A Facile and Efficient Bromination of Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bromination of MWCNTs
2.3. Characterization Methods
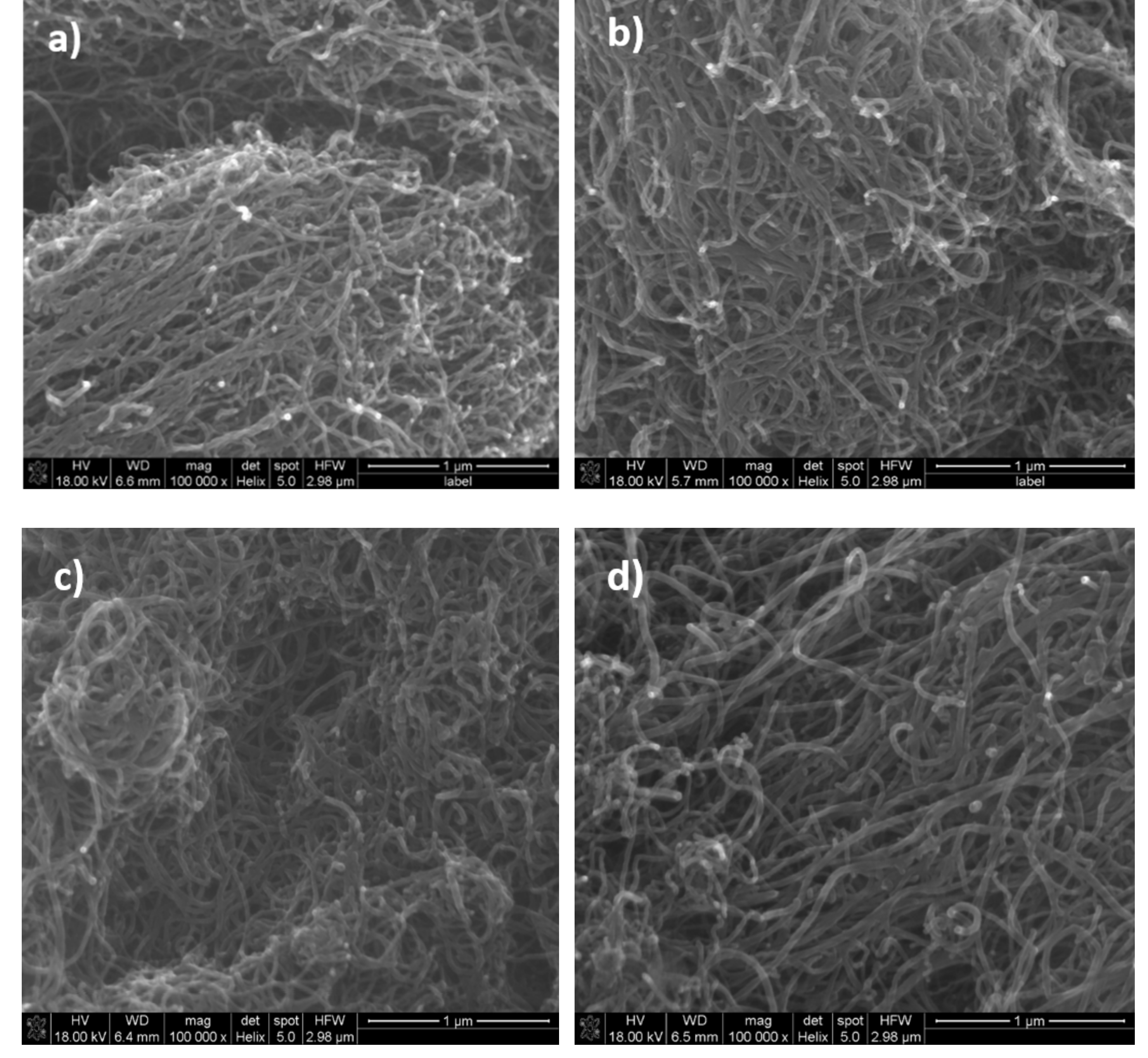
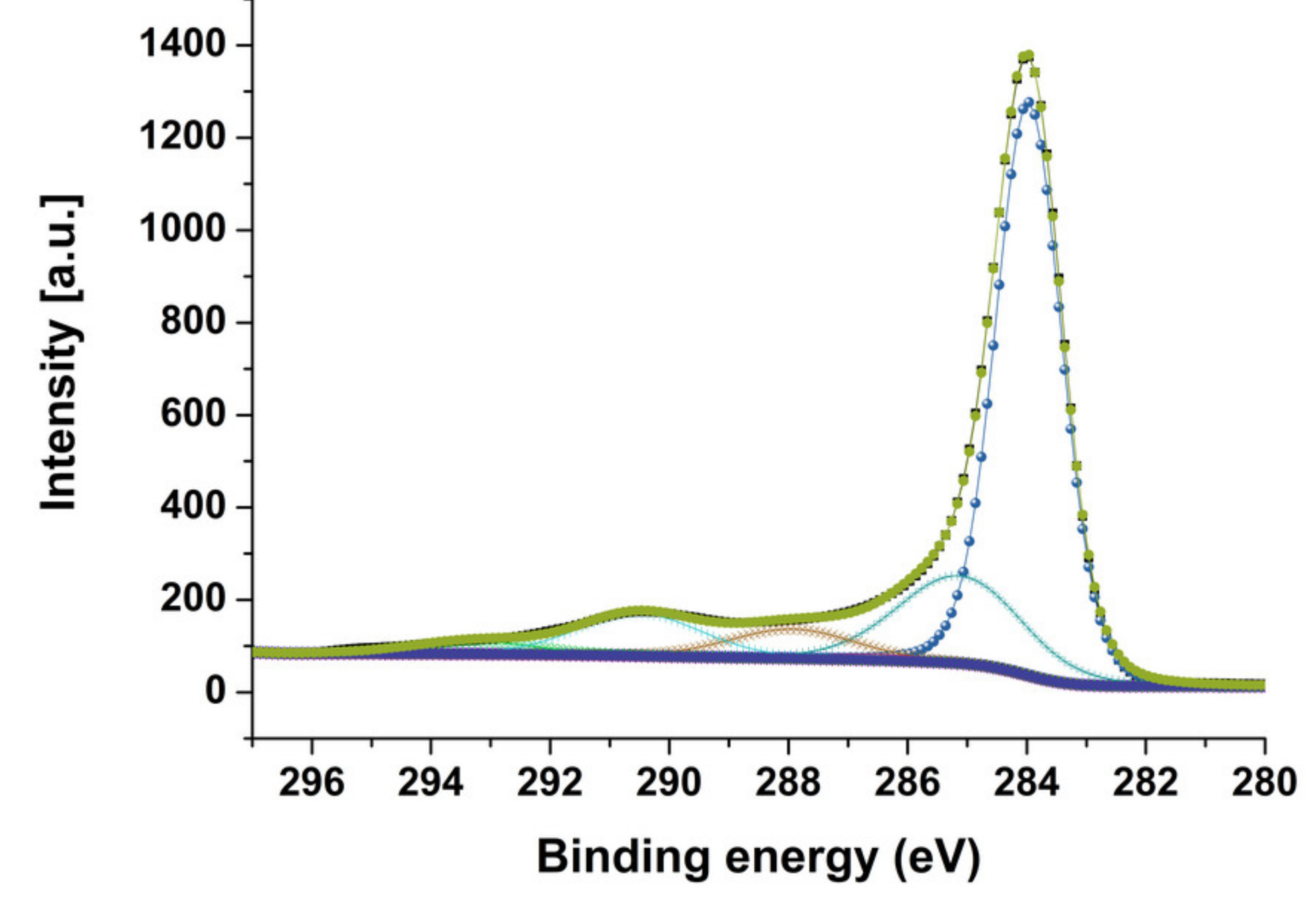
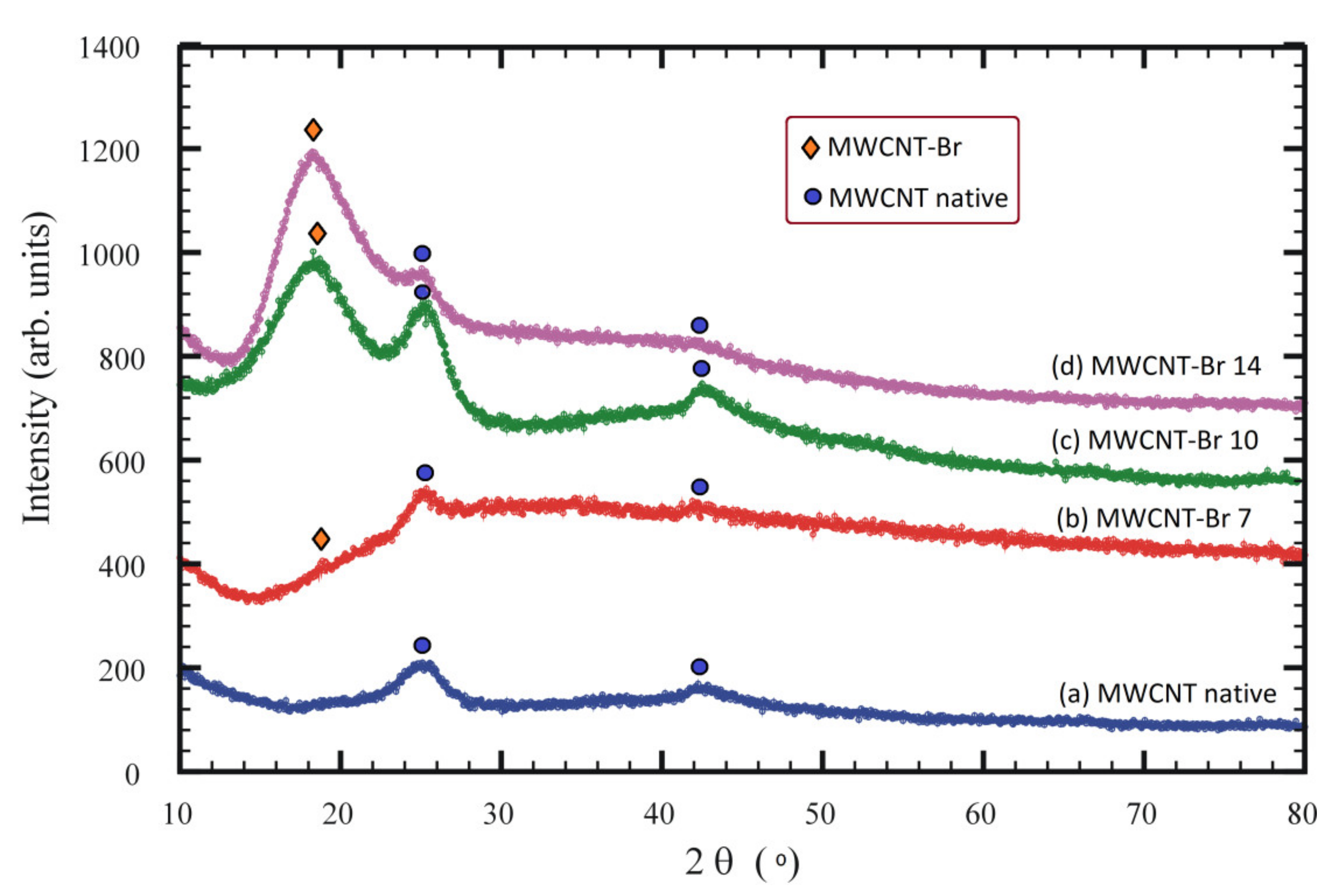
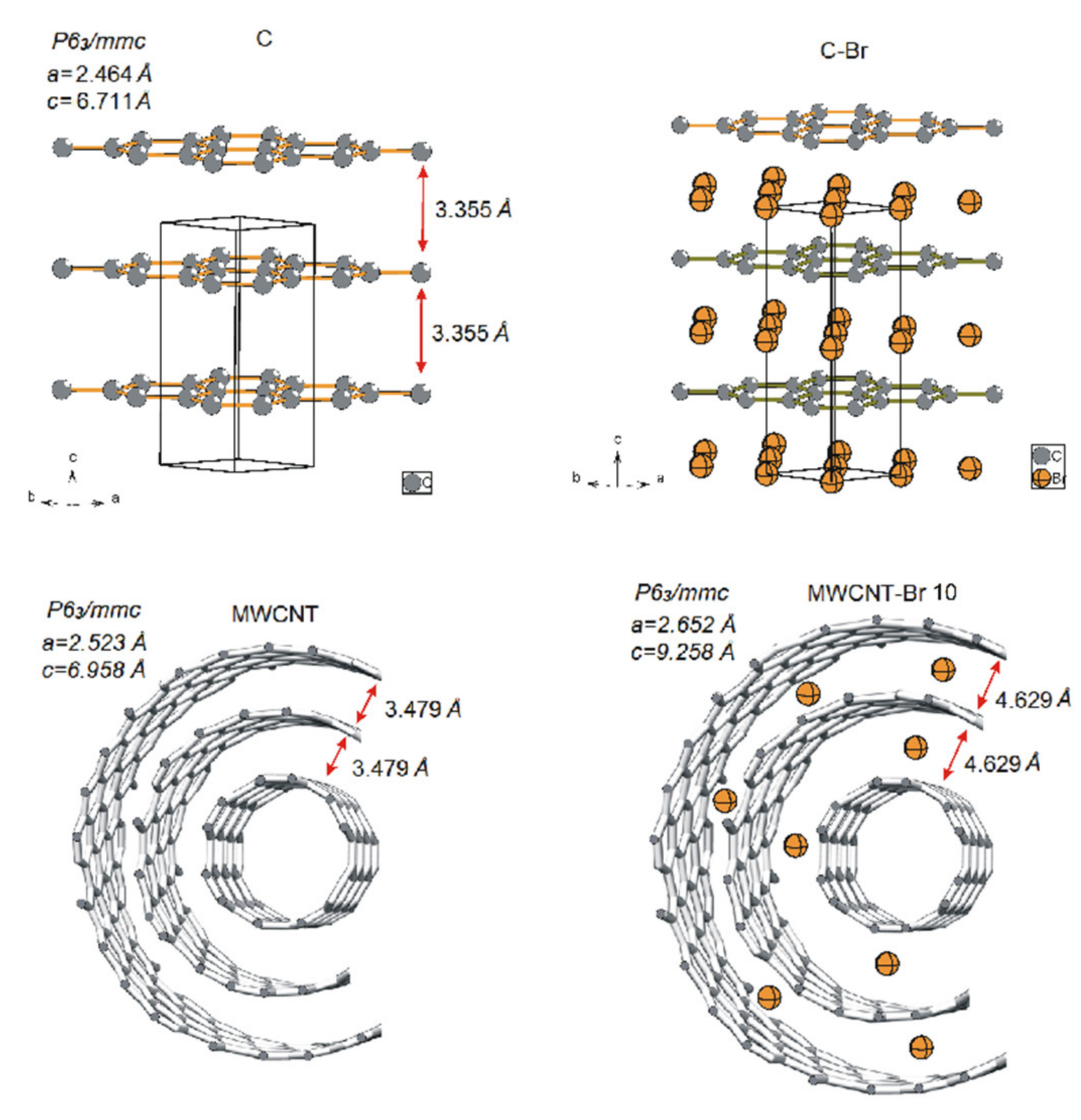
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Huczko, A.; Kurcz, M.; Poplawska, M. Nanorurki Węglowe. Otrzymywanie, Charakterystyka, Zastosowanie, 1st ed.; Wydawnictwa Uniwersytetu Warszawskiego: Warsaw, Poland, 2014. [Google Scholar]
- Afrin, R.; Shah, N.A. Room temperature gas sensors based on carboxyl and thiol functionalized carbon nanotubes buckypapers. Diam. Relat. Mater. 2015, 60, 42–49. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Lii, C.Y.; Stobinski, L.; Tomasik, P.; Liao, C.D. Single-walled carbon nanotube—Potato amylose complex. Carbohydr. Polym. 2003, 51, 93–98. [Google Scholar] [CrossRef]
- Stobinski, L.; Tomasik, P.; Lii, C.Y.; Chan, H.H.; Lin, H.M.; Liu, H.L.; Kao, C.T.; Lu, K.S. Single-walled carbon nanotube–amylopectin complexes. Carbohydr. Polym. 2003, 51, 311–316. [Google Scholar] [CrossRef]
- Polaczek, E.; Tomasik, P.J.; Mazurkiewicz, J.; Wrzalik, R.; Stobinski, L.; Tomasik, P.; Lin, H.M. Interactions of single-walled carbon nanotubes with monosaccharides. J. Nanosci. Nanotechnol. 2005, 5, 479–483. [Google Scholar] [CrossRef]
- Stobinski, L.; Polaczek, E.; Rebilas, K.; Mazurkiewicz, J.; Wrzalik, R.; Lin, H.M.; Tomasik, P. Dextran complexes with single-walled carbon nanotubes. Polimery 2008, 53, 571–575. [Google Scholar] [CrossRef][Green Version]
- Stobinski, L.; Mazurkiewicz, J.; Lin, H.M.; Tomasik, P. Complexes of carbon nanotubes with selected carotenoids. J. Nanosci. Nanotechnol. 2005, 5, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Polaczek, E.; Stobinski, L.; Mazurkiewicz, J.; Tomasik, P.; Koloczek, H.; Lin, H.M. Interactions of anionic polysaccharides with carbon nanotubes. Polimery 2007, 52, 34–38. [Google Scholar] [CrossRef][Green Version]
- Lemek, T.; Mazurkiewicz, J.; Stobinski, L.; Lin, H.M.; Tomasik, P. Non-Covalent functionalization of multi-walled carbon nanotubes with organic aromatic compounds. J. Nanosci. Nanotechnol. 2007, 9, 3081–3088. [Google Scholar] [CrossRef]
- Stobinski, L.; Mazurkiewicz, J.; Tomasik, P.; Peszke, J.; Lin, H.M. Simulated geometry of complexes of CNT with alkanes. Mater. Sci. Poland 2007, 25, 679–686. [Google Scholar]
- Choudhary, V.; Gupta, A. Polymer/Carbon Nanotube Nanocomposites. In Carbon Nanotubes–Polymer Nanocomposites; Yellampalli, S., Ed.; InTechOpen: Rijeka, Croatia, 2011; pp. 65–90. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Terrones, M. Carbon nanotubes: synthesis and properties, electronic devices and other emerging applications. Int. Mater. Rev. 2004, 49, 325–377. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. Functionalization of Carbon Nanotubes. In Functional Molecular Nanostructures. Topics in Current Chemistry, 1st ed.; Schlüter, A.D., Ed.; Springer: Berlin, Germany, 2005; Volume 245, pp. 193–237. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Campidelli, S.; Giordani, S.; Bonifazi, D.; Bianco, A.; Prato, M. Organic functionalisation and characterisation of single-walled carbon nanotubes. Chem. Soc. Rev. 2009, 38, 2214–2230. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Huang, J.L.; Lieber, C.M. Fundamental electronic properties and applications of single-walled carbon nanotubes. Acc. Chem. Res. 2002, 35, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Gu, L.; Meziani, M.J.; Wang, X.; Luo, P.G.; Veca, L.M.; Cao, L.; Sun, Y.P. Advances in bioapplications of carbon nanotubes. Adv. Mater. 2009, 21, 139–152. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; World Scientific Publishing Company: London, UK, 1998. [Google Scholar]
- Zhou, O.; Shimoda, H.; Gao, B.; Oh, S.; Fleming, L.; Yue, G. Materials science of carbon nanotubes: fabrication, integration, and properties of macroscopic structures of carbon nanotubes. Acc. Chem. Res. 2002, 35, 1045–1053. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin. Drug Deliv. 2008, 5, 331–342. [Google Scholar] [CrossRef]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar] [CrossRef]
- Cruz-Silva, E.; Cullen, D.A.; Gu, L.; Romo-Herrera, J.M.; Muñoz-Sandoval, E.; López-Urías, F.; Sumpter, B.G.; Meunier, V.; Charlier, J.C.; Smith, D.J.; et al. Heterodoped nanotubes: theory, synthesis, and characterization of phosphorus−nitrogen doped multiwalled carbon nanotubes. ACS Nano. 2008, 2, 441–448. [Google Scholar] [CrossRef]
- Maciel, O.I.; Campos-Delgado, J.; Cruz-Silva, E.; Pimenta, M.A.; Sumpter, B.G.; Meunier, V.; López-Urías, F.; Muñoz-Sandoval, E.; Terrones, H.; Terrones, M.; et al. Synthesis, electronic structure, and raman scattering of phosphorus-doped single-wall carbon nanotubes. Nano Lett. 2009, 9, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Maciel, O.I.; Anderson, N.; Pimenta, M.A.; Hartschuh, A.; Qian, H.; Terrones, M.; Terrones, H.; Campos-Delgado, J.; Rao, A.M.; Novotny, L.; et al. Electron and phonon renormalization near charged defects in carbon nanotubes. Nat. Mater. 2008, 7, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Romo-Herrera, J.M.; Cullen, D.A.; Cruz-Silva, E.; Ramirez, D.; Sumpter, B.G.; Meunier, V.; Terrones, H.; Smith, D.J.; Terrones, M. The role of sulfur in the synthesis of novel carbon morphologies: from covalent Y-junctions to sea-urchin-like structures. Adv. Funct. Mater. 2009, 19, 1193–1199. [Google Scholar] [CrossRef]
- Drabowicz, J.; Krasowska, D.; Ciesielski, W.; Kulawik, D.; Pyzalska, M.; Zdanowska, S.; Dudzinski, B.; Pokora-Sobczak, P.; Chrzanowski, J.; Makowski, T. Carbon nanotubes functionalized with sulfur, selenium, or phosphorus or substituents containing these elements. Phosphorus Sulfur 2016, 191, 541–547. [Google Scholar] [CrossRef]
- Mazov, I.; Krasnikov, D.; Stadnichenko, A.; Kuznetsov, V.; Romanenko, A.; Anikeeva, O.; Tkachev, E. Direct vapor-phase bromination of multiwall carbon nanotubes. J. Nanotechnol. 2012, 1–5. [Google Scholar] [CrossRef]
- Żarska, S.; Kulawik, D.; Drabowicz, J.; Ciesielski, W. A review of procedures of purification and chemical modification of carbon nanotubes with bromine. Fuller. Nanotub. Carbon Nanostructures 2017, 25, 563–569. [Google Scholar] [CrossRef]
- Unger, E.; Graham, A.; Kreupl, F.; Liebau, M.; Hoenlein, W. Electrochemical functionalization of multi-walled carbon nanotubes for solvation and purification. Curr. Appl. Phys. 2002, 2, 107–111. [Google Scholar] [CrossRef]
- Abdelkader, V.K.; Domingo-Garcia, M.; Melguizo, M.; Lopez-Garzon, R.; Lopez-Garzon, F.J.; Perez-Mendoza, M. Covalent bromination of multi-walled carbon nanotubes by iodine bromide and cold plasma treatments. Carbon 2015, 93, 276–285. [Google Scholar] [CrossRef]
- Moradi, L.; Etesami, I. New route for bromination of multiwalled carbon nanotubes under mild and efficient conditions. Fuller. Nanotub. Carbon Nanostructures 2016, 24, 213–218. [Google Scholar] [CrossRef]
- Hanelt, S.; Friedrich, J.F.; Orts-Gil, G.; Meyer-Plath, A. Study of Lewis acid catalyzed chemical bromination and bromoalkylation of multi-walled carbon nanotubes. Carbon 2012, 50, 1373–1385. [Google Scholar] [CrossRef]
- Jin, Z.X.; Xu, G.Q.; Goh, S.H. A preferentially ordered accumulation of bromine on multi-wall carbon nanotubes. Carbon 2000, 38, 1135–1139. [Google Scholar] [CrossRef]
- Diyuk, V.E.; Zaderko, A.N.; Veselovska, K.I.; Lisnyak, V.V. Functionalization of surface of carbon materials with bromine vapors at mediate high temperature: a thermogravimetric study. J. Therm. Anal. Calorim. 2015, 120, 1665–1678. [Google Scholar] [CrossRef]
- Friedrich, J.F.; Wettmarshausen, S.; Hanelt, S.; Mach, R.; Mix, R.; Zeynalov, E.B.; Meyer-Plath, A. Plasma-chemical bromination of graphitic materials and its use for subsequent functionalization and grafting of organic molecules. Carbon 2010, 48, 3884–3894. [Google Scholar] [CrossRef]
- Chen, Y.K.; Green, M.L.H.; Griffin, J.L.; Hammer, J.; Lago, R.M.; Tsang, S.C. Purification and opening of carbon nanotubes via bromination. Adv. Mater. 1996, 8, 1012–1015. [Google Scholar] [CrossRef]
- Vejpravova, J.; Pacakova, B.; Kalbac, M. Magnetic impurities in single-walled carbon nanotubes and graphene: a review. Analyst 2016, 141, 2639–2656. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Kaufmann, A.; Mukasyan, A.; Varma, A. Single- and multi-wall carbon nanotubes produced using the floating catalyst method: Synthesis, purification and hydrogen up-take. Carbon 2006, 44, 2160–2170. [Google Scholar] [CrossRef]
- Mackeyev, Y.; Bachilo, S.; Hartman, K.B.; Wilson, L.J. The purification of HiPco SWCNTs with liquid bromine at room temperature. Carbon 2007, 45, 1013–1017. [Google Scholar] [CrossRef]
- Hou, P.X.; Bai, S.; Yand, Q.H.; Liu, C.; Cheng, H.M. Multi-step purification of carbon nanotubes. Carbon 2002, 40, 81–85. [Google Scholar] [CrossRef]
- Zeynalov, E.B.; Friedrich, J.F.; Hidde, G.; Ibrahimov, H.J.; Nasibova, G.G. Brominated carbon nanotubes as effective catalysts for petroleum hydrocarbons aerobic oxidation. Oil Gas-Eur Mag. 2012, 38, 45–48. [Google Scholar]
- Zeynalov, E.B.; Friedrich, J.; Meyer-Plath, A.; Hidde, G.; Nuriyev, L.; Aliyeva, A.; Cherepnova, Y. Plasma-chemically brominated single-walled carbon nanotubes as novel catalysts for oil hydrocarbons aerobic oxidation. Appl. Catal. A Gen. 2013, 454, 115–118. [Google Scholar] [CrossRef]
- Zeynalov, E.; Friedrich, J.; Wagner, M.; Hidde, G. Effect of Br-grafted multi-walled carbon nanotubes on the model oxidative environment. Chem. Chem. Technol. 2015, 9, 51–54. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Gevko, P.N.; Koroteev, V.O.; Fedoseeva, Y.V.; Yaya, A.; Ewels, C.P. Bromination of double-walled carbon nanotubes. Chem. Mater. 2012, 24, 2708–2715. [Google Scholar] [CrossRef]
- NC7000™—Technical Data Sheet. Available online: https://www.nanocyl.com/download/tds-nc7000/ (accessed on 17 March 2020).
- Drabowicz, J.; Ciesielski, W.; Kulawik, D. The Process of Preparing the Brominated Multi-Walled Carbon Nanotubes (MWCNT) Containing Bromine Atoms and the Way of Purification. Them. Patent EP3002253, 19 February 2015. [Google Scholar]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangam, N. C sp2/sp3 hybridisations in carbon nanomaterials – XPS and (X)AES study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Loeffen, P. Oxford Diffraction. CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97—WinGX Version; Release 97–2; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Diffr. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Jankovský, O.; Šimek, P.; Klimová, K.; Sedmidubský, D.; Matějková, S.; Pumerac, M.; Sofer, Z. Towards graphene bromide: bromination of graphite oxide. Nanoscale 2014, 6, 6065–6074. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza Filho, A.G.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- DiLeo, R.; Landi, B.; Raffaelle, R. Application of the G’/D Raman Ratio for Purity Assessment of Multi-Walled Carbon Nanotubes. MRS Proc. 2007, 1018, 511. [Google Scholar] [CrossRef]




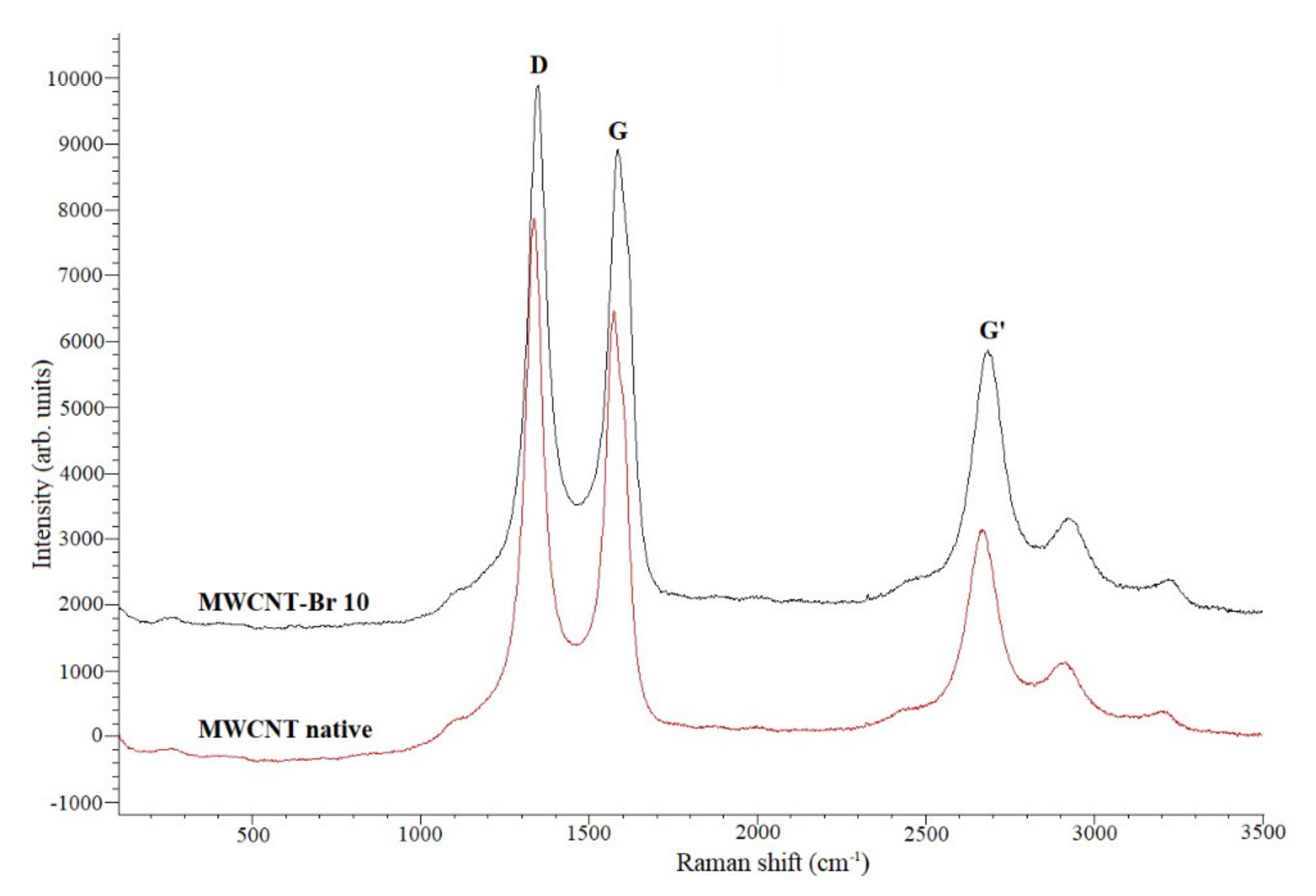
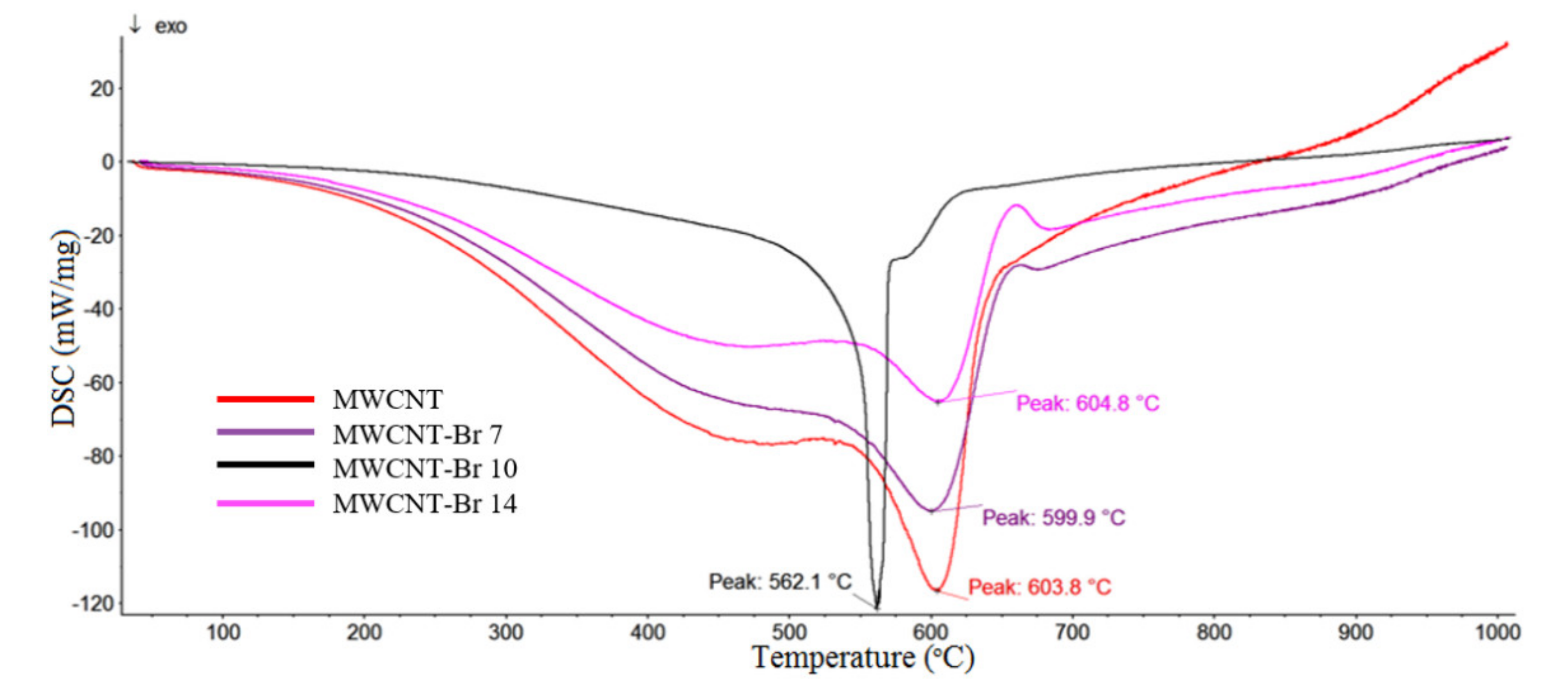

| Samples | C | Br |
|---|---|---|
| MWCNT–Br 7 | 98.2 | 1.8 |
| MWCNT–Br 10 | 97.6 | 2.4 |
| MWCNT–Br 14 | 99.2 | 0.8 |
| Samples | Br | O | C | D-Parameter |
|---|---|---|---|---|
| MWCNT native | 0 | 1.32 | 98.58 | 21.8 |
| MWCNT–Br 7 | 0.74 | 2.18 | 97.07 | 21.8 |
| MWCNT–Br 10 | 0.68 | 3.95 | 95.36 | 20.0 |
| MWCNT–Br 14 | 0.62 | 1.94 | 97.44 | 20.6 |
| Carbon Species | MWCNT Native | MWCNT–Br 7 | MWCNT–Br 10 | MWCNT–Br 14 |
|---|---|---|---|---|
| sp2 (284.0 eV) | 64.2 | 62.6 | 60.2 | 63.8 |
| sp3 (285.0 eV) | 15.5 | 15.0 | 17.4 | 14.0 |
| C–O/C–Br (286.4 eV) | 4.4 | 4.9 | 1.1 | 4.0 |
| C=O (288 eV) | 4.8 | 4.7 | 5.8 | 5.1 |
| O–C=O (289-290 eV) | 7.8 | 6.4 | 8.3 | 7.1 |
| shake-up satellites | 2.4 | 2.3 | 2.5 | 1.9 |
| Bromination Time (Days) | Lattice Parameters (Å) | Interlayer Distances (Å) | Space Group |
|---|---|---|---|
| 0 | a = 2.523 c = 6.958 | 3.479 | P63/mmc |
| 7 | a = 2.625 c = 8.620 | 4.310 | P63/mmc |
| 10 | a = 2.652 c = 9.258 | 4.629 | P63/mmc |
| 14 | a = 2.675 c = 9.420 | 4.710 | P63/mmc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarska, S.; Kulawik, D.; Pavlyuk, V.; Tomasik, P.; Bachmatiuk, A.; Szukiewicz, R.; Ciesielski, W. A Facile and Efficient Bromination of Multi-Walled Carbon Nanotubes. Materials 2021, 14, 3161. https://doi.org/10.3390/ma14123161
Zarska S, Kulawik D, Pavlyuk V, Tomasik P, Bachmatiuk A, Szukiewicz R, Ciesielski W. A Facile and Efficient Bromination of Multi-Walled Carbon Nanotubes. Materials. 2021; 14(12):3161. https://doi.org/10.3390/ma14123161
Chicago/Turabian StyleZarska, Sandra, Damian Kulawik, Volodymyr Pavlyuk, Piotr Tomasik, Alicja Bachmatiuk, Rafał Szukiewicz, and Wojciech Ciesielski. 2021. "A Facile and Efficient Bromination of Multi-Walled Carbon Nanotubes" Materials 14, no. 12: 3161. https://doi.org/10.3390/ma14123161
APA StyleZarska, S., Kulawik, D., Pavlyuk, V., Tomasik, P., Bachmatiuk, A., Szukiewicz, R., & Ciesielski, W. (2021). A Facile and Efficient Bromination of Multi-Walled Carbon Nanotubes. Materials, 14(12), 3161. https://doi.org/10.3390/ma14123161










