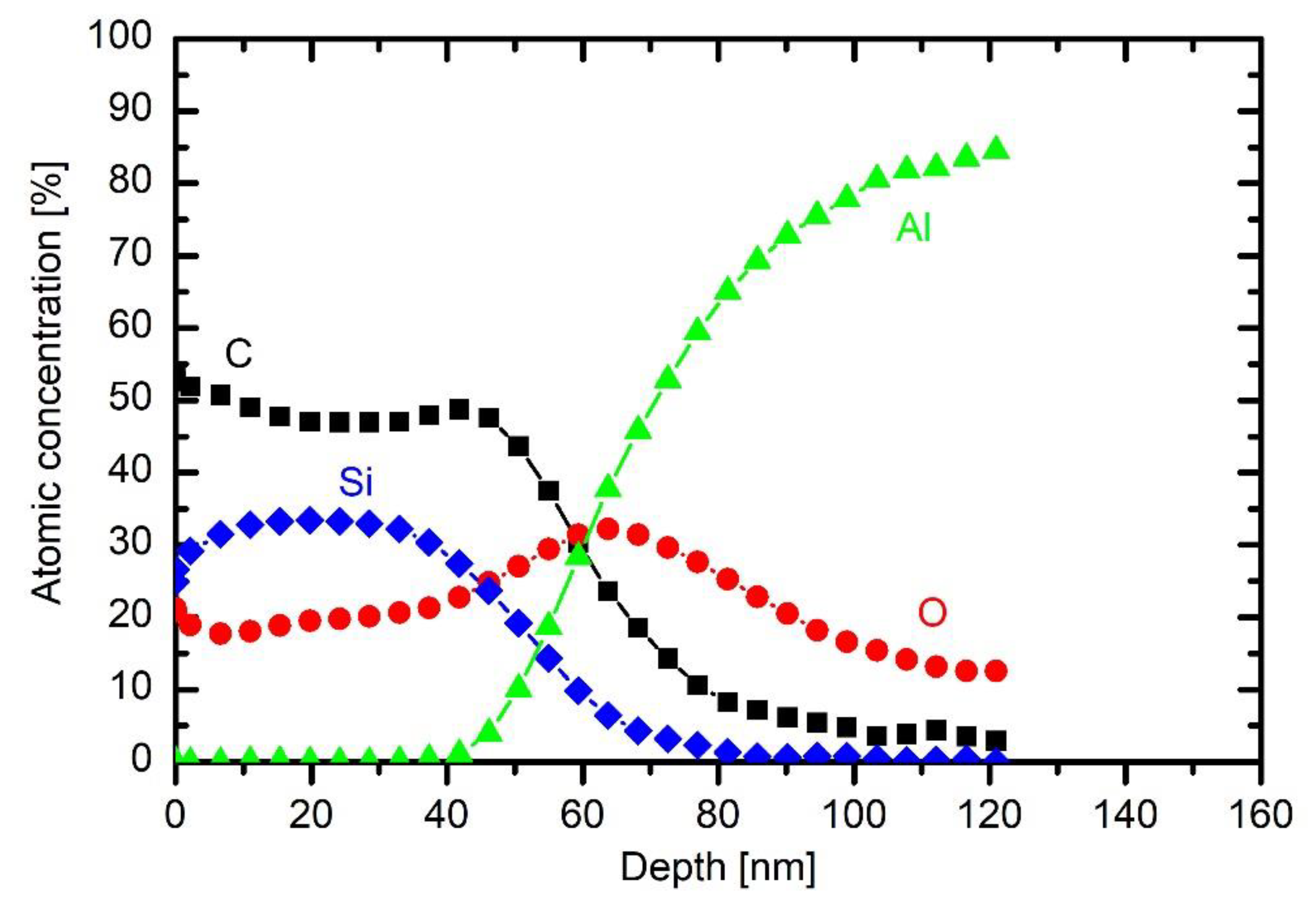
The sample was taken as received, so the aluminium surface is oxidised. The oxygen concentration on the very surface is larger than Al concentration, but after about 10 min of Ar ions etching, the concentrations become equal. The concentration of oxygen slowly decreases with prolonged etching. Such a depth profile is typical for rough metals covered with an oxide film. The slow decrease of the oxygen concentration is explained by rich surface morphology rather than diffusing layer containing partially oxidised aluminium. On top of the oxide layer, there is a layer of organic impurities. The concentration of carbon in the interface between the oxide and bulk aluminium follows the concentration of oxygen, which is again typical for rough substrates. Carbon is therefore not present in the substrate but rather forms a surface film of organic impurities.
3.2.1. Influence of Oxygen Concentration in the Gas Mixture
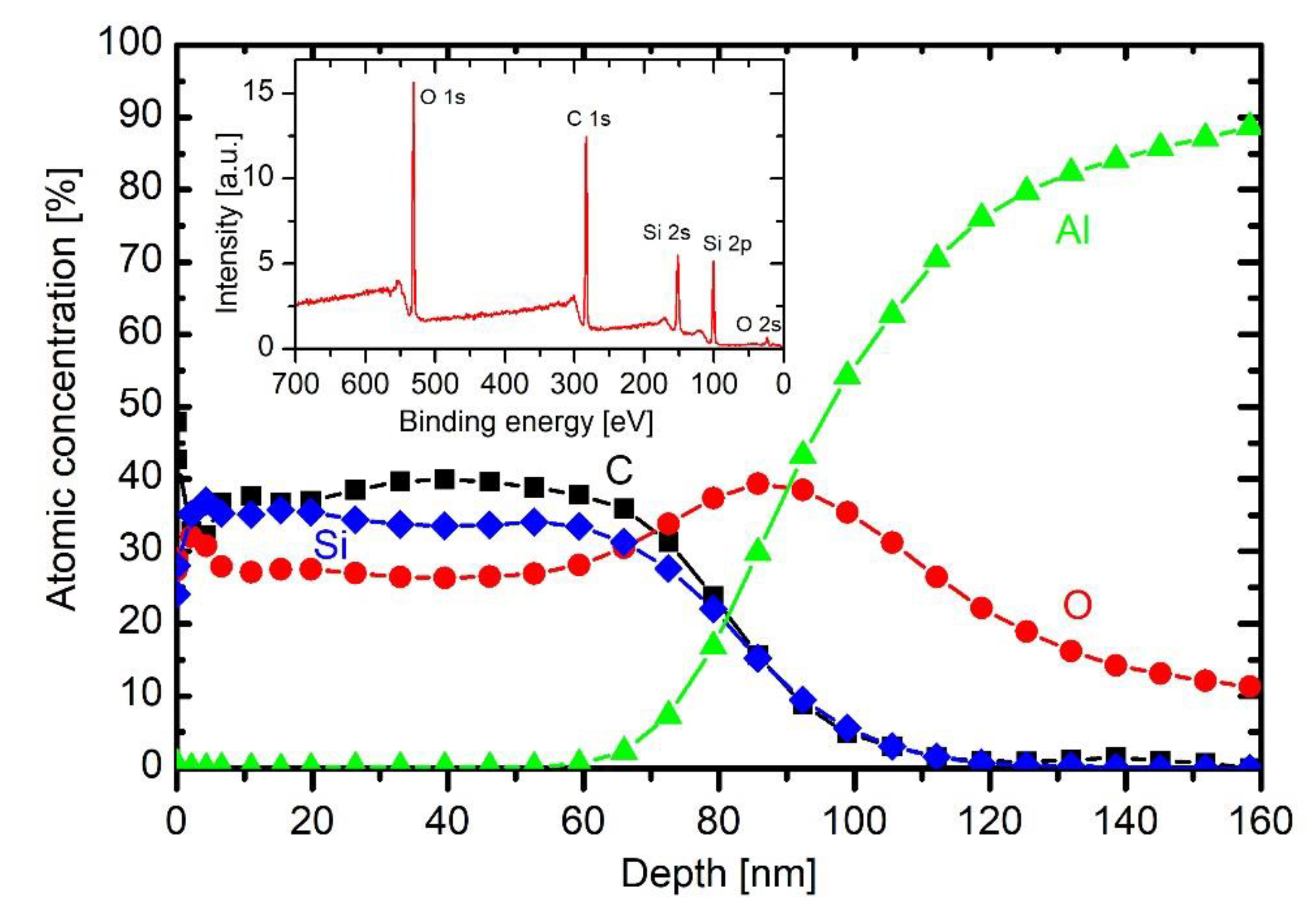
Figure 8 represents a depth profile of a sample treated in HMDSO plasma for half a minute. The broad interface between bulk aluminium and the coating is explained as for the untreated sample whose depth profile is in
Figure 7.
The broad maximum in the oxygen concentration peaking at a depth of about 60 nm is therefore explained by the persistence of the initial oxygen film on the as-received substrate. The coating contains about 50 at.% carbon, 30 at.% silicon and 20 at.% oxygen. Taking into account the accuracy of determination of atomic concentrations from XPS spectra acquired upon Ar ion sputtering, the coating’s composition is close to polydimethylsiloxane ((C2H6OSi)n). The different ratio between Si and O right on the surface observed practically on all samples could be attributed to the differences in the sputtering yields for Si and O with Ar ions (sputtering yield for oxygen is larger than for Si). The composition of the coating is rather uniform throughout the coating, which indicates little change of plasma parameters during the deposition process.
Addition of a small concentration of oxygen into HMDSO plasma causes depletion of the organic component in the coating, as deduced from the results in
Figure 9. This effect is consistent with the results of optical emission. The concentration of about 20% oxygen in the gas mixture enables deposition of the coating with about 40 at.% of carbon, as revealed in
Figure 9. The concentration of oxygen is still lower than silicon, but the difference is smaller than in the case of HMDSO plasma. The broad interface between the coating and the bulk aluminium is practically the same as in
Figure 8. The same applies for interfaces in the depth profiles at larger oxygen concentrations in the original gas mixture.
Figure 9 also shows a typical wide energy range XPS spectrum obtained on the surface of the sample. It contains peaks O 1s, C 1s, Si 2s, Si 2p and O 2s. No other elements were detected on the surface of this coating.
The behaviour of carbon concentration at the interface indicates that the layer of organic impurities persists at the interface, although a substantial concentration of oxygen was added to the gas mixture. From this observation, one can conclude that even 43% oxygen in the gas mixture used for deposition of coatings does not cause significant oxidation of the organic impurities, which are presented on the surface of as-received substrates. Instead, they are buried at the interface by depositing the coating rich in silicon and oxygen.
The concentration of 1/3 oxygen and 2/3 HMDSO causes a significant difference in the composition of the deposited coating. Next to the interface (i.e., between 40 and 70 nm), the composition is as expected, i.e., similar concentrations of Si and O and larger concentration of carbon. On top of this film, however, there is a coating depleted from carbon. Namely, the concentration of Si and O in the film between a few nm and about 25 nm is almost twice as the concentration of carbon. The discharge parameters (gas flows, power) remained the same throughout the deposition process, thus the broad minimum in the C concentration should be explained by changeable plasma parameters without changing the discharge conditions.
This effect is even more pronounced for the case of 43% oxygen and 57% HMDSO. There is a thin film containing Si, O and C on the interface between the coating and the substrate, but the majority of the coating contains little carbon. The depth evolution of Si, O and C is similar to that of 1/3 oxygen and 2/3 HMDSO mixture, except that the depletion of the organic component is more pronounced in the case of 43% oxygen addition. The rather unexpected depletion can be explained with a progressive change of gas-phase or surface kinetics during the deposition.
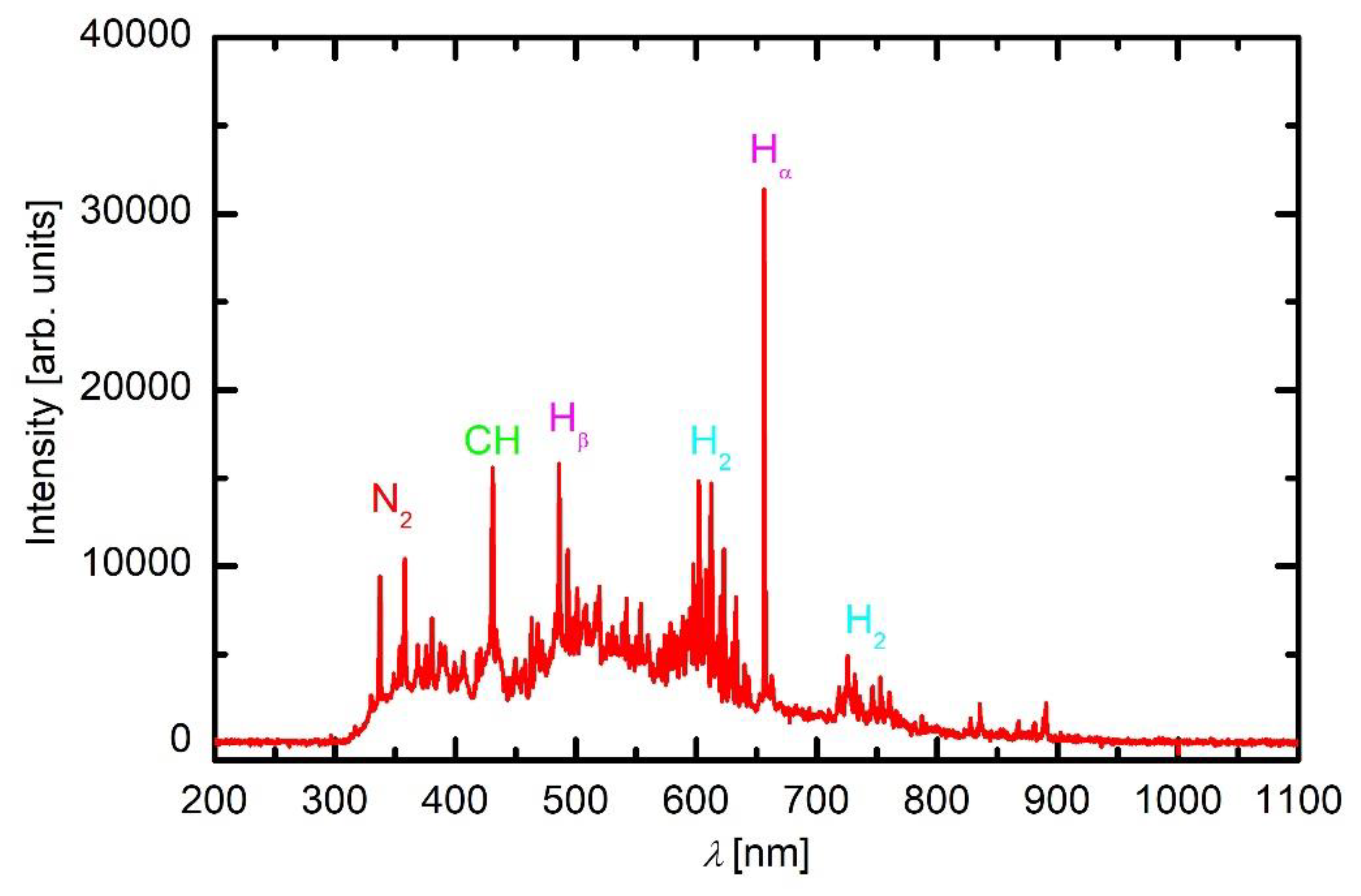
As revealed from the literature survey in the Introduction, the HMDSO plasma tends to develop clusters, often called oligomerisation [
12]. These clusters grow with time and eventually form dust particles [
9]. Once a critical size of particles is achieved, they become negatively charged due to attachment of slow plasma electrons. The negative charge of small particles prevents their contact to any walls of the plasma reactor (including substrates) due to repulsive electric filed in the sheath between the bulk plasma and the wall. The particles remain levitating in the plasma and keep growing as radicals stick to them. A variety of radicals form upon plasma conditions in mixtures of oxygen and HMDSO as confirmed by optical spectra. When the radicals collide with the particles, they may undergo exothermic reactions. Condensation itself is an exothermic event, but the released energy is marginal. On the other hand, there are surface reactions that dissipate significant energy upon interaction. Among them, heterogeneous surface recombination usually prevails in weakly ionised gaseous plasma. There are both oxygen and hydrogen atoms in plasma sustained in a mixture of oxygen and HMDSO, but O atoms are consumed extensively by surface chemical reactions that lead to the formation of CO radicals, as revealed in
Figure 5. Namely, the O-atom peaks at 777 and 845 nm are hardly observable in
Figure 5. Hydrogen atoms are observable in all plasma spectra presented in
Figure 3,
Figure 4 and
Figure 5, while oxygen atoms definitely form in such plasma but are only clearly recognised in
Figure 3 due to the consumption by chemical reactions. The energy released on a particle surface due to surface recombination is about half of the dissociation energy of parent molecules, i.e., about 2 eV per atom recombining on the surface. As a result, any particle levitating in plasma rich in H atoms warms up significantly. Upon steady conditions, the temperature of a particle levitating in plasma depends on the recombination coefficient. The coefficients have never been determined for dust formed in such complex plasma, so it is impossible to speculate about the temperature of the particles, but it is clear that the temperature increases with time. The recombination coefficients usually increase with increasing temperature [
20]. Increasing temperature causes a dramatic increase in the oxidation rate of organic material upon treatment with oxygen atoms [
21]. The final consequence of the interaction between oxygen atoms and particles is, therefore, extensive oxidation of the organic matter of the particle. In fact, the H
α peak in the optical spectrum of plasma rich in oxygen (
Figure 5) is much smaller than peaks of the CO band, indicating qualitatively strong oxidation of organic component. The H
α peak in HMDSO plasma is much larger than any other spectral features (
Figure 4).
The rather unexpected depth profiles of 33% and 43% oxygen addition in gas mixtures can therefore be explained by the time evolution of the oxygen consumption in gaseous plasma. At the beginning of the deposition, there is practically no cluster in plasma so the oxygen, although present in the gas phase in rather large concentration, does not cause chemical reactions in the gas phase, so the coating contains a significant amount of the organic component. As particles appear and grow, more oxygen is used for oxidation of the organic component and the CO bands prevail in optical spectra. Carbon monoxide practically does not stick to surfaces, but the silica-rich particles do upon turning off the discharge. This could be a feasible explanation for the gradual decrease of the carbon concentration at prolonged deposition time. The effect is more pronounced at larger oxygen concentration.
The high concentration of carbon on the very surface (
Figure 8 and
Figure 9) is due to secondary contamination of samples on the way between the plasma lab and XPS characterisation. In fact, the concentration on the very surface is almost the same for all depth profiles.
The coating obtained upon treatment of a sample in HMDSO with a large concentration of oxygen is completely different.
Figure 10 shows a profile at oxygen/HMDSO mixture 2/1.
The coating is free from carbon, and the ratio between oxygen and silicon is close to stoichiometric SiO
2. The result is consistent with the observation reported by other authors [
10]. The carbon at the interface between the substrate and the coating still persists; such oxygen atoms, although rather dense at these conditions, were not able to oxidise the impurity layer before it was buried by the deposit. Although the HMDSO is rich in carbon, the oxygen flux on the surface was obviously large enough to cause immediate oxidation of any organic compound that might have stuck on the surface upon film growth. The film thickness is much smaller than at lower oxygen concentration in the gas mixture due to the absence of an organic component.
The extremely asymmetric capacitive discharge sustained by RF generator, therefore, enables deposition of different coatings, depending on the oxygen addition to the HMDSO precursors, but one should beware of temporal effects of oxygen consumption, as described above.
3.2.2. Influence of Discharge Power
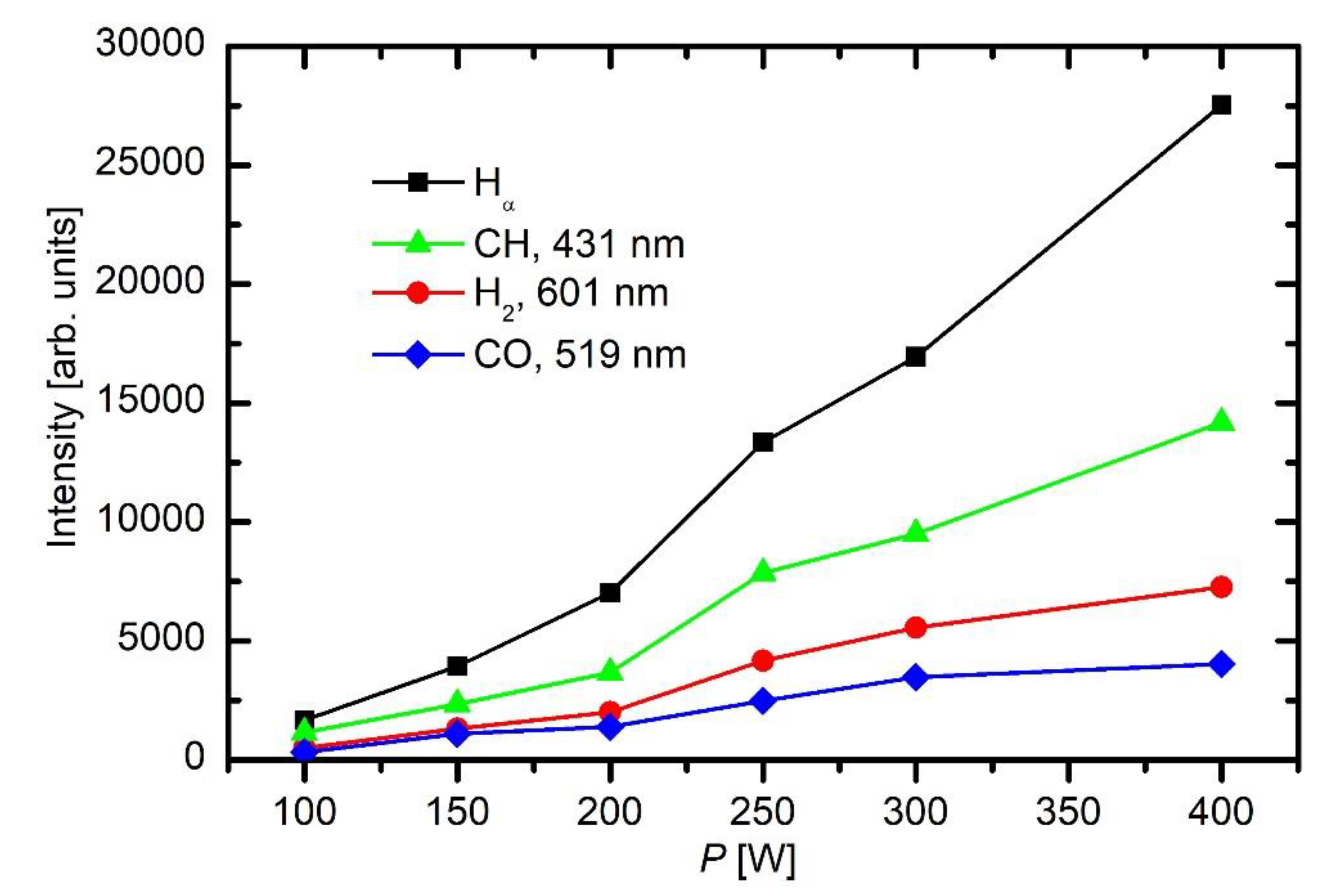
The discharge power governs the plasma parameters, in particular the electron density, which in turn governs the gas-phase reactions and thus the fragmentation of the precursor. A set of experiments was performed at different discharge powers between 50 and 750 W with constant other parameters. The results of these coatings are presented in
Figure 11,
Figure 12 and
Figure 13 and in
Figure 8.
Figure 11 represents the thickness of coatings versus discharge power. The coatings were deposited in 30 s of HMDSO plasma at 10 Pa. It can be observed that, at low powers, the thickness of coatings increases almost linearly with power. However, at higher power, the thickness starts to decrease. This can be explained by a closer inspection of the depth profiles.
The thickness of the coating of a sample treated at the lowest discharge power of 50 W is much smaller than at 200 W (
Figure 8). The observation is explained by poor fragmentation of the precursor upon plasma conditions. As mentioned above, the extremely asymmetric discharge causes practically all available power absorbed in the oscillating sheath next to the powered electrode, so the concentration of electrons in bulk plasma is small. The distribution of carbon is similar to that of an untreated sample (
Figure 7). There is some silicon on the surface, so even this very low discharge power enables deposition of a film rich in organic component, but the deposition rate as estimated from the XPS depth profile is below nm/s.
A double discharge power enables the deposition of a thicker coating as revealed from the depth profile of a sample treated with HMDSO plasma at 100 W. Carbon prevails in the depth profile indicating a large fraction of organic component in the film. The result is similar to that reported by Naddaf [
8] for the case of moderately asymmetric coupling. Aluminium persists right to the very surface so one could speculate that the coating at this power is not uniform enough to shade the substrate completely. Namely, the average thickness of the deposited film is several 10 nm. On the other hand, Si also persists deep from the surface so the speculation may not be correct. The original roughness of this sample at the spot sampled by XPS might be particularly large.
The depth profile of a sample treated at 200 W is shown in
Figure 8 and is discussed above. The depth profile at 500 W is shown in
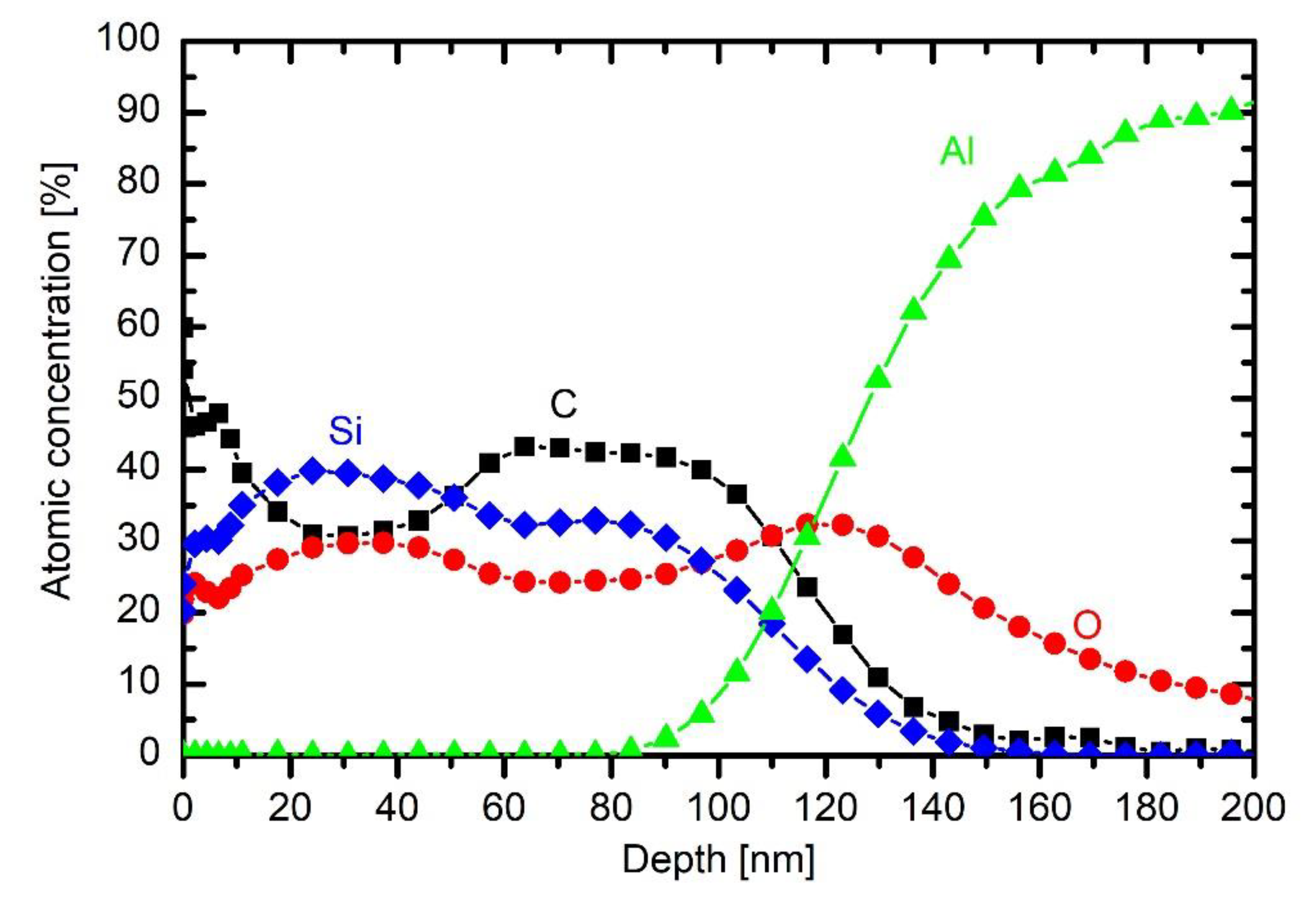
Figure 12. The coating is thicker than at lower powers, indicating that such a rather large power causes significant fragmentation, which is useful for more rapid deposition of the thin film. The composition of the coating on the substrate is somewhere in between those obtained at 200 W without oxygen and 200 W with 2.5 Pa oxygen: roughly 45 at.% C, 28 at.% O and 37 at.% Si. The increased power has therefore similar effect to the coating composition as the addition of a small amount of oxygen to the gas mixture.
More pronounced is the effect at the largest power adopted in this work.
Figure 13 presents the depth profile at 750 W and the same other conditions as in
Figure 8 and
Figure 12. The deposit on the substrate contains a significant fraction of organic compound, but, closer to the surface, the carbon is heavily depleted. The depth profile in
Figure 13, therefore, resembles those of 33% and 43% oxygen addition in HMDSO. Thus, one can conclude that large discharge power results in a coating of similar properties as the addition of moderate amounts of oxygen to the gas mixture. The depletion of the organic compound at such a large discharge power in the extremely asymmetric coupling but in HMDSO plasma could be explained by different effects. The trivial one is the persistence of water vapour in the experimental reactor. As mentioned above, the reactor is only pumped with a rotary pump and never baked, thus the “base pressure” (i.e., the pressure achieved in the reactor before introducing precursor) is rather poor. The base pressure was approximately 1.7 Pa. The pressure increased to 10 Pa upon continuous introduction of the precursor (and also continuous pumping of the system). As mentioned above, the residual atmosphere contains mainly water vapour. The oxygen concentration in the reactor upon deposition of the coating is therefore much less than the concentration of carbon. Even if all oxygen from water vapour interacts with carbon from HMDSO, there should be excessive carbon in the system, thus the negligible concentration of carbon in the surface film down to about 20 nm in
Figure 13 cannot be attributed to complete oxidation of carbon-containing materials.
The poor concentration of carbon in the deposited film is instead explained by partial transformation of HMDSO to light hydrocarbons that are pumped away from the plasma reactor. The formation of methane, acetylene and ethylene has been already confirmed by residual gas analyses for the case of plasma sustained in a moderately asymmetric RF discharge [
14,
22,
23]. The high power as adopted for the experiment whose result is shown in
Figure 13 enables extensive fragmentation of precursor, so dust particles form quickly. The dust particles assume a high temperature so thermal decomposition cannot be neglected. As shown by Pressinger and Bruggemann [
24], the thermal decomposition of HMDSO causes the formation of methane and ethane with some other light hydrocarbons. The remaining composition of hot clusters or dust particles contains silicon and oxygen. Due to the shortage of any other source of oxygen (water vapour), the particles retain a larger concentration of silicon than oxygen. As the discharge is turned off, the potential drop across the sheath between plasma and any surface vanishes so the particles rich in Si and O stick to the surface of the sample. The required condition for such an evolution of gas chemistry is oligomerisation, clustering and final evolution of dust particles. The reactions propagate with time and are faster at higher powers. This is a feasible explanation of why the Si-rich film appears only at high powers at given plasma treatment time and pressure (30 s and 10 Pa in
Figure 11,
Figure 12 and
Figure 13).