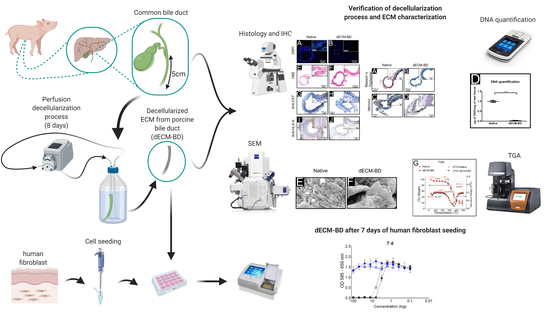
Perfusion Decellularization of Extrahepatic Bile Duct Allows Tissue-Engineered Scaffold Generation by Preserving Matrix Architecture and Cytocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Guidelines
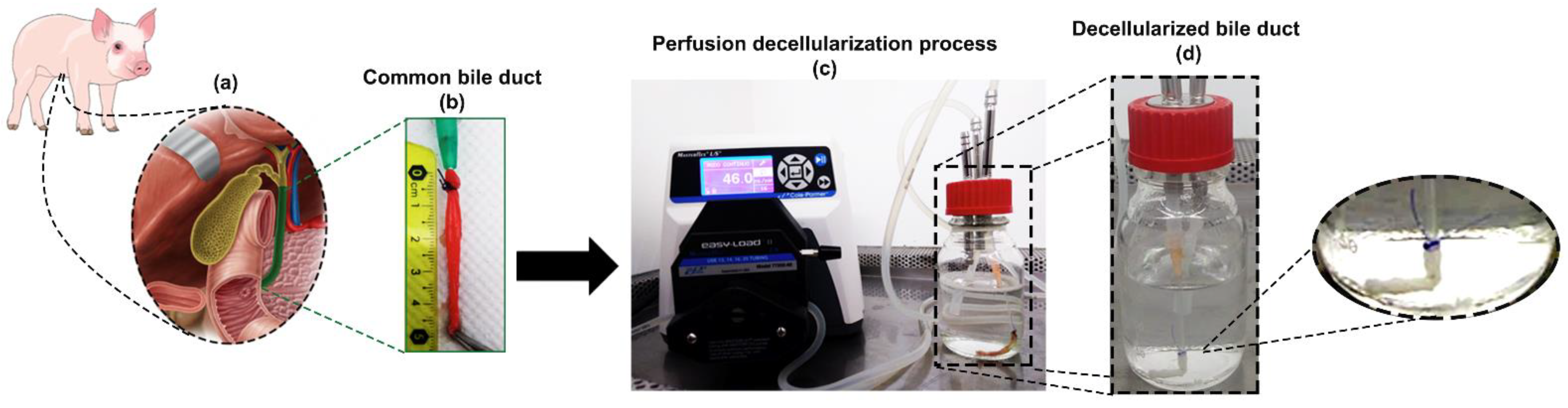
2.2. Bile Duct Harvest
2.3. Decellularization Protocol
2.4. Mesurement of Decellularization
2.4.1. DNA Quantification
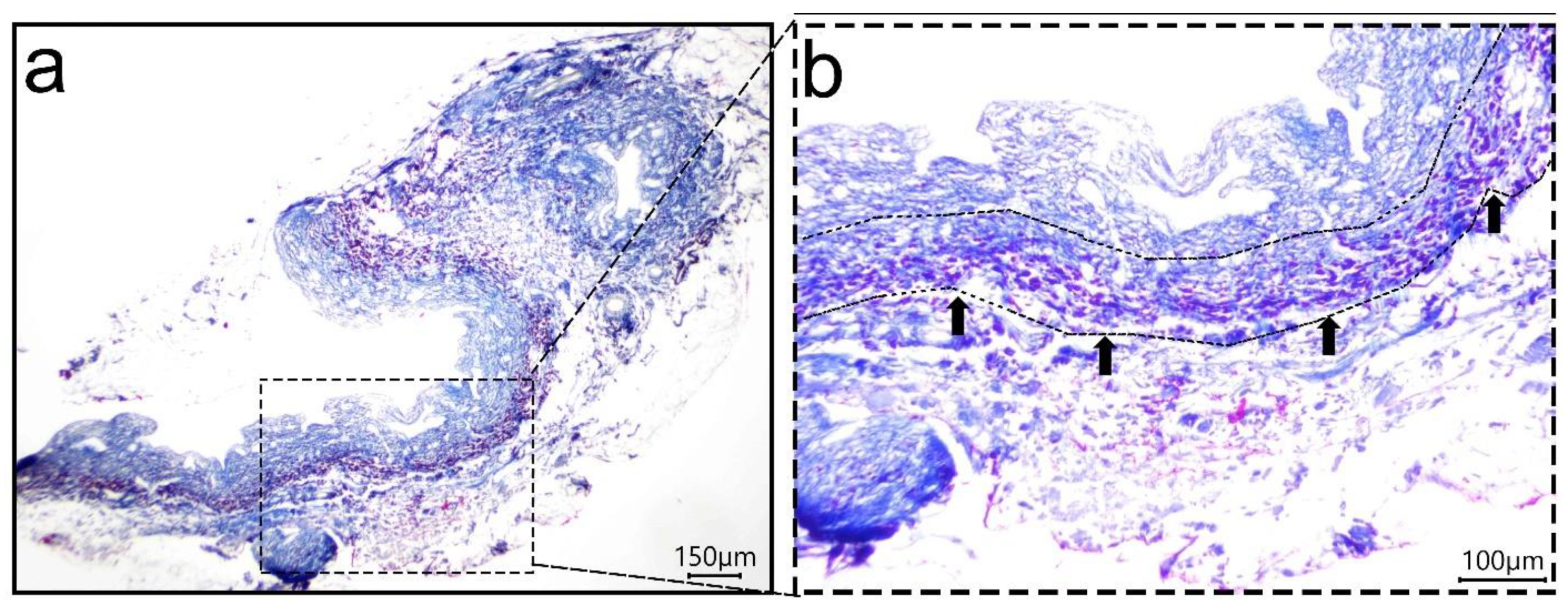
2.4.2. Histological Analyses
2.4.3. Immunohistochemical Analyses
2.4.4. Nuclei Quantification
2.4.5. Positive Stain Area Quantification of Immunohistochemical Images
2.5. Characterization of the Decellularized Extracellular Matrix Scaffold Derived from Porcine Extrahepatic Bile Ducts (dECM-BD)
2.5.1. Microarchitectural Imaging
2.5.2. Thermogravimetric Analysis
2.6. Cytocompatibility of dECM-BD
2.6.1. Detection of Leachable Components and Cytotoxicity in dECM-BD
2.6.2. Cell Infiltration into dECM-BD
2.7. Statistical Analysis
3. Results
3.1. Decellularization Assessment
3.2. ECM Assessment
3.3. Microarchitectural and Thermal Integrity of Decellularized Matrices
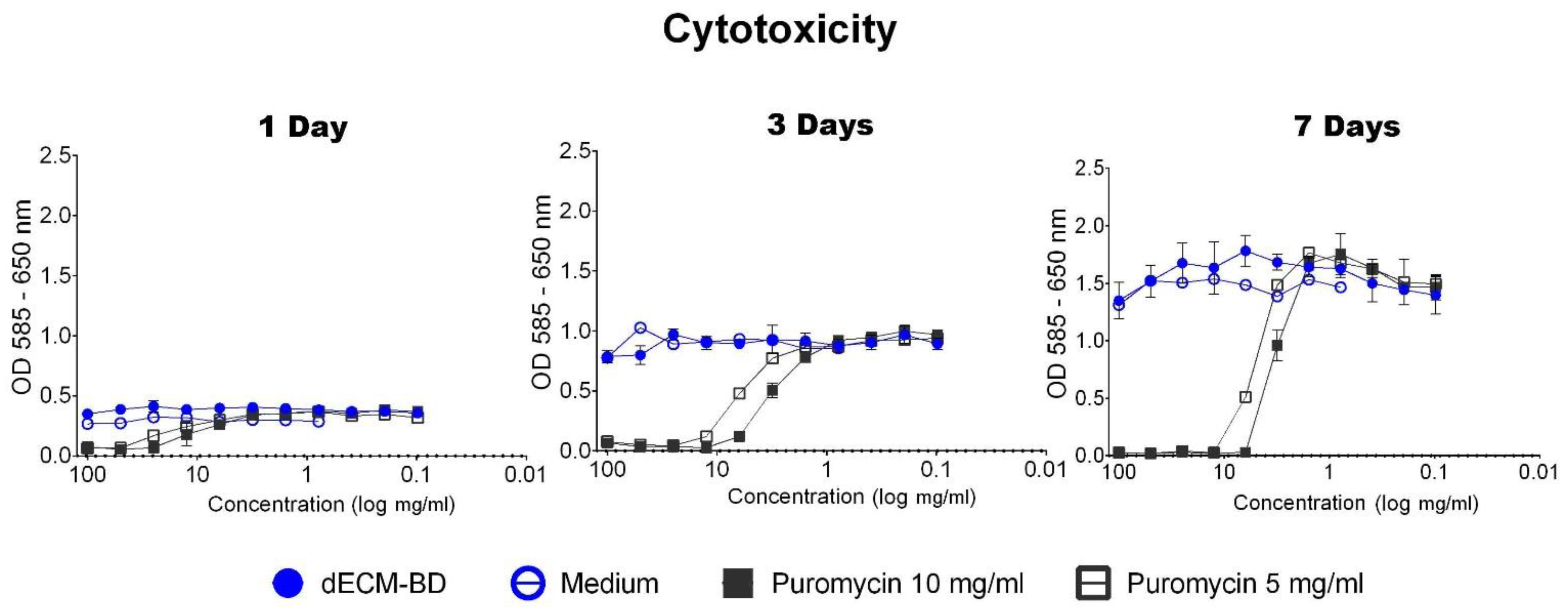
3.4. Cytotoxicity
3.5. Cell Infiltration into dECM-BD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, R.M. Management of failed biliary repairs for major bile duct injuries after laparoscopic cholecystectomy1. J. Am. Coll. Surg. 2004, 199, 192–197. [Google Scholar] [CrossRef]
- Ahrendt, S.A.; Pitt, H.A. Surgical Therapy of Iatrogenic Lesions of Biliary Tract. World J. Surg. 2001, 25, 1360–1365. [Google Scholar] [CrossRef]
- Mercado, M.A. Early versus late repair of bile duct injuries. Surg. Endosc. 2006, 20, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S. Results of a new strategy for reconstruction of biliary injuries having an isolated right-sided component. J. Gastrointest. Surg. 2001, 5, 266–274. [Google Scholar] [CrossRef]
- Mercado, M. Ángel; Chan, C.; Orozco, H.; Villalta, J.M.; Barajas-Olivas, A.; Eraña, J.; Domínguez, I. Long-term evaluation of biliary reconstruction after partial resection of segments IV and V in Iatrogenic Injuries. J. Gastrointest. Surg. 2006, 10, 77–82. [Google Scholar] [CrossRef]
- Wójcicki, M.; Lubikowski, J.; Chmurowicz, T.; Post, M.; Jarosz, K.; Białek, A.; Milkiewicz, P. Liver transplantation as an ultimate step in the management of iatrogenic bile duct injury complicated by secondary biliary cirrhosis. Ann. Transplant. 2012, 17, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Srokowski, E.; Woodhouse, K. Decellularized Scaffolds; Elsevier: Amsterdam, The Netherlands, 2011; pp. 369–386. [Google Scholar]
- Wang, Y.; Cui, C.-B.; Yamauchi, M.; Miguez, P.; Roach, M.; Malavarca, R.; Costello, M.J.; Cardinale, V.; Wauthier, E.; Barbier, C.; et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 2010, 53, 293–305. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiong, X.-Z.; Zhou, R.-X.; Deng, Y.-L.; Jin, Y.-W.; Lu, J.; Li, F.-Y.; Cheng, N.-S. Repair of a common bile duct defect with a decellularized ureteral graft. World J. Gastroenterol. 2016, 22, 10575–10583. [Google Scholar] [CrossRef]
- Nau, P.; Liu, J.; Ellison, E.C.; Hazey, J.W.; Henn, M.; Muscarella, P.; Narula, V.K.; Melvin, W.S. Novel reconstruction of the extrahepatic biliary tree with a biosynthetic absorbable graft. HPB 2011, 13, 573–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, A.J.P.; Rivas, C.D.O.; Romero, I.M.; Garcia, F.J.C.; Poyatos, P.T. Tissue-engineering repair of extrahepatic bile ducts. J. Surg. Res. 2013, 179, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Jave, E.E.; Barrera, G.E.M.; Treviño, A.I.V.; Barba, M.C.P.; Montalvo-Arenas, C.; Mendoza, F.R.; Mancilla, B.L.; Pineda, M.A.G.; Limón, Á.J.; Saavedra, J.A.; et al. Absorbable bioprosthesis for the treatment of bile duct injury in an experimental model. Int. J. Surg. 2015, 20, 163–169. [Google Scholar] [CrossRef]
- Giraldo-Gomez, D.M.; García-López, S.J.; Tamay-De-Dios, L.; Sánchez-Sánchez, R.; Villalba-Caloca, J.; Sotres-Vega, A.; Del Prado-Audelo, M.L.; Gómez-Lizárraga, K.K.; Garciadiego-Cázares, D.; Piña-Barba, M.C. Fast cyclical-decellularized trachea as a natural 3D scaffold for organ engineering. Mater. Sci. Eng. C 2019, 105, 110142. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Agricultura, Ganaderia, Desarrollo Rural, Pesca y Alimentacion: Nom-062-Zoo-1999; Secretaria de Agricultura, Ganaderia, Desarrollo Rural, Pesca y Alimentacion: Mexico City, Mexico, 1999.
- European Union. Directive 2010/63/EU; European Union: Brussels, Belgium, 2010. [Google Scholar]
- Friend, W.G. A polychrome stainfor differentiating precollagen from collagen. Stain. Technol. 1963, 38, 204–206. [Google Scholar] [PubMed]
- Fitzgerald, A.M.P.; Kirkpatrick, J.J.R.; Foo, I.T.H.; Naylor, I.L. A Picropolychrome Staining Technique Applied to Dupuytren’s Tissue. J. Hand Surg. 1995, 20, 519–524. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, N.; Rodríguez-Hernández, A.G.; Enríquez-Jiménez, J.; Alcántara-Quintana, L.E.; Fuentes-Mera, L.; Piña-Barba, M.C.; Zepeda-Rodríguez, A.; Ambrosio, J.R. Nukbone® promotes proliferation and osteoblastic differentiation of mesenchymal stem cells from human amniotic membrane. Biochem. Biophys. Res. Commun. 2013, 434, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Rivera, N.; Romero, S.E.; Ángeles, M.; Zepeda, A.; García, L.E.; Salas, G.; Romero, L.; Malagón, F. Blackwater fever like in murine malaria. Parasitol. Res. 2012, 112, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Getova, V.E.; Van Dongen, J.A.; Brouwer, L.A.; Harmsen, M.C. Adipose tissue-derived ECM hydrogels and their use as 3D culture scaffold. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.L.; Zhang, C.P.; Zhu, H.; Jiang, Y.F.; Fu, X.B. Autofluorescence of collagen fibres in scar. Ski. Res. Technol. 2017, 23, 588–592. [Google Scholar] [CrossRef]
- Curiel-Salgado, M.R. Fundamentos de una técnica policrómica: Tinción de Herovici. Investig. Discapac. 2012, 1, 35–36. Available online: http://www.medigraphic.com/pdfs/invdis/ir-2012/ir121f.pdf. (accessed on 20 September 2020).
- Goh, S.-K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E.; et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the Clearance of Dead Cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [Green Version]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Gieseck, R.L.; De Brito, M.C.; Berntsen, N.L.; Gómez-Vázquez, M.J.; et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–963. [Google Scholar] [CrossRef]
- Oord, J.V.D.; Sciot, R.; Desmet, V. Expression of MHC products by normal and abnormal bile duct epithelium. J. Hepatol. 1986, 3, 310–317. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Tang, R.; Han, C.; Jiang, Y.; Wu, J.; Shao, Y.; Gao, Y.; Yu, J.; Hu, Z.; et al. Fine mapping of the MHC region identifies major independent variants associated with Han Chinese primary biliary cholangitis. J. Autoimmun. 2020, 107, 102372. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Fabris, L. Functional anatomy of normal bile ducts. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2008, 291, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Morell, C.M.; Fabris, L.; Strazzabosco, M. Vascular biology of the biliary epithelium. J. Gastroenterol. Hepatol. 2013, 28, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Kono, N.; Nakanuma, Y. Ultrastructural and immunohistochemical studies of the intrahepatic peribiliary capillary plexus in normal livers and extrahepatic biliary obstruction in human beings. Hepatology 1992, 15, 411–418. [Google Scholar] [CrossRef]
- Deltenre, P.; Valla, D.-C. Ischemic Cholangiopathy. Semin. Liver Dis. 2008, 28, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Skaro, A.I.; Jay, C.L.; Baker, T.B.; Wang, E.; Pasricha, S.; Lyuksemburg, V.; Martin, J.A.; Feinglass, J.M.; Preczewski, L.B.; Abecassis, M.M. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: The untold story. Surgery 2009, 146, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Pietrucha, K. Changes in denaturation and rheological properties of collagen–hyaluronic acid scaffolds as a result of temperature dependencies. Int. J. Biol. Macromol. 2005, 36, 299–304. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef]
- Giraldo-Gomez, D.; Leon-Mancilla, B.; Del Prado-Audelo, M.; Sotres-Vega, A.; Villalba-Caloca, J.; Garciadiego-Cazares, D.; Piña-Barba, M. Trypsin as enhancement in cyclical tracheal decellularization: Morphological and biophysical characterization. Mater. Sci. Eng. C 2016, 59, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Mano, V.; Silva, M.E.S.R.E. Bioartificial polymeric materials based on collagen and poly(N-isopropylacrylamide). Mater. Res. 2007, 10, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Jose, M.V.; Thomas, V.; Dean, D.R.; Nyairo, E. Fabrication and characterization of aligned nanofibrous PLGA/Collagen blends as bone tissue scaffolds. Polymer 2009, 50, 3778–3785. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.C.; Mirmalek-Sani, S.-H.; Deegan, D.B.; Baptista, P.M.; Aboushwareb, T.; Atala, A.; Yoo, J.J. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 2012, 33, 7756–7764. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Anfang, R.; Anandappa, A.; Costa, J.; Javidfar, J.; Wobma, H.M.; Singh, G.; Freytes, D.O.; Bacchetta, M.D.; Sonett, J.R.; et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 2013, 96, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Pang, K.; Du, L.; Wu, X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials 2010, 31, 7257–7265. [Google Scholar] [CrossRef] [PubMed]
- Higuita, M.L.; Griffiths, L.G. Antigen removal process preserves function of small diameter venous valved conduits, whereas SDS-decellularization results in significant valvular insufficiency. Acta Biomater. 2020, 107, 115–128. [Google Scholar] [CrossRef]
- Baiguera, S.; Arkhipva, S.; Yin, D.; Holterman, M.; Macchiarini, P. Rat bile duct decellularization. BioNanoScience 2016, 6, 578–584. [Google Scholar] [CrossRef]
- Willemse, J.; Verstegen, M.M.; Vermeulen, A.; Schurink, I.J.; Roest, H.P.; van der Laan, L.J.; de Jonge, J. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure-controlled perfusion. Mater. Sci. Eng. C 2020, 108, 110200. [Google Scholar] [CrossRef]
- Ahmed, E.; Saleh, T.; Yu, L.; Kwak, H.-H.; Kim, B.-M.; Park, K.-M.; Lee, Y.-S.; Kang, B.-J.; Choi, K.-Y.; Kang, K.-S.; et al. Micro and ultrastructural changes monitoring during decellularization for the generation of a biocompatible liver. J. Biosci. Bioeng. 2019, 128, 218–225. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Volpato, F.Z.; Hutmacher, D.W.; Ivanovski, S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch. Oral Biol. 2018, 88, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.; Mei, S.; Gu, C.; Zheng, L.; Fang, C.; Shi, Y.; Wu, K.; Lu, T.; Jin, Y.; Lin, X.; et al. Decellularized cartilage as a prospective scaffold for cartilage repair. Mater. Sci. Eng. C 2019, 101, 588–595. [Google Scholar] [CrossRef]
- Hoshiba, T.; Yokoyama, N. Decellularized extracellular matrices derived from cultured cells at stepwise myogenic stages for the regulation of myotube formation. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1867, 118658. [Google Scholar] [CrossRef]
- Syed, O.; Walters, N.J.; Day, R.M.; Kim, H.-W.; Knowles, J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014, 10, 5043–5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilpin, S.E.; Guyette, J.P.; Gonzalez, G.; Ren, X.; Asara, J.M.; Mathisen, D.J.; Vacanti, J.P.; Ott, H.C. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J. Heart Lung Transplant. 2014, 33, 298–308. [Google Scholar] [CrossRef]
- Jank, B.; Xiong, L.; Moser, P.T.; Guyette, J.P.; Ren, X.; Cetrulo, C.L.; Leonard, D.A.; Fernandez, L.; Fagan, S.P.; Ott, H.C. Engineered composite tissue as a bioartificial limb graft. Biomaterials 2015, 61, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, A.; Yang, Y. Decellularization strategies for regenerative medicine: From processing techniques to applications. BioMed Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, R.M. Bile Flow. In Encyclopedia of Gastroenterology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 188–192. [Google Scholar]
- Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Damink, L.O.; Hoopen, H.T.; Feijen, J. Methylcellulose cell culture as a new cytotoxicity test system for biomaterials. J. Mater. Sci. Mater. Electron. 1991, 2, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Echeverry-Rendon, M.; Echeverria, F.; Harmsen, M.C. Interaction of different cell types with magnesium modified by plasma electrolytic oxidation. Colloids Surf. B Biointerfaces 2020, 193, 111153. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, J.; Petersen, A.; Zuidema, J.; Van Beuge, M.; Rodriguez, S.; Lathuile, A.; Veldhuis, G.; Steendam, R.; Bank, R.; Popa, E.; et al. Microsphere size influences the foreign body reaction. eCM 2014, 28, 335–347. [Google Scholar] [CrossRef]
- Luo, L.; Eswaramoorthy, R.; Mulhall, K.J.; Kelly, D.J. Decellularization of porcine articular cartilage explants and their subsequent repopulation with human chondroprogenitor cells. J. Mech. Behav. Biomed. Mater. 2016, 55, 21–31. [Google Scholar] [CrossRef]
- Whitelock, J.M.; Melrose, J. Adhesion of cells to biomaterials. Wiley Encycl. Biomed. Eng. 2006. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Marín, Y.; Abad-Contreras, D.E.; Ustarroz-Cano, M.; Pérez-Gallardo, N.S.; Villafuerte-García, L.; Puente-Guzmán, D.M.; Villar-Velasco, J.L.d.; Rodríguez-López, L.A.; Torres-Villalobos, G.; Mercado, M.Á.; et al. Perfusion Decellularization of Extrahepatic Bile Duct Allows Tissue-Engineered Scaffold Generation by Preserving Matrix Architecture and Cytocompatibility. Materials 2021, 14, 3099. https://doi.org/10.3390/ma14113099
Ramírez-Marín Y, Abad-Contreras DE, Ustarroz-Cano M, Pérez-Gallardo NS, Villafuerte-García L, Puente-Guzmán DM, Villar-Velasco JLd, Rodríguez-López LA, Torres-Villalobos G, Mercado MÁ, et al. Perfusion Decellularization of Extrahepatic Bile Duct Allows Tissue-Engineered Scaffold Generation by Preserving Matrix Architecture and Cytocompatibility. Materials. 2021; 14(11):3099. https://doi.org/10.3390/ma14113099
Chicago/Turabian StyleRamírez-Marín, Yolik, David Eduardo Abad-Contreras, Martha Ustarroz-Cano, Norma S. Pérez-Gallardo, Lorena Villafuerte-García, Dulce Maria Puente-Guzmán, Jorge Luna del Villar-Velasco, Leonardo Alejandro Rodríguez-López, Gonzalo Torres-Villalobos, Miguel Ángel Mercado, and et al. 2021. "Perfusion Decellularization of Extrahepatic Bile Duct Allows Tissue-Engineered Scaffold Generation by Preserving Matrix Architecture and Cytocompatibility" Materials 14, no. 11: 3099. https://doi.org/10.3390/ma14113099
APA StyleRamírez-Marín, Y., Abad-Contreras, D. E., Ustarroz-Cano, M., Pérez-Gallardo, N. S., Villafuerte-García, L., Puente-Guzmán, D. M., Villar-Velasco, J. L. d., Rodríguez-López, L. A., Torres-Villalobos, G., Mercado, M. Á., Tapia-Jurado, J., Martínez-García, F. D., Harmsen, M. C., Piña-Barba, M. C., & Giraldo-Gomez, D. M. (2021). Perfusion Decellularization of Extrahepatic Bile Duct Allows Tissue-Engineered Scaffold Generation by Preserving Matrix Architecture and Cytocompatibility. Materials, 14(11), 3099. https://doi.org/10.3390/ma14113099