Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle
Abstract
1. Introduction
- High degree-of-freedom in motion.
- High resolution and accuracy.
- The ability to dispense various bioink solutions simultaneously.
- Ease of use.
- Compact size.
- Ease of sterilization.
- Full-automation capability.
- Affordability.
- Versatility.
2. Materials and Methods
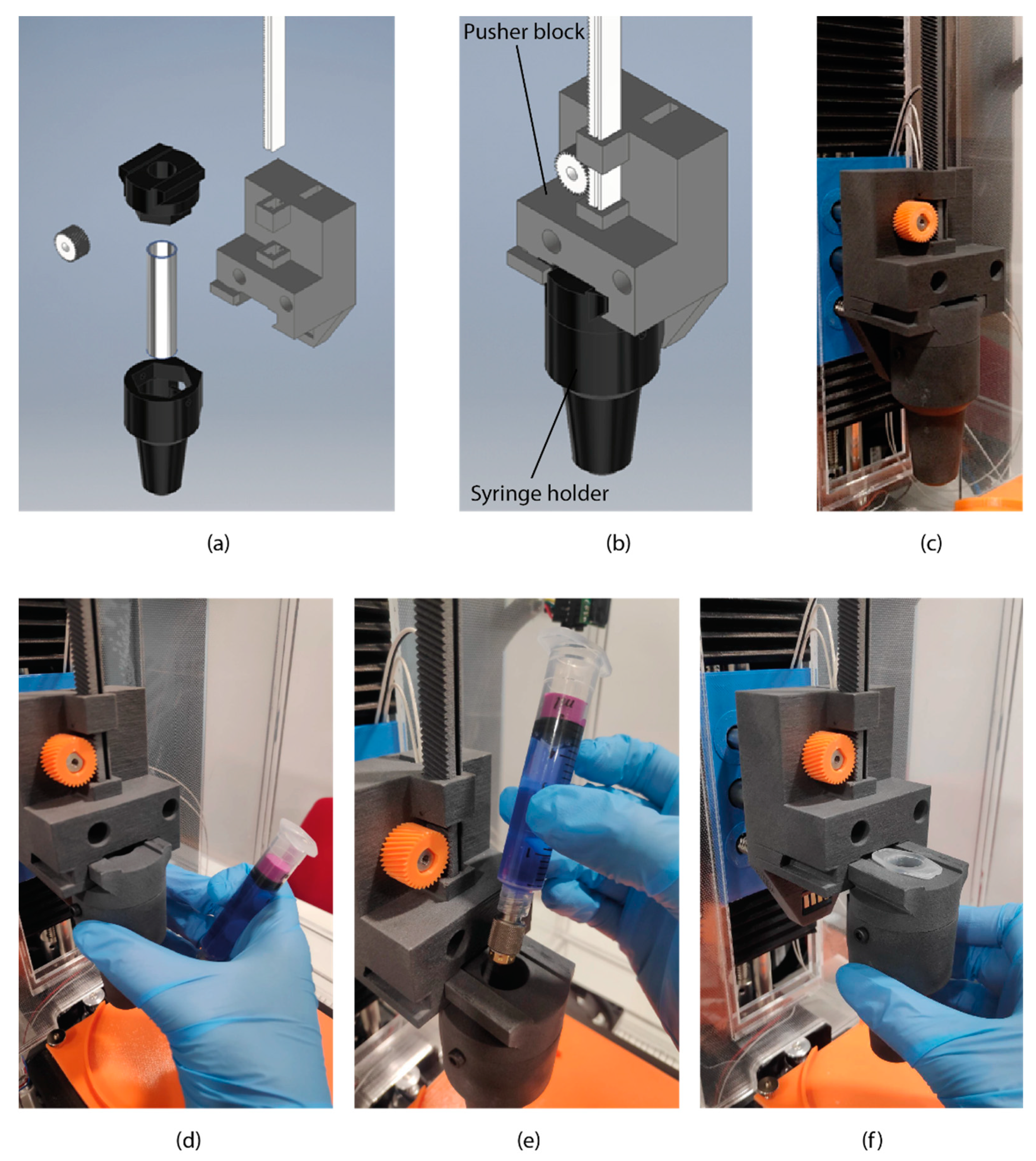
2.1. Bioprinter Design
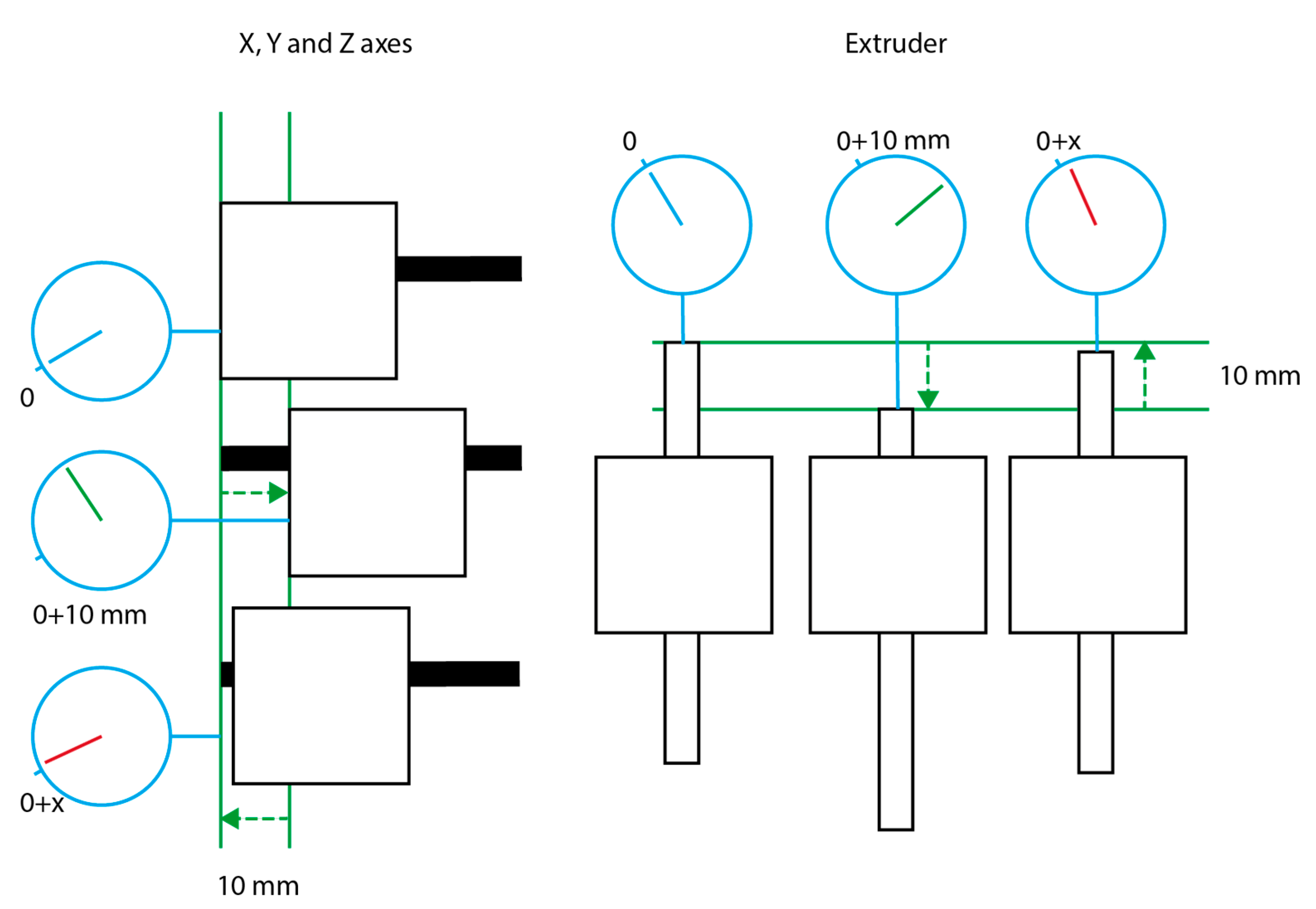
2.2. Accuracy Measurements
2.3. Cell Culture and Bioink Preparation
2.4. Bioprinting
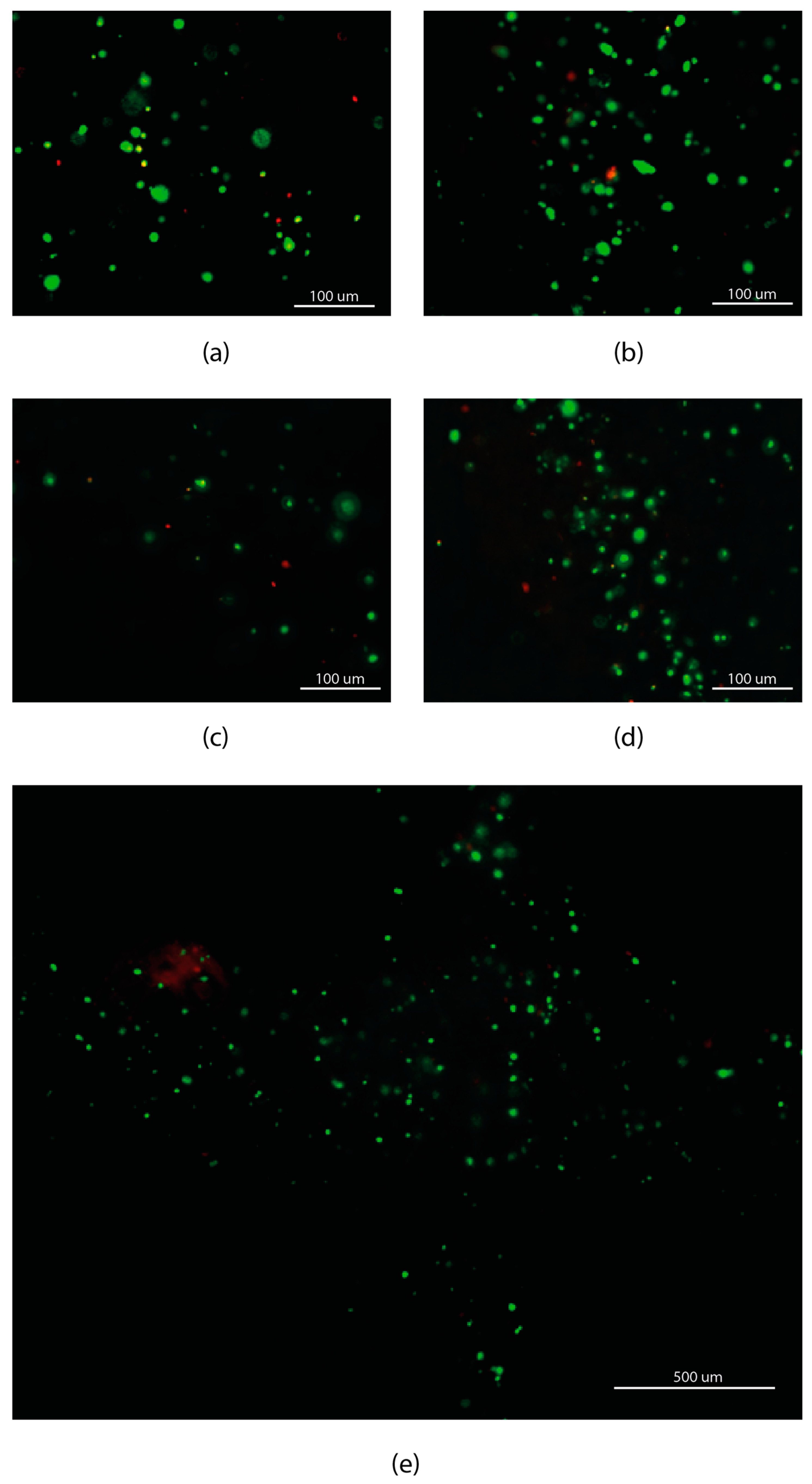
2.5. Cell Viability Evaluation Assay
3. Results and Discussion
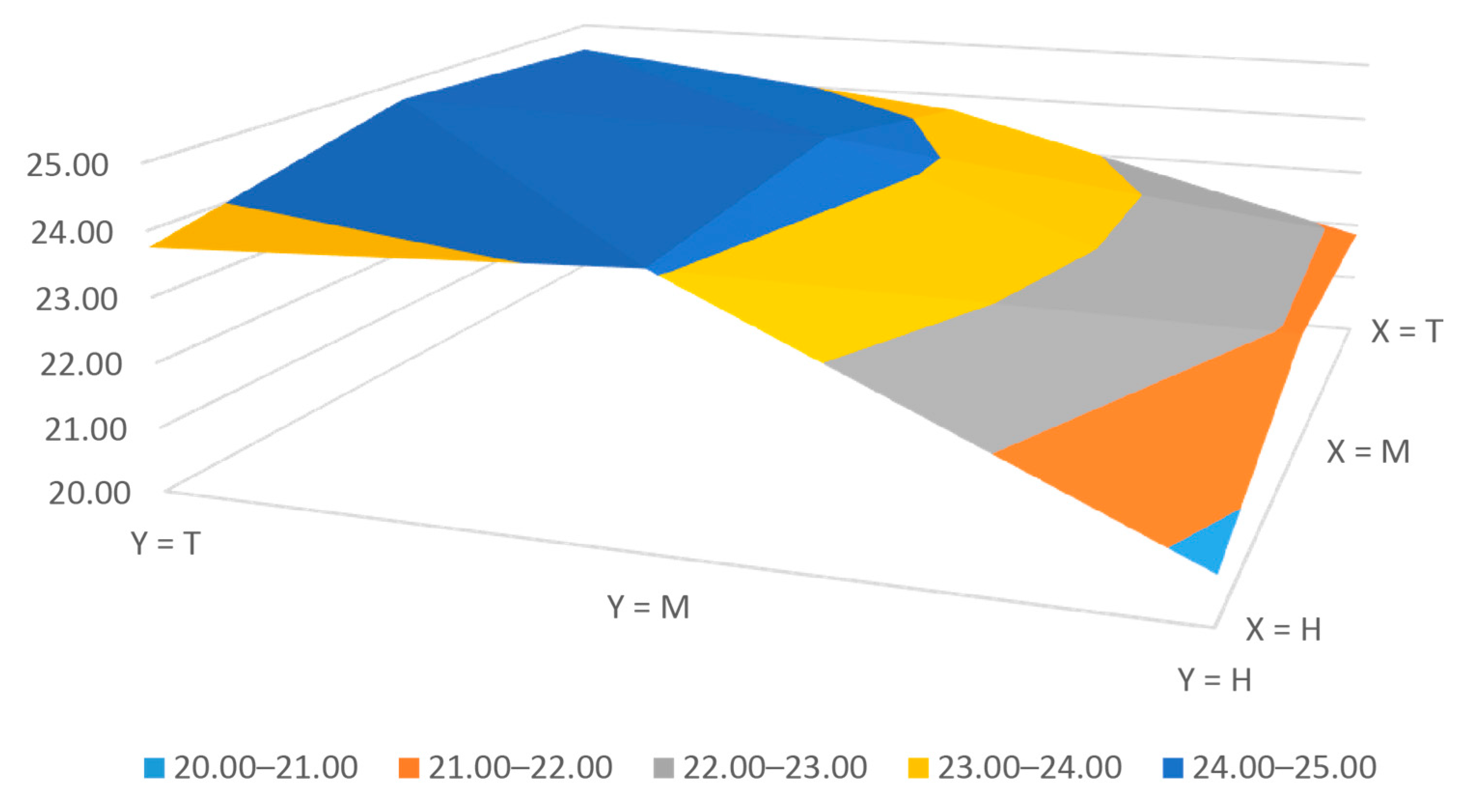
3.1. Bioprinter Accuracy
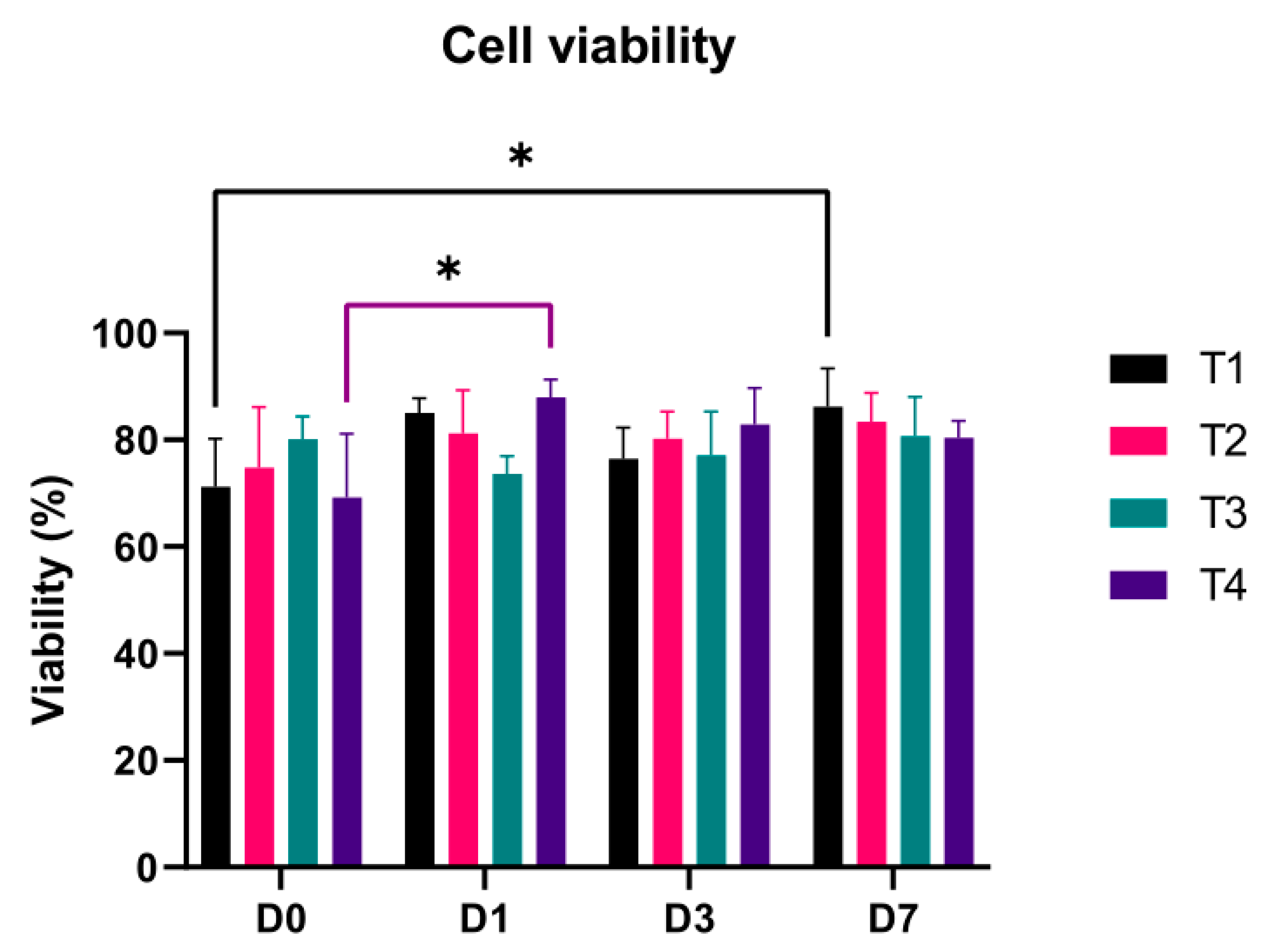
3.2. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eswaramoorthy, S.D.; Ramakrishna, S.; Rath, S.N. Recent advances in three-dimensional bioprinting of stem cells. J. Tissue Eng. Regen. Med. 2019, 13, 908–924. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.F. Print Me an Organ! Why We Are Not There Yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Chen, H.; Yu, Y. Development of ‘Multi-arm Bioprinter’ for hybrid biofabrication of tissue engineering constructs. Robot. Comput. Integr. Manuf. 2014, 30, 295–304. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1–47. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Mancha, E.; Gómez-Blanco, J.; López-Nieto, E.; García-Casado, J.; Macías, A.; Díaz-Díez, M.; Carrasco-Amador, J.; Torrejón, D.; Sanchez-Margallo, F.; Pagador, J. Hydrogels for bioprinting: A systematic review of hydrogels synthesis, bioprinting parameters and bioprinted structures behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Pan, H.M.; Chen, S.; Jang, T.-S.; Han, W.T.; Jung, H.; Li, Y.; Song, J. Plant seed-inspired cell protection, dormancy, and growth for large-scale biofabrication. Biofabrication 2019, 11, 025008. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Z.; Li, J.; Zhang, Y.; Yao, B.; Liu, Y.; Song, W.; Fu, X.; Wu, X.; Huang, S. An approach for mechanical property optimization of cell-laden alginate–gelatin composite bioink with bioactive glass nanoparticles. J. Mater. Sci. Mater. Med. 2020, 31, 103. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef]
- CELLINK BIO X-CELLINK. Available online: https://www.cellink.com/product/cellink-bio-x/ (accessed on 12 April 2021).
- Poietis Poietis—4D Bioprinting Next Generation. Available online: https://poietis.com/bioprinters/ (accessed on 12 April 2021).
- RegenHU Biofactory. Available online: https://www.regenhu.com/3d-bioprinters#biofactory (accessed on 12 April 2021).
- Matamoros, M.; Gómez-Blanco, J.C.; Sánchez, Á.J.; Mancha, E.; Marcos, A.C.; Carrasco-Amador, J.P.; Pagador, J.B. Temperature and Humidity PID Controller for a Bioprinter Atmospheric Enclosure System. Micromachines 2020, 11, 999. [Google Scholar] [CrossRef]
- Aydın, L.; Küçük, S.; Kenar, H. Design and Construction of a Novel Micro-Extrusion System for Bio-printing Applications. Int. J. Appl. Math. Electron. Comput. 2016, 4, 52. [Google Scholar] [CrossRef]
- Goldstein, T.A.; Epstein, C.J.; Schwartz, J.; Krush, A.; Lagalante, D.J.; Mercadante, K.P.; Zeltsman, D.; Smith, L.P.; Grande, D.A. Feasibility of Bioprinting with a Modified Desktop 3D Printer. Tissue Eng. Part C Methods 2016, 22. [Google Scholar] [CrossRef]
- Bessler, N.; Ogiermann, D.; Buchholz, M.B.; Santel, A.; Heidenreich, J.; Ahmmed, R.; Zaehres, H.; Brand-Saberi, B. Nydus One Syringe Extruder (NOSE): A Prusa i3 3D printer conversion for bioprinting applications utilizing the FRESH-method. HardwareX 2019, 6, e00069. [Google Scholar] [CrossRef]
- Ioannidis, K.; Danalatos, R.I.; Champeris Tsaniras, S.; Kaplani, K.; Lokka, G.; Kanellou, A.; Papachristou, D.J.; Bokias, G.; Lygerou, Z.; Taraviras, S. A Custom Ultra-Low-Cost 3D Bioprinter Supports Cell Growth and Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 580889. [Google Scholar] [CrossRef] [PubMed]
- Kahl, M.; Gertig, M.; Hoyer, P.; Friedrich, O.; Gilbert, D.F. Ultra-low-cost 3D bioprinting: Modification and application of an off-the-shelf desktop 3D-printer for biofabrication. Front. Bioeng. Biotechnol. 2019, 7, 184. [Google Scholar] [CrossRef]
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible bioprinting: Adaptation of a low-cost 3D-printer for precise cell placement and stem cell differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef]
- Campbell, J.; McGuinness, I.; Wirz, H.; Sharon, A.; Sauer-Budge, A.F. Multimaterial and Multiscale Three-Dimensional Bioprinter. J. Nanotechnol. Eng. Med. 2015, 6, 021005. [Google Scholar] [CrossRef]
- McElheny, C.; Hayes, D.; Devireddy, R. Design and Fabrication of a low-cost three-dimensional bioprinter. J. Med. Devices Trans. ASME 2017, 11, 041001. [Google Scholar] [CrossRef]
- Wijnen, B.; Hunt, E.J.; Anzalone, G.C.; Pearce, J.M. Open-source syringe pump library. PLoS ONE 2014, 9, e107216. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.; Shiakolas, P.S.; Oberg, J.C.; Faizee, S.; Batra, A. On the Development of a Modular 3D Bioprinter for Research in Biomedical Device Fabrication. In Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2017; pp. 1–9. [Google Scholar]
- Yenilmez, B.; Temirel, M.; Knowlton, S.; Lepowsky, E.; Tasoglu, S. Development and characterization of a low-cost 3D bioprinter. Bioprinting 2019, 13, e00044. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Gómez-Blanco, J.C.; Mancha-Sánchez, E.; Marcos, A.C.; Matamoros, M.; Díaz-Parralejo, A.; Pagador, J.B. Bioink temperature influence on shear stress, pressure and velocity using computational simulation. Processes 2020, 8, 865. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Kozinski, J.A.; Chen, X.; Hwang, D.K. Modeling Mechanical Cell Damage in the Bioprinting Process Employing a Conical Needle. J. Mech. Med. Biol. 2015, 15, 1550073. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Magalhães, I.P.; de Oliveira, P.M.; Dernowsek, J.; Casas, E.B.L.; Casas, M.S.L. Investigation of the effect of nozzle design on rheological bioprinting properties using computational fluid dynamics. Rev. Mater. 2019, 24. [Google Scholar] [CrossRef]
- Martanto, W.; Baisch, S.M.; Costner, E.A.; Prausnitz, M.R.; Smith, M.K. Fluid dynamics in conically tapered microneedles. AIChE J. 2005, 51, 1599–1607. [Google Scholar] [CrossRef]
- Smith, C.; Oldt, G. Multiaxial Bio-Printer Head; Mechanical Engineering Rowan University: Glassboro, NJ, USA, 2018. [Google Scholar]
- Gómez-Blanco, J.; Pagador, J.; Galván-Chacon, V.; Sánchez-Peralta, L.; Matamoros, M.; Marcos, A.; Sánchez-Margallo, F. Computational simulation-based comparative analysis of standard FDM 3D printing and conical nozzles for pneumatic and piston-driven bioprinting. Addit. Manuf. 2021. under review. [Google Scholar]
- Marinaro, F.; Macías-García, B.; Sánchez-Margallo, F.M.; Blázquez, R.; Álvarez, V.; Matilla, E.; Hernández, N.; Gómez-Serrano, M.; Jorge, I.; Vázquez, J.; et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine model. Biol. Reprod. 2019, 100, 1180–1192. [Google Scholar] [CrossRef]
- Hesuani, Y.D.; Pereira, F.D.A.S.; Parfenov, V.; Koudan, E.; Mitryashkin, A.; Replyanski, N.; Kasyanov, V.; Knyazeva, A.; Bulanova, E.; Mironov, V. Design and Implementation of Novel Multifunctional 3D Bioprinter. 3D Print. Addit. Manuf. 2016, 3, 65–68. [Google Scholar] [CrossRef]
- Ning, L.; Betancourt, N.; Schreyer, D.J.; Chen, X. Characterization of Cell Damage and Proliferative Ability during and after Bioprinting. ACS Biomater. Sci. Eng. 2018, 4, 3906–3918. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.K.; Schmid, R.; Arkudas, A.; Kengelbach-Weigand, A.; Bosserhoff, A.K. Tumor Cells Develop Defined Cellular Phenotypes after 3D-Bioprinting in Different Bioinks. Cells 2019, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, A.; Sodupe-Ortega, E.; Pernía-Espinoza, A.; Shimizu, T.; Escobedo-Lucea, C. A versatile open-source printhead for low-cost 3d microextrusion-based bioprinting. Polymers 2020, 12, 2346. [Google Scholar] [CrossRef]

| Test | Nozzle | Flow Rate (mm3/s) | Printing Velocity (mm/s) |
|---|---|---|---|
| T1 | 22G conical tip | 10 | 8.55 |
| T2 | E3D v6 standard nozzle | 10 | 8.55 |
| T3 | E3D v6 standard nozzle | 15 | 12.83 |
| T4 | E3D v6 standard nozzle | 20 | 17.11 |
| Sample | X Error (μm) | Y Error (μm) | Z Error (μm) | Extruder Error (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | M | T | H | M | T | H | M | T | ||
| 1 | 30 | 40 | 20 | 10 | 30 | 20 | 10 | 20 | 10 | 20 |
| 2 | 0 | 0 | 0 | 10 | 40 | 10 | 10 | 30 | 10 | 0 |
| 3 | 20 | 10 | 20 | 20 | 40 | 30 | 10 | 40 | 10 | 0 |
| 4 | 0 | 10 | 0 | 20 | 30 | 10 | 10 | 20 | 20 | 0 |
| 5 | 20 | 10 | 10 | 0 | 0 | 20 | 0 | 10 | 20 | 0 |
| 6 | 20 | 20 | 20 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| 7 | 20 | 10 | 20 | 20 | 0 | 10 | 0 | 10 | 10 | 10 |
| 8 | 0 | 20 | 0 | 20 | 10 | 20 | 0 | 0 | 0 | 10 |
| 9 | 0 | 10 | 20 | 20 | 0 | 30 | 0 | 10 | 0 | 10 |
| 10 | 0 | 20 | 0 | 20 | 10 | 30 | 0 | 0 | 0 | 0 |
| Axis | Studied Bioprinter | Khal et al. [23] | Reid et al. [24] | Hesuani et al. [39] |
|---|---|---|---|---|
| X | 11.67 | 12.00 | 13.00 | 10.00 |
| Y | 10.85 | 12.00 | 13.00 | 10.00 |
| Z | 11.98 | 4.00 | - | - |
| Extruder | 5.00 | - | - | - |
| Hand-Made Bioprinters | Measurement Time | Cell Line | Cell Viability |
|---|---|---|---|
| New extruder | 0, 1, 3 and 7 day | endMSCs | 69.20 to 87. 93% |
| Campbell et al. [25] | 1 day | HUVECs | Decreased in time |
| Bessler et al. [21] | 0 day | mESC and HEK293 | 60 to 95% |
| Kahl et al. [23] | 0, 2, 5 and 7 day | HEK 293 | RFU–increased in time |
| Ozbolat et al. [4] | 1 and 7 day | CPCs | 43.92 to 92.87% |
| Roehm et al. [42] | 5 day | IMR-32 | 60 to 80% |
| Sanz-García et al. [43] | 0 and 1 day | hASCs | 90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Blanco, J.C.; Galván-Chacón, V.; Patrocinio, D.; Matamoros, M.; Sánchez-Ortega, Á.J.; Marcos, A.C.; Duarte-León, M.; Marinaro, F.; Pagador, J.B.; Sánchez-Margallo, F.M. Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle. Materials 2021, 14, 3100. https://doi.org/10.3390/ma14113100
Gómez-Blanco JC, Galván-Chacón V, Patrocinio D, Matamoros M, Sánchez-Ortega ÁJ, Marcos AC, Duarte-León M, Marinaro F, Pagador JB, Sánchez-Margallo FM. Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle. Materials. 2021; 14(11):3100. https://doi.org/10.3390/ma14113100
Chicago/Turabian StyleGómez-Blanco, Juan C., Victor Galván-Chacón, David Patrocinio, Manuel Matamoros, Álvaro J. Sánchez-Ortega, Alfonso C. Marcos, María Duarte-León, Federica Marinaro, José B. Pagador, and Francisco M. Sánchez-Margallo. 2021. "Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle" Materials 14, no. 11: 3100. https://doi.org/10.3390/ma14113100
APA StyleGómez-Blanco, J. C., Galván-Chacón, V., Patrocinio, D., Matamoros, M., Sánchez-Ortega, Á. J., Marcos, A. C., Duarte-León, M., Marinaro, F., Pagador, J. B., & Sánchez-Margallo, F. M. (2021). Improving Cell Viability and Velocity in μ-Extrusion Bioprinting with a Novel Pre-Incubator Bioprinter and a Standard FDM 3D Printing Nozzle. Materials, 14(11), 3100. https://doi.org/10.3390/ma14113100









