Synthesis of Transition Metal Complexes and Their Effects on Combustion Properties of Semi-Rigid Polyvinyl Chloride
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Transition Metal Ion Complexes
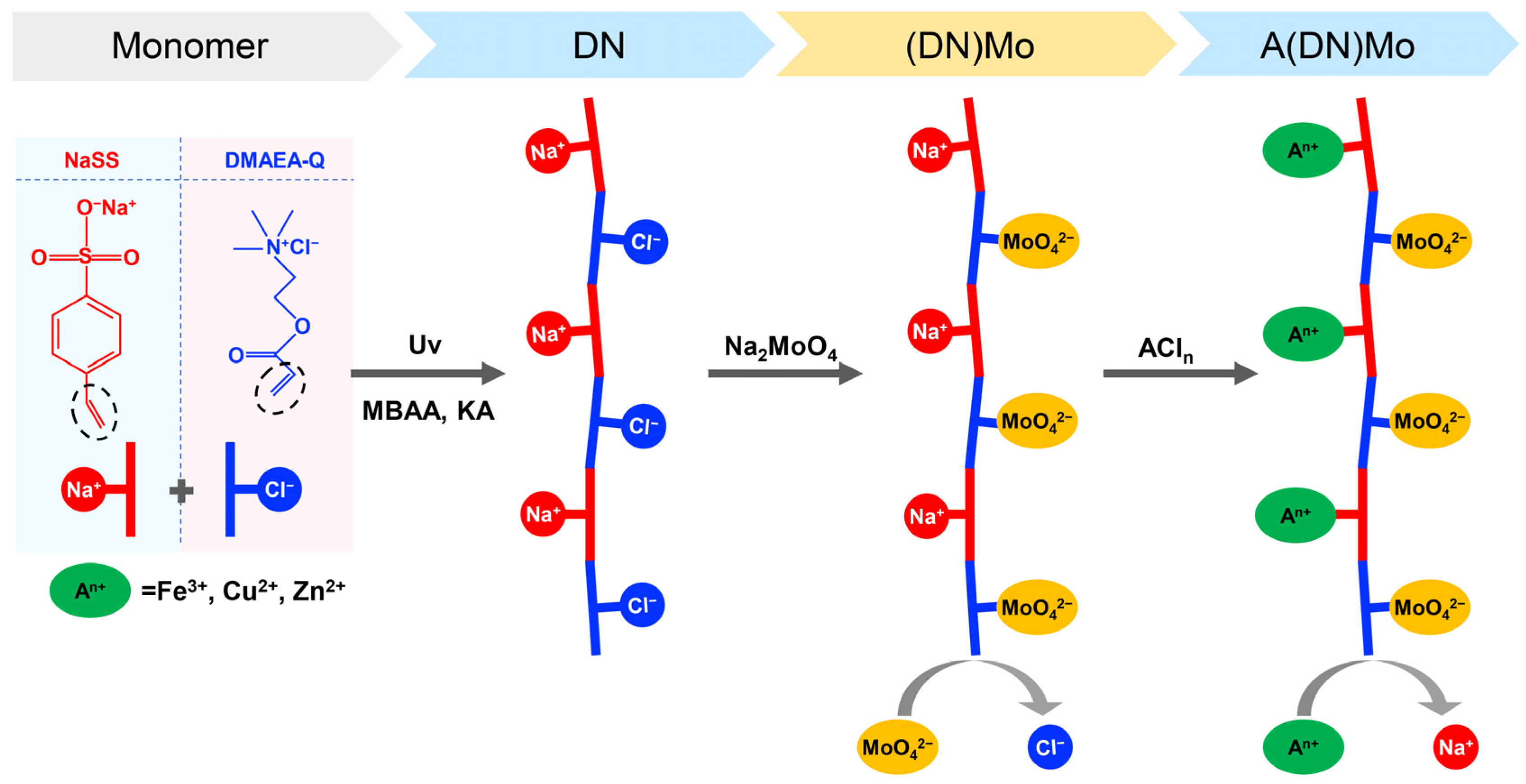
2.2.1. Synthesis of an Amphoteric Polymer (DN)
2.2.2. Synthesis of Molybdate Ion Complex ((DN)Mo)
2.2.3. Synthesis of Transition Metal Complexes (A(DN)Mo)
2.3. Preparation of PVC Composites
2.4. Characterization
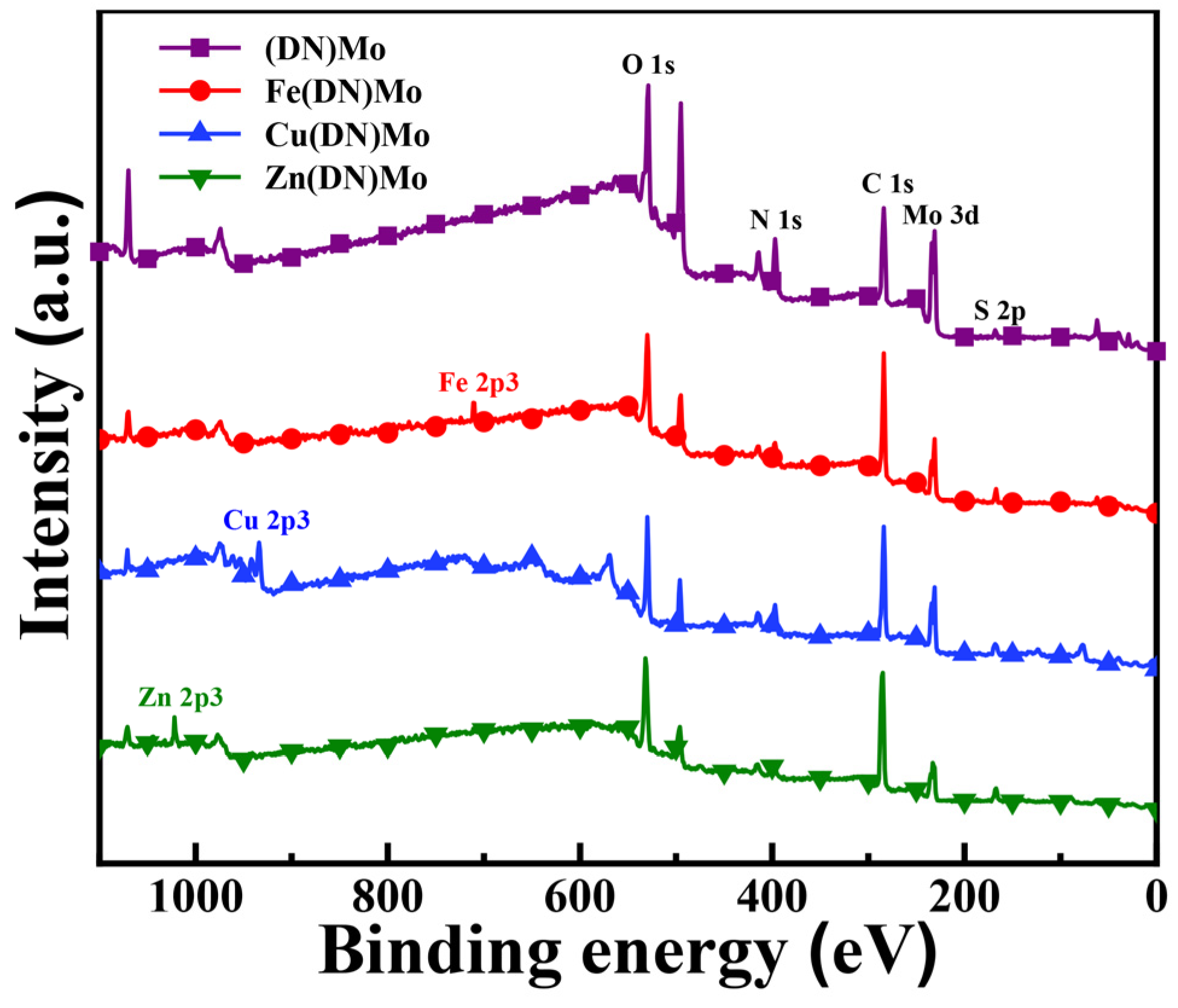
2.4.1. X-ray Photoelectron Spectroscopy Test
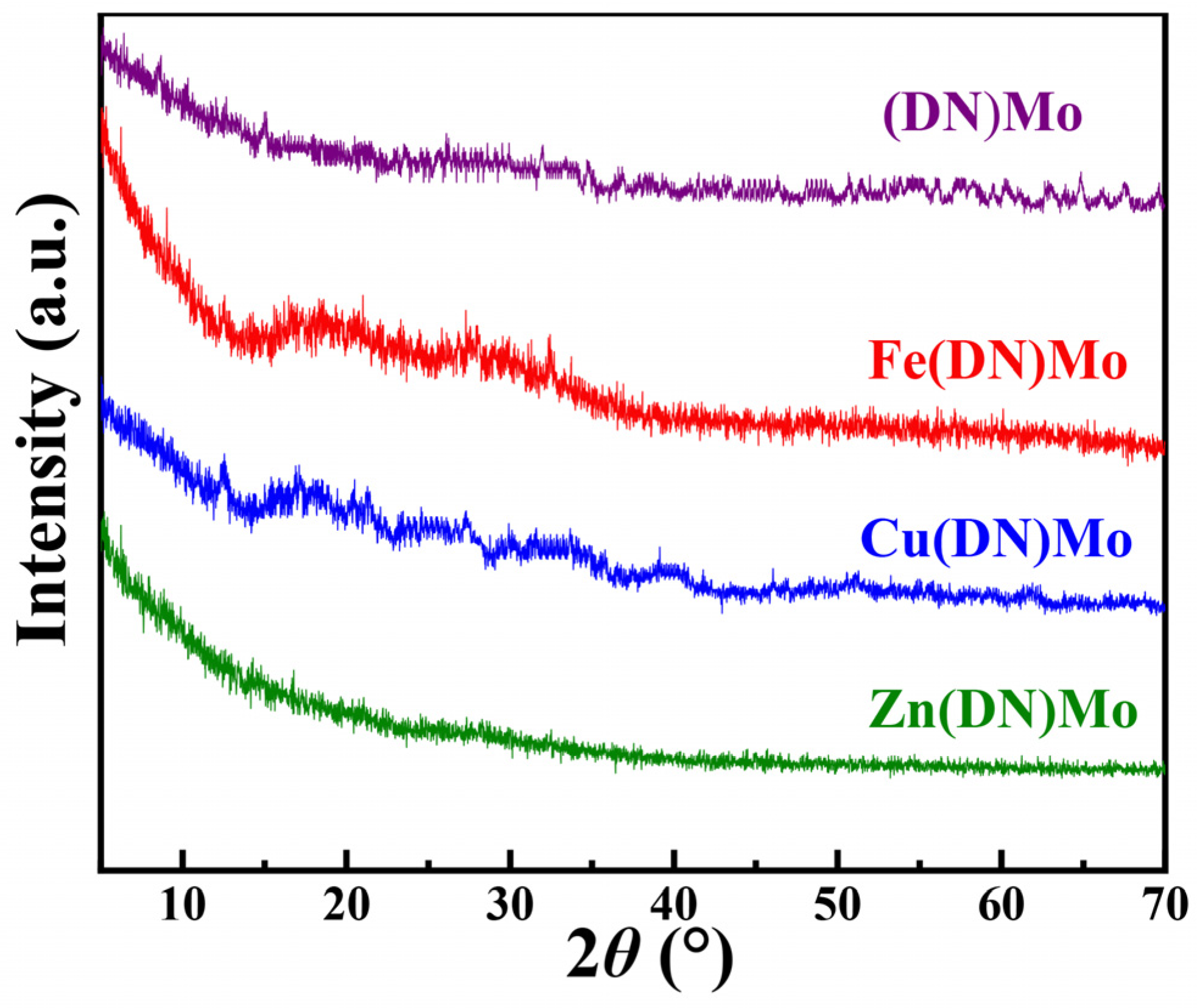
2.4.2. X-ray Diffraction Test
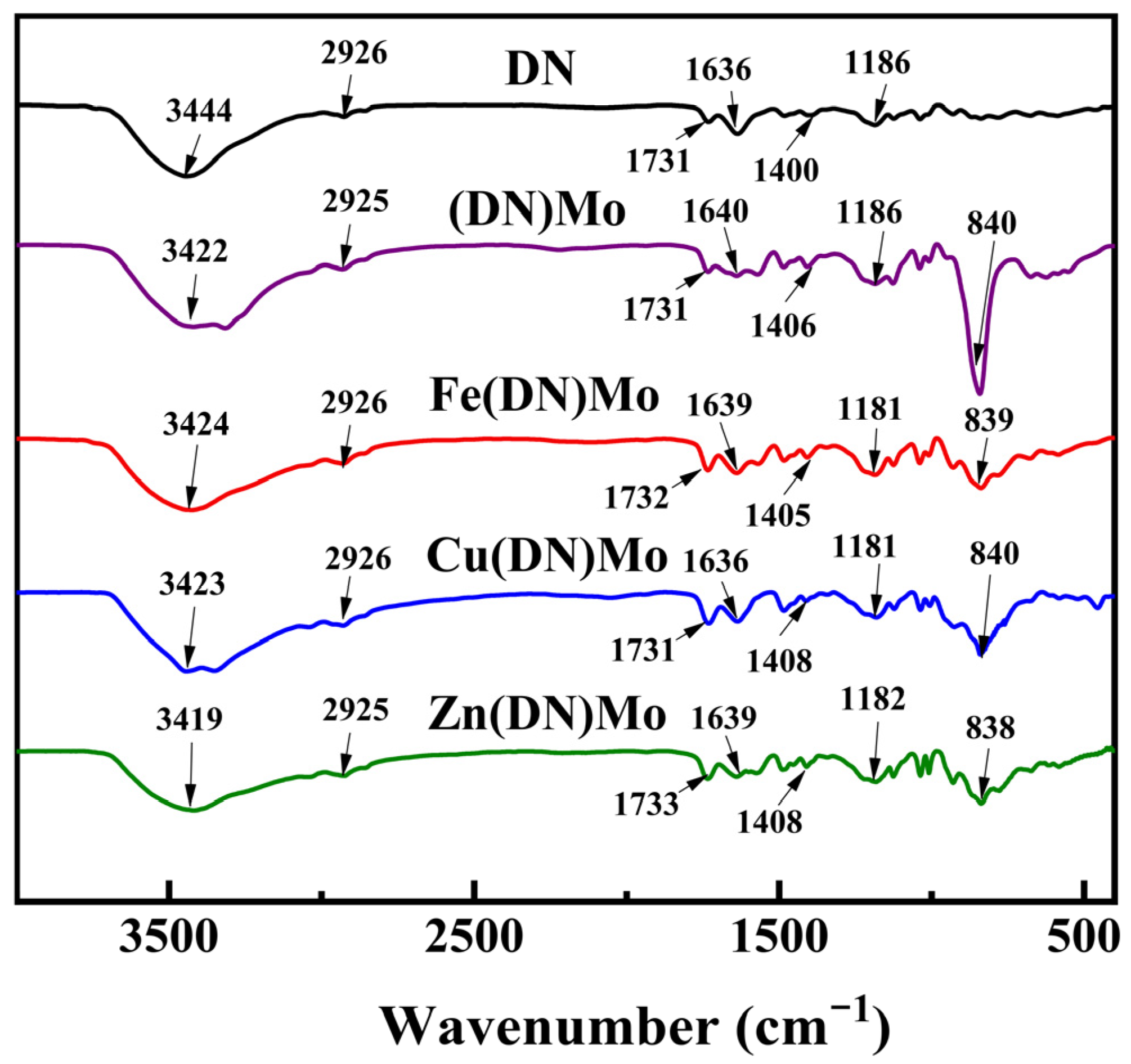
2.4.3. Fourier Transform Infrared Spectroscopy Test
2.4.4. Thermogravimetric Analyses
2.4.5. Scanning Electron Microscopy
2.4.6. Smoke Density Test
2.4.7. Limiting Oxygen Index
2.4.8. Microcalorimetry Test
2.4.9. Mechanical Properties
3. Results and Discussion
3.1. Characterization of Flame Retardants and Smoke Suppressants
3.2. Characterization of Smoke Density for PVC Composites
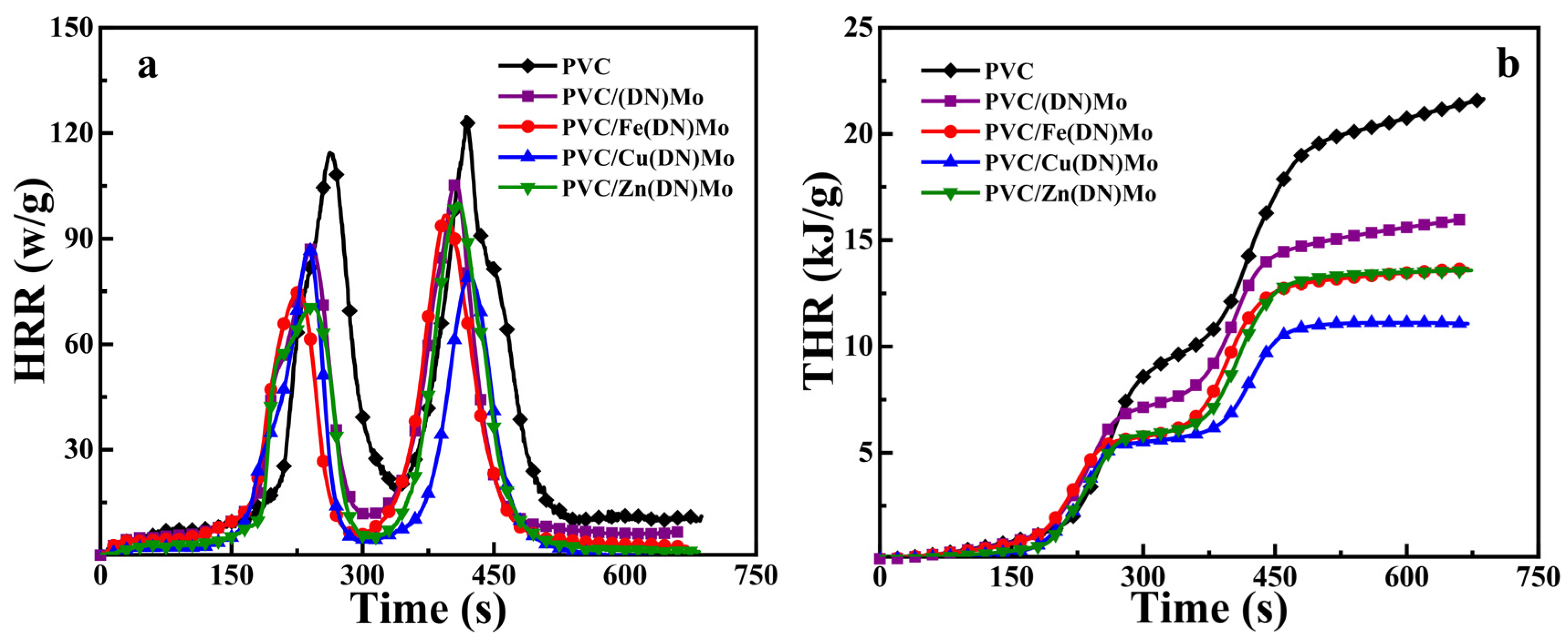
3.3. Characterization of Flame Retardancy for PVC Composites
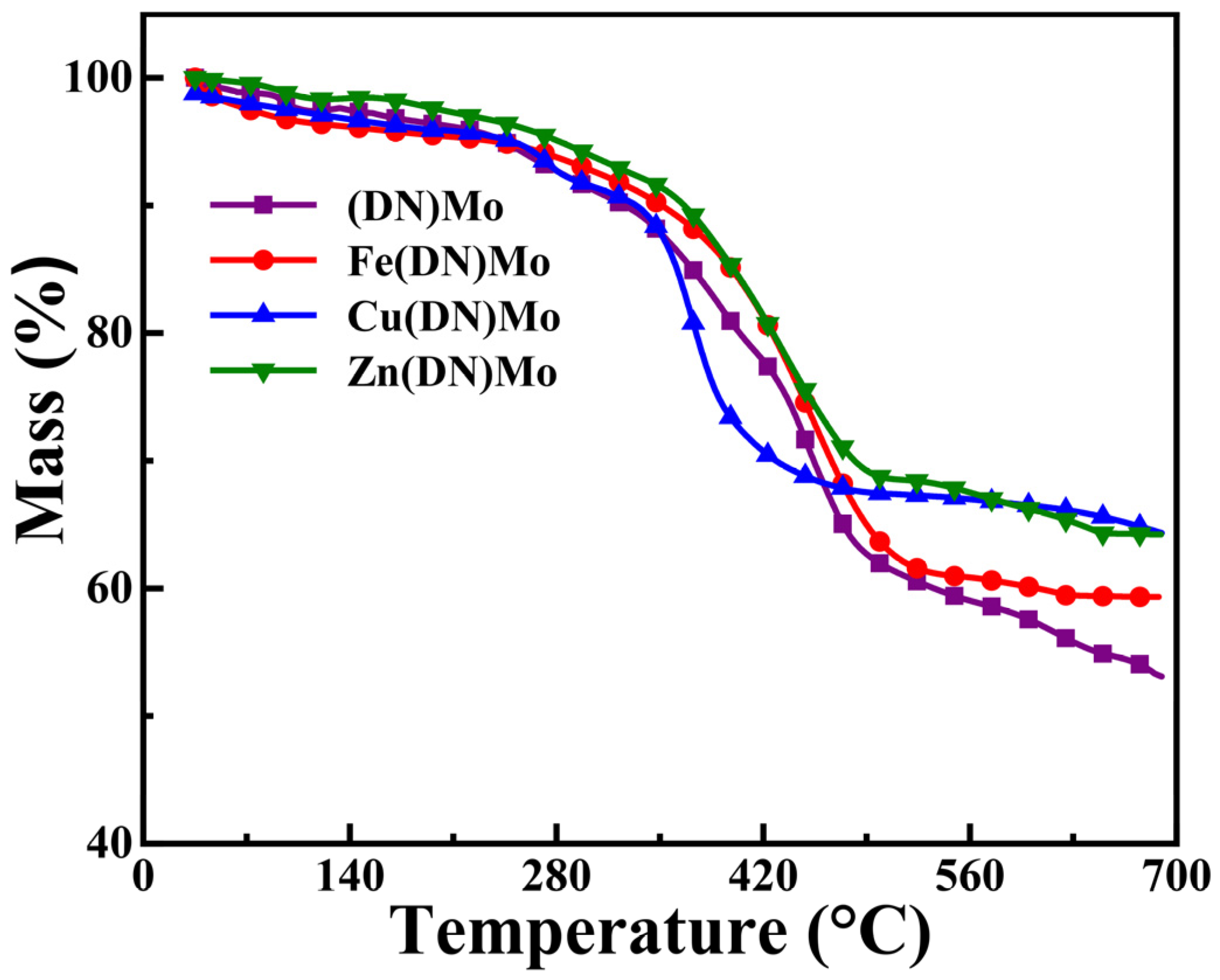
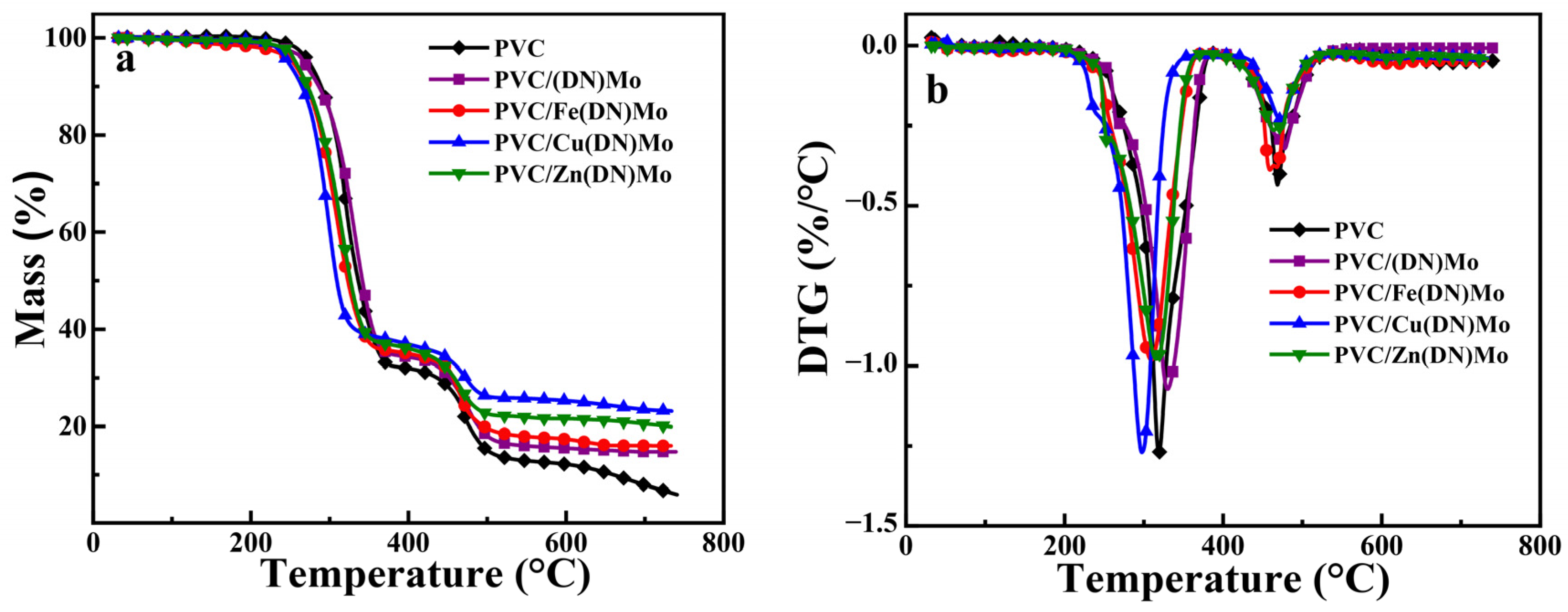
3.4. Thermal Stability of PVC Composites
3.5. Morphology Analysis of Char Residue
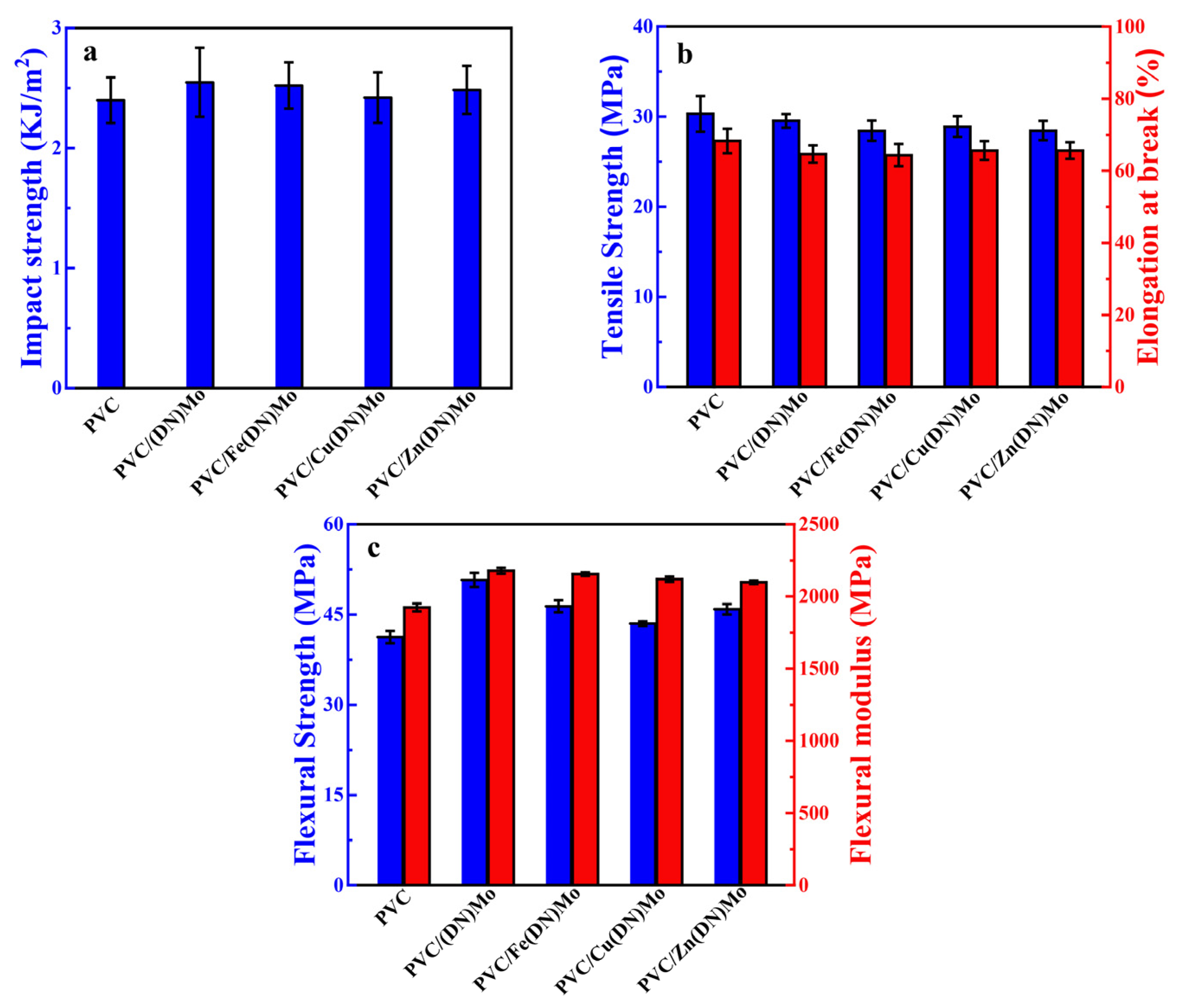
3.6. Mechanical Properties of PVC Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.-H.; Wu, W.-H.; Yang, G.; Jiao, Y.-H.; Qu, H.-Q.; Xu, J.-Z. Two Kinds of Activated Carbon Spheres-Supported Metal Oxides for Reducing Smoke Release Volume and Fire Hazard in Flexible poly(vinyl Chloride). J. Therm. Anal. Calorim. 2019, 135, 2101–2110. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.; Qu, H.; Sun, J.; Xu, J. Flame-Retarding and Mechanical Properties of Flexible Polyvinyl Chloride With Surface-Treated Lamellar Magnesium Hydroxide. J. Macromol. Sci. Part B 2015, 55, 115–127. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Wang, D.-Y. Fabrication of Low-Fire-Hazard Flexible Poly (vinyl Chloride) via Reutilization of Heavy Metal Biosorbents. J. Hazard. Mater. 2017, 339, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Sang, B.; Li, Z.; Yu, L.; Li, X.; Zhang, Z. Preparation of Zinc Hydroxystannate-Titanate Nanotube Flame Retardant and Evaluation Its Smoke Suppression Efficiency for Flexible Polyvinyl Chloride Matrix. Mater. Lett. 2017, 204, 133–137. [Google Scholar] [CrossRef]
- Ning, Y.; Guo, S. Flame-Retardant and Smoke-Suppressant Properties of Zinc Borate and Aluminum Trihydrate-Filled Rigid PVC. J. Appl. Polym. Sci. 2000, 77, 3119–3127. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Overview of the Recent Literature on Flame Retardancy and Smoke Suppression in PVC. Polym. Adv. Technol. 2005, 16, 707–716. [Google Scholar] [CrossRef]
- Basfar, A.A. Effect of Various Combinations of Flame-Retardant Fillers on Flammability of Radiation Cross-Linked poly(vinyl Chloride) (PVC). Polym. Degrad. Stab. 2003, 82, 333–340. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Qu, H.; Tian, C. Zinc Hydroxystannate and Zinc Stannate As Flame-Retardant Agents for Flexible poly(vinyl Chloride). J. Appl. Polym. Sci. 2005, 98, 1469–1475. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Han, J. RETRACTED ARTICLE: Preparation of Multilayers Zinc Hydroxystannate Microcapsules and Its Application in Flame-Retardant PVC Composites. Silicon 2018, 10, 2845–2854. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Yang, L.; Wang, Z.; Chen, Z.; Fan, W. Study of the Fire Performance of Magnesium Hydroxide Sulfate Hydrate Whisker Flame Retardant Polyethylene. Macromol. Mater. Eng. 2004, 289, 984–989. [Google Scholar] [CrossRef]
- Garcia, D.; Balart, R.; Crespo, J.E.; López, J.; Garcia-Sanoguera, D. Mechanical Properties of Recycled PVC Blends With Styrenic Polymers. J. Appl. Polym. Sci. 2006, 101, 2464–2471. [Google Scholar] [CrossRef]
- Li, B.; Wang, J. Effect of Curprous Oxide in Combination With Molybdenum Trioxide on Smoke Suppression in Rigid poly(vinyl Chloride). J. Vinyl Addit. Technol. 2001, 7, 37–42. [Google Scholar] [CrossRef]
- Liu, L.; Wu, W.H.; Xue, H.H.; Qu, H.Q. A Series of Metal Molybdates As Flame-Retardants and Smoke Suppressants for Flexible PVC. Adv. Mater. Res. 2013, 634–638, 1881–1885. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, G.Q.; Guo, F.; Gao, Y.J.; Tao, H.J. Effect of DPK Flame Retardant on Combustion Characteristics and Fire Safety of PVC Membrane. Case Stud. Therm. Eng. 2017, 10, 656–663. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Bo, C.; Zhou, Y. Thermal Degradation and Flame Retardant Mechanism of poly(vinyl Chloride) Plasticized With a Novel Chlorinated Phosphate Based on Soybean Oil. Thermochim. Acta 2015, 613, 113–120. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, W.; Meng, W.; Xu, S.; Han, H.; Yu, Y.; Qu, H.; Xu, J. Application of Metallic Phytates to poly(vinyl Chloride) As Efficient Biobased Phosphorous Flame Retardants. J. Appl. Polym. Sci. 2018, 135, 46601. [Google Scholar] [CrossRef]
- Bin Ihsan, A.; Sun, T.L.; Kuroda, S.; Haque, A.; Kurokawa, T.; Nakajima, T.; Gong, J.P. A Phase Diagram of Neutral Polyampholyte – from Solution to Tough Hydrogel. J. Mater. Chem. B 2013, 1, 4555–4562. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Zhou, K.; Yang, W.; Hu, Y.; Gong, X. The Influence of manganese–cobalt oxide/Graphene on Reducing Fire Hazards of poly(butylene Terephthalate). J. Hazard. Mater. 2014, 278, 391–400. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Feng, X.; Yue, G.; Yang, W. Fabrication of Superhydrophobicity on Cotton Fabric by sol–gel. Appl. Surf. Sci. 2012, 258, 8134–8138. [Google Scholar] [CrossRef]
- Song, Q.; Wu, H.; Liu, H.; Wang, T.; Meng, W.; Qu, H. Chitosan-Regulated Inorganic Oxyacid Salt Flame Retardants: Preparation and Application in PVC Composites. J. Therm. Anal. Calorim. 2020, 1–11. [Google Scholar] [CrossRef]
- Dong, J.; Wen, Y.; Miao, Y.; Xie, Z.; Zhang, Z.; Yang, H. A Nanoporous Zirconium Phytate Film for Immobilization of Redox Protein and the Direct Electrochemical Biosensor. Sens. Actuators B Chem. 2010, 150, 141–147. [Google Scholar] [CrossRef]
- Sahebjamee, N.; Soltanieh, M.; Mousavi, S.M.; Heydarinasab, A. Removal of Cu2+, Cd2+ and Ni2+ Ions from Aqueous Solution Using a Novel chitosan/Polyvinyl Alcohol Adsorptive Membrane. Carbohydr. Polym. 2019, 210, 264–273. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Li, X.; Zhou, J.-A. A Nano Graphene oxide/α-Zirconium Phosphate Hybrid for Rigid Polyvinyl Chloride Foams With Simultaneously Improved Mechanical Strengths, Smoke Suppression, Flame Retardancy and Thermal Stability. Compos. Part A Appl. Sci. Manuf. 2019, 121, 180–188. [Google Scholar] [CrossRef]
- Zhang, B.; Han, J. Synthesis of Microencapsulated Zinc Stannate and Its Application in Flame-Retardant poly(vinyl Chloride) Membrane Material. Fire Mater. 2018, 42, 109–118. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr.; Pike, R.D.; Cole, J.R.; Doyal, A.S.; Kimlin, E.J.; Lee, J.T.; Murry, P.J.; Quinlan, R.A.; Zhang, J. Cone Calorimetric Study of Copper-Promoted Smoke Suppression and Fire Retardance of poly(vinyl chloride). Polym. Degrad. Stab. 2003, 82, 15–24. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Zhang, B.; Hu, Y.; Xu, B. Effect of Molybdenum Trioxide-Loaded Graphene and Cuprous Oxide-Loaded Graphene on Flame Retardancy and Smoke Suppression of Polyurethane Elastomer. Ind. Eng. Chem. Res. 2016, 55, 4930–4941. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ugaz, V.M.; North, S.W.; Rogers, W.J.; Mannan, M.S. Development of a Miniature Calorimeter for Identification and Detection of Explosives and Other Energetic Compounds. J. Hazard. Mater. 2007, 142, 662–668. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.; Liu, T. Effects of Copper-Containing Layered Double Hydroxide on Thermal and Smoke Behavior of poly(vinyl Chloride). J. Appl. Polym. Sci. 2011, 122, 273–281. [Google Scholar] [CrossRef]
- Qu, H.; Wu, W.; Jiao, Y.; Xu, J. Thermal Behavior and Flame Retardancy of Flexible poly(vinyl Chloride) Treated With Al(OH)3 and ZnO. Polym. Int. 2005, 54, 1469–1473. [Google Scholar] [CrossRef]
- Tian, C.M.; Qu, H.Q.; Wu, W.H.; Guo, H.Z.; Xu, J.Z. Metal Chelates As Flame Retardants and Smoke Suppressants for Flexible Poly (Vinyl Chloride). J. Fire Sci. 2004, 22, 41–51. [Google Scholar] [CrossRef]
- Jakić, M.; Vrandečić, N.S.; Klarić, I. Thermal Degradation of poly(vinyl chloride)/Poly(ethylene Oxide) Blends: Thermogravimetric Analysis. Polym. Degrad. Stab. 2013, 98, 1738–1743. [Google Scholar] [CrossRef]

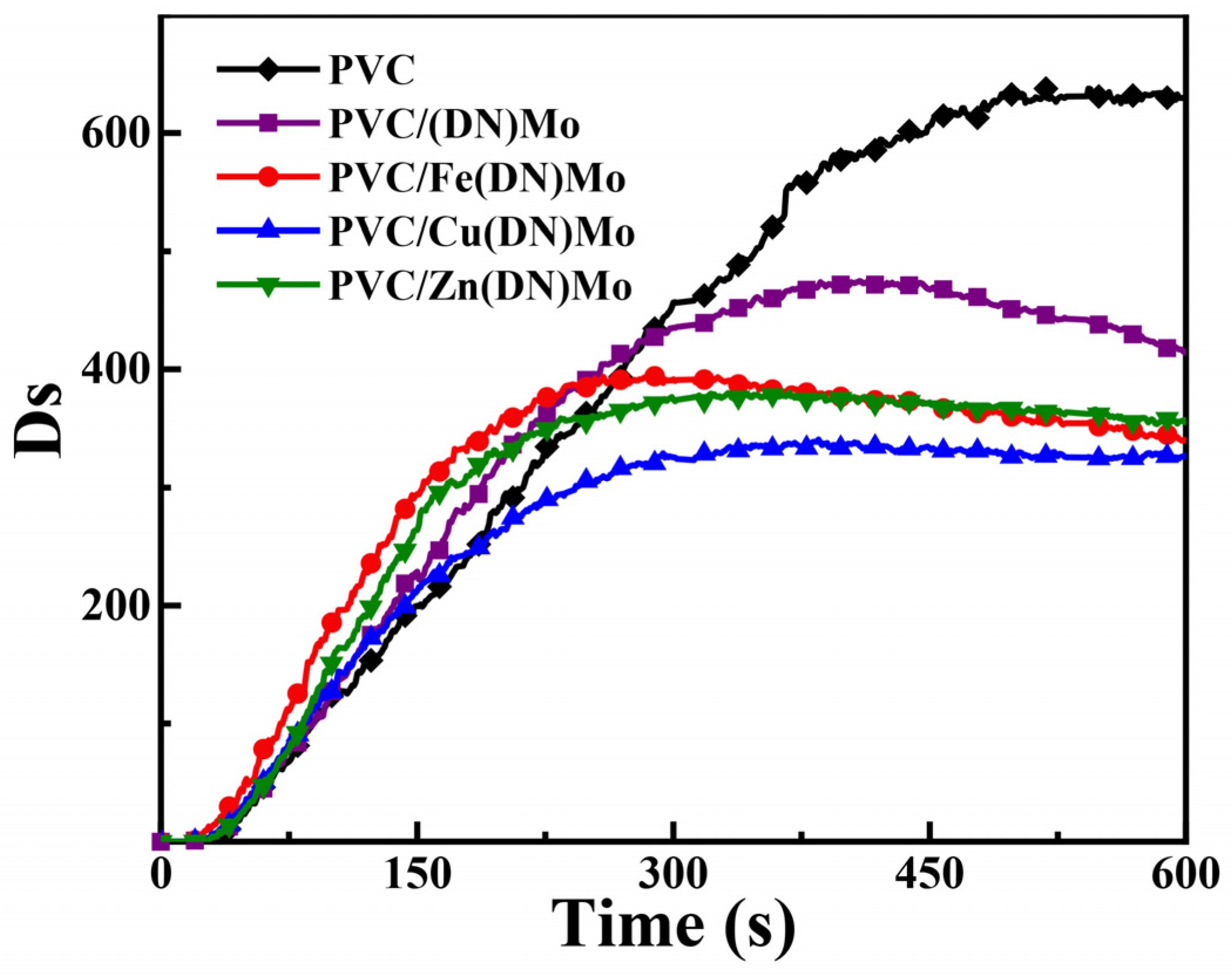


| Sample | Dsmax | Ds600 |
|---|---|---|
| PVC | 637.7 | 633.4 |
| PVC/(DN)Mo | 474.8 | 404.9 |
| PVC/Fe(DN)Mo | 396.7 | 335.0 |
| PVC/Cu(DN)Mo | 340.3 | 324.7 |
| PVC/Zn(DN)Mo | 380.2 | 346.5 |
| Sample | First Stage | Second Stage | THR (kJ/g) | LOI (%) | ||
|---|---|---|---|---|---|---|
| pHRR (w/g) | Tp (s) | pHRR (w/g) | Tp (s) | |||
| PVC | 114.43 | 262.5 | 124.64 | 418.5 | 21.66 | 35.2 |
| PVC/(DN)Mo | 87.19 | 240 | 106.08 | 407 | 15.97 | 37.2 |
| PVC/Fe(DN)Mo | 74.87 | 225.5 | 96.61 | 394 | 13.66 | 38.6 |
| PVC/Cu(DN)Mo | 86.93 | 237.5 | 79.21 | 422.5 | 11.07 | 39.1 |
| PVC/Zn(DN)Mo | 70.93 | 242.5 | 100.05 | 409.5 | 13.57 | 38.9 |
| Sample | T5% (°C) | First Stage | Second Stage | Char Residue (%) | ||
|---|---|---|---|---|---|---|
| Mass (%) | Tmax (°C) | Mass (%) | Tmax (°C) | |||
| PVC | 273.71 | 66.05 | 319.02 | 27.12 | 468.66 | 5.94 |
| PVC/(DN)Mo | 270.12 | 62.16 | 332.81 | 23.01 | 480.06 | 14.78 |
| PVC/Fe(DN)Mo | 262.63 | 60.66 | 318.28 | 22.91 | 467.85 | 16.01 |
| PVC/Cu(DN)Mo | 254.92 | 58.27 | 305.75 | 18.43 | 478.63 | 23.14 |
| PVC/Zn(DN)Mo | 265.14 | 60.01 | 324.05 | 19.93 | 472.29 | 19.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, P.; Xu, J.; Li, B.; Liu, W.; Zhao, J.; Ke, Q.; Bi, S.; Chen, X. Synthesis of Transition Metal Complexes and Their Effects on Combustion Properties of Semi-Rigid Polyvinyl Chloride. Materials 2021, 14, 2634. https://doi.org/10.3390/ma14102634
Xiang P, Xu J, Li B, Liu W, Zhao J, Ke Q, Bi S, Chen X. Synthesis of Transition Metal Complexes and Their Effects on Combustion Properties of Semi-Rigid Polyvinyl Chloride. Materials. 2021; 14(10):2634. https://doi.org/10.3390/ma14102634
Chicago/Turabian StyleXiang, Pei, Jun Xu, Biao Li, Weiqi Liu, Jinshun Zhao, Qining Ke, Siwen Bi, and Xuhuang Chen. 2021. "Synthesis of Transition Metal Complexes and Their Effects on Combustion Properties of Semi-Rigid Polyvinyl Chloride" Materials 14, no. 10: 2634. https://doi.org/10.3390/ma14102634
APA StyleXiang, P., Xu, J., Li, B., Liu, W., Zhao, J., Ke, Q., Bi, S., & Chen, X. (2021). Synthesis of Transition Metal Complexes and Their Effects on Combustion Properties of Semi-Rigid Polyvinyl Chloride. Materials, 14(10), 2634. https://doi.org/10.3390/ma14102634






