CrossRef]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRµCT slices. Eur. Cells Mater. 2012, 23, 237–248. [Google Scholar] [CrossRef]
- Jiřík, M.; Bartoš, M.; Tomášek, P.; Malečková, A.; Kural, T.; Horáková, J.; Lukáš, D.; Suchý, T.; Kochová, P.; Kalbacova, M.H.; et al. Generating standardized image data for testing and calibrating quantification of volumes, surfaces, lengths, and object counts in fibrous and porous materials using X-ray microtomography. Microsc. Res. Tech. 2018, 81, 551–568. [Google Scholar] [CrossRef]
- Passlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Zentek, J. Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium, and lead in the liver and kidneys of dogs according to age, gender, and the occurrence of chronic kidney disease. J. Vet. Sci. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, W.; Huang, H.; Pei, J.; Qu, H.; Yuan, G.; Li, Y. The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: A 20-month study. Acta Biomater. 2018, 69, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Bentley, P.J.; Grubb, B.R. Effects of a zinc-deficient diet on tissue zinc concentrations in rabbits. J. Anim. Sci. 1991, 69, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Bulat, Z.; Dukić-Ćosić, D.; Antonijević, B.; Bulat, P.; Vujanović, D.; Buha, A.; Matovic, V. Effect of Magnesium Supplementation on the Distribution Patterns of Zinc, Copper, and Magnesium in Rabbits Exposed to Prolonged Cadmium Intoxication. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Lin, S.; Ran, X.; Yan, X.; Yan, W.; Wang, Q.; Yin, T.; Zhou, J.G.; Hu, T.; Wang, G. Corrosion behavior and biocompatibility evaluation of a novel zinc-based alloy stent in rabbit carotid artery model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1814–1823. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2006, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Kubásek, J.; Pinc, J.; Drahokoupil, J.; Čavojský, M.; Vojtěch, D. Extrusion of the biodegradable ZnMg0.8Ca0.2 alloy—The influence of extrusion parameters on microstructure and mechanical characteristics. J. Mech. Behav. Biomed. Mater. 2020, 108, 103796. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.-X.; Zheng, Y.; Zhu, D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, J.; Dargusch, M. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Bostman, O. Osteolytic changes accompanying degradation of absorbable fracture fixation implants. J. Bone Jt. Surgery. Br. Vol. 1991, 73-B, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Goodman, S. Wear particles and osteolysis. In Orthopaedic Bone Cements; Elsevier: Amsterdam, The Netherlands, 2008; pp. 140–163. [Google Scholar]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef]
- Moonga, B.S.; Dempster, D.W. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J. Bone Miner. Res. 2009, 10, 453–457. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Shang, Z.; Zhao, B.; Jiao, M.; Liu, W.; Cheng, M.; Zhai, B.; Guo, Y.; Liu, B.; et al. Biodegradable metals for bone defect repair: A systematic review and meta-analysis based on animal studies. Bioact. Mater. 2021, 6, 4027–4052. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Su, Y.; Yang, H.; Gao, J.; Qin, Y.; Zheng, Y.; Zhu, D. Interfacial Zinc Phosphate is the Key to Controlling Biocompatibility of Metallic Zinc Implants. Adv. Sci. 2019, 6, 1900112. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y.; Qin, L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Toyosaki-Maeda, T.; Takano, H.; Tomita, T.; Tsuruta, Y.; Maeda-Tanimura, M.; Shimaoka, Y.; Takahashi, T.; Itoh, T.; Suzuki, R.; Ochi, T. Differentiation of monocytes into multinucleated giant bone-resorbing cells: Two-step differentiation induced by nurse-like cells and cytokines. Arthritis Res. 2001, 3, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Zinc, Fact Sheet for Health Professionals. Available online: Https://Ods.Od.Nih.Gov/Factsheets/Zinc-HealthProfessional (accessed on 2 June 2021).
- Magnesium, Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 2 June 2021).
- Strontium in Drinking Water—Guideline Technical Document for Public Consultation. Available online: Ttps://Www.Canada.ca/En/Health-Canada/Programs/Consultation-Strontium-Drinking-Water/Document.Html (accessed on 2 June 2021).
- Vormann, J. Magnesium: Nutrition and Homoeostasis. AIMS Public Health 2016, 3, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yang, H.; Yu, Z.; Jia, B.; Qiao, H.; Zheng, Y.; Dai, K. Serum zinc levels and multiple health outcomes: Implications for zinc-based biomaterials. Bioact. Mater. 2020, 5, 410–422. [Google Scholar] [CrossRef]
- Fonseca, J.E.; Brandi, M.L. Mechanism of action of strontium ranelate: What are the facts? Clin. Cases Miner. Bone Metab. 2010, 7, 17–18. [Google Scholar] [PubMed]
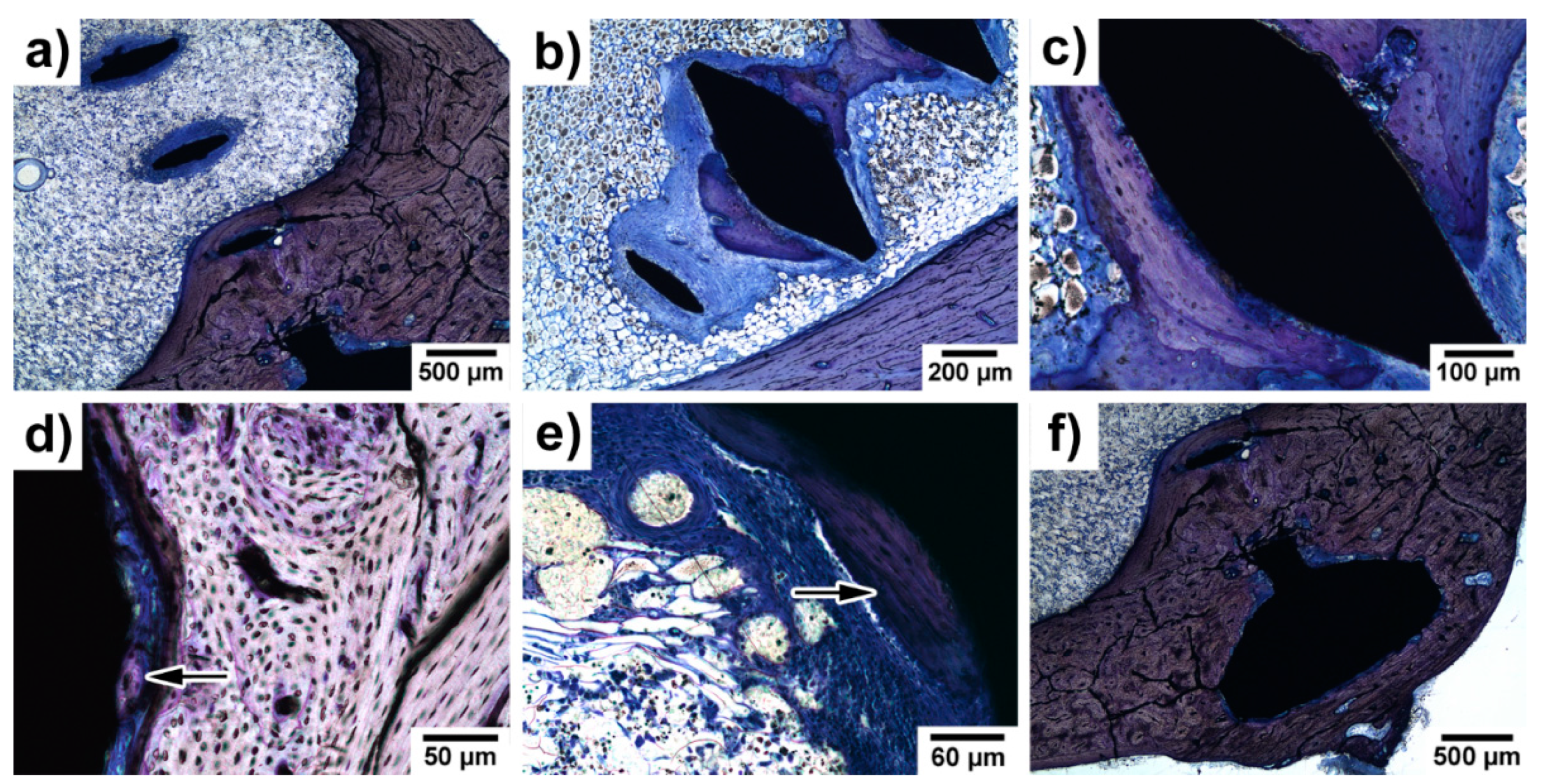
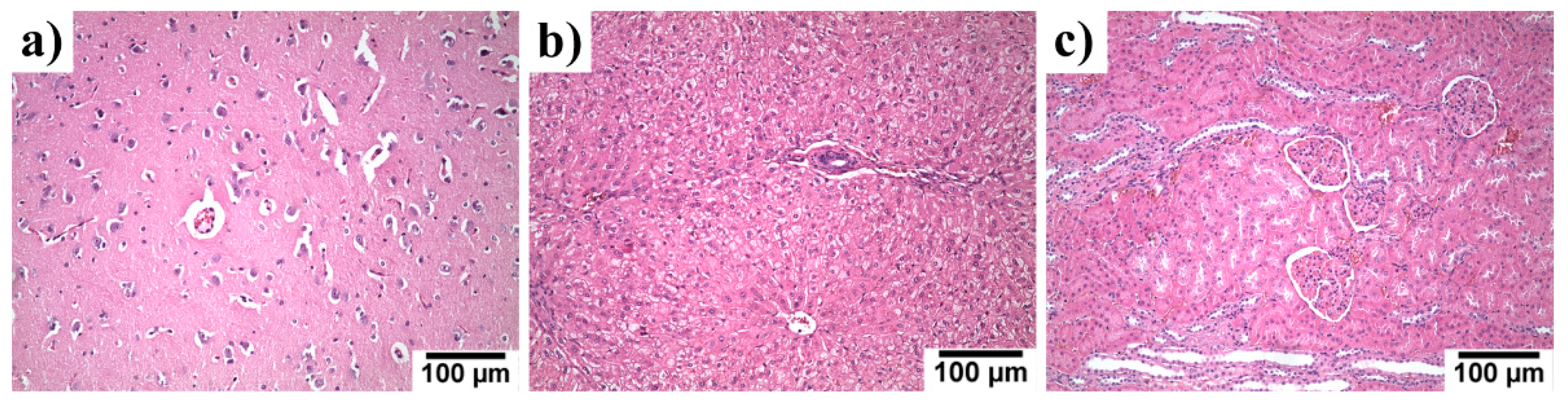
| No. of Animals, Experimental Time | Setting | Zn/Mg Alloy | Methods of Analysis | Results | Ref. |
|---|---|---|---|---|---|
| 30 rats, 4, 8, 12 weeks | Femoral condyle defect, with 99.99% Ti (N = 15), Zn–0.8Sr (wt.%), N = 15 | Zn–0.8Sr (wt.%) Ti 99.99% | microCT, Histomorphometry, SEM | Good biosafety. Osteogenesis time related: greater after 12 weeks than after 4 weeks. Biodegradation products greater around Zn–0.8Sr alloy. Heart, liver, spleen, lung, and kidneys—no abnormalities in the Zn–0.8Sr alloy group in comparison with the pure Ti group. The concentration of Zn2+ and Sr2+ in the blood and organs of the Zn–0.8Sr group was not higher than the pure Ti implant group | [22] |
| 54 rats, 8 weeks | Experimental alloys implanted into femoral bone of rats | Zn 99.99% Zn–0.1Sr Zn–0.1Mn Zn–0.4Cu Zn–0.4Fe Zn–0.2Li Zn–0.8Mg Zn–0.8Ca Zn–2.0Ag | microCT, Histology, SEM, blood analysis of Zn concentration | No gas, no obvious degradation after 8 weeks. Circumferential osteogenesis. Volume of pure Zn decreased to 95.12 ± 1.39% after 8 weeks and degradation rate was 0.14 ± 0.05 mm/year. Zn–0.4Cu alloys had higher rate of degradation: 0.26 ± 0.03 mm/year. Faster degradation: Zn–2.0Ag, Zn–0.4Li, Zn–0.4Fe, and Zn–0.8Mg. Same degradation rate as pure Zn: Zn–0.1Mn, Zn–0.8Ca, and Zn–0.8Sr | [23] |
| 27 beagle dogs, 8, 12, 24 weeks | Experimental alloy used for treatment of mandibular fractures of beagle dogs. Control group: Ti 99.99%, PLLA (poly-l-lactic acid) | Zn–2.0Mg–2.0Fe PPLA Ti 99.99% | 3-point bending test, X-ray, microCT, histology, analysis of Zn concentration in blood, before surgery, 4, 12, and 24 weeks after surgery | Zinc alloy: good mechanical properties. Increasing in vivo degradation rate of the zinc alloy implants: 0.033 ± 0.015 mm/year at 4 weeks, 0.079 ± 0.009 mm/year at 12 weeks, 0.095 ± 0.009 mm/year at 24 weeks. Good biocompatibility: no difference between the pre-op and post-op blood and biochemical results. Serum zinc value in the zinc alloy group was slightly higher after implantation than before, no statistically significant difference | [24] |
| 12 rabbits, 24 operated sites, 16 weeks | Fracture of ulnar bone and its osteosynthesis | 99.9% Mg (wt.%) | Mechanical testing, X-ray, microCT, histology | Well tolerated. Corrosion product formation and gas formation. Histologically normal bone properties. All fractures healed. Bone growth over and around degrading Mg devices. Good mechanical properties of healed bone. Facilitated fracture healing while stimulating local bone growth | [3] |
| 32 rats, 24 weeks | Femoral pin implanted bilaterally | ZX50 *, WZ21 ** | Histology, microCT | Volume loss: ZX50: 1.2%/day, WZ21: 0.5%/day. ZX50: massive gas production. WZ21: new bone production, good osteoconductivity and osteoinductivity of Mg. Excellent bone recovery | [2] |
| 36 rats, 4, 8, 12 weeks | Ulnar bone defect replaced by a metallic experimental alloy tube | Mg–3Zn–1Ca–0.5REE ***/ hydrothermally (HT) coated and uncoated alloy | Histology, microCT, X-ray, serum Mg2+ and Ca2+ concentrations | Mg2+ normal levels in all groups. Ca2+ levels higher than normal, but not significantly. No organ histological pathology. HT coated alloy with better biocompatibility and higher resistance to corrosion | [11] |
| 8 rabbits, 9 months to 3.5 years | Cylindrical alloy implant in a medullary cavity | LAE442 **** | Histology, microCT, mechanical testing, organ corrosion products testing | Good biocompatibility, no gas formation, no toxicity in vital organs. Slow resorption: 2–2.5% in 9 months, 5% in 3.5 years. Accumulation of REE *** in implant site and vital organs | [12] |
| 18 rabbits, 16 weeks | Alloy plate and 2 screws implanted into femoral bone | 99.9% Mg Mg–1Sr (wt.%) | Histology, blood count, Sr concentration in implanted site and vital organs | Corrosion rate slower in group with Sr. Good biocompatibility in the group with Sr. Highest increase of Sr2+ concentration in liver (388 ± 25 μg/kg) at 4w post-implantation and returned to 32 ± 2 μg/kg after 16 weeks implantation. Increase in the Sr2+ ion concentration in the blood from 2–8 weeks post-implantation | [25] |
| 48 humans, 12 months | Treatment of osteonecrosis of femoral head by vascularized bone grafting. Two groups: (i) Mg screw (ii) without fixation | 99.9 Mg (wt.%) § | X-ray, CT, functional recovery, Harris hip score (HHS), serum levels of Mg, Ca, and P | Mg screw group 25% volume reduction in 12 months. Normal levels of Mg, Ca, P in both groups. HHS was significantly improved in the Mg screw group | [26] |
| 23 goats, 4, 8, 12, 48 weeks | Femoral neck fractures. Control group: no treatment/empty defect | Mg 99.99 wt.% § | Histology, microCT, X-ray, serum Mg and Ca ion, liver and kidney functions pre-op and at 2, 4, 8, 12, and 48 weeks after surgery. | Normal levels of ions all weeks/all groups. Normal organ function & histology. All fractures healed. Degradation of metal: 10% at 4 weeks, 38.8% at 12 weeks and 45.3% at 48 weeks. No cytotoxicity. Good bone production around screws. No gas production observed | [27] |
| 36 rabbits, 6, 12, 24 weeks | Reconstruction of anterior cruciate knee ligament by Mg alloy and titanium screws | MgYREEZr and Ti–6Al–4V (wt.%) | Histology, microCT, blood testing for alloy elements | Good biocompatibility, no inflammatory changes, no necrosis. Similar results to titanium alloy. Gas production decreased within 24 weeks. Very low levels of alloy elements in blood | [28] |
| 18 rats, 4, 26, and 52 weeks | Pins into femoral bone of a rat, one group treated by ZX10, other by ZX20 | ZX10 and ZX20 §§ ZX20 | Histology, microCT | Higher degradation rate of ZX20. Histologically ZX10 and ZX20 are well tolerated. Good implant-bone contact from 4 weeks post-op Gas production: insignificant between pure Mg (99.999%) and ZX10, significantly higher gas production of ZX20, compared to ZX10 and pure Mg | [29] |
| Parameter | Score | Interpretation | 120 Days of Implantation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gas bubbles | 0 | No | 0 | 0 | 0 | 0 | 0 | 0 | NI |
| — | 1 | Yes | — | — | — | — | — | — | — |
| Overall impression of bone structure (BS) | 0 | Smooth | 0 | 0 | — | — | 0 | — | — |
| 1 | Irregular | — | — | 1 | 1 | — | 1 | NI | |
| Bone cavities (BC) | 0 | ≤3 osteon-like cavities | 0 | 0 | 0 | 0 | 0 | 0 | NI |
| 1 | 4–6 osteon-like cavities or ≤10 smaller | — | — | — | — | — | — | — | |
| 2 | 7–10 osteon-like cavities or 11–20 smaller | — | — | — | — | — | — | — | |
| Periosteal remodelling (PR) | 0 | No | — | — | NA | 1 | — | — | NI |
| 1 | ≥1/4 periosteal bone 1 osteon thick | 2 | 2 | — | — | 2 | 2 | — | |
| 2 | ≥1/4 periosteal bone 2 osteon thick | — | — | — | — | — | — | — | |
| 3 | ≥1/4 periosteal bone 3 osteon thick | — | — | — | — | — | — | — | |
| Endosteal remodelling (ER) | 0 | No | — | 0 | — | NC | NC | NC | NI |
| 1 | ≥1/4 endosteal bone 1 osteon thick | — | — | — | — | — | — | — | |
| 2 | ≥1/4 endosteal bone 2 osteons thick | — | — | 2 | — | — | — | — | |
| 3 | ≥1/4 endosteal bone 3 osteons thick | 3 | — | — | — | — | — | — | |
| Periosteal apposition (PA) | 0 | No | — | — | NA | — | — | — | NI |
| 1 | Yes | 1 | 1 | 1 | 1 | 1 | — | ||
| Peri-implant bone formation (PIF) | 0 | No | — | — | — | — | — | — | NI |
| 1 | Yes | 1 | 1 | NI | 1 | 1 | 1 | ||
| Peri-implant fibrosis (PF) | 0 | No | — | — | — | — | — | — | NI |
| 1 | ≤25% implant surface | — | — | — | — | — | — | — | |
| 2 | 25–50% implant surface | — | — | — | — | 2 | — | — | |
| 3 | ≥51% implant surface | 3 | 3 | NI | 3 | — | 3 | — | |
| [mm] | max. thickness | 0.10 | 0.10 | 0.05 | 0.04 | 0.05 | 0.05 | ||
| Lymphoplasmacellular reaction (LYM) | 0 | <30 cells per section | 0 | 0 | 0 | 0 | 0 | 0 | NI |
| 1 | 30–50 cells per section | — | — | — | — | — | — | — | |
| 2 | 51–100 cells per section | — | — | — | — | — | — | — | |
| 3 | >100 cells per section | — | — | — | — | — | — | — | |
| Macrophages (MPH) | 0 | <3 cells per section | — | 0 | 0 | 0 | 0 | 0 | NI |
| 1 | 3–20 cells per section | 1 | — | — | — | — | — | — | |
| 2 | >20 cells per section | — | — | — | — | — | — | — | |
| Giant cells (GC) | 0 | No | 0 | 0 | 0 | 0 | 0 | 0 | NI |
| 1 | 1–10 cells per section | — | — | — | — | — | — | — | |
| 2 | >10 cells per section | — | — | — | — | — | — | — | |
| Interface—features of material corrosion | 0 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | Yes | — | — | — | — | — | — | — | |
| Experimental Group—This Study | Control Groups (For Details See References) | |||||
|---|---|---|---|---|---|---|
| Tissue | Zinc [mg/kg] of Tissue | Magnesium [mg/kg] of Tissue | Strontium [μg/kg] of Tissue | Zinc [mg/kg] of Tissue | Magnesium [mg/kg] of Tissue | Strontium [μg/kg] of Tissue |
| Brain | 10.4 ± 0.99 | 144 ± 5 | 120 ± 35 | ~11.3 [40] ~11.0 [41] ~16.7 [42] | ~152 [40] ~257 [42] | - |
| Kidneys | 24.27 ± 1.18 | 177 ± 14 | 50 ± 5 | ~23.4 [40] ~10.2 [43] ~44.3 [42] | ~182 [40] ~345 [42] | ~19 [25] |
| Liver | 31.57 ± 1.97 | 174 ± 6 | 28 ± 19 | ~32.1 [40] ~24.6 [41] ~14.7 [43] ~41.9 [42] | ~151 [40] ~349 [42] | ~32 [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klíma, K.; Ulmann, D.; Bartoš, M.; Španko, M.; Dušková, J.; Vrbová, R.; Pinc, J.; Kubásek, J.; Ulmannová, T.; Foltán, R.; et al. Zn–0.8Mg–0.2Sr (wt.%) Absorbable Screws—An In-Vivo Biocompatibility and Degradation Pilot Study on a Rabbit Model. Materials 2021, 14, 3271. https://doi.org/10.3390/ma14123271
Klíma K, Ulmann D, Bartoš M, Španko M, Dušková J, Vrbová R, Pinc J, Kubásek J, Ulmannová T, Foltán R, et al. Zn–0.8Mg–0.2Sr (wt.%) Absorbable Screws—An In-Vivo Biocompatibility and Degradation Pilot Study on a Rabbit Model. Materials. 2021; 14(12):3271. https://doi.org/10.3390/ma14123271
Chicago/Turabian StyleKlíma, Karel, Dan Ulmann, Martin Bartoš, Michal Španko, Jaroslava Dušková, Radka Vrbová, Jan Pinc, Jiří Kubásek, Tereza Ulmannová, René Foltán, and et al. 2021. "Zn–0.8Mg–0.2Sr (wt.%) Absorbable Screws—An In-Vivo Biocompatibility and Degradation Pilot Study on a Rabbit Model" Materials 14, no. 12: 3271. https://doi.org/10.3390/ma14123271
APA StyleKlíma, K., Ulmann, D., Bartoš, M., Španko, M., Dušková, J., Vrbová, R., Pinc, J., Kubásek, J., Ulmannová, T., Foltán, R., Brizman, E., Drahoš, M., Beňo, M., & Čapek, J. (2021). Zn–0.8Mg–0.2Sr (wt.%) Absorbable Screws—An In-Vivo Biocompatibility and Degradation Pilot Study on a Rabbit Model. Materials, 14(12), 3271. https://doi.org/10.3390/ma14123271






