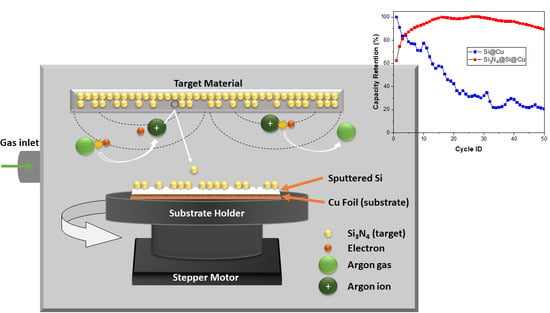
Fabrication of Si3N4@Si@Cu Thin Films by RF Sputtering as High Energy Anode Material for Li-Ion Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Thin Film Deposition
2.3. Characterization Techniques
2.4. Coin Cell Preparation
2.5. Electrochemical Testing
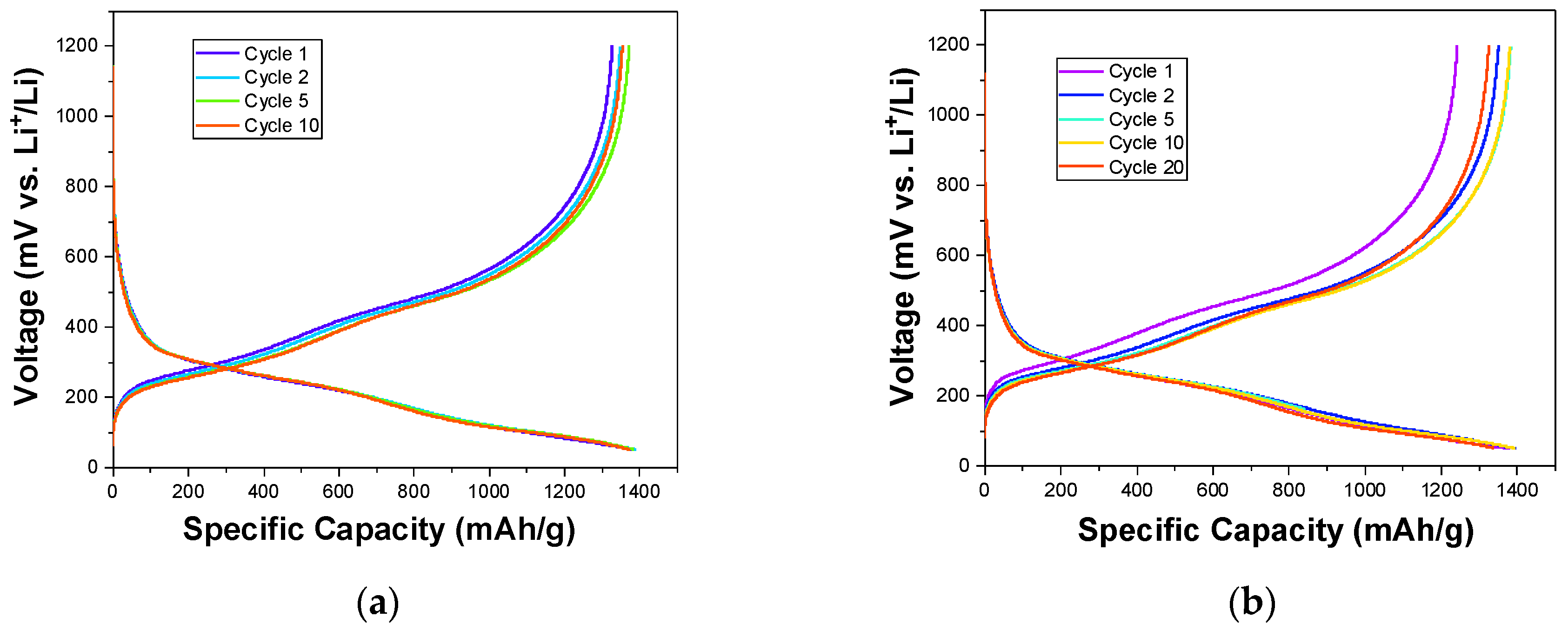
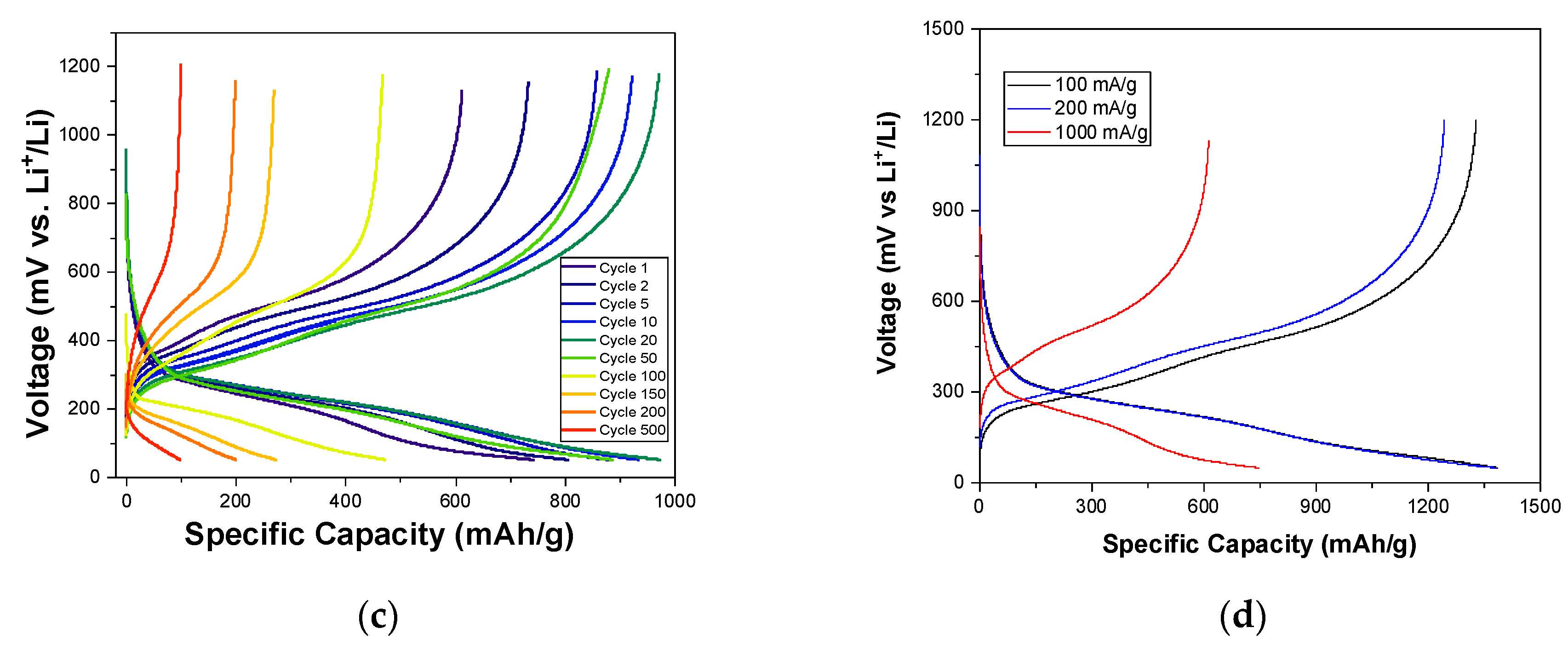
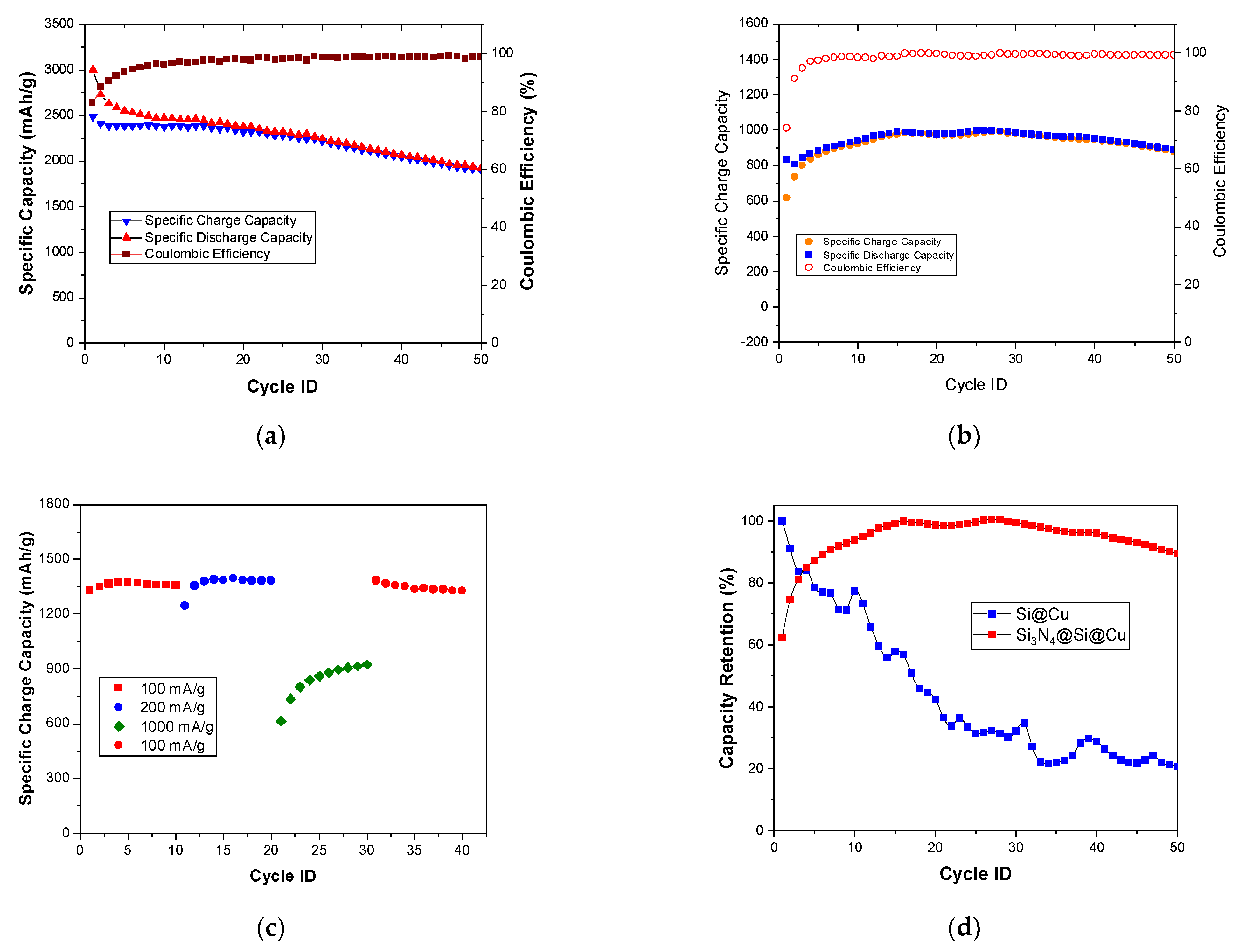
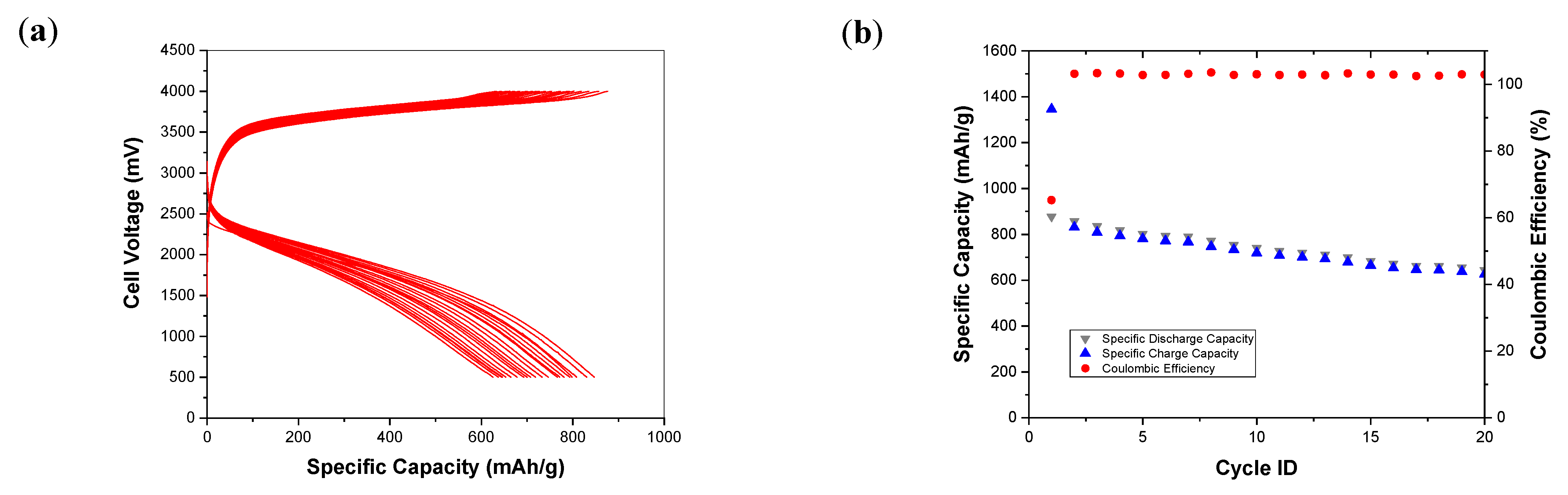
3. Results and Discussion
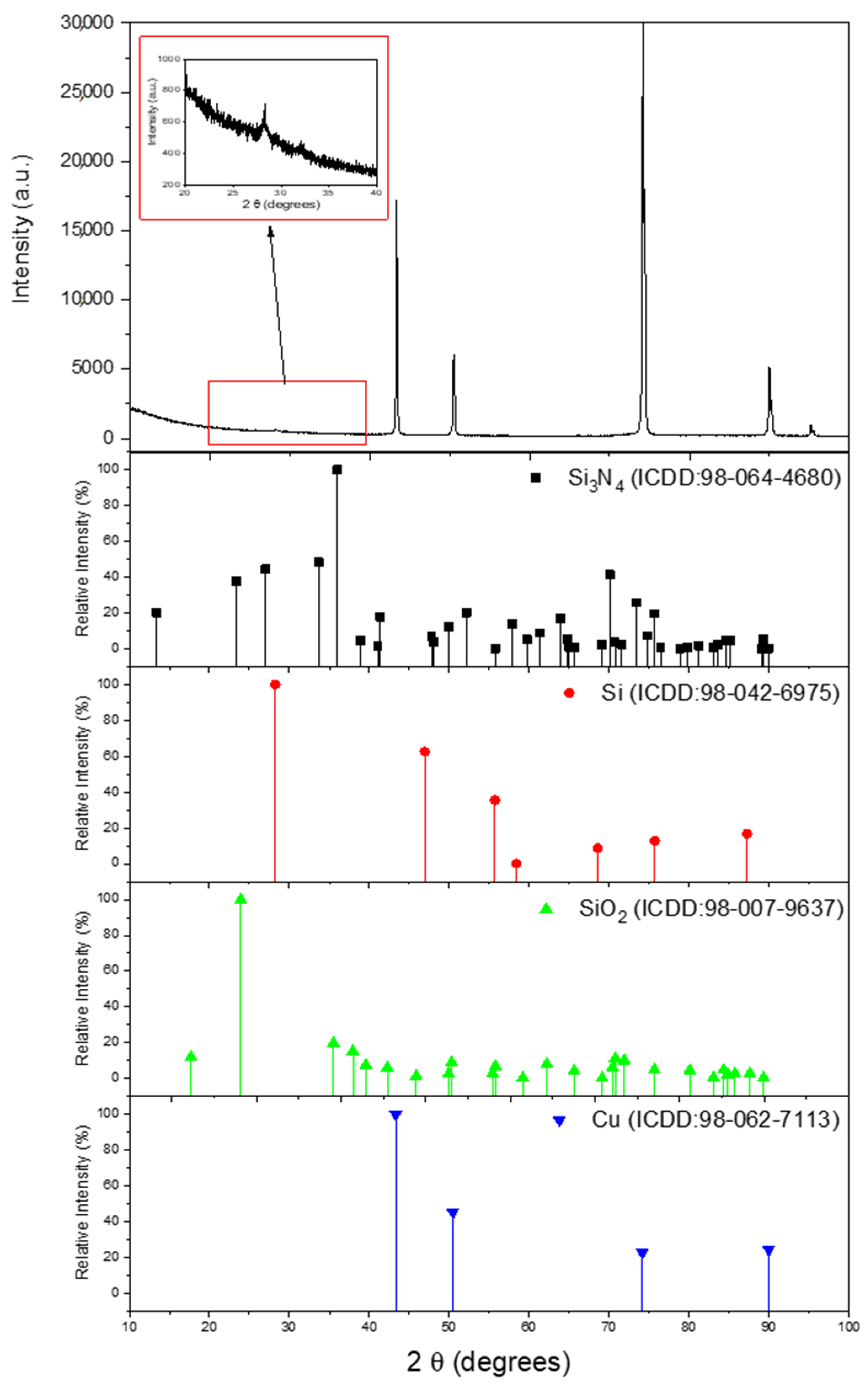
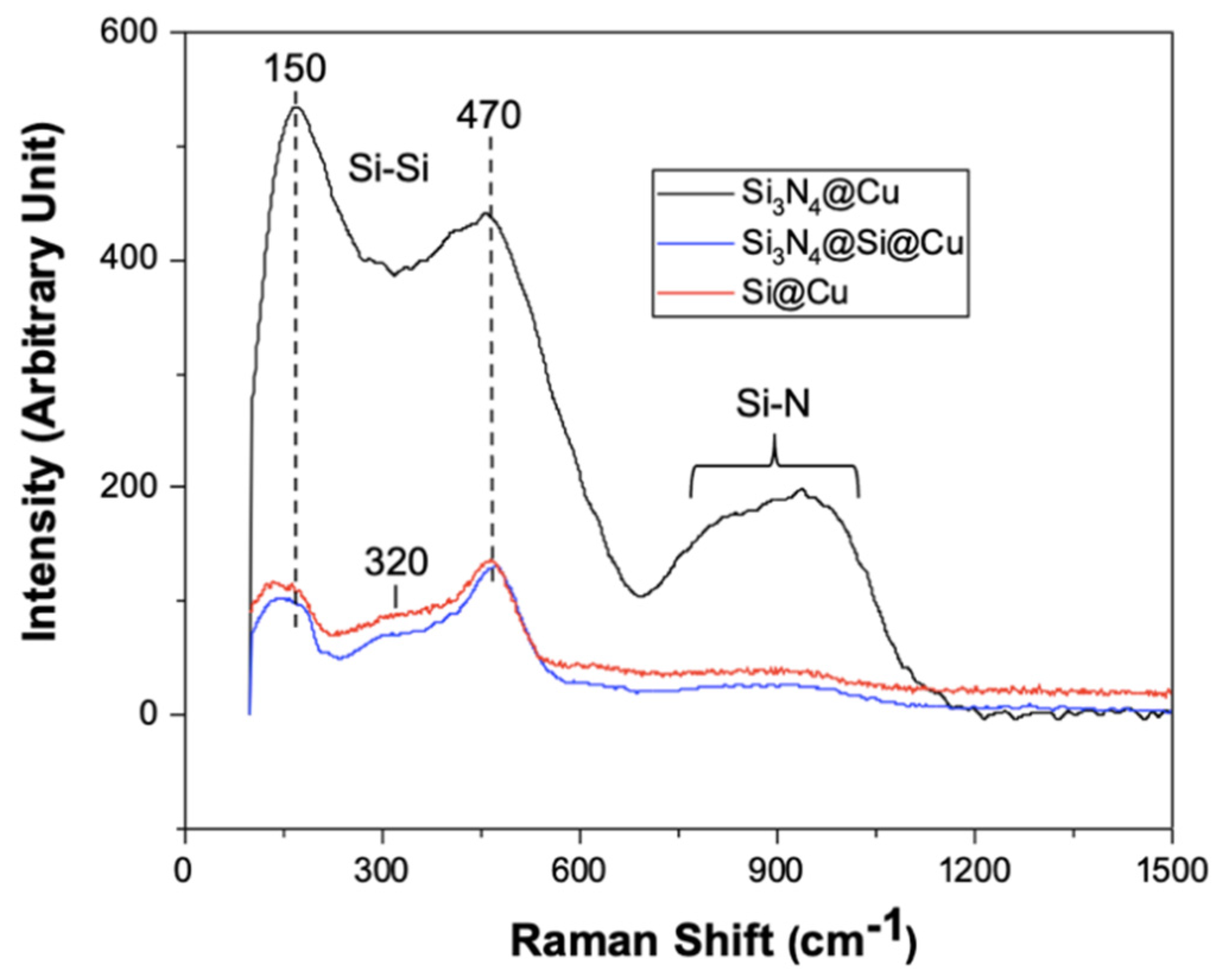
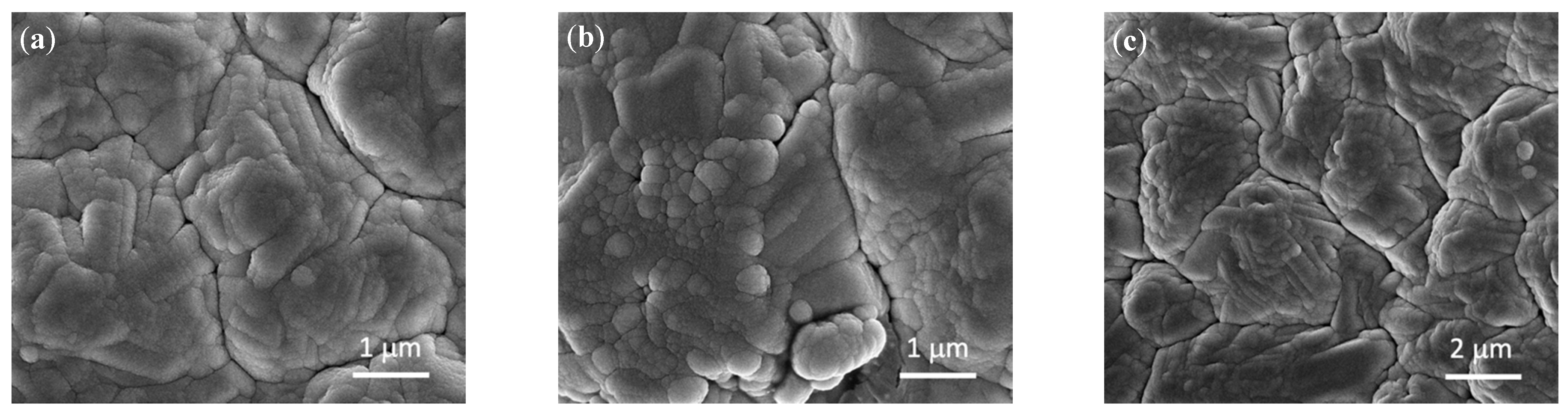
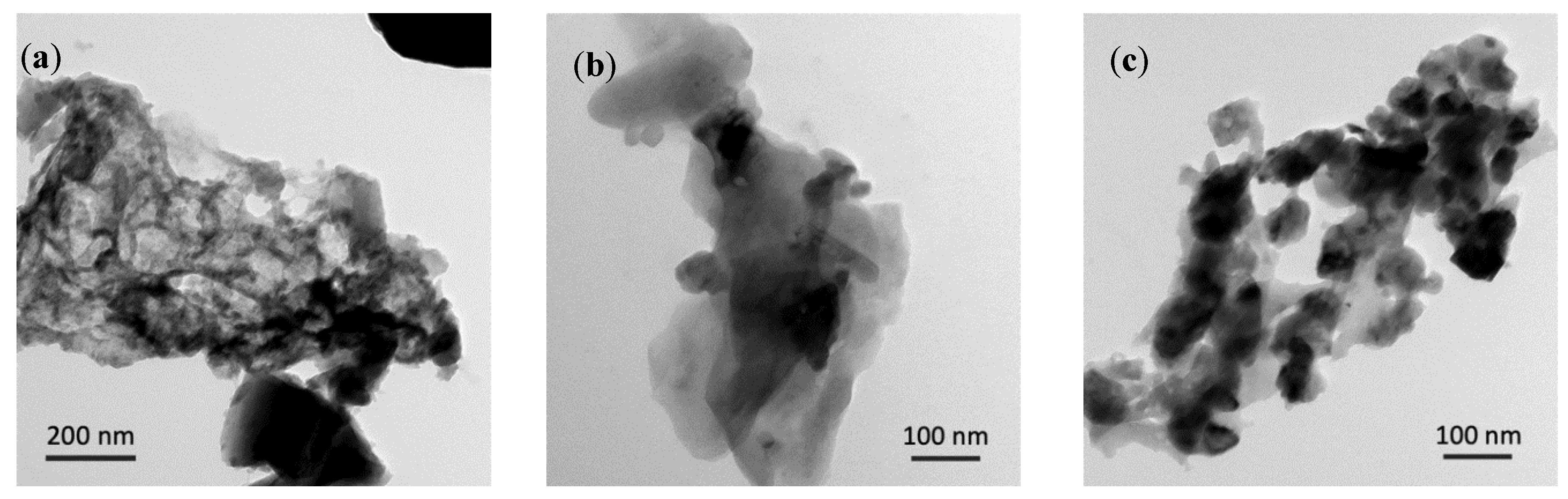
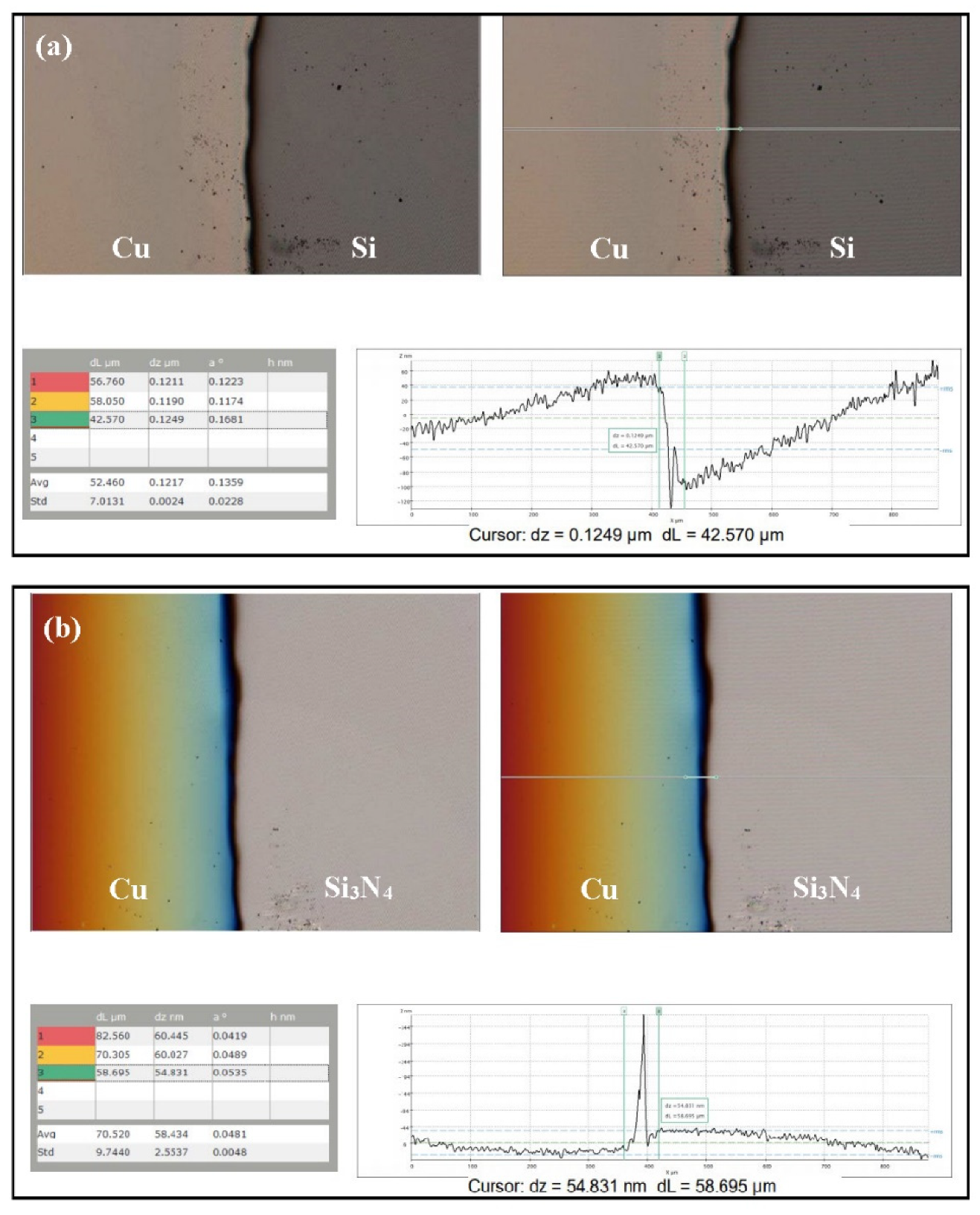
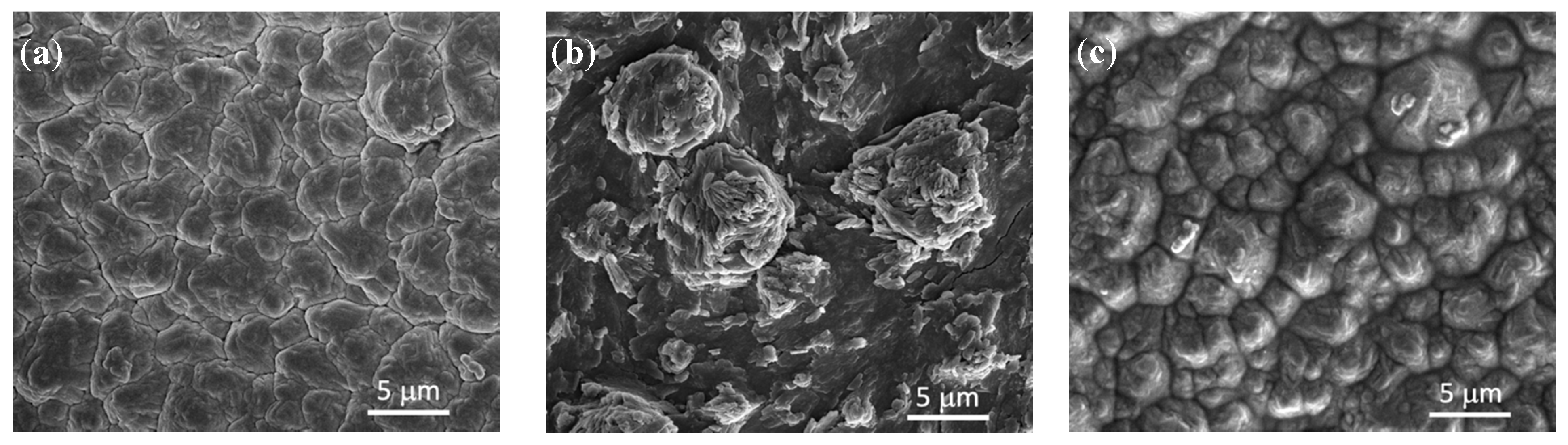
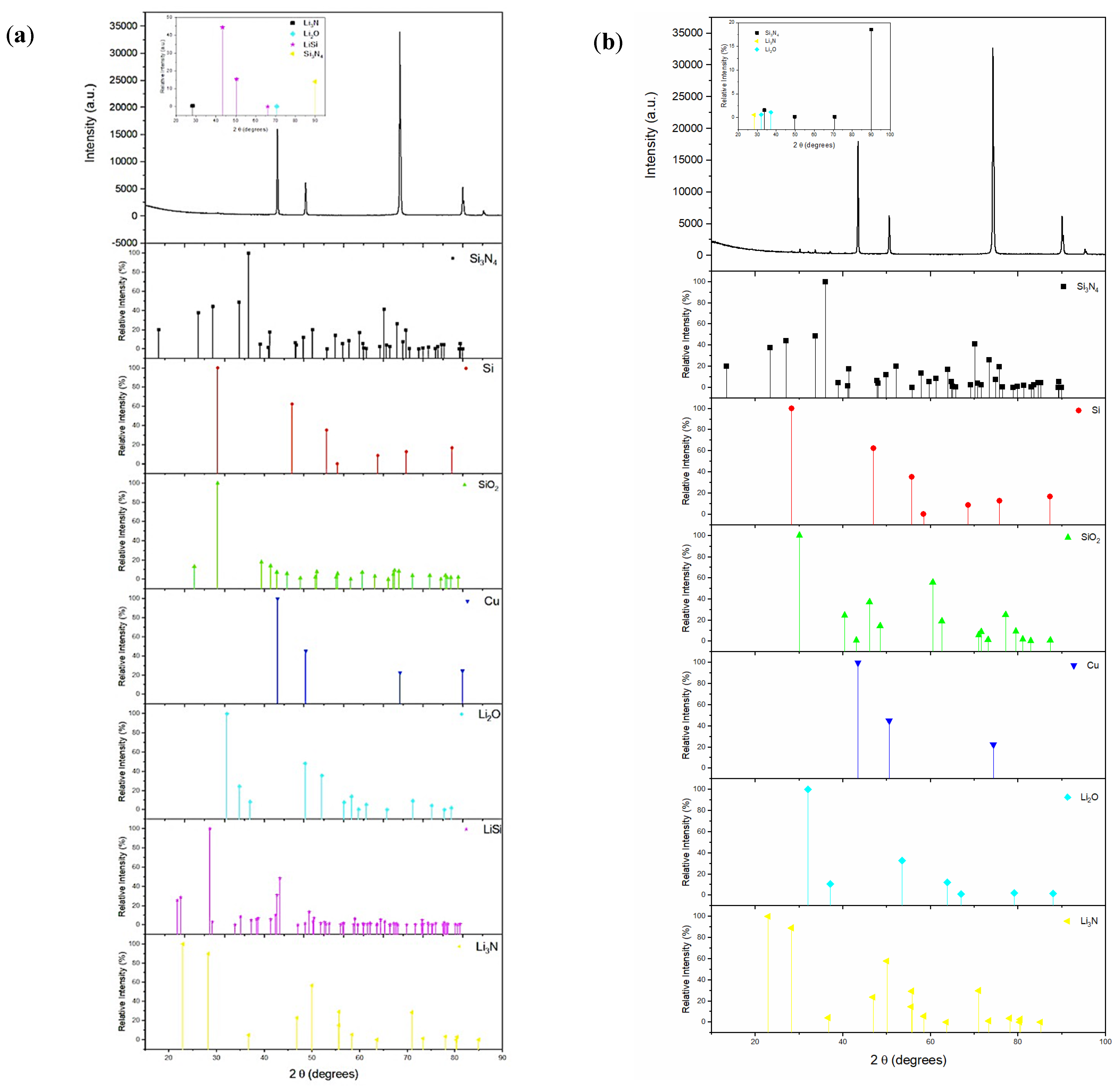
3.1. Characterization of As-Prepared Anode Materials
3.2. Electrochemical Tests
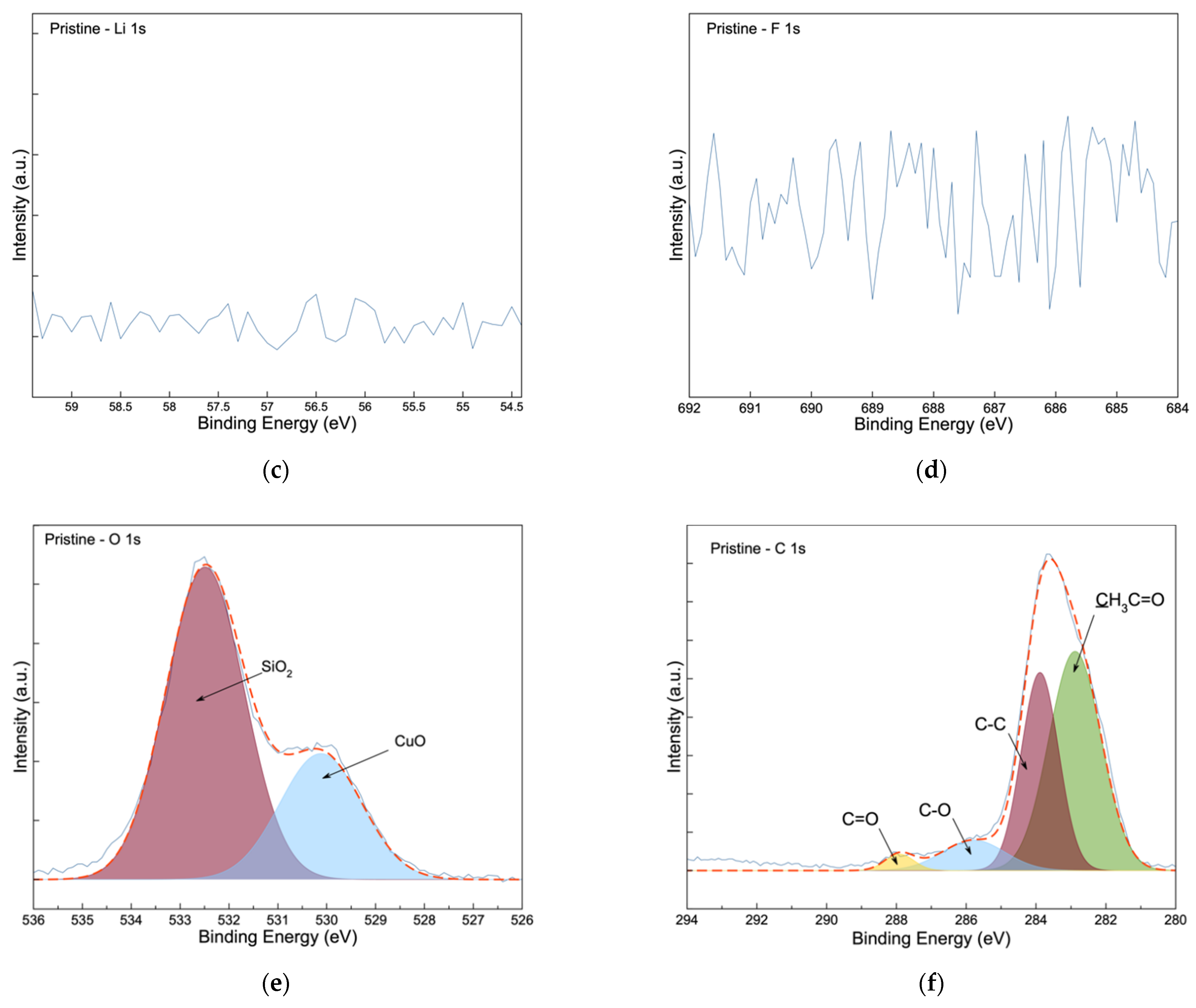
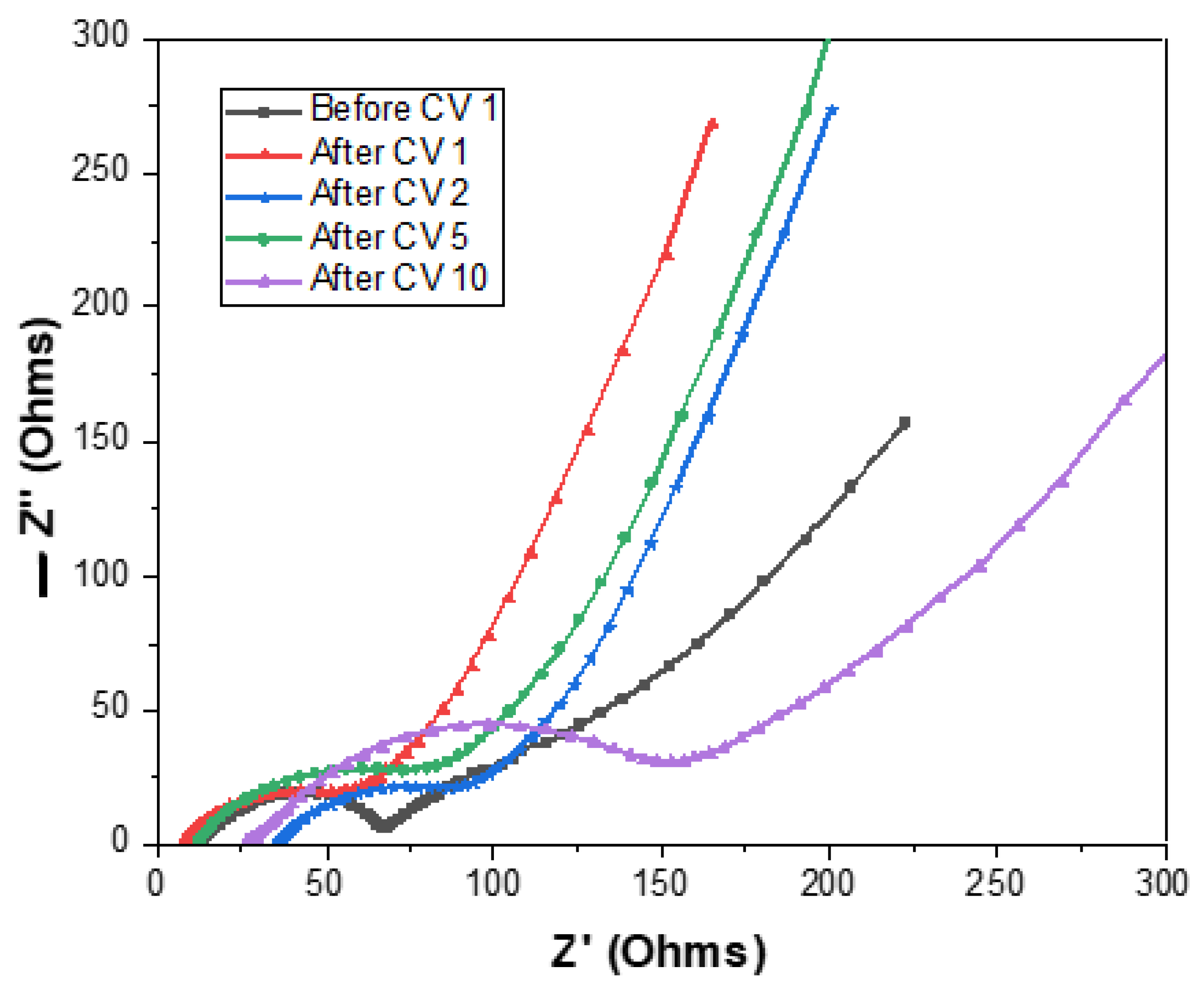
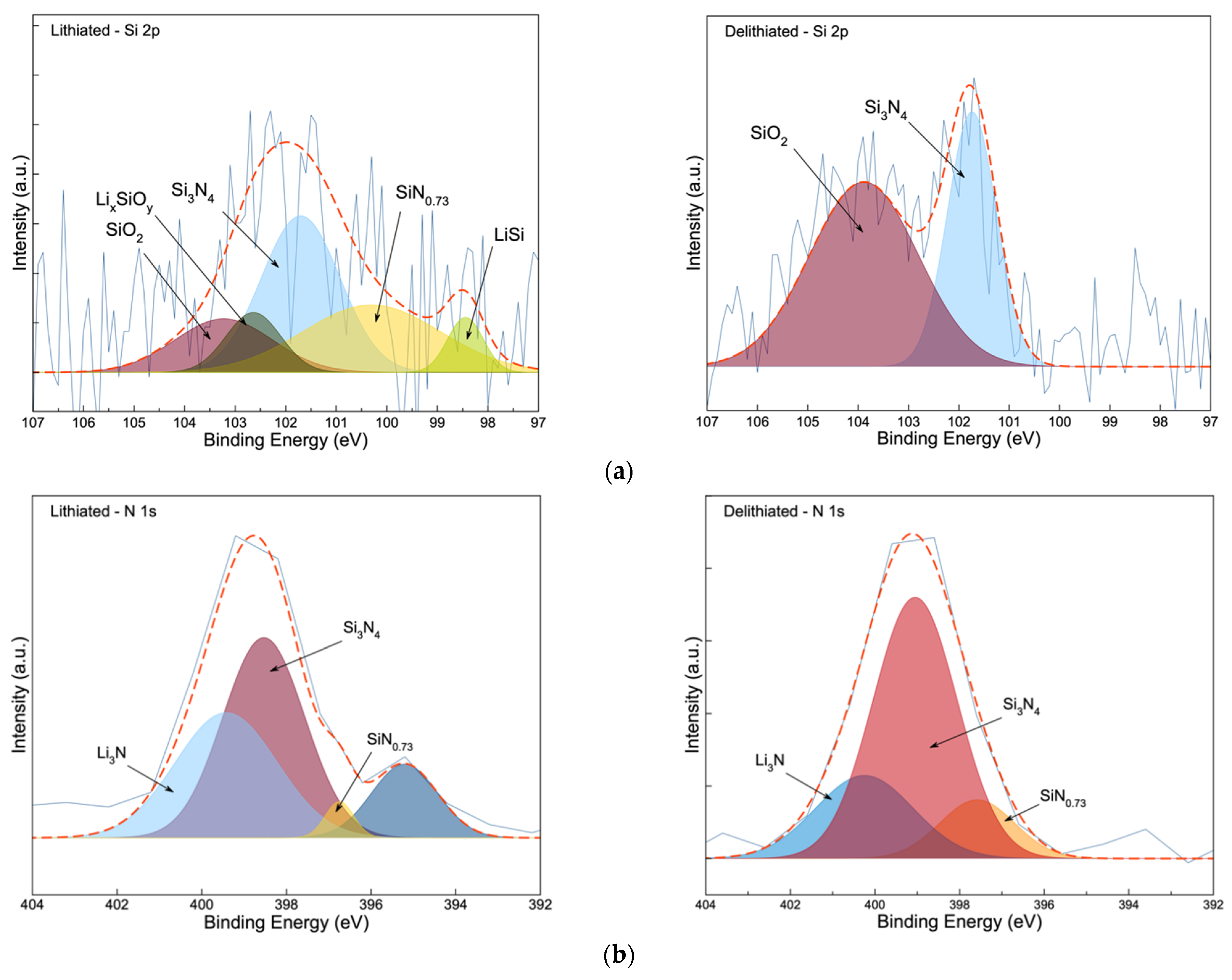
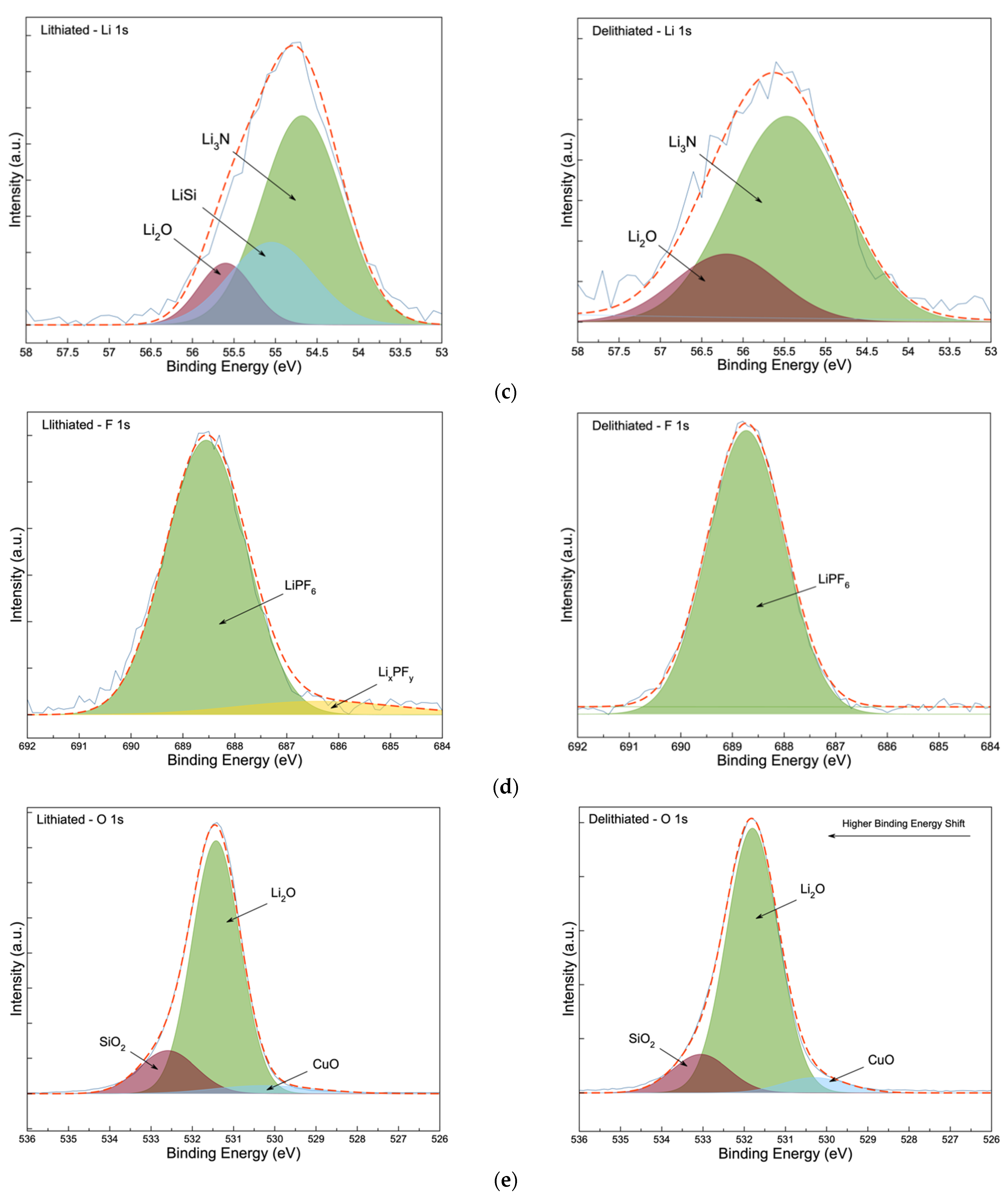
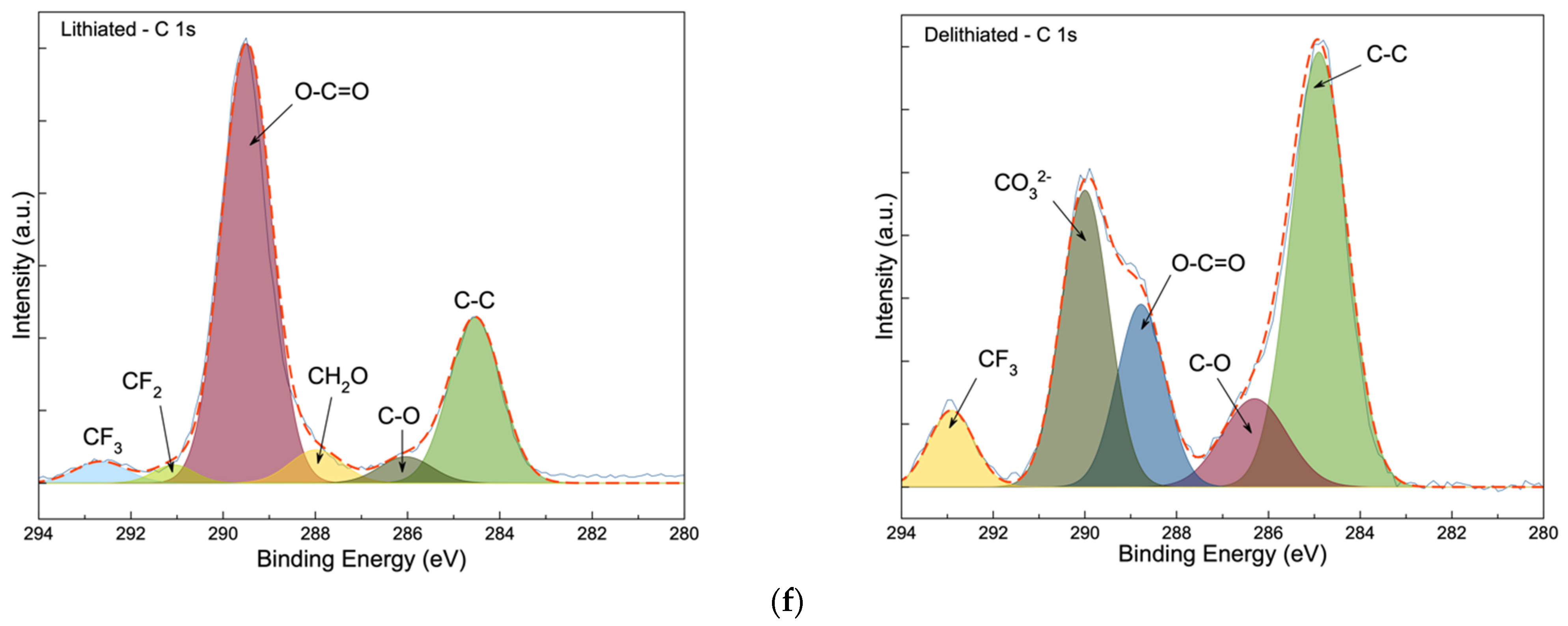
3.3. Post-Mortem Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.B. Intercalation electrodes. In Materials for Advanced Batteries; Murphy, D.W., Broadhead, J., Steele, B.C.H., Eds.; Springer: Boston, MA, USA, 1980; pp. 145–161. [Google Scholar]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. Lixcoo2 (0 < x <−1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David WI, F.; Bruce, P.G.; Goodenough, J.B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 2001, 1, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, C.; Kim, N.; Hwang, J.-Y.; Sun, Y.-K. Rational design of silicon-based composites for high-energy storage devices. J. Mater. Chem. A 2016, 4, 5366–5384. [Google Scholar] [CrossRef]
- Teki, R.; Datta, M.K.; Krishnan, R.; Parker, T.C.; Lu, T.-M.; Kumta, P.N.; Koratkar, N. Nanostructured Silicon Anodes for Lithium Ion Rechargeable Batteries. Small 2009, 5, 2236–2242. [Google Scholar] [CrossRef]
- Yim, C.-H.; Courtel, F.M.; Abu-Lebdeh, Y. A high capacity silicon–graphite composite as anode for lithium-ion batteries using low content amorphous silicon and compatible binders. J. Mater. Chem. A 2013, 1, 8234–8243. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2007, 3, 31–35. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Dimov, N.; Ogumi, Z. Carbon-Coated Si as a Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2002, 149, A1598–A1603. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Ma, Y.; Chen, Y. Latest development of nanostructured Si/C materials for lithium anode studies and applications. Energy Storage Mater. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Goldman, J.L.; Long, B.R.; Gewirth, A.A.; Nuzzo, R.G. Strain Anisotropies and Self-Limiting Capacities in Single-Crystalline 3D Silicon Microstructures: Models for High Energy Density Lithium-Ion Battery Anodes. Adv. Funct. Mater. 2011, 21, 2412–2422. [Google Scholar] [CrossRef]
- Maranchi, J.P.; Hepp, A.F.; Evans, A.G.; Nuhfer, N.T.; Kumta, P.N. Interfacial Properties of the a-Si/Cu:Active–Inactive Thin-Film Anode System for Lithium-Ion Batteries. J. Electrochem. Soc. 2006, 153, A1246–A1253. [Google Scholar] [CrossRef]
- Wu, X.-L.; Guo, Y.-G.; Wan, L.-J. Rational Design of Anode Materials Based on Group IVA Elements (Si, Ge, and Sn) for Lithium-Ion Batteries. Chem. Asian J. 2013, 8, 1948–1958. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Q.; Fan, Y.; Wang, X.; Susantyoko, R.A. Soft silicon anodes for lithium ion batteries. Energy Environ. Sci. 2014, 7, 2261–2268. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.J.; Li, X.; Xie, W.; Wang, Q.; Liu, Q.; Fu, Y.; He, D. Group IVA Element (Si, Ge, Sn)-Based Alloying/Dealloying Anodes as Negative Electrodes for Full-Cell Lithium-Ion Batteries. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Yushin, G. High-Capacity Anode Materials for Lithium-Ion Batteries: Choice of Elements and Structures for Active Particles. Part. Part. Syst. Charact. 2014, 31, 317–336. [Google Scholar] [CrossRef]
- Dimov, N.; Kugino, S.; Yoshio, M. Carbon-coated silicon as anode material for lithium ion batteries: Advantages and limitations. Electrochim. Acta 2003, 48, 1579–1587. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Kowolik, K.; Srinivasan, V. Increased cycling efficiency and rate capability of copper-coated silicon anodes in lithium-ion batteries. J. Power Sources 2011, 196, 393–398. [Google Scholar] [CrossRef]
- Suzuki, N.; Cervera, R.B.; Ohnishi, T.; Takada, K. Silicon nitride thin film electrode for lithium-ion batteries. J. Power Sources 2013, 231, 186–189. [Google Scholar] [CrossRef]
- Ulvestad, A.; Andersen, H.F.; Jensen, I.J.; Mongstad, T.T.; Mæhlen, J.P.; Prytz, Ø.; Kirkengen, M. Substoichiometric Silicon Nitride—An Anode Material for Li-ion Batteries Promising High Stability and High Capacity. Sci. Rep. 2018, 8, 8634. [Google Scholar] [CrossRef]
- De Guzman, R.C.; Yang, J.; Cheng, M.M.-C.; Salley, S.O.; Ng, K.S. High capacity silicon nitride-based composite anodes for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14577–14584. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, J.W.; Sung, Y.-E.; Oh, S.M. Failure Modes of Silicon Powder Negative Electrode in Lithium Secondary Batteries. Electrochem. Solid State Lett. 2004, 7, A306. [Google Scholar] [CrossRef]
- Datta, M.K.; Maranchi, J.; Chung, S.J.; Epur, R.; Kadakia, K.; Jampani, P.; Kumta, P.N. Amorphous silicon–carbon based nano-scale thin film anode materials for lithium ion batteries. Electrochim. Acta 2011, 56, 4717–4723. [Google Scholar] [CrossRef]
- Hong, W.-E.; Ro, J.-S. Kinetics of solid phase crystallization of amorphous silicon analyzed by Raman spectroscopy. J. Appl. Phys. 2013, 114, 073511. [Google Scholar] [CrossRef]
- Volodin, V.A.; Koshelev, D.I. Quantitative analysis of hydrogen in amorphous silicon using Raman scattering spectroscopy. J. Raman Spectrosc. 2013, 44, 1760–1764. [Google Scholar] [CrossRef]
- Bandet, J.; Despax, B.; Caumont, M. Nitrogen bonding environments and local order in hydrogenated amorphous silicon nitride films studied by Raman spectroscopy. J. Appl. Phys. 1999, 85, 7899–7904. [Google Scholar] [CrossRef]
- Liu, R.; Canonico, M. Applications of UV-Raman Spectroscopy to Microelectronic Materials and Devices. AIP Conf. Proc. 2003, 683, 738–743. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chang, C.-C.; Duh, J.-G. Silicon nitride coated silicon thin film on three dimensions current collector for lithium ion battery anode. J. Power Sources 2016, 325, 64–70. [Google Scholar] [CrossRef]
- Jerliu, B.; Hüger, E.; Dörrer, L.; Seidlhofer, B.K.; Steitz, R.; Horisberge, M.; Schmidt, H. Lithium insertion into silicon electrodes studied by cyclic voltammetry and operando neutron reflectometry. Phys. Chem. Chem. Phys. 2018, 20, 23480–23491. [Google Scholar] [CrossRef]
- Salihoglu, O.; Kahlout, Y.E. Doped Silicon Nanowires for Lithium Ion Battery Anodes. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Korake, P.V.; Gaikwad, A.G. Capture of carbon dioxide over porous solid adsorbents lithium silicate, lithium aluminate and magnesium aluminate at pre-combustion temperatures. Front. Chem. Sci. Eng. 2010, 5, 215–226. [Google Scholar] [CrossRef]
- Hashemi, A.; Bahari, A. Structural and dielectric characteristic of povidone–silica nanocomposite films on the Si (n) substrate. Appl. Phys. A 2017, 123, 535. [Google Scholar] [CrossRef]
- Wood, K.N.; Teeter, G. XPS on Li-Battery-Related Compounds: Analysis of Inorganic SEI Phases and a Methodology for Charge Correction. ACS Appl. Energy Mater. 2018, 1, 4493–4504. [Google Scholar] [CrossRef]
- Somerville, L.; Bareño, J.; Jennings, P.; McGordon, A.; Lyness, C.; Bloom, I. The Effect of Pre-Analysis Washing on the Surface Film of Graphite Electrodes. Electrochim. Acta 2016, 206, 70–76. [Google Scholar] [CrossRef]
- Dan, Z.; Yang, Y.; Qin, F.; Wang, H.; Chang, H. Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange. Materials 2018, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S.; Thoss, F.; Zier, M.; Hoffmann, M.; Jaumann, T.; Herklotz, M.; Nikolowski, K.; Scheiba, F.; Kohl, M.; Giebeler, L.; et al. Binding Energy Referencing for XPS in Alkali Metal-Based Battery Materials Research (II): Application to Complex Composite Electrodes. Batteries 2018, 4, 36. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merabet, H.; De Luna, Y.; Mohamed, K.; Bensalah, N. Fabrication of Si3N4@Si@Cu Thin Films by RF Sputtering as High Energy Anode Material for Li-Ion Batteries. Materials 2021, 14, 2824. https://doi.org/10.3390/ma14112824
Merabet H, De Luna Y, Mohamed K, Bensalah N. Fabrication of Si3N4@Si@Cu Thin Films by RF Sputtering as High Energy Anode Material for Li-Ion Batteries. Materials. 2021; 14(11):2824. https://doi.org/10.3390/ma14112824
Chicago/Turabian StyleMerabet, Hocine, Yannis De Luna, Khadiga Mohamed, and Nasr Bensalah. 2021. "Fabrication of Si3N4@Si@Cu Thin Films by RF Sputtering as High Energy Anode Material for Li-Ion Batteries" Materials 14, no. 11: 2824. https://doi.org/10.3390/ma14112824
APA StyleMerabet, H., De Luna, Y., Mohamed, K., & Bensalah, N. (2021). Fabrication of Si3N4@Si@Cu Thin Films by RF Sputtering as High Energy Anode Material for Li-Ion Batteries. Materials, 14(11), 2824. https://doi.org/10.3390/ma14112824