Effects of the Interface between Inorganic and Organic Components in a Bi2Te3–Polypyrrole Bulk Composite on Its Thermoelectric Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Characterizations
2.3.1. Material Characterizations
2.3.2. Thermoelectric Characterizations
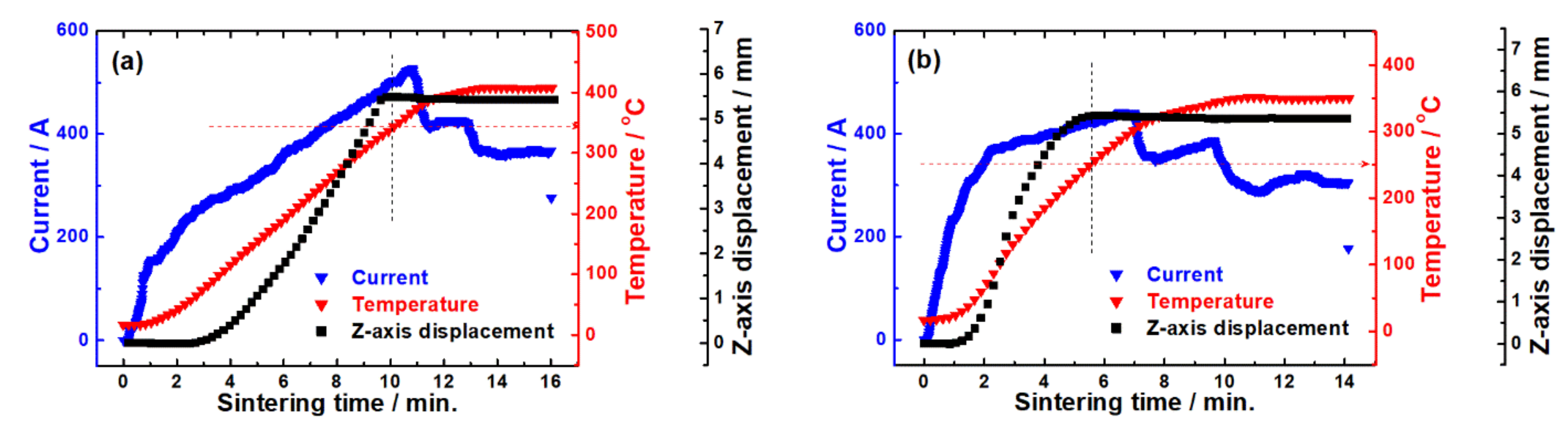
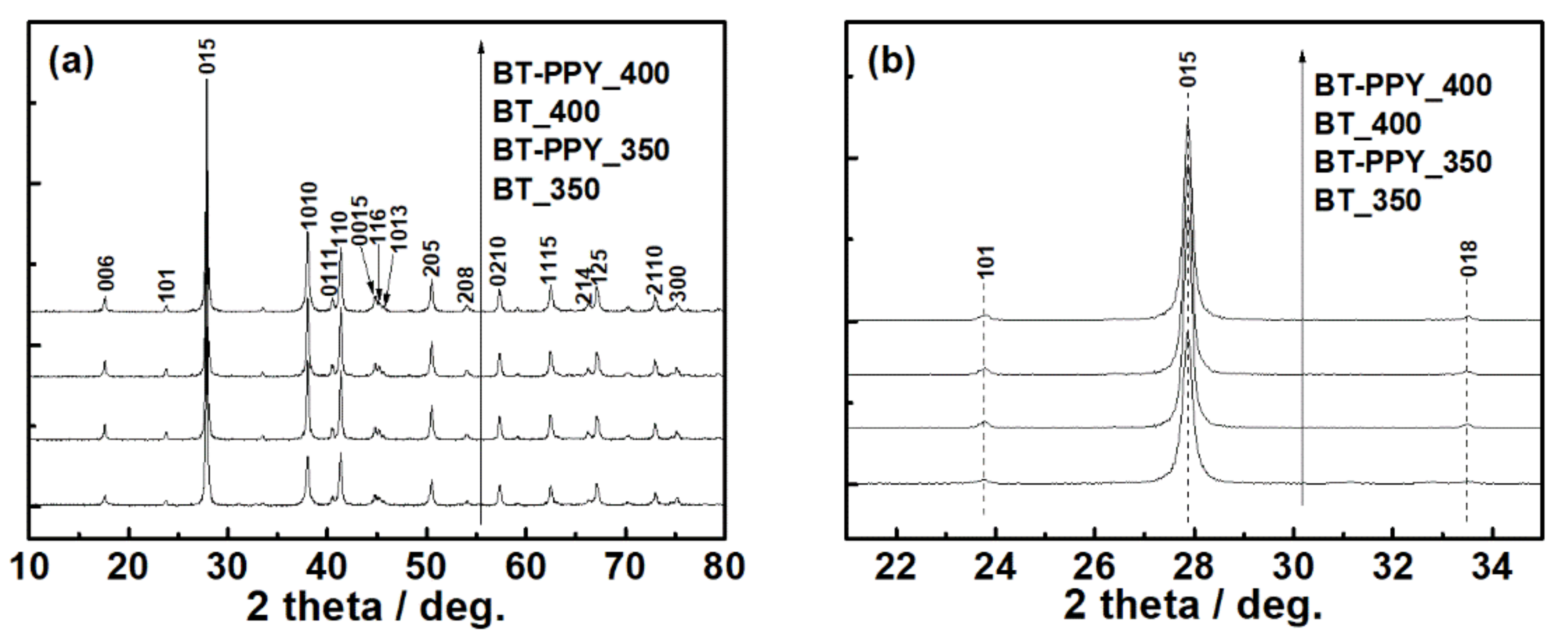
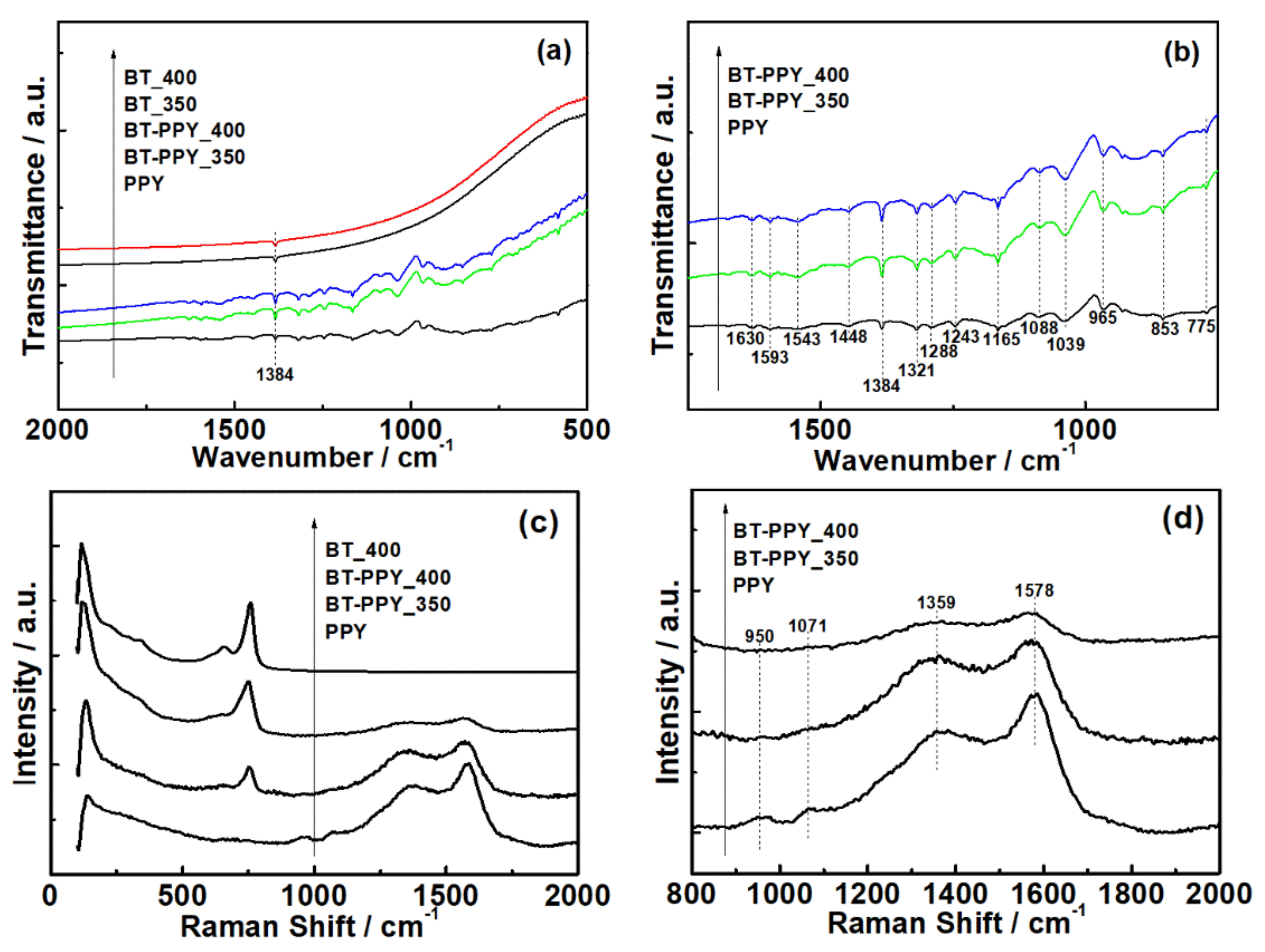
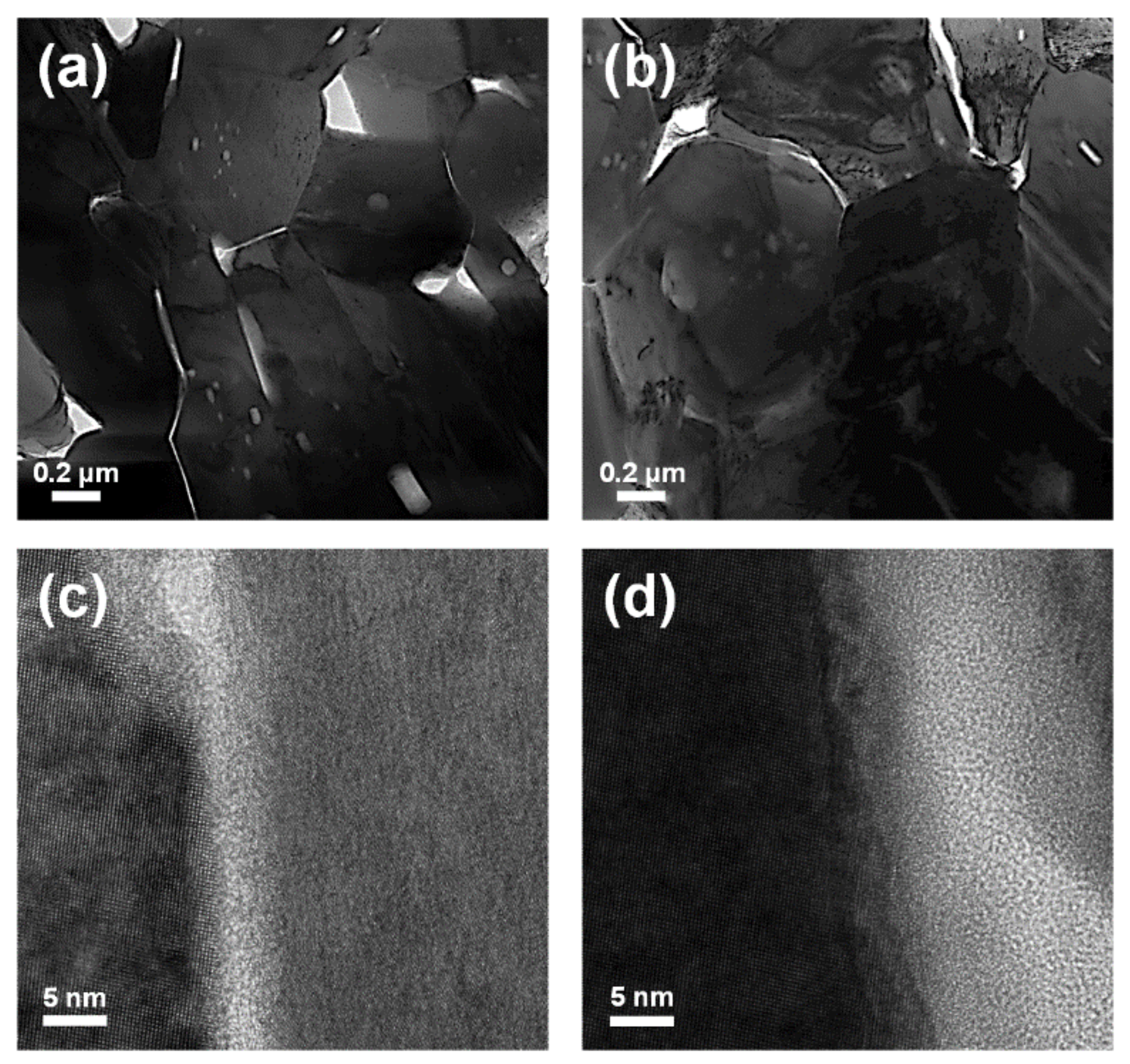
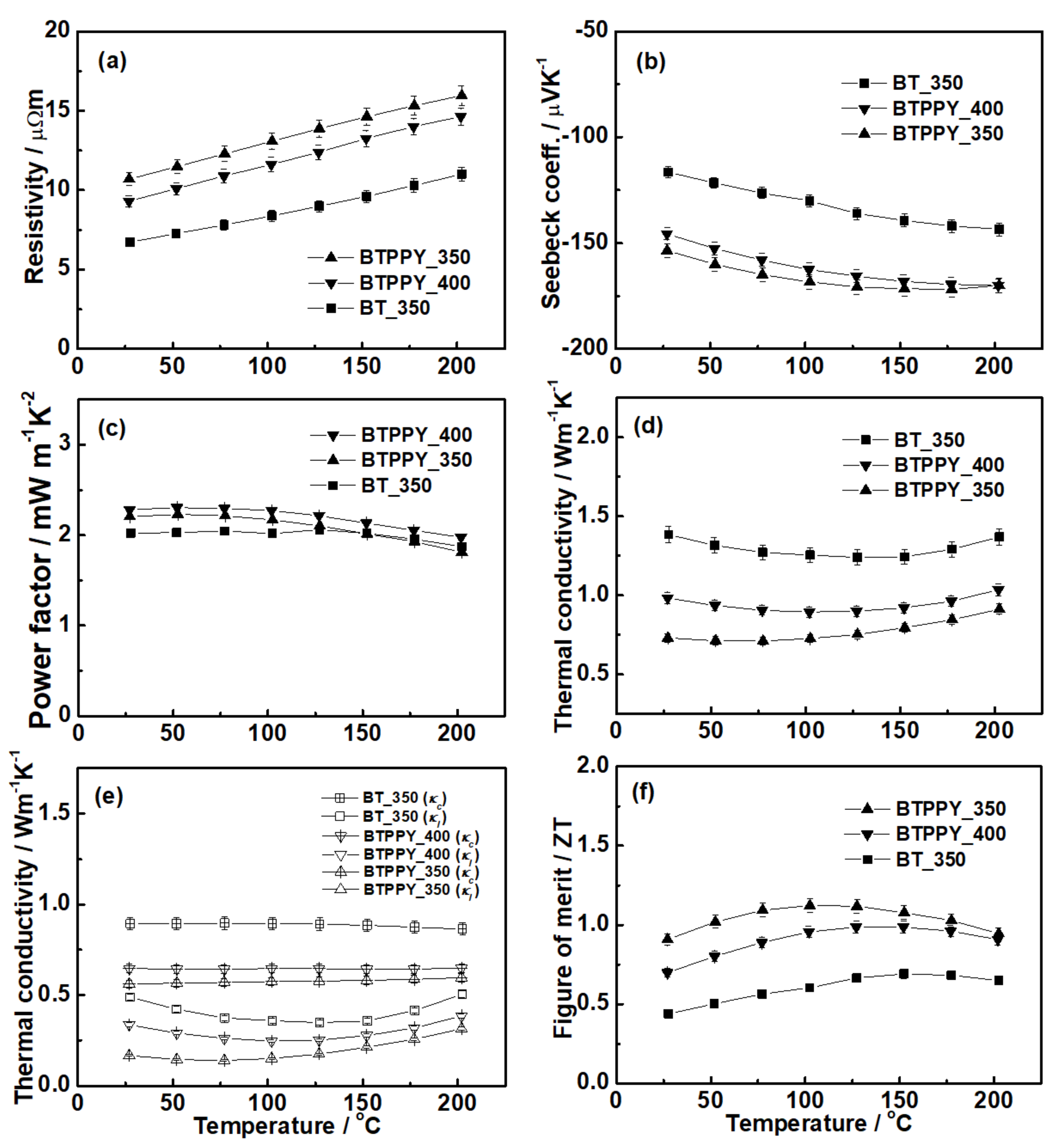
3. Results
4. Discussion
5. Conclusions
- The composite exhibited a decrease in the carrier concentration, possibly due to carrier offsetting at the interface consisting of the energy band junction. The composite showed a slight increase in the power factor compared to pristine Bi2Te3 because of the decrease in the carrier concentration.
- The composite recorded lower thermal conductivity than pristine Bi2Te3 because the former exhibited lower carrier and lattice thermal conductivities, which resulted from the decrease in the carrier concentration and the phonon scattering effect at the interface, respectively.
- The decoupling effect of the electrical and thermal properties resulted in remarkably enhanced ZT values, which may be evaluated as the highest performance among n-type binary Bi2Te3. Because of the superior ZT values at low temperatures, the composite may be highly applicable to thermoelectric operations at low temperatures, such as energy-harvesting devices and systems.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.-P.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmS-bTe2+m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef] [PubMed]
- Goldsmid, H.J. Introduction to Thermoelectricity; Springer: Berlin, Germany, 2009; pp. 72–77. [Google Scholar] [CrossRef]
- Rowe, D.M. Thermoelectrics Handbook: Macro to Nano; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 1–10. Available online: https://www.routledge.com/Thermoelectrics-Handbook-Macro-to-Nano/Rowe/p/book/9780849322648 (accessed on 6 March 2021).
- Ji, X.; Zhang, B.; Tritt, T.M.; Kolis, J.W.; Kumbhar, A. Solution-Chemical Syntheses of Nano-Structured Bi2Te3 and PbTe Thermoelectric Materials. J. Electron. Mater. 2007, 36, 721–726. [Google Scholar] [CrossRef]
- Takabatake, T. Thermoelectric Nanomaterials: Materials Design and Applications; Koumoto, K., Mori, T., Eds.; Springer: Berlin, Germany, 2009; pp. 33–49. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Han, G.; Yang, L.; Cheng, L.; Zou, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. 2012, 22, 535–549. [Google Scholar] [CrossRef]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef]
- Ghosh, S.; Valiyaveettil, S.M.; Shankar, G.; Maity, T.; Chen, K.-H.; Biswas, K.; Suwas, S.; Mallik, R.C. Enhanced Thermoelectric Properties of In-Filled Co4Sb12 with InSb Nanoinclusions. ACS Appl. Energy Mater. 2019, 3, 635–646. [Google Scholar] [CrossRef]
- Yazdani, S.; Pettes, M.T. Nanoscale self-assembly of thermoelectric materials: A review of chemistry-based approaches. Nanotechnology 2018, 29, 432001. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.; Xiao, Y.; Fu, L.; Luo, Y.; Zhang, D.; Liu, M.; Li, W.; Zhang, M. Effect of TiC Nanoinclusions on Thermoelectric and Mechanical Performance of Polycrystalline In4 Se2.65. J. Am. Ceram. Soc. 2015, 98, 3813–3817. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.E.; Fang, F.; Opila, R.L. Promising Thermoelectric Properties of Commercial PEDOT:PSS Materials and Their Bi2Te3 Powder Composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef]
- Kim, C.; Yang, Y.; Baek, J.Y.; Lopez, D.H.; Kim, D.H.; Kim, H. Concurrent defects of intrinsic tellurium and extrinsic silver in an n-type Bi2Te2.88Se0.15 thermoelectric material. Nano Energy 2019, 60, 26–35. [Google Scholar] [CrossRef]
- Kim, C.; Lopez, D.H.; Kim, D.H.; Kim, H. Dual defect system of tellurium antisites and silver interstitials in off-stoichiometric Bi2(Te,Se)3+ycausing enhanced thermoelectric performance. J. Mater. Chem. A 2018, 7, 791–800. [Google Scholar] [CrossRef]
- Xu, S.; Hong, M.; Shi, X.-L.; Wang, Y.; Ge, L.; Bai, Y.; Wang, L.; Dargusch, M.; Zou, J.; Chen, Z.-G. High-Performance PEDOT:PSS Flexible Thermoelectric Materials and Their Devices by Triple Post-Treatments. Chem. Mater. 2019, 31, 5238–5244. [Google Scholar] [CrossRef]
- Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Adv. Electron. Mater. 2019, 5, 1800769-1–1800769-23. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D. Conductive polymers for thermoelectric power generation. Prog. Mater. Sci. 2018, 93, 270–310. [Google Scholar] [CrossRef]
- Yusupov, K.; Vomiero, A. Polymer-Based Low-Temperature Thermoelectric Composites. Adv. Funct. Mater. 2020, 30, 2002015-1–2002015-26. [Google Scholar] [CrossRef]
- Thongkham, W.; Lertsatitthanakorn, C.; Jiramitmongkon, K.; Tantisantisom, K.; Boonkoom, T.; Jitpukdee, M.; Sinthiptharakoon, K.; Klamchuen, A.; Liangruksa, M.; Khanchaitit, P. Self-Assembled Three-Dimensional Bi2Te3 Nanowire–PEDOT:PSS Hybrid Nanofilm Network for Ubiquitous Thermoelectrics. ACS Appl. Mater. Interfaces 2019, 11, 6624–6633. [Google Scholar] [CrossRef]
- Bae, E.J.; Kang, Y.H.; Jang, K.-S.; Lee, C.; Cho, S.Y. Solution synthesis of telluride-based nano-barbell structures coated with PEDOT:PSS for spray-printed thermoelectric generators. Nanoscale 2016, 8, 10885–10890. [Google Scholar] [CrossRef]
- Kim, C.; Baek, J.Y.; Lopez, D.H.; Kim, D.H.; Kim, H. Decoupling effect of electrical and thermal properties of Bi2Te3-polypyrrole hybrid material causing remarkable enhancement in thermoelectric performance. J. Ind. Eng. Chem. 2019, 71, 119–126. [Google Scholar] [CrossRef]
- Sales, B.C.; Mandrus, D.; Chakoumakos, B.C.; Keppens, V.; Thompson, J.R. Filled Skutterudite Antimonides: Electron Crys-tals and Phonon Glasses. Phys. Rev. B 1997, 56, 15081–15089. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Yu, D.; He, J.; Liu, Z.; Xu, B.; Tian, Y. Enhanced thermoelectric figure of merit in nanocrystalline Bi2Te3 bulk. J. Appl. Phys. 2009, 105, 094303. [Google Scholar] [CrossRef]
- Qi, Z.; Pickup, P.G. Size Control of Polypyrrole Particles. Chem. Mater. 1997, 9, 2934–2939. [Google Scholar] [CrossRef]
- Lei, J.; Cai, Z.; Martin, C.R. Effect of reagent concentrations used to synthesize polypyrrole on the chemical characteristics and optical and electronic properties of the resulting polymer. Synth. Met. 1992, 46, 53–69. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, C.; Song, H.; Xu, J.; Mo, D.; Shi, H.; Wang, Z.; Jiang, F.; Lu, B.; Zhu, Z. Free-standing PEDOT:PSS Film as Elec-trode for the Electrodeposition of Bismuth Telluride and Its Thermoelectric Performance. Int. J. Electrochem. Sci. 2014, 9, 7540–7551. [Google Scholar]
- Dallas, P.; Niarchos, D.; Vrbanic, D.; Boukos, N.; Pejovnik, S.; Trapalis, C.; Petridis, D. Interfacial polymerization of pyrrole and in situ synthesis of polypyrrole/silver nanocomposites. Polymer 2007, 48, 2007–2013. [Google Scholar] [CrossRef]
- Jia, Y.; Xiao, P.; He, H.; Yao, J.; Liu, F.; Wang, Z.; Li, Y. Photoelectrochemical properties of polypyrrole/TiO2 nanotube arrays nanocomposite under visible light. Appl. Surf. Sci. 2012, 258, 6627–6631. [Google Scholar] [CrossRef]
- Singh, A.; Salmi, Z.; Jha, P.; Joshi, N.; Kumar, A.; Decorse, P.; Lecoq, H.; Lau-Truong, S.; Aswal, D.K.; Gupta, S.K.; et al. One step synthesis of highly ordered free standing flexible polypyrrole-silver nanocomposite films at air–water interface by photopolymerization. RSC Adv. 2013, 3, 13329–13336. [Google Scholar] [CrossRef]
- Gu, Z.; Li, C.; Wang, G.; Zhang, L.; Li, X.; Wang, W.; Jin, S. Synthesis and characterization of polypyrrole/graphite oxide composite by in situ emulsion polymerization. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1329–1335. [Google Scholar] [CrossRef]
- Han, M.-K.; Sung-Jin, K.; Lee, D.-H.; Kim, S.-J. Thermoelectric Properties of Bi2Te3: CuI and the Effect of Its Doping with Pb Atoms. Materials 2017, 10, 1235. [Google Scholar] [CrossRef]
- Carroll, E.; Buckley, D.; Mogili, N.V.V.; McNulty, D.; Moreno, M.S.; Glynn, C.; Collins, G.; Holmes, J.D.; Razeeb, K.M.; O’Dwyer, C. 2D Nanosheet Paint from Solvent-Exfoliated Bi2Te3 Ink. Chem. Mater. 2017, 29, 7390–7400. [Google Scholar] [CrossRef]
- Mathys, G.; Truong, V.-T. Spectroscopic study of thermo-oxidative degradation of polypyrrole powder by FT-IR. Synth. Met. 1997, 89, 103–109. [Google Scholar] [CrossRef]
- Sak-Bosnar, M.; Budimir, M.V.; Kovac, S.; Kukulj, D.; Duic, L. Chemical and electrochemical characterization of chemically synthesized conducting polypyrrole. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1609–1614. [Google Scholar] [CrossRef]
- Wu, F.; Song, H.; Gao, F.; Shi, W.; Jia, J.; Hu, X. Effects of Different Morphologies of Bi2Te3 Nanopowders on Thermoelectric Properties. J. Electron. Mater. 2013, 42, 1140–1145. [Google Scholar] [CrossRef]
- Xu, B.; Feng, T.; Agne, M.T.; Zhou, L.; Ruan, X.; Snyder, G.J.; Wu, Y. Highly Porous Thermoelectric Nanocomposites with Low Thermal Conductivity and High Figure of Merit from Large-Scale Solution-Synthesized Bi2 Te2.5 Se0.5 Hollow Nanostructures. Angew. Chem. Int. Ed. 2017, 56, 3546–3551. [Google Scholar] [CrossRef]
- Hong, M.; Chasapis, T.C.; Chen, Z.-G.; Yang, L.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. n-Type Bi2Te3–xSex Nanoplates with Enhanced Thermoelectric Efficiency Driven by Wide-Frequency Phonon Scatterings and Synergistic Carrier Scatterings. ACS Nano 2016, 10, 4719–4727. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Lopez, D.H. Effects of the Interface between Inorganic and Organic Components in a Bi2Te3–Polypyrrole Bulk Composite on Its Thermoelectric Performance. Materials 2021, 14, 3080. https://doi.org/10.3390/ma14113080
Kim C, Lopez DH. Effects of the Interface between Inorganic and Organic Components in a Bi2Te3–Polypyrrole Bulk Composite on Its Thermoelectric Performance. Materials. 2021; 14(11):3080. https://doi.org/10.3390/ma14113080
Chicago/Turabian StyleKim, Cham, and David Humberto Lopez. 2021. "Effects of the Interface between Inorganic and Organic Components in a Bi2Te3–Polypyrrole Bulk Composite on Its Thermoelectric Performance" Materials 14, no. 11: 3080. https://doi.org/10.3390/ma14113080
APA StyleKim, C., & Lopez, D. H. (2021). Effects of the Interface between Inorganic and Organic Components in a Bi2Te3–Polypyrrole Bulk Composite on Its Thermoelectric Performance. Materials, 14(11), 3080. https://doi.org/10.3390/ma14113080





