Yttrium Oxide Freeze-Casts: Target Materials for Radioactive Ion Beams
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
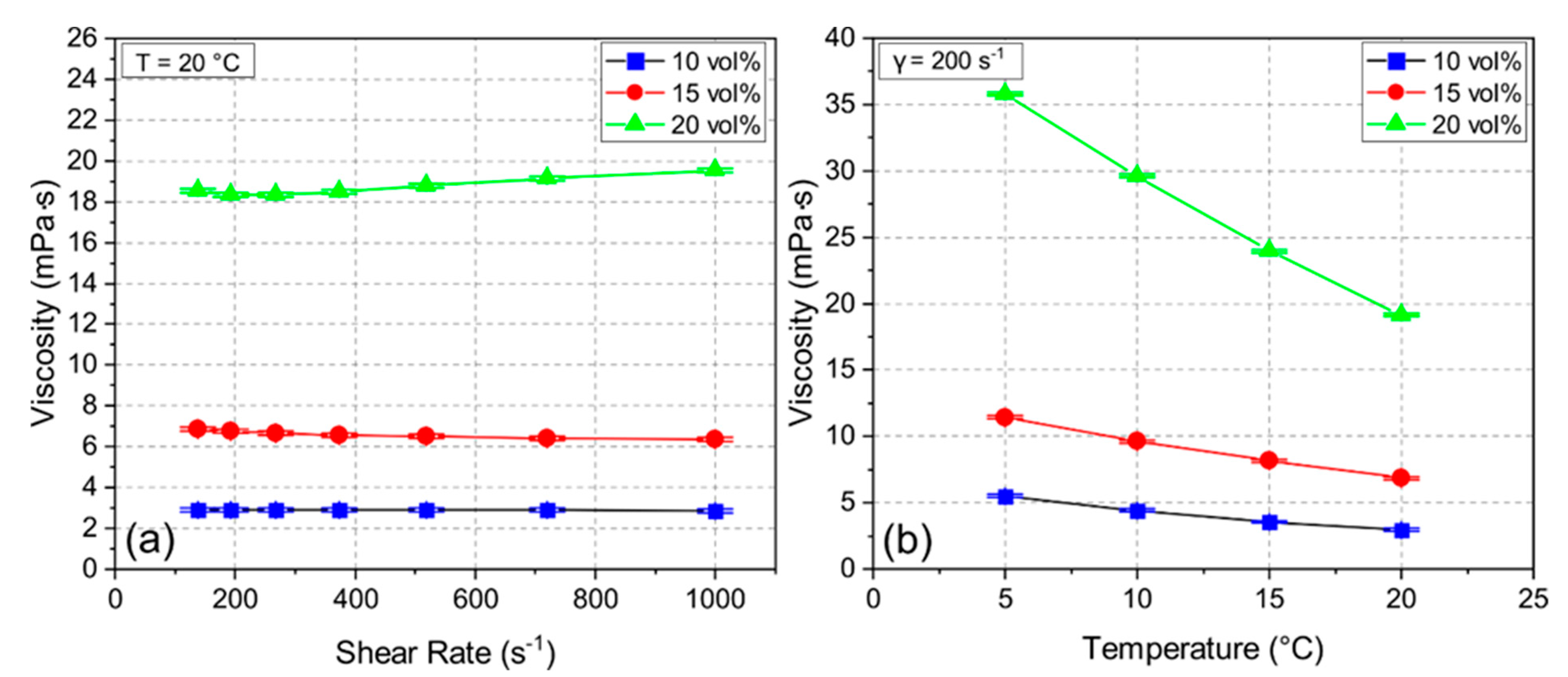
3.1. Suspension Properties and Sintering Shrinkage
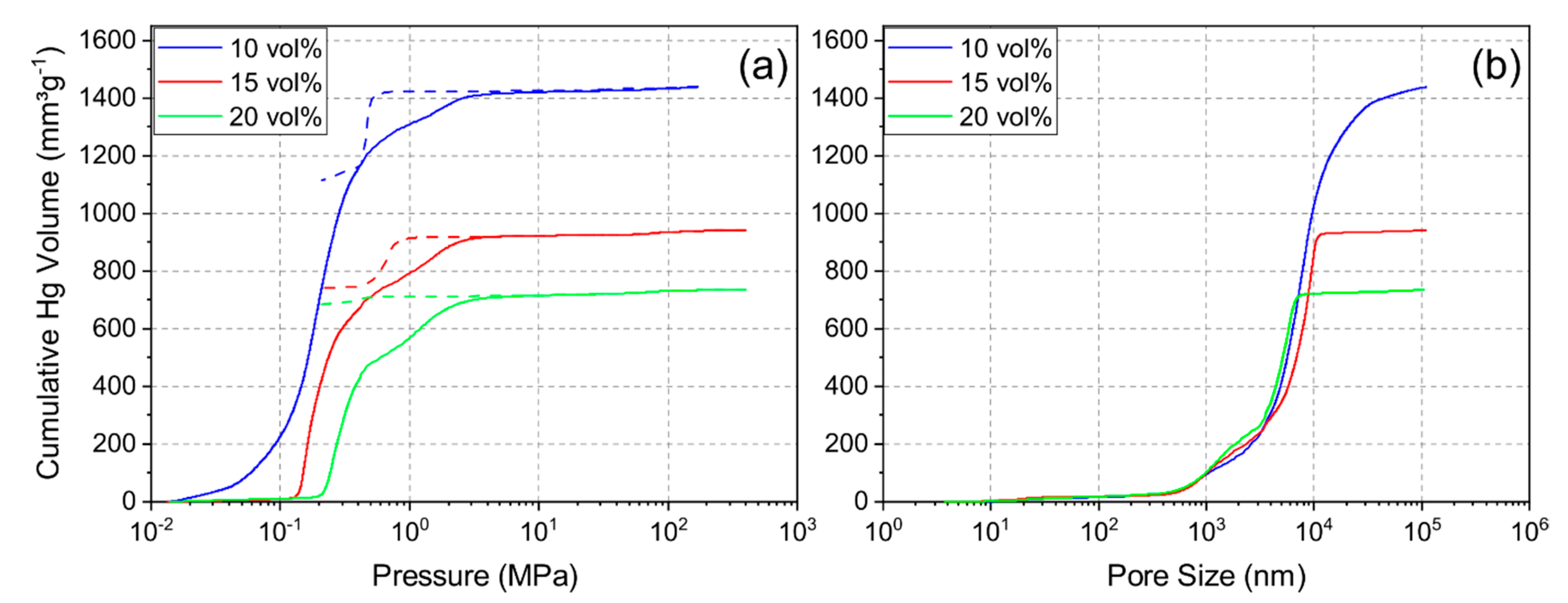
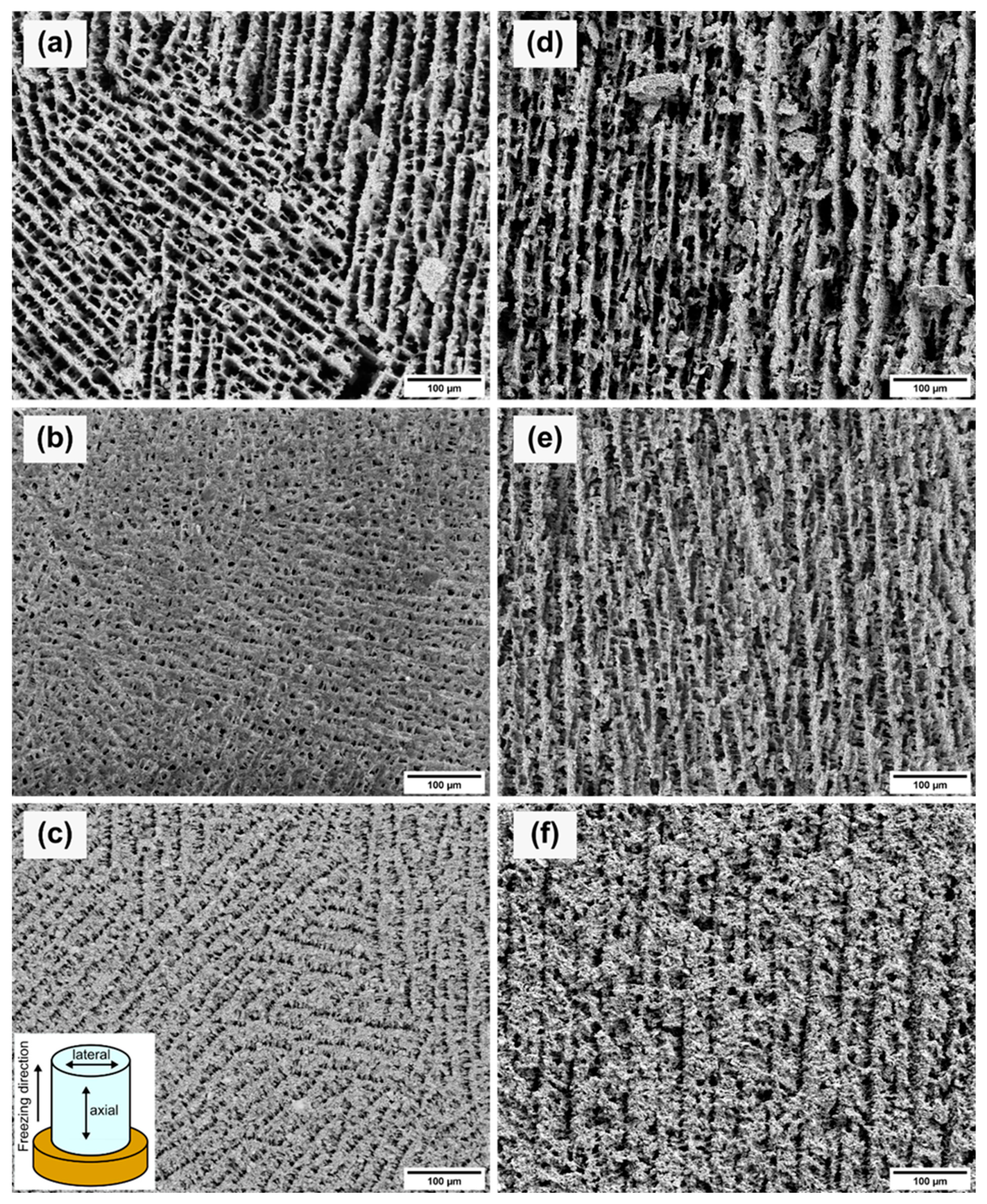
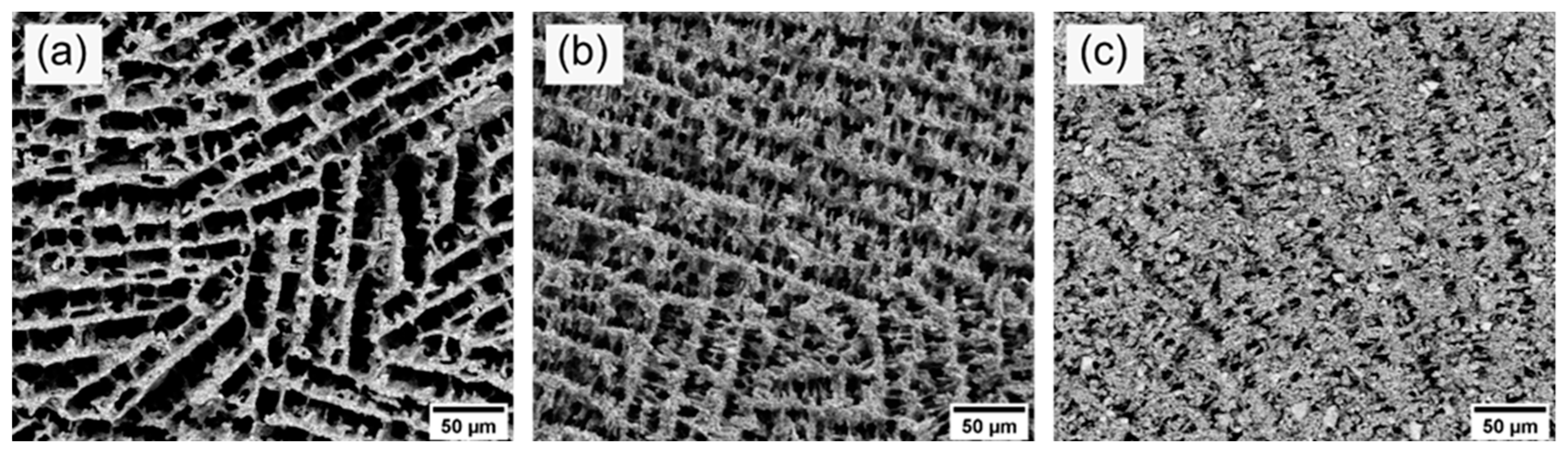
3.2. Effect of Solid Loading
3.3. Effect of Thermal Aging
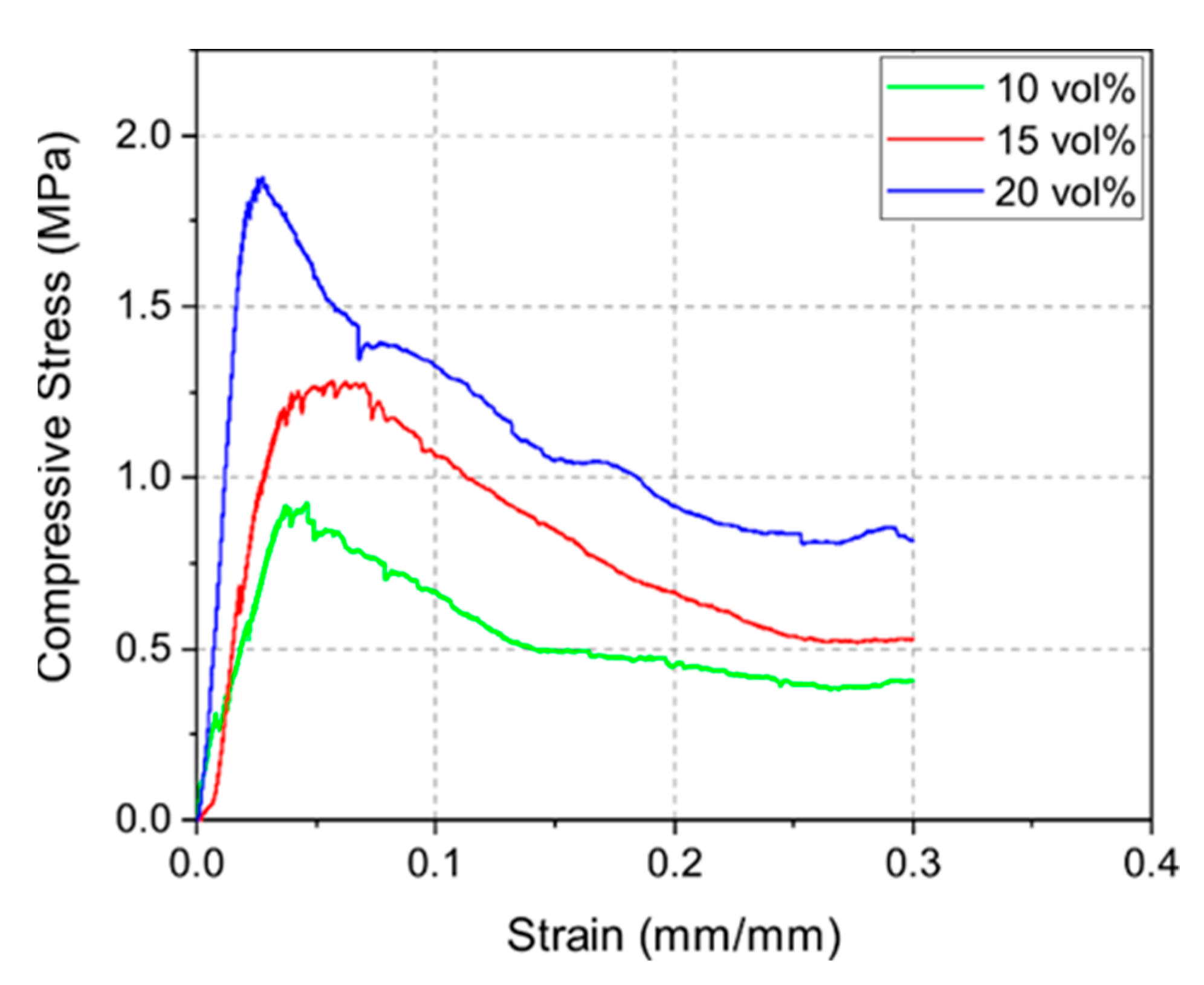
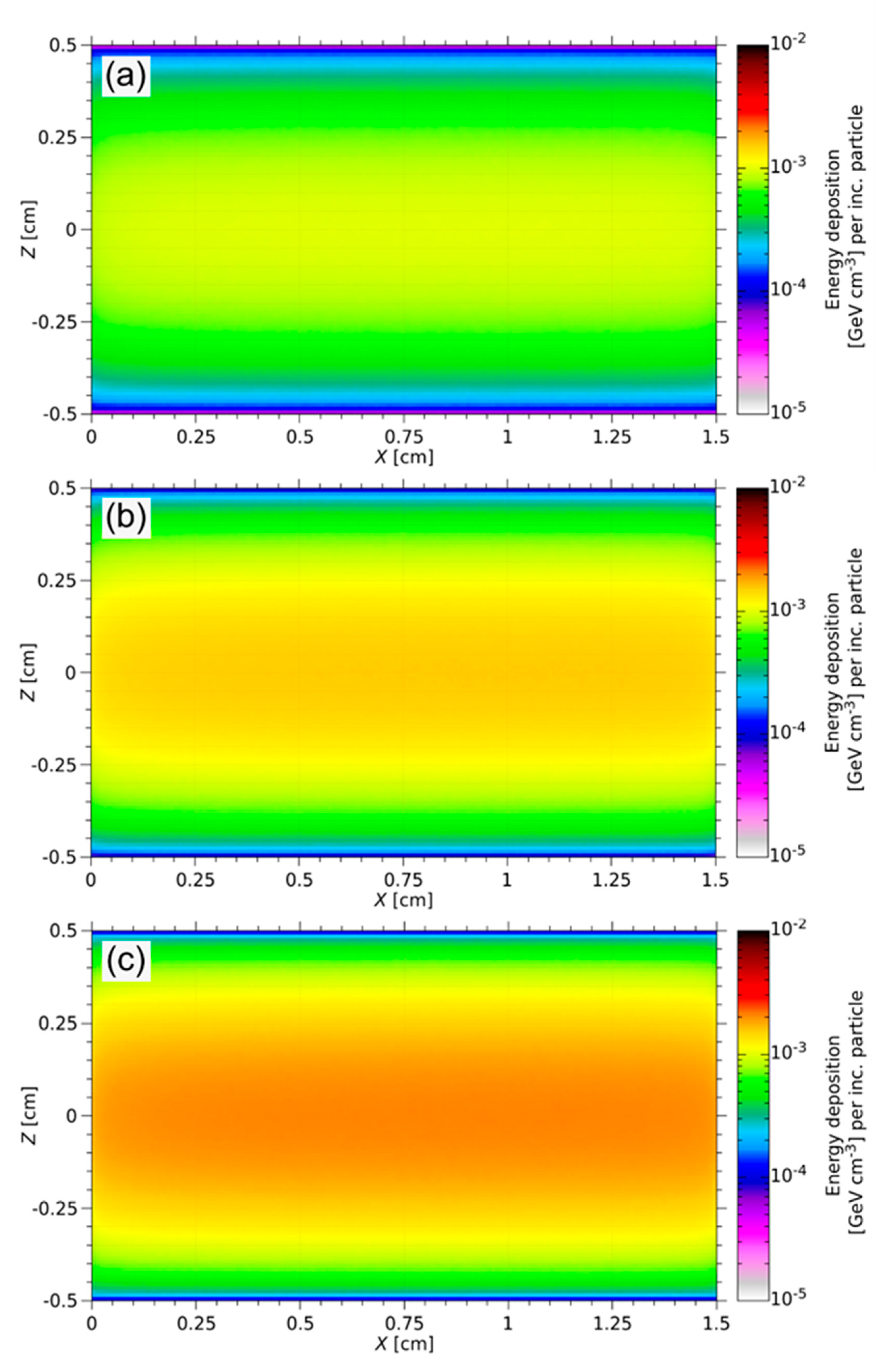
3.4. Thermo-Mechanical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catherall, R.; Andreazza, W.; Breitenfeldt, M.; Dorsival, A.; Focker, G.J.; Gharsa, T.P.; Giles, T.J.; Grenard, J.-L.; Locci, F.; Martins, P.; et al. The ISOLDE facility. J. Phys. G: Nucl. Part. Phys. 2017, 44, 094002. [Google Scholar] [CrossRef]
- Fernandes, S.; Bruetsch, R.; Catherall, R.; Groeschel, F.; Guenther-Leopold, I.; Lettry, J.; Manfrin, E.; Marzari, S.; Noah, E.; Sgobba, S.; et al. Microstructure evolution of nanostructured and submicrometric porous refractory ceramics induced by a continuous high-energy proton beam. J. Nucl. Mater. 2011, 416, 99–110. [Google Scholar] [CrossRef]
- Ramos, J.P. Thick solid targets for the production and online release of radioisotopes: The importance of the material charac-teristics—A review. Nucl. Instrum. Methods Phys. Res. B 2020, 463, 201–210. [Google Scholar] [CrossRef]
- Gottberg, A. Target materials for exotic ISOL beams. Nucl. Instrum. Methods Phys. Res. B 2016, 376, 8–15. [Google Scholar] [CrossRef]
- Stegemann, S.; Ballof, J.; Cocolios, T.; Correia, J.-G.; Dockx, K.; Poleshchuk, O.; Ramos, J.; Schell, J.; Stora, T.; Vleugels, J. A porous hexagonal boron nitride powder compact for the production and release of radioactive 11C. J. Eur. Ceram. Soc. 2020, 41, 4086–4097. [Google Scholar] [CrossRef]
- Kirchner, R. On the release and ionization efficiency of catcher-ion-source systems in isotope separation on-line. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1992, 70, 186–199. [Google Scholar] [CrossRef]
- Deville, S. Freeze-Casting of Porous Ceramics: A Review of Current Achievements and Issues. Adv. Eng. Mater. 2008, 10, 155–169. [Google Scholar] [CrossRef]
- Czapski, M.; Stora, T.; Tardivat, C.; Deville, S.; Augusto, R.S.; Leloup, J.; Bouville, F.; Luis, R.F. Porous silicon carbide and aluminum oxide with unidirectional open porosity as model target materials for radioisotope beam production. Nucl. Instrum. Methods Phys. Res. B 2013, 317, 385–388. [Google Scholar] [CrossRef]
- Deville, S. Ice-templating, freeze casting: Beyond materials processing. J. Mater. Res. 2013, 28, 2202–2219. [Google Scholar] [CrossRef]
- Naleway, S.E.; Fickas, K.C.; Maker, Y.N.; Meyers, M.A.; McKittrick, J. Reproducibility of ZrO2-based freeze casting for bio-materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Wu, Y.; Lv, S.; Lu, K. Microstructure of TiO2 porous ceramics by freeze casting of nanoparticle suspensions. Ceram. Int. 2017, 43, 14593–14598. [Google Scholar] [CrossRef]
- Sofie, S.W.; Dogan, F. Freeze Casting of Aqueous Alumina Slurries with Glycerol. J. Am. Ceram. Soc. 2004, 84, 1459–1464. [Google Scholar] [CrossRef]
- Stora, T. Recent developments of target and ion sources to produce ISOL beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 317, 402–410. [Google Scholar] [CrossRef]
- Yoshida, H.; Morita, K.; Kim, B.-N.; Soga, K.; Yamamoto, T. Production of transparent yttrium oxide ceramics by the combi-nation of low temperature spark plasma sintering and zinc cation-doping. J. Eur. Ceram. Soc. 2018, 38, 1972–1980. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Ning, K.; Luo, D.; Yang, H.; Yin, D.; Tang, D.; Kong, L.B. Densification of Yttria Transparent Ceramics: The Utilization of Activated Sintering. J. Am. Ceram. Soc. 2016, 99, 1671–1675. [Google Scholar] [CrossRef]
- Szymanska, J.; Malek, M.; Wisniewski, P.; Jaroslaw, M. Rheological properties of ceramic slurries based on yttrium III oxide for ceramic shell moulds preparation. Aluminium 2000, 2015. [Google Scholar] [CrossRef]
- Santos, S.C.; Setz, L.F.G.; Yamagata, C.; de Mello-Castanho, S.R.H. Rheological Study of Yttrium Oxide Aqueous Suspensions. MSF 2010, 660–661, 712–717. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; He, Y. Fabrication of highly porous Y2O3:Ho,Yb ceramic and its thermometric applications. J. Alloy. Compd. 2018, 741, 1158–1162. [Google Scholar] [CrossRef]
- Brook, R.J.; Cahn, R.W.; Bever, M.B. Concise Encyclopedia of Advanced Ceramic Materials, 1st ed.; Pergamon Press: Oxford, MI, USA, 1991. [Google Scholar]
- Ferrari, A.; Sala, P.R.; Fassò, A.; Ranft, J. FLUKA: A Multi-Particle Transport Code; CERN: Geneva, Switzerland, 2005. [Google Scholar]
- Böhlen, T.; Cerutti, F.; Chin, M.; Fassò, A.; Ferrari, A.; Ortega, P.; Mairani, A.; Sala, P.; Smirnov, G.; Vlachoudis, V. The FLUKA Code: Developments and Challenges for High Energy and Medical Applications. Nucl. Data Sheets 2014, 120, 211–214. [Google Scholar] [CrossRef]
- Hunter, R.J.; Ottewill, R.H.; Rowell, R.L. Zeta Potential in Colloid Science: Principles and Applications, 1st ed.; Academic Press: London, UK, 1988. [Google Scholar]
- Preiss, A.; Su, B.; Collins, S.; Simpson, D. Tailored graded pore structure in zirconia toughened alumina ceramics using dou-ble-side cooling freeze casting. J. Eur. Ceram. Soc. 2012, 32, 1575–1583. [Google Scholar] [CrossRef]
- Rahaman, M.N. Sintering of Ceramics, 1st ed.; Taylor and Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements, 1st ed.; CRC Press: London, UK, 2019. [Google Scholar]
- Giesche, H. Mercury Porosimetry: A General (Practical) Overview. Part. Part. Syst. Charact. 2006, 23, 9–19. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Shen, X.; Gurlo, A. Freeze Casting: From Low-Dimensional Building Blocks to Aligned Porous Structures-A Review of Novel Materials, Methods, and Applications. Adv. Mater. 2020, 32, 1907176. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.H.; Zeng, Y.-P.; Jiang, D. Properties of Microstructure-Controllable Porous Yttria-Stabilized Ziroconia Ceramics Fab-ricated by Freeze Casting. Int. J. Appl. Ceram. Technol. 2008, 5, 198–203. [Google Scholar] [CrossRef]
- Liu, X.; Xue, W.; Shi, C.; Sun, J. Fully interconnected porous Al2O3 scaffolds prepared by a fast cooling freeze casting method. Ceram. Int. 2015, 41, 11922–11926. [Google Scholar] [CrossRef]
- Gibson, L.J. Mechanical Behavior of Metallic Foams. Annu. Rev. Mater. Res. 2000, 30, 191–227. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Doğan, F.; Bal, B.S. Freeze casting of porous hydroxyapatite scaffolds. II. Sintering, microstructure, and mechanical behavior. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.E. Properties of Yttrium Oxide Ceramics. J. Am. Ceram. Soc. 1957, 40, 274–278. [Google Scholar] [CrossRef]


| Sample Labelling | 10Y | 15Y | 20Y |
|---|---|---|---|
| Solid Loading (vol%) | 10 | 15 | 20 |
| PVA (wt%) | 3 | 3 | 3 |
| PEG (wt%) | 2 | 2 | 2 |
| Dispersant (wt%) | 1.5 | 1.5 | 1.5 |
| Sample | Diameter (%) | Height (%) |
|---|---|---|
| 10Y | 6.5 ± 1.2 | 8.4 ± 0.7 |
| 15Y | 6.5 ± 0.8 | 8.0 ± 0.5 |
| 20Y | 5.9 ± 0.4 | 7.4 ± 1.3 |
| Sample | Average Pore Size | |
|---|---|---|
| Before Aging (µm) | After Aging (µm) | |
| 10Y | 11.8 ± 3.6 | 11.1 ± 3.5 |
| 15Y | 6.5 ± 2.0 | 7.1 ± 2.1 |
| 20Y | 4.4 ± 1.4 | 5.2 ± 1.8 |
| Sample | Edep (J cm−3) | P (W) | ΔT (K) | Eyoung (MPa) | σ (MPa) | σs (MPa) |
|---|---|---|---|---|---|---|
| 10Y | 8.07 | 9.51 | 24.73 | 3.53 | 8.12 × 10−3 | 0.84 |
| 15Y | 1.24 | 14.61 | 25.05 | 4.99 | 1.16 × 10−2 | 1.28 |
| 20Y | 1.62 | 19.10 | 24.48 | 11.52 | 2.66 × 10−2 | 1.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kröll, E.; Vadalà, M.; Schell, J.; Stegemann, S.; Ballof, J.; Rothe, S.; Lupascu, D.C. Yttrium Oxide Freeze-Casts: Target Materials for Radioactive Ion Beams. Materials 2021, 14, 2864. https://doi.org/10.3390/ma14112864
Kröll E, Vadalà M, Schell J, Stegemann S, Ballof J, Rothe S, Lupascu DC. Yttrium Oxide Freeze-Casts: Target Materials for Radioactive Ion Beams. Materials. 2021; 14(11):2864. https://doi.org/10.3390/ma14112864
Chicago/Turabian StyleKröll, Eva, Miriana Vadalà, Juliana Schell, Simon Stegemann, Jochen Ballof, Sebastian Rothe, and Doru C. Lupascu. 2021. "Yttrium Oxide Freeze-Casts: Target Materials for Radioactive Ion Beams" Materials 14, no. 11: 2864. https://doi.org/10.3390/ma14112864
APA StyleKröll, E., Vadalà, M., Schell, J., Stegemann, S., Ballof, J., Rothe, S., & Lupascu, D. C. (2021). Yttrium Oxide Freeze-Casts: Target Materials for Radioactive Ion Beams. Materials, 14(11), 2864. https://doi.org/10.3390/ma14112864







