Designing Carbon-Enriched Alumina Films Possessing Visible Light Absorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodization
2.3. Characterization
3. Results and Discussion
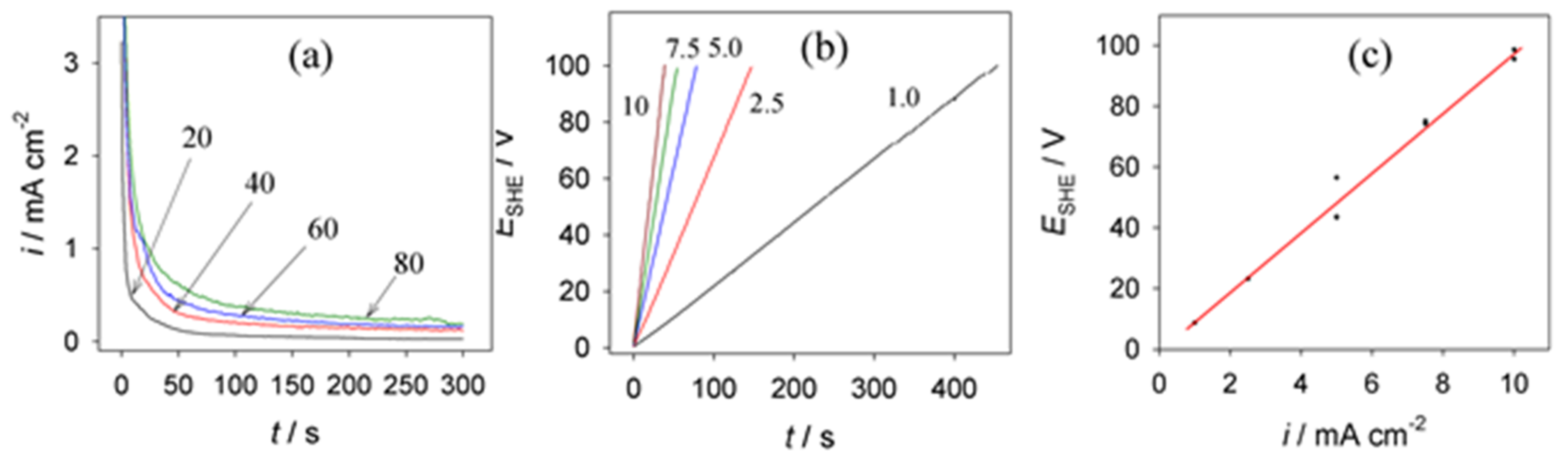
3.1. Anodic Behavior of Aluminum in the Vanadate Solutions
3.2. Anodic Behavior of Aluminum in HCOOH Solutions
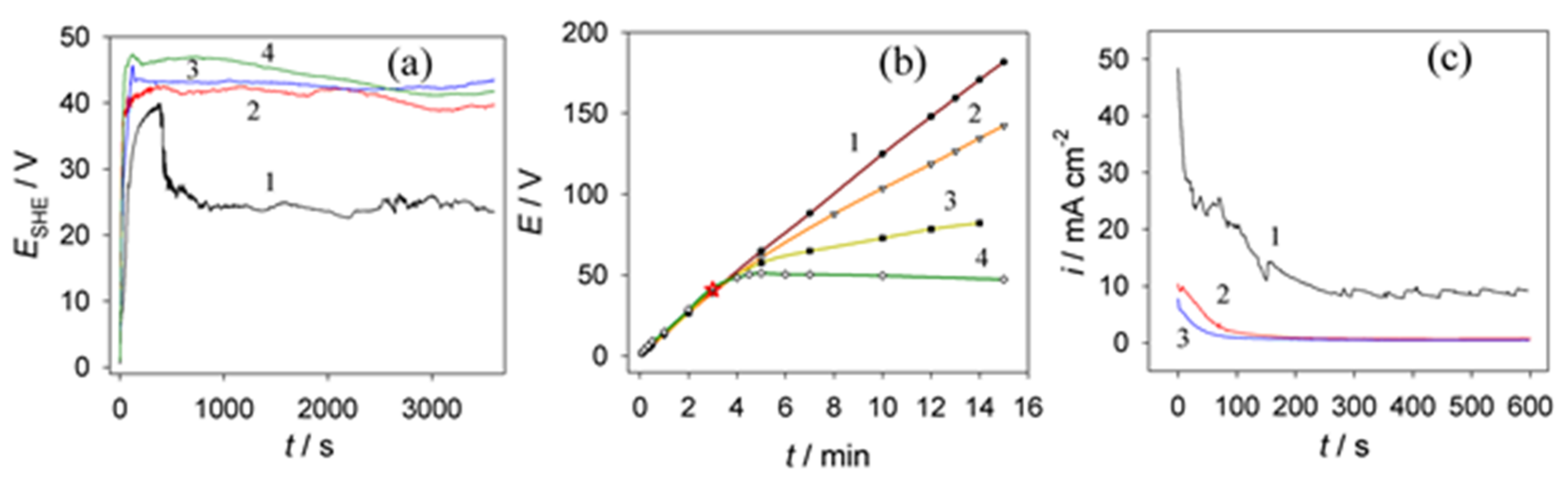
3.3. Anodic Behavior of Aluminum in NaVO3–HCOOH–HCOONa Solution
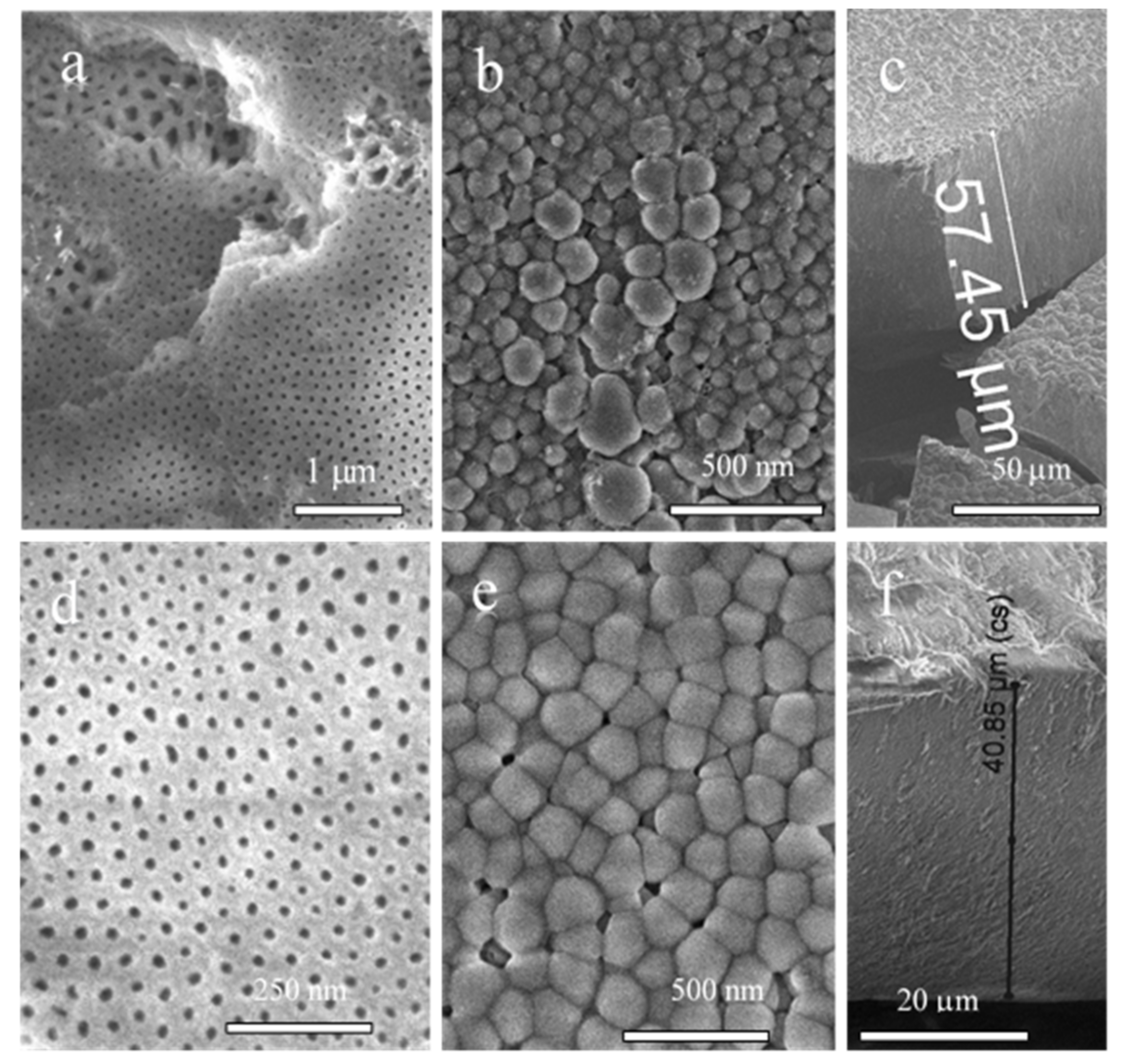
3.4. Films
3.5. The Composition of Films
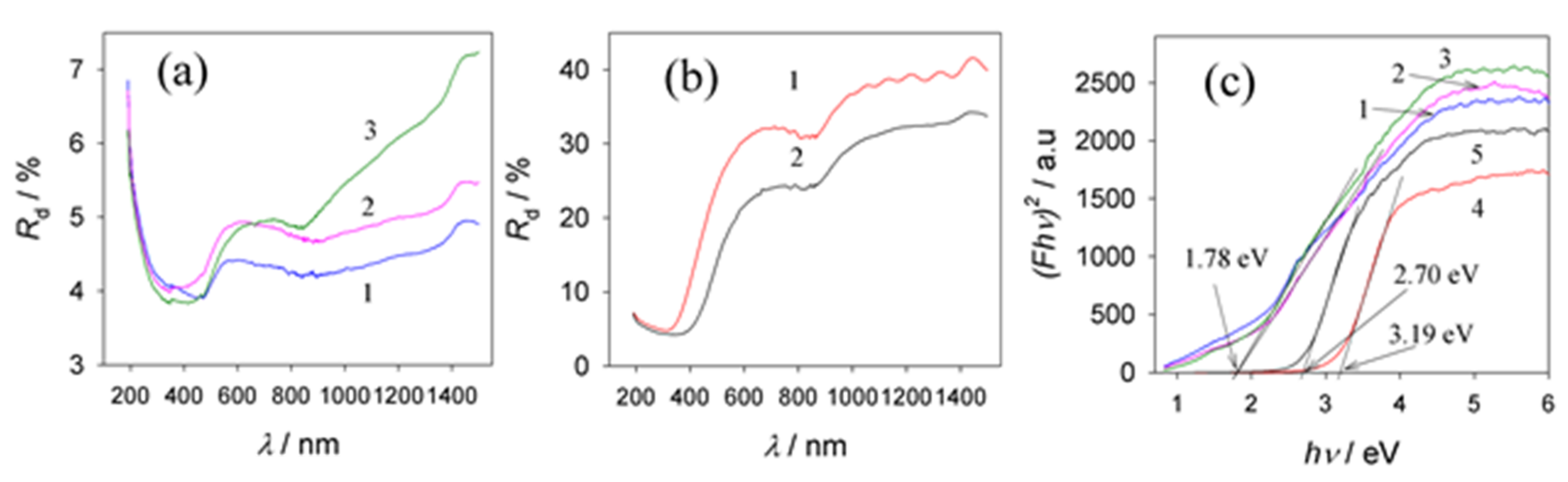
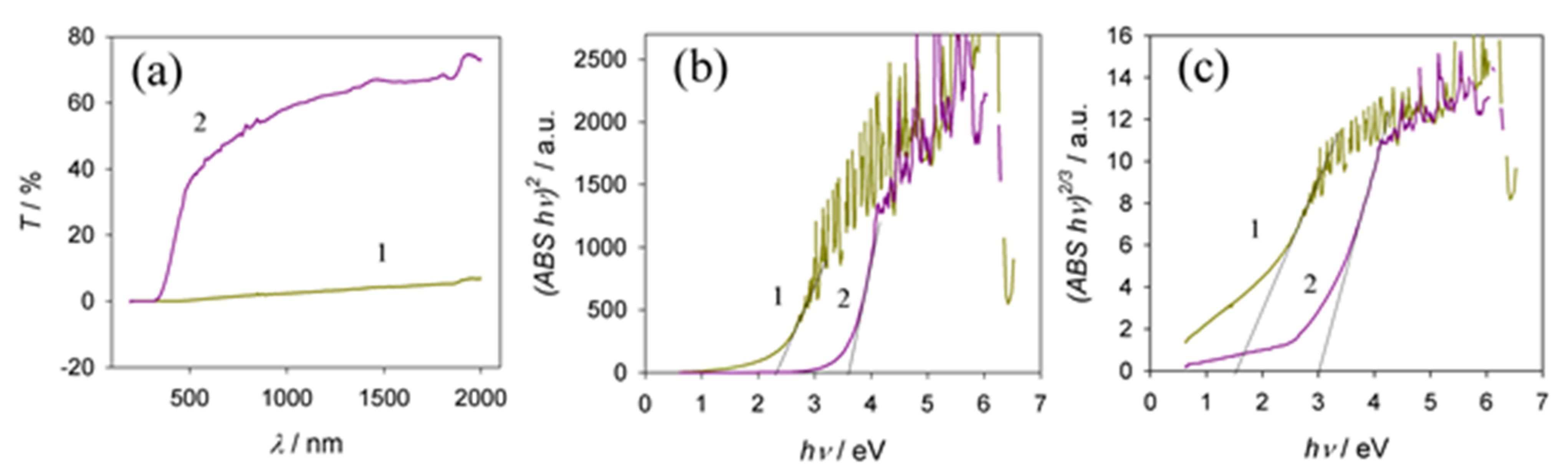
3.6. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ono, S.; Saito, M.; Asoh, H. Self-Ordering of Anodic Porous Alumina Formed in Organic Acid Electrolytes. Electrochim. Acta 2005, 51, 827–833. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In Nanostructured Materials in Electrochemistry; Effekhari, A., Ed.; Willey-VCH: Weinheim, Germany, 2008; pp. 1–116. [Google Scholar]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–198. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; Govyadinov, A.N.; Mukhurov, N.I. Thermo treatment of anodic alumina membranes. J. Membr. Sci. 1995, 98, 131–142. [Google Scholar] [CrossRef]
- O’Sullivan, J.P.; Wood, G.C. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. R. Soc. Lond. Ser. A 1970, 317, 511–534. [Google Scholar]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gosele, U. Self-ordering Regimes of Porous Alumina: The 10 Porosity Rule. Nano Lett. 2002, 2, 677–681. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gosele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Cheng, C.; Ngan, A.H.W. Fast fabrication of self-ordered anodic porous alumina on oriented aluminum grains by high acid concentration and high temperature anodization. Nanotechnology 2013, 24, 215602. [Google Scholar] [CrossRef]
- Norek, M.; Stępniovski, W.J.; Siemiaszko, D. Effect of ethylene glycol on morphology of anodic alumina prepared in hard anodization. J. Electroanal. Chem. 2016, 762, 20–28. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-ordering anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef]
- Li, Y.; Ling, Z.Y.; Chen, S.S.; Wang, J.C. Fabrication of novel porous anodic alumina membranes by two-step hard anodization. Nanotechnology 2008, 19, 225604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, M.; Ma, L.; Shen, W. Fabrication of highly ordered nanoporous alumina films by stable high-field anodization. Nanotechnology 2006, 17, 5101. [Google Scholar] [CrossRef]
- Chernyakova, K.; Karpicz, R.; Zavadski, S.; Poklonskaya, O.; Jagminas, A.; Vrublevsky, I. Structures and fluorescence characterization of anodic alumina/carbon composite formed in tartaric acid solution. J. Lumin. 2017, 182, 233–239. [Google Scholar] [CrossRef]
- Jagminas, A.; Kaciulis, S.; Klimas, V.; Rėza, A.; Mickevicius, S.; Soltani, P. Fabrication of Graphene-Alumina Heterostructured Films with Nanotube Morphology. J. Phys. Chem. C 2016, 120, 9490–9497. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Evidence for electrohydrodynamic convection as a source of spontaneous self-ordering in porous anodic alumina films. Phys. Chem. Chem. Phys. 2016, 18, 6946–6953. [Google Scholar] [CrossRef][Green Version]
- Paschanka, M. Multilevel self-organization on anodized aluminium: Discovering hierarchical honeycomb structures from nanometre to sub-millimetre scale. Phys. Chem. Chem. Phys. 2020, 22, 15867–15875. [Google Scholar] [CrossRef]
- Ralston, K.D.; Chrisanti, S.; Young, T.L.; Buchheita, R.G. Corrosion Inhibition of Aluminum Alloy 2024-T3 by Aqueous Vanadium Species. J. Electrochem. Soc. 2008, 155, C350–C359. [Google Scholar] [CrossRef]
- Liu, S.; Thompson, G.E.; Skeldon, P. Vanadate post-treatments of anodised aluminium and AA 2024 T3 alloy for corrosion protection. Trans. Inst. Met. Finish. 2018, 96, 137–144. [Google Scholar] [CrossRef]
- Lemerle, J.; Nejem, L.; Lefebvre, J. Condensation process in polyvanadic acid solutions. J. Inorg. Nucl. Chem. 1980, 42, 17. [Google Scholar] [CrossRef]
- Ifezue, D. Certified reference materials for quantification of vanadate species in anodic alumina Rf-GDOES depth profiles. J. Anal. At. Spectrom. 2013, 28, 1311–1319. [Google Scholar] [CrossRef]
- Tajima, S. Luminescence, breakdown and colouring of anodic oxide films on aluminium. Electrochim. Acta 1977, 22, 995. [Google Scholar] [CrossRef]
- Fukushima, T.; Fukuda, Y.; Ito, G.; Sato, Y. Anodic Oxidation and Local Corrosion of Aluminum in Mono-carboxylic Acids. J. Met. Finish. Soc. Jpn. 1970, 21, 319. [Google Scholar] [CrossRef]
- Noguchi, H.; Yoshimura, C. Effect of Fluoride on Anodization of Aluminum in Mono-Carboxylic Acid Baths. J. Met. Finish. Soc. Jpn. 1987, 38, 133. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Origin of self-organisation in porous anodic alumina films derived from analogy with Rayleigh–Bénard convection cells. J. Mater. Chem. 2011, 21, 18761. [Google Scholar] [CrossRef]
- Moshfegh, A.Z.; Ignatiev, A. Formation and characterization of thin film vanadium oxides: Auger electron spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, scanning electron microscopy, and optical reflectance studies. Thin Solid Films 1991, 198, 251–268. [Google Scholar] [CrossRef]
- Aerts, T.; De Graeve, I.; Terryn, H. Study of initiation and development of local burning phenomena during anodizing of aluminium under controlled conversion. Electrochim. Acta 2008, 54, 270. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7555. [Google Scholar] [CrossRef]
- Wood, G.C.; Skeldon, P.; Thompson, G.E.; Shimizu, K. A Model for the Incorporation of Electrolyte Species into Anodic Alumina. J. Electrochem. Soc. 1996, 143, 74–83. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+)’. J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887. [Google Scholar] [CrossRef]
- Kaciulis, S. Spectroscopy of Carbon: From Diamond to Nitride Films. Surf. Interface Anal. 2012, 44, 1155–1161. [Google Scholar] [CrossRef]
- Mezzi, A.; Kaciulis, S. Surface Investigations of Carbon Films: From Diamond to Graphite. Surf. Interface Anal. 2010, 42, 1082–1084. [Google Scholar] [CrossRef]
- Mizokawa, Y.; Miyasato, T.; Nakamura, S.; Geib, K.M.; Wilmsen, C.W. Comparison of the CKLL first-derivative auger spectra from XPS and AES using diamond, graphite, SiC and diamond-like-carbon films. Surf. Sci. 1987, 182, 431–438. [Google Scholar] [CrossRef]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Katsuta, Y.; Yasumori, A. Large-Scale Fabrication of Ordered Nanoporous Alumina Films with Arbitrary Pore Intervals by Critical-Potential Anodization. J. Electrochem. Soc. 2006, 153, B384–B391. [Google Scholar] [CrossRef]
- Shamala, K.S.; Murthy, L.C.S.; Narasimha Rao, K. Studies on optical and dielectric properties of Al2O3 thin films prepared by electron beam evaporation and spray pyrolysis method. Mater. Sci. Eng. B 2004, 106, 269–274. [Google Scholar] [CrossRef]
- Reddy, P.R.; Ajith, K.M.; Udayashankar, N.K. Optical and mechanical studies on freestanding amorphous anodic porous alumina formed in oxalic and sulphuric acid. Appl. Phys. A 2018, 124, 765. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.W.; Li, Y.; Liu, W.M. Optical constants of anodic aluminum oxide films formed in oxalic acid solution. Thin Solid Films 2008, 516, 7689–7694. [Google Scholar] [CrossRef]
- Yan, H.; Lemmens, P.; Wulferding, D.; Shi, J.; Becker, K.D.; Lin, C.; Lak, A.; Schilling, M. Tailoring defect structure and optical absorption of porous anodic aluminum oxide membranes. Mat. Chem. Phys. 2012, 135, 206–211. [Google Scholar] [CrossRef]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M.; Chusuei, C.C.; Julien, C.M. Correlation between growth conditions, microstructure, and optical properties in pulsed-laser-deposited V2O5 thin films. Chem. Mater. 2005, 17, 1213–1219. [Google Scholar] [CrossRef]
- Bullot, J.; Cordier, P.; Gallais, O.; Gauthier, M.; Babonneau, F. Thin layers deposited from V2O5 gels: II. An optical absorption study. J. Non-Cryst. Solids 1984, 68, 135–146. [Google Scholar] [CrossRef]
- Rakić, A.D. Algorithm for the determination of intrinsic optical constants of metal films: Application to aluminum. Appl. Opt. 1995, 34, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
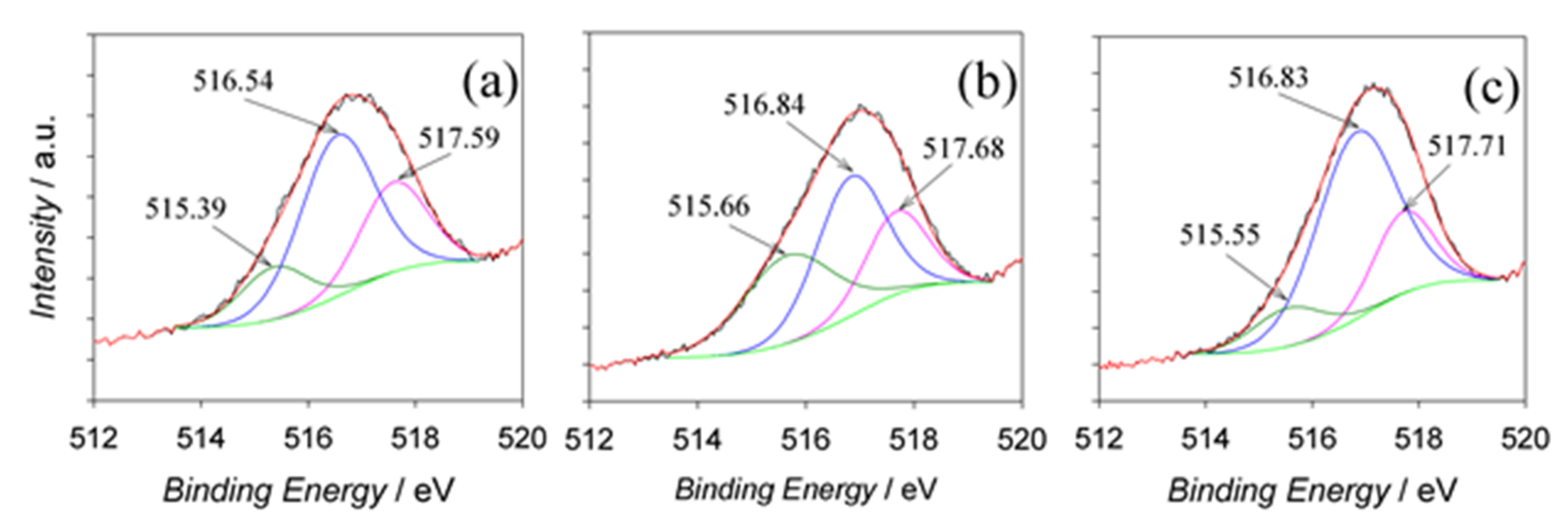



| Ea, V | Name | Peak BE | FWHM eV | Area (P) CPS.eV | Atomic % | Q | SF |
|---|---|---|---|---|---|---|---|
| 60 | V2p | 516.74 | 2.52 | 25,150 | 2.26 | 1 | 6.330 |
| O1s | 531.66 | 3.17 | 267,785 | 53.51 | 1 | 2.850 | |
| C1s | 284.73 | 2.28 | 30,885 | 17.08 | 1 | 1.000 | |
| - | Al2p | 74.24 | 2.04 | 28,750 | 27.16 | 1 | 0.574 |
| 80 | V2p | 516.89 | 2.57 | 29,080 | 2.45 | 1 | 6.330 |
| - | O1s | 531.61 | 3.19 | 281,965 | 52.80 | 1 | 2.850 |
| - | C1s | 284.59 | 2.19 | 33,005 | 17.11 | 1 | 1.000 |
| - | Al2p | 74.19 | 2.09 | 31,225 | 27.64 | 1 | 0.574 |
| 100 | V2p | 517.04 | 2.34 | 29,354 | 2.71 | 1 | 6.330 |
| - | O1s | 531.70 | 3.20 | 261,951 | 53.75 | 1 | 2.850 |
| - | C1s | 284.67 | 2.19 | 30,490 | 17.31 | 1 | 1.000 |
| - | Al2p | 74.27 | 2.03 | 27,040 | 26.23 | 1 | 0.574 |
| Anodizing Voltage, V | V3+ | V4+ | V5+ |
| 60 | 0.166 | 0.555 | 0.278 |
| 80 | 0.353 | 0.423 | 0.224 |
| 100 | 0.121 | 0.663 | 0.215 |
| Anodizing Voltage, V | 284.49–284.60 eV (C–C, C–H) | 285.68–286.03 eV (C–OH) | 286.72–287.13 eV (C–O) | 289.01–289.12 eV (C–OOH) |
| 60 | 0.572 | 0.146 | 0.019 | 0.263 |
| 80 | 0.589 | 0.070 | 0.090 | 0.253 |
| 100 | 0.539 | 0.149 | 0.077 | 0.235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagminas, A.; Klimas, V.; Chernyakova, K.; Jasulaitiene, V. Designing Carbon-Enriched Alumina Films Possessing Visible Light Absorption. Materials 2022, 15, 2700. https://doi.org/10.3390/ma15072700
Jagminas A, Klimas V, Chernyakova K, Jasulaitiene V. Designing Carbon-Enriched Alumina Films Possessing Visible Light Absorption. Materials. 2022; 15(7):2700. https://doi.org/10.3390/ma15072700
Chicago/Turabian StyleJagminas, Arunas, Vaclovas Klimas, Katsiaryna Chernyakova, and Vitalija Jasulaitiene. 2022. "Designing Carbon-Enriched Alumina Films Possessing Visible Light Absorption" Materials 15, no. 7: 2700. https://doi.org/10.3390/ma15072700
APA StyleJagminas, A., Klimas, V., Chernyakova, K., & Jasulaitiene, V. (2022). Designing Carbon-Enriched Alumina Films Possessing Visible Light Absorption. Materials, 15(7), 2700. https://doi.org/10.3390/ma15072700






