Characterization, Antimicrobial Effects, and Cytocompatibility of a Root Canal Sealer Produced by Pozzolan Reaction between Calcium Hydroxide and Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing of the Material
2.2. Characterization of the Material
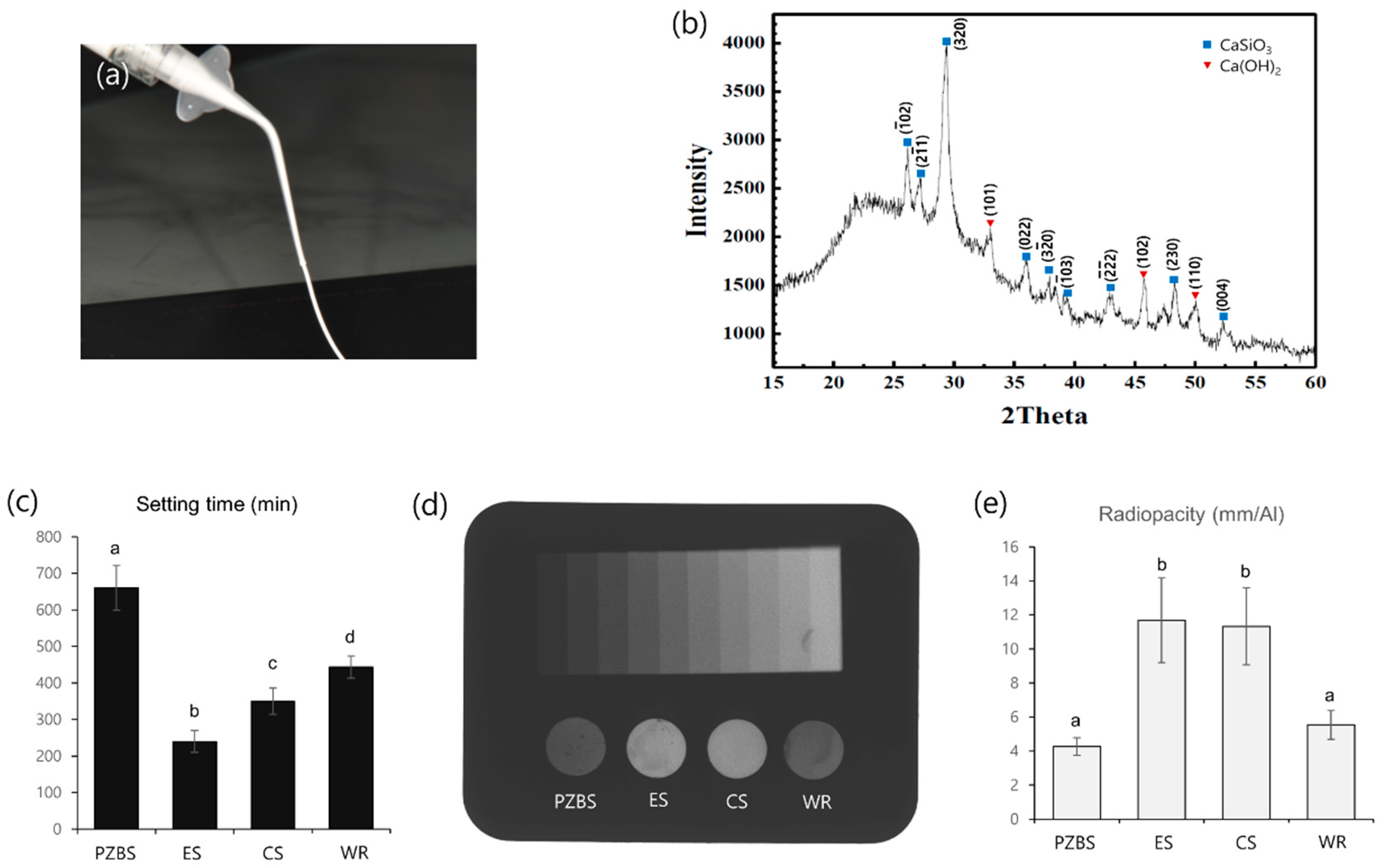
2.2.1. XRD Analysis
2.2.2. Initial Setting Time
2.2.3. Radiopacity
2.3. Antimicrobial Evaluation
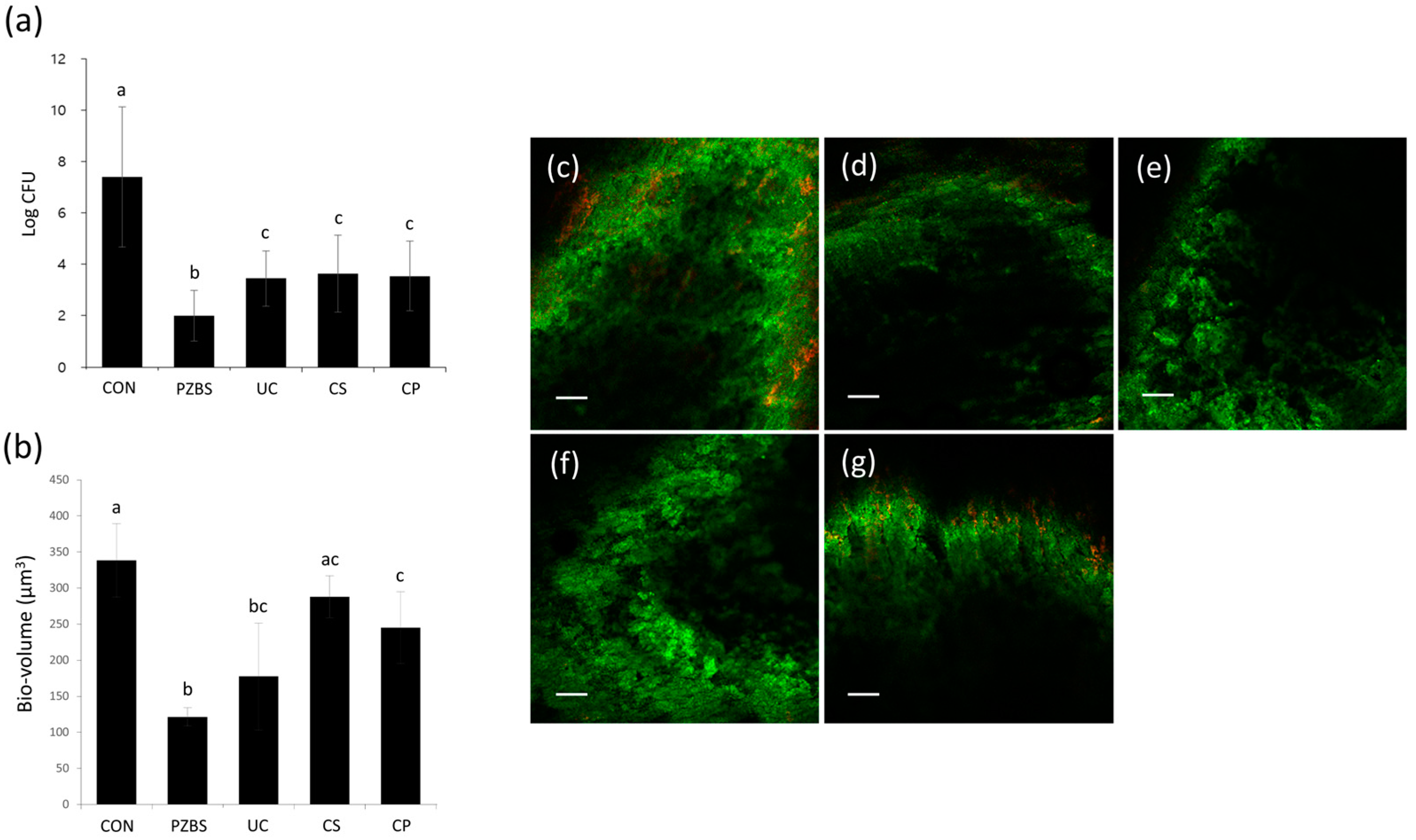
2.3.1. Intracanal Biofilm Formation
2.3.2. Counting of CFUs
2.3.3. Confocal Laser Scanning Microscopic (CLSM) Analysis
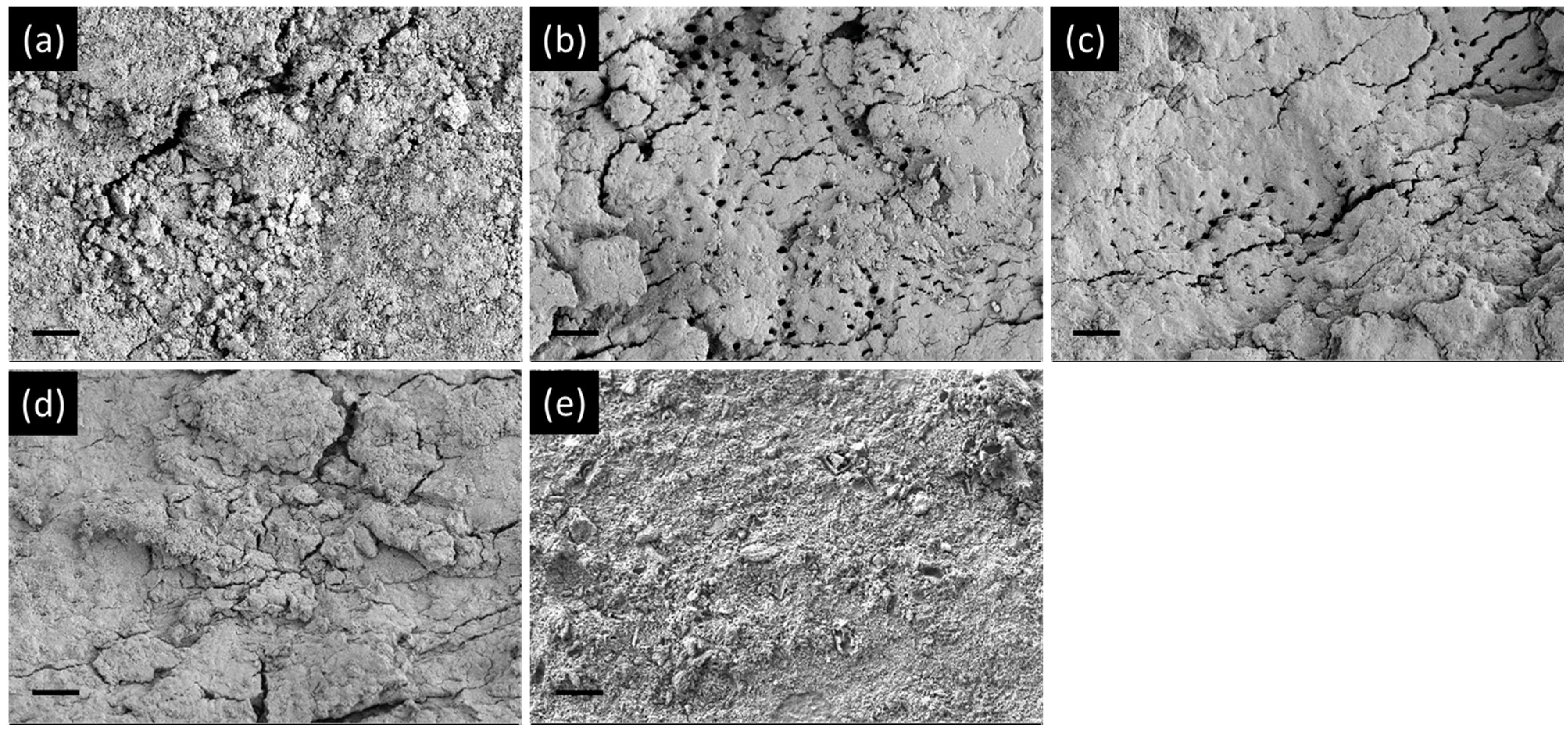
2.3.4. Field-Emission Scanning Electron Microscopy (FE-SEM) Observations
2.4. Cytocompatibility Evaluation
2.4.1. Preparation of Material Extracts
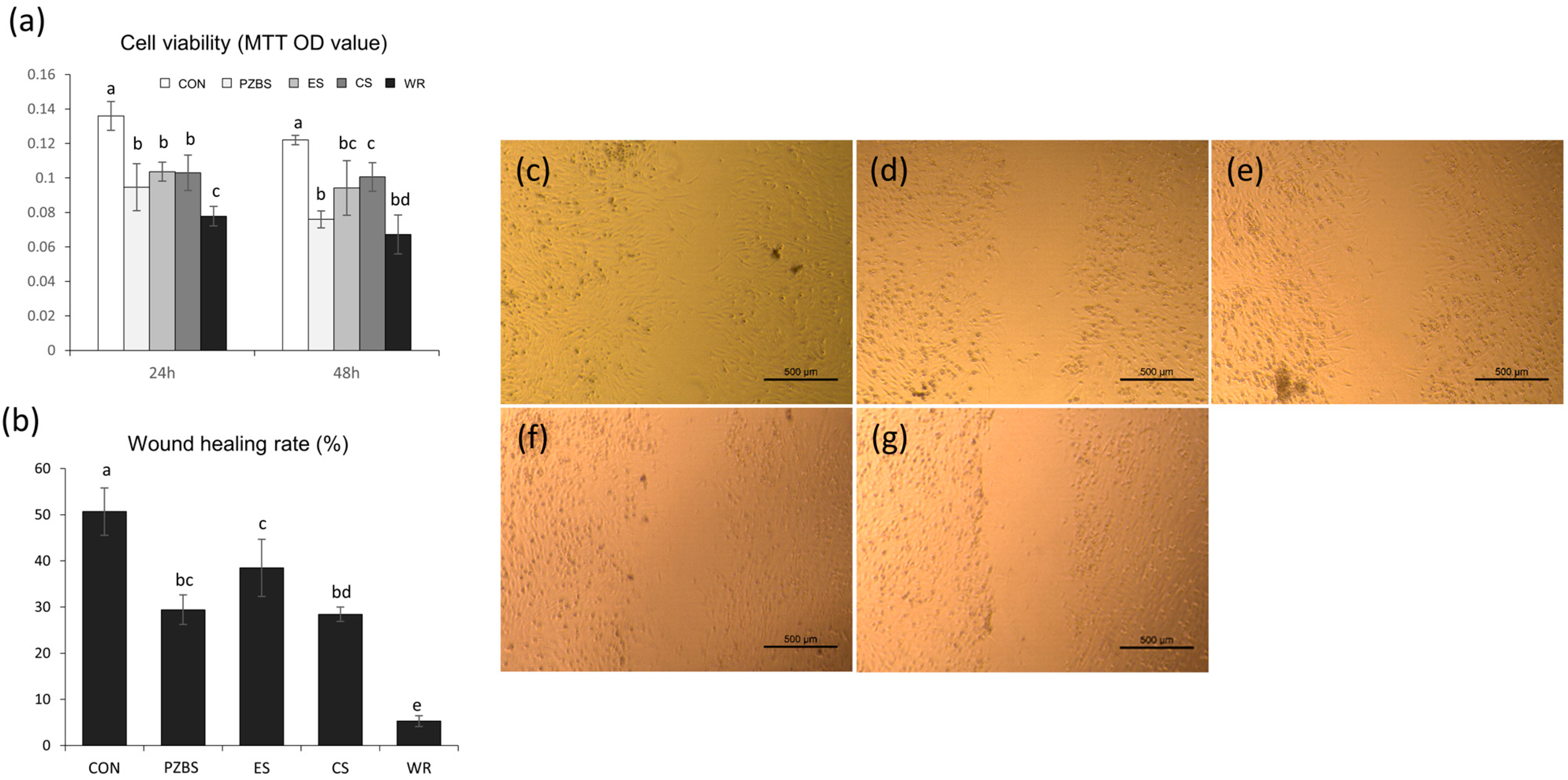
2.4.2. Cell Viability Test
2.4.3. Cell Migration Assay
2.4.4. Cell Morphological Observations Using SEM
2.5. Statistical Analysis
3. Results
3.1. Characterization, Setting Time, and Radiopacity
3.2. Antibacterial Effects
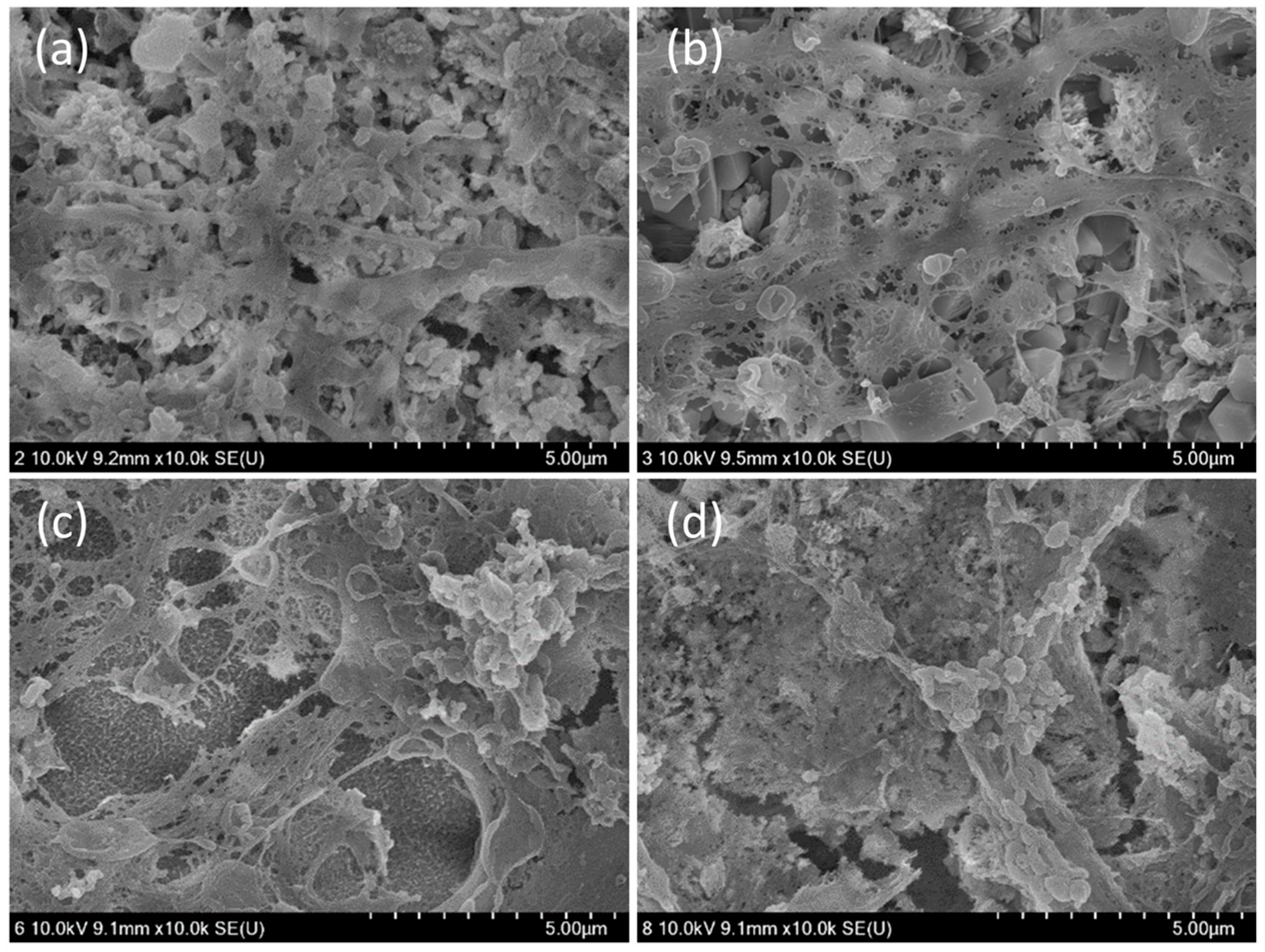
3.3. Cytocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, E.S.; Park, Y.B.; Kwon, Y.S.; Shon, W.J.; Lee, K.W.; Min, K.S. Physical properties and biocompatibility of an injectable calcium-silicate-based root canal sealer: In vitro and in vivo study. BMC Oral Health 2015, 15, 129. [Google Scholar] [CrossRef]
- Viola, N.V.; Tanomaru-Filho, M.; Cerri, P.S. MTA versus Portland cement: Review of literature. RSBO 2011, 8, 446–452. [Google Scholar]
- Ozlek, E.; Gündüz, H.; Akkol, E.; Neelakantan, P. Dentin moisture conditions strongly influence its interactions with bioactive root canal sealers. Restor. Dent. Endod. 2020, 45, e24. [Google Scholar] [CrossRef]
- Oh, H.; Kim, E.; Lee, S.; Park, S.; Chen, D.; Shin, S.J.; Kim, E.; Kim, S. Comparison of biocompatibility of calcium silicate-based sealers and epoxy resin-based sealer on human periodontal ligament stem cells. Materials 2020, 13, 5242. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; de Azevedo Queiroz, Í.O.; Oliveira, P.H.C.; Conti, L.C.; Azuma, M.M.; Oliveira, S.H.P.; Cintra, L.T.A. Cytotoxicity and biocompatibility of a new bioceramic endodontic sealer containing calcium hydroxide. Braz. Oral Res. 2019, 33, e042. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Jung, C.; Shin, D.H.; Cho, Y.B.; Song, M. Calcium silicate-based root canal sealers: A literature review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Lim, M.J.; Jang, H.J.; Yu, M.K.; Lee, K.W.; Min, K.S. Removal efficacy and cytotoxicity of a calcium hydroxide paste using N-2-methyl-pyrrolidone as a vehicle. Restor. Dent. Endod. 2017, 42, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J. Ancient and modern concretes: What is the real difference. Concr. Int. 1987, 9, 12–23. [Google Scholar]
- Lee, C.Y.; Lee, H.K.; Lee, K.M. Strength and microstructural characteristics of chemically activated fly ash-cement systems. Cem. Concr. Res. 2003, 33, 425–431. [Google Scholar] [CrossRef]
- Yu, M.K.; Kim, M.A.; Rosa, V.; Hwang, Y.C.; Del Fabbro, M.; Sohn, W.J.; Min, K.S. Role of extracellular DNA in Enterococcus faecalis biofilm formation and its susceptibility to sodium hypochlorite. J. Appl. Oral Sci. 2019, 27, e20180699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Zhang, C.F.; Chu, C.H.; Zhu, X.F. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int. J. Oral Sci. 2012, 4, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, A.; Jayasree, R.; Vidhya, S.; Mahalaxmi, S.; Sujatha, V.; Kumar, T.S.S. Comparative evaluation of micron- and nano-sized intracanal medicaments on penetration and fracture resistance of root dentin—An in vitro study. Int. J. Biol. Macromol. 2017, 104 Pt B, 1866–1873. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, Q.; Bai, D.; Liu, Y.; Chen, L.; Jin, S.; Wu, Y.; Duan, K. Potential use of dimethyl sulfoxide in treatment of infections caused by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 7159–7169. [Google Scholar] [PubMed]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Subtractive protein profiling of Salmonella typhimurium biofilm treated with DMSO. Proteins 2017, 36, 286–298. [Google Scholar] [CrossRef]
- Lindblad, R.M.; Lassila, L.V.; Vallittu, P.K.; Tjäderhane, L. The effect of chlorhexidine and dimethyl sulfoxide on long-term sealing ability of two calcium silicate cements in root canal. Dent. Mater. 2021, 37, 328–335. [Google Scholar] [CrossRef]
- Lindblad, R.M.; Lassila, L.V.; Vallittu, P.K.; Tjäderhane, L. The effect of chlorhexidine and dimethyl sulfoxide on long- term microleakage of two different sealers in root canals. Eur. Endod. J. 2019, 4, 38–44. [Google Scholar] [CrossRef]
- Mestieri, L.B.; Zaccara, I.M.; Pinheiro, L.S.; Barletta, F.B.; Kopper, P.M.P.; Grecca, F.S. Cytocompatibility and cell proliferation evaluation of calcium phosphate-based root canal sealers. Restor. Dent. Endod. 2020, 45, e2. [Google Scholar] [CrossRef]
- Collado-Gonzalez, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Ortolani-Seltenerich, P.S.; Lozano, A.; Forner, L.; Llena, C.; Rodríguesz-Lozano, F.J. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int. Endod. J. 2017, 50, 875–884. [Google Scholar] [CrossRef]
- Rodríguesz-Lozano, F.J.; García-Bernal, D.; Oñate-Sánchez, R.E.; Ortolani-Seltenerich, P.S.; Forner, L.; Moraleda, J.M. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int. Endod. J. 2017, 50, 67–76. [Google Scholar] [CrossRef]
- Seo, D.G.; Lee, D.; Kim, Y.M.; Song, D.; Kim, S.Y. Biocompatibility and mineralization activity of three calcium silicate-based root canal sealers compared to conventional resin-based sealer in human dental pulp stem cells. Materials 2019, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Prot. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-A.; Rosa, V.; Neelakantan, P.; Hwang, Y.-C.; Min, K.-S. Characterization, Antimicrobial Effects, and Cytocompatibility of a Root Canal Sealer Produced by Pozzolan Reaction between Calcium Hydroxide and Silica. Materials 2021, 14, 2863. https://doi.org/10.3390/ma14112863
Kim M-A, Rosa V, Neelakantan P, Hwang Y-C, Min K-S. Characterization, Antimicrobial Effects, and Cytocompatibility of a Root Canal Sealer Produced by Pozzolan Reaction between Calcium Hydroxide and Silica. Materials. 2021; 14(11):2863. https://doi.org/10.3390/ma14112863
Chicago/Turabian StyleKim, Mi-Ah, Vinicius Rosa, Prasanna Neelakantan, Yun-Chan Hwang, and Kyung-San Min. 2021. "Characterization, Antimicrobial Effects, and Cytocompatibility of a Root Canal Sealer Produced by Pozzolan Reaction between Calcium Hydroxide and Silica" Materials 14, no. 11: 2863. https://doi.org/10.3390/ma14112863
APA StyleKim, M.-A., Rosa, V., Neelakantan, P., Hwang, Y.-C., & Min, K.-S. (2021). Characterization, Antimicrobial Effects, and Cytocompatibility of a Root Canal Sealer Produced by Pozzolan Reaction between Calcium Hydroxide and Silica. Materials, 14(11), 2863. https://doi.org/10.3390/ma14112863








