Adjustment of Mechanical Properties of Medium Manganese Steel Produced by Laser Powder Bed Fusion with a Subsequent Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Atomization
2.2. Stacking Fault Energy
2.3. Laser Powder Bed Fusion Process
2.4. Heat Treatment and Mechanical Testing
2.5. Microstructure Characterization
3. Results
3.1. Powder Characterization
3.2. LPBF
3.3. Mechanical Properties
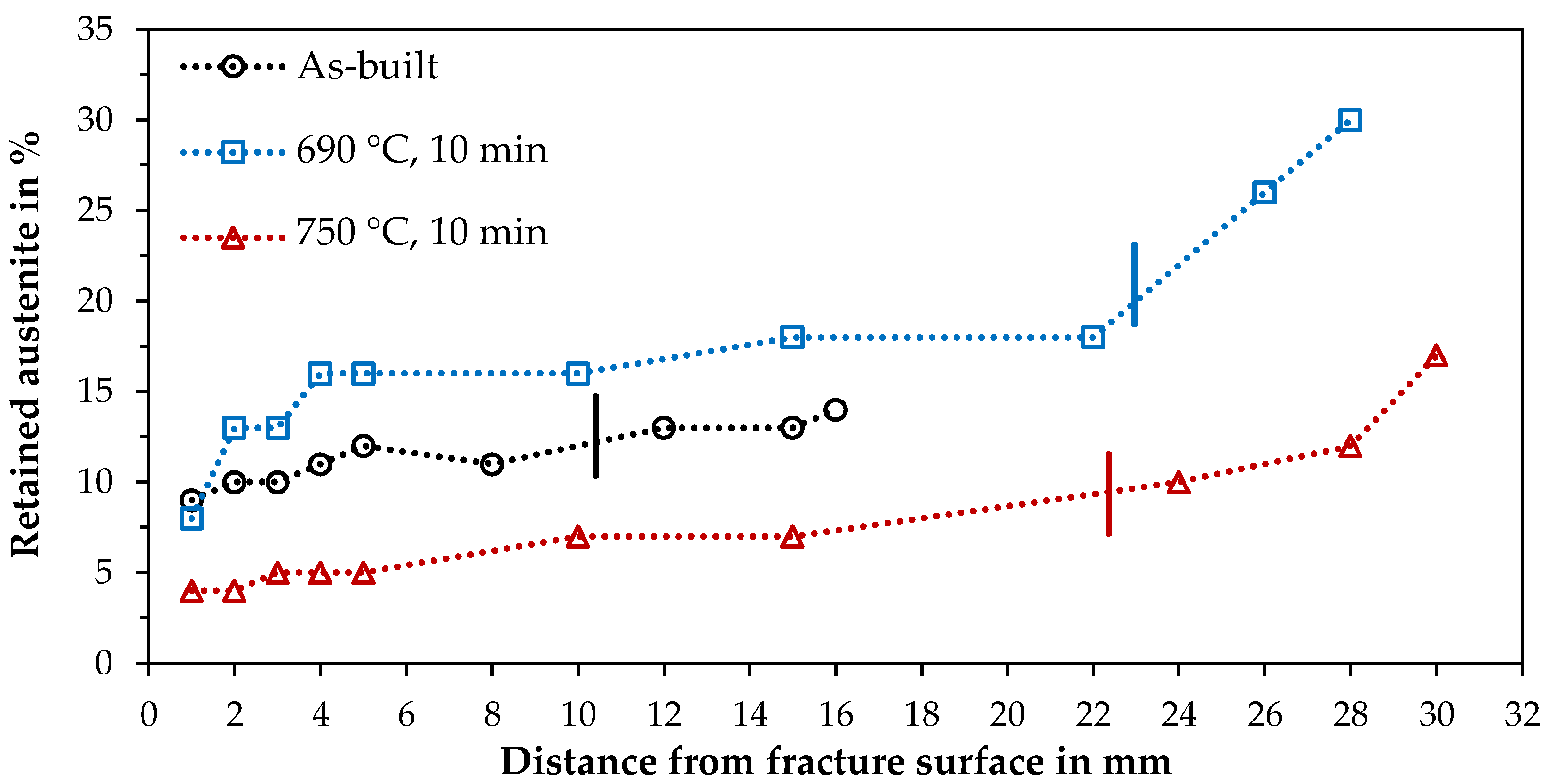
3.4. Hardness and XRD
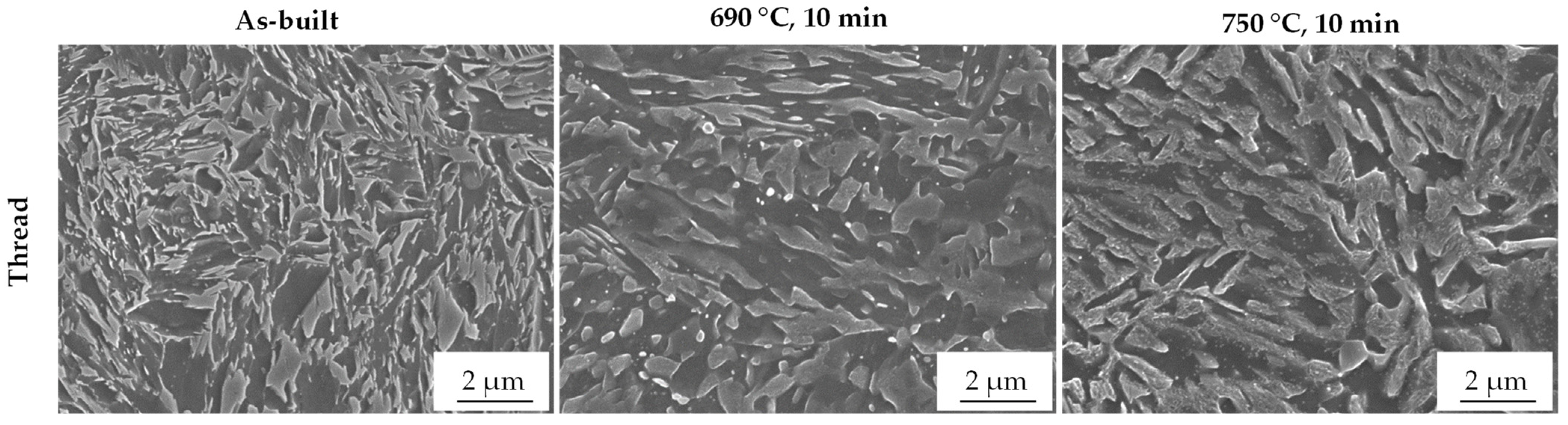
3.5. Microstructure and EBSD
4. Summary and Discussion
5. Conclusions
- The composed medium manganese steel alloy was successfully gas atomized with a high powder quality. Burn-off of manganese during atomization and LPBF needs to be taken into account (21.4% in total).
- The powder could be processed by LPBF, reaching densities up to 99.9%. However, melt spatter occurred and led to bonding errors, resulting in high standard deviations of mechanical properties, especially in elongation. The effect of Si and Al on process instability needs to be further investigated.
- As-built microstructure is suitable to activate transformation induced plasticity (TRIP) in the LPBF-samples by intercrititical annealing, and mechanical behaviour was similar to conventionally produced medium manganese steel processed at comparable annealing temperature and duration.
- Austenite transformation could be detected by XRD and EBSD. The results suggest that metallographic grinding may already cause a transformation, and therefore, ablation by an etchant is preferable if possible.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Value | Unit | Reference |
|---|---|---|---|
| D | 2 | µm | estimated |
| −1828.4 + 4.686 × T | J·mol−1 | [49] | |
| 3970–1.6667 × T | J·mol−1 | [50] | |
| 5481.04–1.79912 × T | J·mol−1 | [49] | |
| −560 + 8 × T | J·mol−1 | [51] | |
| −22,166 | J·mol−1 | [23] | |
| −9135.5 + 15,282.1 × XMn | J·mol−1 | [52] | |
| 3326.28 | J·mol−1 | [43] | |
| 1780 | J·mol−1 | [53] | |
| 42,500 | J·mol−1 | [54] | |
| 2.966 × 10−5 | mol·m−2 | [55] | |
| 10 | mJ·m−2 | estimated | |
| T | 293 | K |
References
- Schmidt, M.; Merklein, M.; Bourell, D.; Dimitrov, D.; Hausotte, T.; Wegener, K.; Overmeyer, L.; Vollertsen, F.; Levy, G.N. Laser based additive manufacturing in industry and academia. Cirp Ann. 2017, 66, 561–583. [Google Scholar] [CrossRef]
- Salman, O.; Gammer, C.; Eckert, J.; Salih, M.; Abdulsalam, E.; Prashanth, K.; Scudino, S. Selective laser melting of 316L stainless steel: Influence of TiB2 addition on microstructure and mechanical properties. Mater. Today Commun. 2019, 21, 100615. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Klahn, C.; Leutenecker, B.; Meboldt, M. Design strategies for the process of additive manufacturing. Procedia Cirp 2015, 36, 230–235. [Google Scholar] [CrossRef]
- Haase, C.; Bültmann, J.; Hof, J.; Ziegler, S.; Bremen, S.; Hinke, C.; Schwedt, A.; Prahl, U.; Bleck, W. Exploiting process-related advantages of selective laser melting for the production of high-manganese steel. Materials 2017, 10, 56. [Google Scholar] [CrossRef]
- Ardila, L.; Garciandia, F.; González-Díaz, J.; Álvarez, P.; Echeverria, A.; Petite, M.; Deffley, R.; Ochoa, J. Effect of IN718 recycled powder reuse on properties of parts manufactured by means of selective laser melting. Phys. Procedia 2014, 56, 99–107. [Google Scholar] [CrossRef]
- Kies, F.; Köhnen, P.; Wilms, M.B.; Brasche, F.; Pradeep, K.G.; Schwedt, A.; Richter, S.; Weisheit, A.; Schleifenbaum, J.H.; Haase, C. Design of high-manganese steels for additive manufacturing applications with energy-absorption functionality. Mater. Des. 2018, 160, 1250–1264. [Google Scholar] [CrossRef]
- Knoop, D.; Lutz, A.; Mais, B.; von Hehl, A. A tailored AlSiMg alloy for laser powder bed fusion. Metals 2020, 10, 514. [Google Scholar] [CrossRef]
- Springer, H.; Baron, C.; Mostaghimi, F.; Poveleit, J.; Mädler, L.; Uhlenwinkel, V. Additive manufacturing of high modulus steels: New possibilities for lightweight design. Addit. Manuf. 2020, 32, 101033. [Google Scholar] [CrossRef]
- Mohr, G.; Johannsen, J.; Knoop, D.; Gärtner, E.; Hummert, K.; Emmelmann, C. Processing of a high-strength Al-Fe-Ni alloy using laser beam melting and its potential for in-situ graded mechanical properties. In Proceedings of the Lasers in Manufacturing Conference, Munich, Germany, 26–29 June 2017. [Google Scholar]
- Liverani, E.; Toschi, S.; Ceschini, L.; Fortunato, A. Effect of selective laser melting (SLM) process parameters on microstructure and mechanical properties of 316L austenitic stainless steel. J. Mater. Process. Technol. 2017, 249, 255–263. [Google Scholar] [CrossRef]
- Deirmina, F.; Peghini, N.; AlMangour, B.; Grzesiak, D.; Pellizzari, M. Heat treatment and properties of a hot work tool steel fabricated by additive manufacturing. Mater. Sci. Eng. A 2019, 753, 109–121. [Google Scholar] [CrossRef]
- Niendorf, T.; Brenne, F. Steel showing twinning-induced plasticity processed by selective laser melting—An additively manufactured high performance material. Mater. Charact. 2013, 85, 57–63. [Google Scholar] [CrossRef]
- Grajcar, A.; Kalinowska-Ozgowicz, E.; Opiela, M.; Grzegorczyk, B.; Gołombek, K. Effects of Mn and Nb on the macro-and microsegregation in high-Mn high-Al content TRIP steels. Arch. Mater. Sci. Eng. 2011, 49, 5–14. [Google Scholar]
- Grajcar, A. Segregation behaviour of third generation advanced high-strength Mn-Al steels. Arch. Foundry Eng. 2012, 12, 123–128. [Google Scholar] [CrossRef]
- Bambach, M.; Conrads, L.; Daamen, M.; Güvenç, O.; Hirt, G. Enhancing the crashworthiness of high-manganese steel by strain-hardening engineering, and tailored folding by local heat-treatment. Mater. Des. 2016, 110, 157–168. [Google Scholar] [CrossRef]
- Matlock, D.K.; Speer, J.G.; De Moor, E.; Gibbs, P.J. Recent developments in advanced high strength sheet steels for automotive applications: An overview. Jestech 2012, 15, 1–12. [Google Scholar]
- Sander, J.; Hufenbach, J.; Bleckmann, M.; Giebeler, L.; Wendrock, H.; Oswald, S.; Gemming, T.; Eckert, J.; Kühn, U. Selective laser melting of ultra-high-strength TRIP steel: Processing, microstructure, and properties. J. Mater. Sci. 2017, 52, 4944–4956. [Google Scholar] [CrossRef]
- Droste, M.; Günther, J.; Kotzem, D.; Walther, F.; Niendorf, T.; Biermann, H. Cyclic deformation behavior of a damage tolerant CrMnNi TRIP steel produced by electron beam melting. Int. J. Fatigue 2018, 114, 262–271. [Google Scholar] [CrossRef]
- Günther, J.; Lehnert, R.; Wagner, R.; Burkhardt, C.; Wendler, M.; Volkova, O.; Biermann, H.; Niendorf, T. Effect of compositional variation induced by EBM processing on deformation behavior and phase stability of austenitic Cr-Mn-Ni TRIP steel. J. Miner. Met. Mater. Soc. 2020, 72, 1052–1064. [Google Scholar] [CrossRef]
- Jacob, R.; Sankaranarayanan, S.R.; Babu, S.K. Recent advancements in manganese steels–A review. Mater. Today Proc. 2020, 27, 2852–2858. [Google Scholar] [CrossRef]
- Facchini, L.; Vicente, N., Jr.; Lonardelli, I.; Magalini, E.; Robotti, P.; Molinari, A. Metastable austenite in 17–4 precipitation-hardening stainless steel produced by selective laser melting. Adv. Eng. Mater. 2010, 12, 184–188. [Google Scholar] [CrossRef]
- Allain, S.; Chateau, J.-P.; Bouaziz, O.; Migot, S.; Guelton, N. Correlations between the calculated stacking fault energy and the plasticity mechanisms in Fe–Mn–C alloys. Mater. Sci. Eng. A 2004, 387, 158–162. [Google Scholar] [CrossRef]
- Sato, K.; Ichinose, M.; Hirotsu, Y.; Inoue, Y. Effects of deformation induced phase transformation and twinning on the mechanical properties of austenitic Fe–Mn–Al alloys. ISIJ Int. 1989, 29, 868–877. [Google Scholar] [CrossRef]
- Saeed-Akbari, A.; Mosecker, L.; Schwedt, A.; Bleck, W. Characterization and prediction of flow behavior in high-manganese twinning induced plasticity steels: Part I. Mechanism maps and work-hardening behavior. Metall. Mater. Trans. A 2012, 43, 1688–1704. [Google Scholar] [CrossRef]
- Zambrano, O. Stacking fault energy maps of Fe–Mn–Al–C–Si steels: Effect of temperature, grain size, and variations in compositions. J. Eng. Mater. Technol. 2016, 138. [Google Scholar] [CrossRef]
- Zambrano, O. A general perspective of Fe–Mn–Al–C steels. J. Mater. Sci. 2018, 53, 14003–14062. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Han, J. Current opinion in medium manganese steel. Mater. Sci. Technol. 2015, 31, 843–856. [Google Scholar] [CrossRef]
- De Cooman, B. High Mn TWIP Steel and Medium Mn Steel. In Automotive Steels; Woodhead Publishing: Cambridge, UK, 2017; pp. 317–385. [Google Scholar]
- Gibbs, P.J.; De Cooman, B.; Brown, D.W.; Clausen, B.; Schroth, J.G.; Merwin, M.J.; Matlock, D.K. Strain partitioning in ultra-fine grained medium-manganese transformation induced plasticity steel. Mater. Sci. Eng. A 2014, 609, 323–333. [Google Scholar] [CrossRef]
- Ding, W.; Wang, R.; Li, Y.; Wang, B. High elongation of medium-manganese steel containing 1.0 wt.% Al after a short intercritical annealing time. J. Mater. Res. Technol. 2020, 9, 7262–7272. [Google Scholar] [CrossRef]
- Mueller, J.J.; Hu, X.; Sun, X.; Ren, Y.; Choi, K.; Barker, E.; Speer, J.; Matlock, D.; De Moor, E. Austenite formation and cementite dissolution during intercritical annealing of a medium-manganese steel from a martensitic condition. Mater. Des. 2021, 203, 109598. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, Z.; Tang, D.; Ye, N.; Yang, S.; Liu, W. Improved microstructural homogeneity and mechanical property of medium manganese steel with Mn segregation banding by alternating lath matrix. Mater. Sci. Eng. A 2018, 711, 175–181. [Google Scholar] [CrossRef]
- Jiang, H.-t.; Ding, W.; Tang, D.; Huang, W. Mechanical property and microstructural characterization of C-Mn-Al-Si hot dip galvanizing TRIP steel. J. Iron Steel Res. Int. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Gibbs, P.; De Moor, E.; Merwin, M.; Clausen, B.; Speer, J.; Matlock, D. Austenite stability effects on tensile behavior of manganese-enriched-austenite transformation-induced plasticity steel. Metall. Mater. Trans. A 2011, 42, 3691–3702. [Google Scholar] [CrossRef]
- Li, Y.; Huyan, F.; Ding, W. Microstructure and tensile properties of a 0.20 C–4.86 Mn steel after short intercritical-annealing times. Mater. Sci. Technol. 2019, 35, 220–230. [Google Scholar] [CrossRef]
- Dutta, A.; Ponge, D.; Sandlöbes, S.; Raabe, D. Strain partitioning and strain localization in medium manganese steels measured by in situ microscopic digital image correlation. Materialia 2019, 5, 100252. [Google Scholar] [CrossRef]
- Zapf, H.; Höfemann, M.; Emmelmann, C. Laser welding of additively manufactured medium manganese steel alloy with conventionally manufactured dual-phase steel. Procedia Cirp 2020, 94, 655–660. [Google Scholar] [CrossRef]
- Höfemann, M.; Schmale, H.C.; Epperlein, L.; Heemann, L.; Mostaghimi, F.; Schob, B.; Zapf, H.; Mahr, J.; Paul, C.; Höfner, L. Ein Niedriglegierter Stahlwerkstoff für die Laseradditive Fertigung–Prozesskette und Eigenschaften. In Konstruktion für die Additive Fertigung 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 29–41. [Google Scholar]
- Schwenck, D.; Ellendt, N.; Mädler, L.; Fischer-Bühner, J.; Hofmann, P.; Uhlenwinkel, V. Generation of small batch high quality metal powder. Powder Metall. 2014, 57, 171–175. [Google Scholar] [CrossRef]
- Schwenck, D.; Ellendt, N.; Fischer-Bühner, J.; Hofmann, P.; Uhlenwinkel, V. A novel convergent–divergent annular nozzle design for close-coupled atomisation. Powder Metall. 2017, 60, 198–207. [Google Scholar] [CrossRef]
- Olson, G.B.; Cohen, M. A general mechanism of martensitic nucleation: Part I. General concepts and the FCC→ HCP transformation. Metall. Trans. A 1976, 7, 1897–1904. [Google Scholar]
- Yang, W.; Wan, C. The influence of aluminium content to the stacking fault energy in Fe-Mn-Al-C alloy system. J. Mater. Sci. 1990, 25, 1821–1823. [Google Scholar] [CrossRef]
- Inden, G. Experimental Determination of Phase Diagrams. In Statics and Dynamics of Alloy Phase Transformations; Springer: Boston, MA, USA, 1994; pp. 17–43. [Google Scholar]
- Jin, J.-E.; Jung, M.; Lee, C.-Y.; Jeong, J.; Lee, Y.-K. Néel temperature of high Mn austenitic steels. Met. Mater. Int. 2012, 18, 419–423. [Google Scholar] [CrossRef]
- Krell, J.; Röttger, A.; Geenen, K.; Theisen, W. General investigations on processing tool steel X40CrMoV5-1 with selective laser melting. J. Mater. Process. Technol. 2018, 255, 679–688. [Google Scholar] [CrossRef]
- Azizi, H.; Ghiaasiaan, R.; Prager, R.; Ghoncheh, M.; Samk, K.A.; Lausic, A.; Byleveld, W.; Phillion, A. Metallurgical and mechanical assessment of hybrid additively-manufactured maraging tool steels via selective laser melting. Addit. Manuf. 2019, 27, 389–397. [Google Scholar] [CrossRef]
- Mostaghimi, F.; Fischer-Bühner, J.; Heemann, L.; Hofmann, P.; Von Hehl, A.; Uhlenwinkel, V. Anti-Satellite System for the Improvement of Powder Quality in Additive Manufacturing Using a FeMnAlSi Alloy. In Proceedings of the European PMPM2018 Conference, Ljubljana, Slovenia, 10–14 October 2018; p. 28. [Google Scholar]
- Dew-Hughes, D.; Kaufman, L. Ternary phase diagrams of the manganese-titanium-iron and the aluminum-titanium-iron systems: A comparison of computer calculations with experiment. Calphad 1979, 3, 175–203. [Google Scholar] [CrossRef]
- Breedis, J.; Kaufman, L. Formation of Hcp and Bcc phases in auslenitic iron alloys. Metall. Mater. Trans. B 1971, 2, 3249. [Google Scholar] [CrossRef]
- Dumay, A.; Chateau, J.-P.; Allain, S.; Migot, S.; Bouaziz, O. Influence of addition elements on the stacking-fault energy and mechanical properties of an austenitic Fe–Mn–C steel. Mater. Sci. Eng. A 2008, 483, 184–187. [Google Scholar] [CrossRef]
- Saeed-Akbari, A.; Imlau, J.; Prahl, U.; Bleck, W. Derivation and variation in composition-dependent stacking fault energy maps based on subregular solution model in high-manganese steels. Metall. Mater. Trans. A 2009, 40, 3076–3090. [Google Scholar] [CrossRef]
- Grässel, O.; Frommeyer, G.; Derder, C.; Hofmann, H. Phase transformations and mechanical properties of Fe-Mn-Si-Al TRIP-steels. Le J. De Phys. Iv 1997, 7, C5-383–C5-388. [Google Scholar] [CrossRef]
- Adler, P.; Olson, G.; Owen, W. Strain hardening of Hadfield manganese steel. Metall. Mater. Trans. A 1986, 17, 1725–1737. [Google Scholar] [CrossRef]
- Charles, J.; Berghezan, A.; Lutts, A. High manganese-aluminum austenitic steels for cryogenic applications, some mechanical and physical properties. Le J. De Phys. Colloq. 1984, 45, C1-619–C1-623. [Google Scholar] [CrossRef]
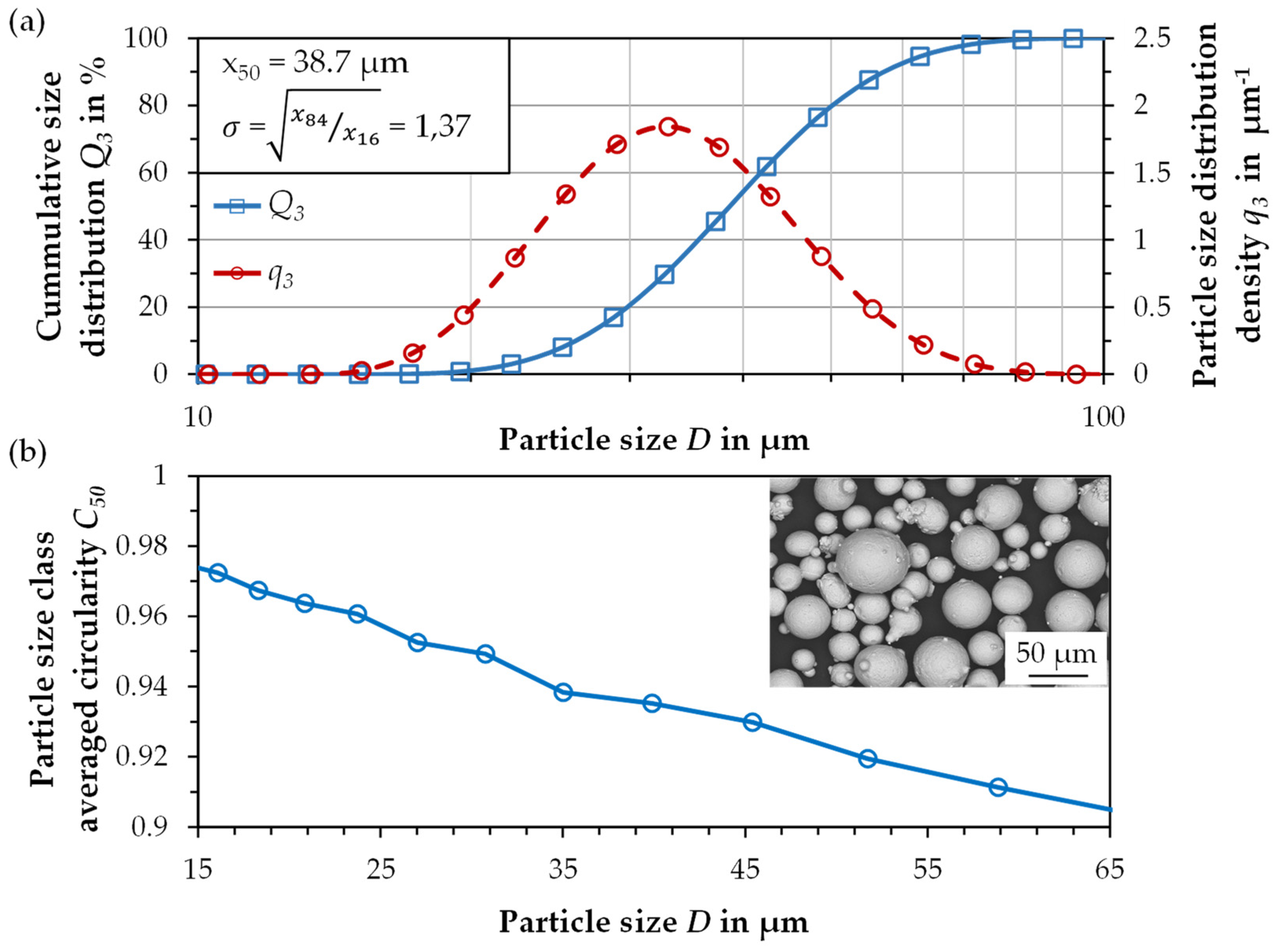
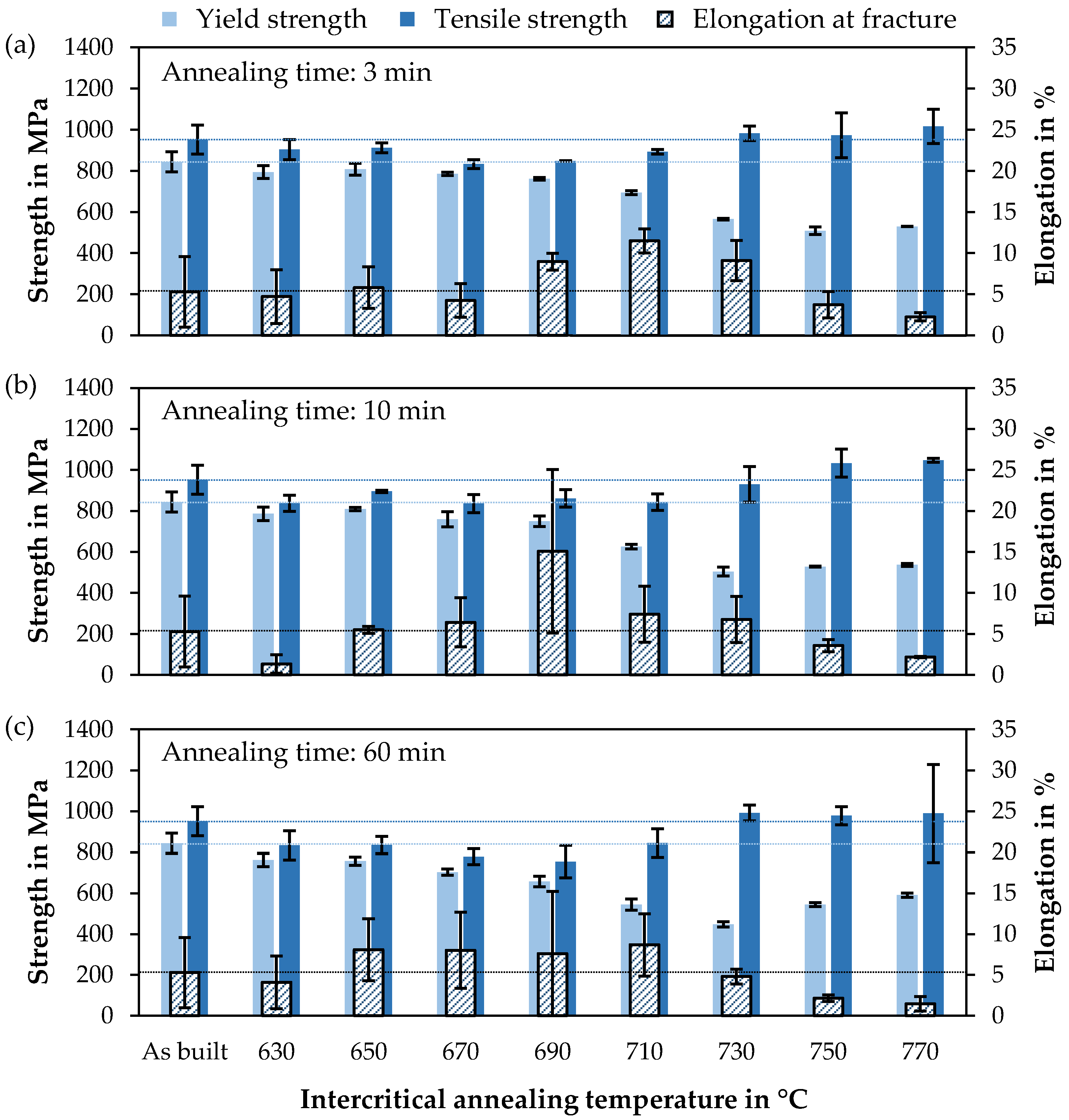
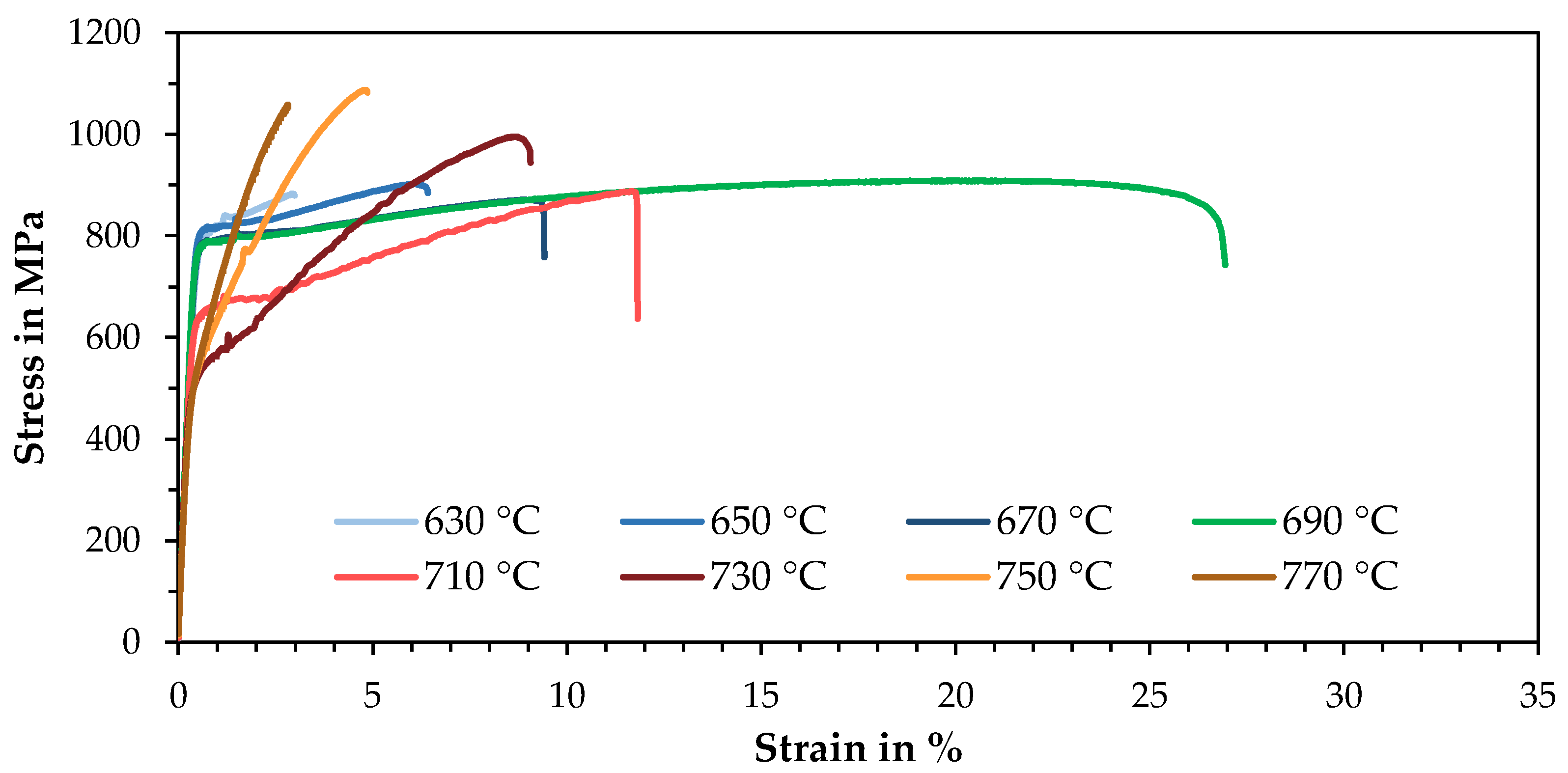
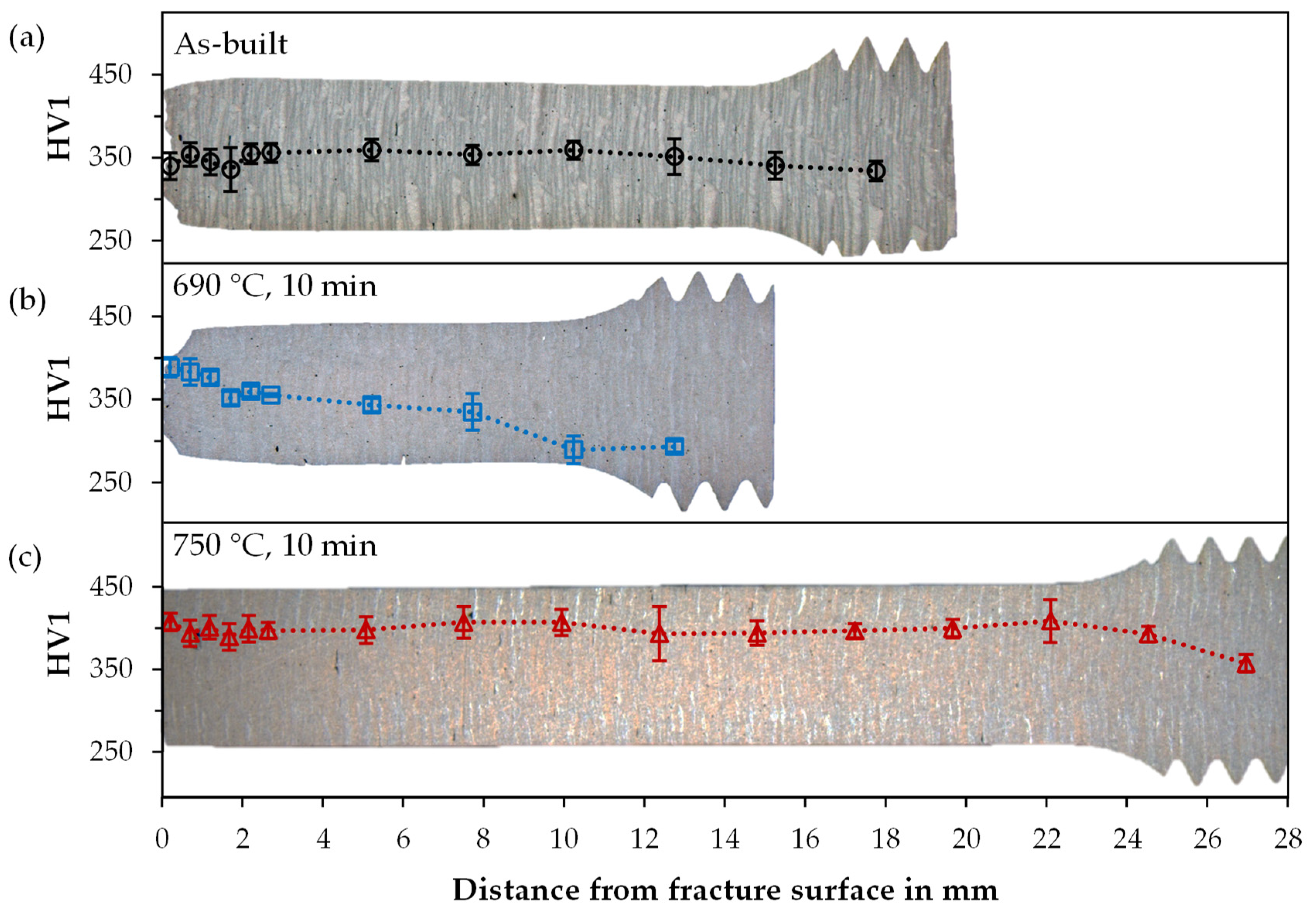

| Parameter | Symbol | Unit | Value |
|---|---|---|---|
| Gas | — | — | Argon |
| Gas temperature | TG | °C | 23 |
| Gas pressure | PG | bar | 12 |
| Gas mass flow rate | kg h−1 | 583 | |
| Melt nozzle diameter | dm | Mm | 2.5 |
| Melt temperature | Tm | °C | 1715 |
| Mean melt flow rate | kg h−1 | 265 | |
| Gas-to-melt ratio | GMR | — | 2.2 |
| Fe | C | Mn | Si | Al | |
|---|---|---|---|---|---|
| Raw material | bal. | 0.20 | 5.00 | 0.50 | 2.00 |
| Powder | bal. | 0.21 | 4.61 | 0.50 | 1.89 |
| LPBF-sample | bal. | 0.23 | 3.93 | 0.51 | 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heemann, L.; Mostaghimi, F.; Schob, B.; Schubert, F.; Kroll, L.; Uhlenwinkel, V.; Steinbacher, M.; Toenjes, A.; von Hehl, A. Adjustment of Mechanical Properties of Medium Manganese Steel Produced by Laser Powder Bed Fusion with a Subsequent Heat Treatment. Materials 2021, 14, 3081. https://doi.org/10.3390/ma14113081
Heemann L, Mostaghimi F, Schob B, Schubert F, Kroll L, Uhlenwinkel V, Steinbacher M, Toenjes A, von Hehl A. Adjustment of Mechanical Properties of Medium Manganese Steel Produced by Laser Powder Bed Fusion with a Subsequent Heat Treatment. Materials. 2021; 14(11):3081. https://doi.org/10.3390/ma14113081
Chicago/Turabian StyleHeemann, Lena, Farhad Mostaghimi, Bernd Schob, Frank Schubert, Lothar Kroll, Volker Uhlenwinkel, Matthias Steinbacher, Anastasiya Toenjes, and Axel von Hehl. 2021. "Adjustment of Mechanical Properties of Medium Manganese Steel Produced by Laser Powder Bed Fusion with a Subsequent Heat Treatment" Materials 14, no. 11: 3081. https://doi.org/10.3390/ma14113081
APA StyleHeemann, L., Mostaghimi, F., Schob, B., Schubert, F., Kroll, L., Uhlenwinkel, V., Steinbacher, M., Toenjes, A., & von Hehl, A. (2021). Adjustment of Mechanical Properties of Medium Manganese Steel Produced by Laser Powder Bed Fusion with a Subsequent Heat Treatment. Materials, 14(11), 3081. https://doi.org/10.3390/ma14113081









