Recent Developments in the Flame-Retardant System of Epoxy Resin
Abstract
1. Introduction
2. Research Progress on Flame Retardants for Epoxy Resin
2.1. Phosphorus Flame Retardants
2.1.1. Inorganic Phosphorus
2.1.2. Organic Phosphorus
2.1.3. DOPO
2.1.4. Phosphorus/Silicon Synergistic Flame Retardants
2.1.5. Phosphorus/Nitrogen Synergistic Flame Retardant
2.2. Carbon-Based Materials
2.2.1. Graphene
2.2.2. Carbon Nanotubes
2.2.3. Expandable Graphite
2.3. Silicon Flame Retardants
2.3.1. Siloxane
2.3.2. Silica
2.3.3. POSS
2.4. Nanocomposites
2.5. Metal-Containing Compounds
3. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- De Farias, M.A.; Coelho, L.A.F.; Pezzin, S.H. Hybrid Nanocomposites Based on Epoxy/silsesquioxanes Matrices Reinforced with Multi-walled Carbon Nanotubes. Mater. Res. 2015, 18, 1304–1312. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.; Liew, K.M.; Song, L.; Wang, C.; Hu, Y. Highly Effective P–P Synergy of a Novel DOPO-Based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.; Dong, Y.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Wang, D.-Y. Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J. Mater. Chem. A 2018, 6, 6376–6386. [Google Scholar] [CrossRef]
- Martins, M.; Schartel, B.; Magalhães, F.D.; Pereira, C.M.C. The effect of traditional flame retardants, nanoclays and carbon nanotubes in the fire performance of epoxy resin composites. Fire Mater. 2016, 41, 111–130. [Google Scholar] [CrossRef]
- Toldy, A.; Anna, P.; Csontos, I.; Szabó, A.; Marosi, G. Intrinsically flame retardant epoxy resin—Fire performance and background—Part I. Polym. Degrad. Stab. 2007, 92, 2223–2230. [Google Scholar] [CrossRef]
- Shen, D.; Xu, Y.-J.; Long, J.-W.; Shi, X.; Chen, L.; Wang, Y.-Z. Epoxy resin flame-retarded via a novel melamine-organophosphinic acid salt: Thermal stability, flame retardance and pyrolysis behavior. J. Anal. Appl. Pyrolysis 2017, 128, 54–63. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stab. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Zheng, T.; Ni, X. Loading an organophosphorous flame retardant into halloysite nanotubes for modifying UV-curable epoxy resin. RSC Adv. 2016, 6, 57122–57130. [Google Scholar] [CrossRef]
- Zhuang, R.-C.; Yang, J.; Wang, D.-Y.; Huang, Y.-X. Simultaneously enhancing the flame retardancy and toughness of epoxy by lamellar dodecyl-ammonium dihydrogen phosphate. RSC Adv. 2015, 5, 100049–100053. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wang, D.-Y. Renewable Cardanol-Based Surfactant Modified Layered Double Hydroxide as a Flame Retardant for Epoxy Resin. ACS Sustain. Chem. Eng. 2015, 3, 3281–3290. [Google Scholar] [CrossRef]
- Zotti, A.; Borriello, A.; Ricciardi, M.; Antonucci, V.; Giordano, M.; Zarrelli, M. Effects of sepiolite clay on degradation and fire behaviour of a bisphenol A-based epoxy. Compos. Part B Eng. 2015, 73, 139–148. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Meenakshi, K.S.; Sudhan, E.P.J.; Kumar, S.A.; Umapathy, M. Development and characterization of novel DOPO based phosphorus tetraglycidyl epoxy nanocomposites for aerospace applications. Prog. Org. Coatings 2011, 72, 402–409. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb; Thomas, S.; Saeb, M.R. Flame Retardant Epoxy Composites on the Road of Innovation: An Analysis with Flame Retardancy Index for Future Development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. [Google Scholar] [CrossRef]
- Qian, L.; Ye, L.; Qiu, Y.; Qu, S. Thermal degradation behavior of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymers 2011, 52, 5486–5493. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymers 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Wagner, J.; Deglmann, P.; Fuchs, S.; Ciesielski, M.; Fleckenstein, C.A.; Doring, M. A flame retardant synergism of organic disulfides and phosphorous compounds. Polym. Degrad. Stab. 2016, 129, 63–76. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Cheng, L.; Wang, M. Preparation and flame retardancy of an intumescent flame-retardant epoxy resin system constructed by multiple flame-retardant compositions containing phosphorus and nitrogen heterocycle. Polym. Degrad. Stab. 2015, 119, 251–259. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Peng, H.; Liao, J.; Wang, X. Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym. Degrad. Stab. 2015, 118, 120–129. [Google Scholar] [CrossRef]
- Ma, C.; Yu, B.; Hong, N.; Pan, Y.; Hu, W.; Hu, Y. Facile Synthesis of a Highly Efficient, Halogen-Free, and Intumescent Flame Retardant for Epoxy Resins: Thermal Properties, Combustion Behaviors, and Flame-Retardant Mechanisms. Ind. Eng. Chem. Res. 2016, 55, 10868–10879. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chou, C.-I. The effect of silicon sources on the mechanism of phosphorus–silicon synergism of flame retardation of epoxy resins. Polym. Degrad. Stab. 2005, 90, 515–522. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G. The novel silicon-containing epoxy/PEPA phosphate flame retardant for transparent intumescent fire resistant coating. Appl. Surf. Sci. 2016, 385, 453–463. [Google Scholar] [CrossRef]
- Gu, J.; Liang, C.; Zhao, X.; Gan, B.; Qiu, H.; Guo, Y.; Yang, X.; Zhang, Q.; Wang, D.-Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Kalali, E.N.; Wang, X. Functionalized layered double hydroxide-based epoxy nanocomposites with improved flame retardancy and mechanical properties. J. Mater. Chem. A 2015, 3, 6819–6826. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Wang, M.; Liu, P.; Pan, Y.; Liu, D. Synergistic effect of an aromatic boronic acid derivative and magnesium hydroxide on the flame retardancy of epoxy resin. Polym. Degrad. Stab. 2016, 130, 257–263. [Google Scholar] [CrossRef]
- Guan, F.-L.; Gui, C.-X.; Zhang, H.-B.; Jiang, Z.; Jiang, Y.; Yu, Z.-Z. Enhanced thermal conductivity and satisfactory flame retardancy of epoxy/alumina composites by combination with graphene nanoplatelets and magnesium hydroxide. Compos. Part B Eng. 2016, 98, 134–140. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Brominated flame retardants and the formation of dioxins and furans in fires and combustion. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Babu, H.V.; Llorca, J.; Wang, D.-Y. Impact of halogen-free flame retardant with varied phosphorus chemical surrounding on the properties of diglycidyl ether of bisphenol-A type epoxy resin: Synthesis, fire behaviour, flame-retardant mechanism and mechanical properties. RSC Adv. 2016, 6, 59226–59236. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Preparation and investigation of flame-retardant epoxy resin modified with a novel halogen-free flame retardant containing phosphaphenanthrene, triazine-trione, and organoboron units. J. Appl. Polymers Sci. 2017, 134, 45291. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Chen, C.; Ye, Y.S.; Zeng, H.; Qu, H.; Liu, J.W.; Zhou, X.; Long, S.; Xie, X. Highly thermally conductive flame retardant epoxy nanocomposites with multifunctional ionic liquid flame retardant-functionalized boron nitride nanosheets. J. Mater. Chem. A 2018, 6, 20500–20512. [Google Scholar] [CrossRef]
- Javaid, A.; Afzal, A. Carbon fiber reinforced modified bisphenol-a diglycidylether epoxy composites for flame retardant applications. Mater. Res. Express 2018, 5, 065703. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q.; Qin, X.-Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef]
- Takano, N.; Fukuda, T.; Miyatake, M. Prepreg for printed circuit board and metal-clad laminate. Eur. Pat. Appl. 2002, 1197514. [Google Scholar]
- Honda, N.; Sugiama, T. Halogen-free flame-retardant epoxy resin composition (to Toshiba). U.S. Patent 5,994,429, 1999. [Google Scholar]
- Weferling, N.; Schmitz, H.P. Process for preparing arylalphosphinic acids (to Clariant). U.S. Patent 6,242,642, 2001. [Google Scholar]
- Wang, C.S.; Shieh, J.Y. Phosphorus-containing dihydric phenol or naphthol-advanced epoxy resin or cured (to Nat Science Council). U.S. Patent 6,291,626, 2002. [Google Scholar]
- Hörold, S. Phosphorus flame retardants in thermoset resins. Polym. Degrad. Stab. 1999, 64, 427–431. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Di Cortemiglia, M.L. Overview of fire retardant mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Wu, Q.; Lu, J.; Qu, B. Preparation and characterization of microcapsulated red phosphorus and its flame-retardant mechanism in halogen-free flame retardant polyolefins. Polym. Int. 2003, 52, 1326–1331. [Google Scholar] [CrossRef]
- Van Der Veen, I.; De Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q. Melamine cyanurate-microencapsulated red phosphorus flame retardant unreinforced and glass fiber reinforced polyamide 66. Polym. Degrad. Stab. 2006, 91, 3103–3109. [Google Scholar] [CrossRef]
- Qiu, S.; Ma, C.; Wang, X.; Zhou, X.; Feng, X.; Yuen, K.K.R.; Hu, Y. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Huang, Z.; Cao, Y.; Li, L. Continuous flame-retardant actions of two phosphate esters with expandable graphite in rigid polyurethane foams. Polym. Degrad. Stab. 2016, 130, 97–102. [Google Scholar] [CrossRef]
- Rao, W.-H.; Xu, H.-X.; Xu, Y.-J.; Qi, M.; Liao, W.; Xu, S.; Wang, Y.-Z. Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem. Eng. J. 2018, 343, 198–206. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Xie, C.; Du, J.; Dong, Z.; Sun, S.; Zhao, L.; Dai, L. Improving thermal and flame-retardant properties of epoxy resins by a new imine linkage phosphorous-containing curing agent. Polym. Eng. Sci. 2016, 56, 441–447. [Google Scholar] [CrossRef]
- Zhu, Z.-M.; Wang, L.-X.; Dong, L.-P. Influence of a novel P/N-containing oligomer on flame retardancy and thermal degradation of intumescent flame-retardant epoxy resin. Polym. Degrad. Stab. 2019, 162, 129–137. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A Review of Recent Progress in Phosphorus-based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Flame retardancy of thermoplastic polyesters?a review of the recent literature. Polym. Int. 2004, 54, 11–35. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Yu, L.-X.; Xu, Y.-J.; Rao, W.-H.; Chen, L.; Wang, Y.-Z. Polyethyleneimine modified ammonium polyphosphate toward polyamine-hardener for epoxy resin: Thermal stability, flame retardance and smoke suppression. Polym. Degrad. Stab. 2016, 131, 62–70. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsiue, G.-H.; Lee, R.-H.; Chiu, Y.-S. Phosphorus-containing epoxy for flame retardant. III: Using phosphorylated diamines as curing agents. J. Appl. Polym. Sci. 1997, 63, 895–901. [Google Scholar] [CrossRef]
- White, B.; Yin, M.; Hall, A.; Le, D.; Stolbov, S.; Rahman, T.; Turro, N.; O’Brien, S.P. Complete CO Oxidation over Cu2O Nanoparticles Supported on Silica Gel. Nano Lett. 2006, 6, 2095–2098. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lin, Y.-C.; Wang, X.-N.; Zhong, L.; Li, Q.-L.; Liu, Z.-G. Influence of Cuprous Oxide on Enhancing the Flame Retardancy and Smoke Suppression of Epoxy Resins Containing Microencapsulated Ammonium Polyphosphate. Ind. Eng. Chem. Res. 2015, 54, 12705–12713. [Google Scholar] [CrossRef]
- Gao, M.; Yang, S. A novel intumescent flame-retardant epoxy resins system. J. Appl. Polym. Sci. 2010, 115, 2346–2351. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chen, J.; Cai, S.; Hu, C. Novel flame-retardant epoxy composites containing aluminium β-carboxylethylmethylphosphinate. Polym. Eng. Sci. 2014, 55, 657–663. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Peng, S.; Peng, P.; Zou, L.; Chen, J.; Liu, J. Flexible transparent flame-retardant membrane based on a novel UV-curable phosphorus-containing acrylate. Fire Mater. 2017, 42, 99–108. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Li, S.; Sun, J. Flame retardant epoxy resins from bisphenol-A epoxy cured with hyperbranched polyphosphate ester. J. Polym. Res. 2011, 18, 2229–2237. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Hyperbranched phosphorus flame retardants: Multifunctional additives for epoxy resins. Polym. Chem. 2019, 10, 4346–4358. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Sulfur’s role in the flame retardancy of thio-ether–linked hyperbranched polyphosphoesters in epoxy resins. Eur. Polym. J. 2020, 122, 109390. [Google Scholar] [CrossRef]
- Zhang, J.; Mi, X.; Chen, S.; Xu, Z.; Zhang, D.; Miao, M.; Wang, J. A bio-based hyperbranched flame retardant for epoxy resins. Chem. Eng. J. 2020, 381, 122719. [Google Scholar] [CrossRef]
- Carja, I.-D.; Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Coroaba, A.; Lisa, G.; López, C.G.; Soriano, M.F.; Pérez, V.F.; Sánchez, M.D.R. A straightforward, eco-friendly and cost-effective approach towards flame retardant epoxy resins. J. Mater. Chem. A 2014, 2, 16230–16241. [Google Scholar] [CrossRef]
- Yang, J.-W.; Wang, Z. Synthesis of aluminum ethylphenylphosphinate flame retardant and its application in epoxy resin. Fire Mater. 2018, 42, 638–644. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Sakai, E. Thermal stability and fire behavior of aluminum diethylphosphinate-epoxy resin nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 28, 18–27. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Cai, S.-J. Comparative study of aluminum diethylphosphinate and aluminum methylethylphosphinate-filled epoxy flame-retardant composites. Polym. Compos. 2012, 33, 918–926. [Google Scholar] [CrossRef]
- Ren, H.; Sun, J.; Wu, B.; Zhou, Q. Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar] [CrossRef]
- Spontón, M.; Ronda, J.C.; Galià, M.; Cádiz, V. Flame retardant epoxy resins based on diglycidyl ether of (2,5-dihydroxyphenyl)diphenyl phosphine oxide. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2142–2151. [Google Scholar] [CrossRef]
- Mu, X.; Wang, N.; Pan, Y.; Cai, W.; Song, L.; Hu, Y. A facile approach to prepare phosphorus and nitrogen containing macromolecular covalent organic nanosheets for enhancing flame retardancy and mechanical property of epoxy resin. Compos. Part B Eng. 2019, 164, 390–399. [Google Scholar] [CrossRef]
- Huang, W.; He, W.; Long, L.; Yan, W.; He, M.; Qin, S.; Yu, J. Thermal degradation kinetics of flame-retardant glass-fiber-reinforced polyamide 6T composites based on bridged DOPO derivatives. Polym. Bull. 2018, 76, 2061–2080. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, F.-L.; Park, S.-J. Synthesis of a novel phosphorus-nitrogen-containing intumescent flame retardant and its application to fabrics. J. Ind. Eng. Chem. 2015, 27, 40–43. [Google Scholar] [CrossRef]
- Sun, D.; Yao, Y. Synthesis of three novel phosphorus-containing flame retardants and their application in epoxy resins. Polym. Degrad. Stab. 2011, 96, 1720–1724. [Google Scholar] [CrossRef]
- Gu, L.; Chen, G.; Yao, Y. Two novel phosphorus–nitrogen-containing halogen-free flame retardants of high performance for epoxy resin. Polym. Degrad. Stab. 2014, 108, 68–75. [Google Scholar] [CrossRef]
- Schartel, B.; Balabanovich, A.; Braun, U.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.; Altstädt, V. Pyrolysis of epoxy resins and fire behavior of epoxy resin composites flame-retarded with 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide additives. J. Appl. Polym. Sci. 2007, 104, 2260–2269. [Google Scholar] [CrossRef]
- Buczko, A.; Stelzig, T.; Bommer, L.; Rentsch, D.; Heneczkowski, M.; Gaan, S. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stab. 2014, 107, 158–165. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Doring, M.; Kramer, J.; Altstädt, V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur. Polym. J. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Huo, S.; Zhang, B.; Yang, S. Preparation and flame retardancy of DOPO-based epoxy resin containing bismaleimide. High Perform. Polym. 2016, 28, 1090–1095. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrolysis 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, Z.; Chen, R.; Wang, J.; Zhou, H. Synthesis of DOPO-based pyrazine derivative and its effect on flame retardancy and thermal stability of epoxy resin. Polym. Adv. Technol. 2019, 30, 2331–2339. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Fan, H.; Yang, R. Study on mechanism of phosphorus–silicon synergistic flame retardancy on epoxy resins. Polym. Degrad. Stab. 2012, 97, 2241–2248. [Google Scholar] [CrossRef]
- Lu, L.; Zeng, Z.; Qian, X.; Shao, G.; Wang, H. Thermal degradation and combustion behavior of flame-retardant epoxy resins with novel phosphorus-based flame retardants and silicon particles. Polym. Bull. 2018, 76, 3607–3619. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, S. A novel phosphorus−silicon containing epoxy resin with enhanced thermal stability, flame retardancy and mechanical properties. Polym. Degrad. Stab. 2019, 164, 36–45. [Google Scholar] [CrossRef]
- Hsiue, G.-H.; Liu, Y.-L.; Tsiao, J. Phosphorus-containing epoxy resins for flame retardancy V: Synergistic effect of phosphorus-silicon on flame retardancy. J. Appl. Polym. Sci. 2000, 78, 1–7. [Google Scholar] [CrossRef]
- Raimondo, M.; Guadagno, L.; Chirico, S.; Mariconda, A.; Bonnaud, L.; Murariu, O.; Russo, S.; Longo, P.; Dubois, P. Effect of incorporation of POSS compounds and phosphorous hardeners on thermal and fire resistance of nanofilled aeronautic resins. RSC Adv. 2015, 5, 10974–10986. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, W.; Luo, J.; Meng, X.; Xin, Z.; Wu, J.; Jiang, Z. Flame Retardancy and Mechanism of Novel Phosphorus-Silicon Flame Retardant Based on Polysilsesquioxane. Polymers 2019, 11, 1304. [Google Scholar] [CrossRef]
- Luo, J.; Li, R.; Zou, H.; Chen, Y.; Liang, M. High char yields of DOPO/organosilicon-modified epoxy resins and phosphorus–silicon synergism of thermal stability. High Perform. Polym. 2018, 31, 800–809. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, R. Novel flame retardancy effects of DOPO-POSS on epoxy resins. Polym. Degrad. Stab. 2011, 96, 2167–2173. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, T.; Xiang, H.; Li, Z.; Xu, Z.; Kong, Q.; Zhang, J.; Li, Z.; Pan, Y.; Wang, D. Simultaneously improving flame retardancy and dynamic mechanical properties of epoxy resin nanocomposites through synergistic effect of zirconium phenylphosphate and POSS. J. Therm. Anal. Calorim. 2018, 135, 2117–2124. [Google Scholar] [CrossRef]
- Song, S.; Ma, J.; Cao, K.; Chang, G.; Huang, Y.; Yang, J. Synthesis of a novel dicyclic silicon-/phosphorus hybrid and its performance on flame retardancy of epoxy resin. Polym. Degrad. Stab. 2014, 99, 43–52. [Google Scholar] [CrossRef]
- Leu, T.-S.; Wang, C.-S. Synergistic effect of a phosphorus-nitrogen flame retardant on engineering plastics. J. Appl. Polym. Sci. 2004, 92, 410–417. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, D.-Y.; Liang, W.-J.; Li, F.; Wang, J.-S.; Liu, Y.-Q. Bi-phase flame-retardant actions of water-blown rigid polyurethane foam containing diethyl-N,N-bis(2-hydroxyethyl) phosphoramide and expandable graphite. J. Anal. Appl. Pyrolysis 2017, 124, 247–255. [Google Scholar] [CrossRef]
- Januszewski, R.; Dutkiewicz, M.; Kubicki, M.; Dutkiewicz, G.; Maciejewski, H.; Marciniec, B. Synthesis and characterization of new (dimethylsilyl)phenoxy and (dimethyl(vinyl)silyl)phenoxy substituted cyclotriphosphazenes. J. Organomet. Chem. 2017, 853, 64–67. [Google Scholar] [CrossRef]
- Januszewski, R.; Dutkiewicz, M.; Orwat, B.; Maciejewski, H.; Marciniec, B. A library of multisubstituted cyclotriphosphazenes—Molecular scaffolds for hybrid materials. New J. Chem. 2018, 42, 15552–15555. [Google Scholar] [CrossRef]
- Ai, L.; Chen, S.; Zeng, J.; Liu, P.; Liu, W.; Pan, Y.; Liu, D. Synthesis and flame retardant properties of cyclophosphazene derivatives containing boron. Polym. Degrad. Stab. 2018, 155, 250–261. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, D. Novel Cyclolinear Cyclotriphosphazene-Linked Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Characterization, and Flammability Characteristics. Ind. Eng. Chem. Res. 2012, 51, 15064–15074. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Wang, X.; Wu, D. Synthesis, characterization and curing properties of a novel cyclolinear phosphazene-based epoxy resin for halogen-free flame retardancy and high performance. RSC Adv. 2012, 2, 5789. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wu, D. Novel Spirocyclic Phosphazene-Based Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Curing Behaviors, and Flammability Characteristics. ACS Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef]
- Liang, W.-J.; Zhao, B.; Zhao, P.-H.; Zhang, C.-Y.; Liu, Y.-Q. Bisphenol-S bridged penta(anilino)cyclotriphosphazene and its application in epoxy resins: Synthesis, thermal degradation, and flame retardancy. Polym. Degrad. Stab. 2017, 135, 140–151. [Google Scholar] [CrossRef]
- Xu, M.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, C.; Zhai, X.; Qu, L.; Mu, J. Effect of hexaphenoxycyclotriphosphazene combined with octapropylglycidylether polyhedral oligomeric silsesquioxane on thermal stability and flame retardancy of epoxy resin. High Perform. Polym. 2014, 26, 744–752. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, C.; Zhang, C.; Bai, X.; Mu, J. Octasilsesquioxane-reinforced TMBP epoxy nanocomposites: Characterization of thermal, flame-retardant, and morphological properties. High Perform. Polym. 2012, 24, 747–755. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Doring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.-J. Chemically modified graphene: Flame retardant or fuel for combustion? J. Mater. Chem. 2011, 21, 3277–3279. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Liao, S.-H.; Liu, P.-L.; Hsiao, M.-C.; Teng, C.-C.; Wang, C.-A.; Ger, M.-D.; Chiang, C.-L. One-Step Reduction and Functionalization of Graphene Oxide with Phosphorus-Based Compound to Produce Flame-Retardant Epoxy Nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Bai, Z.-M.; Tang, G.; Song, L.; Stec, A.A.; Hull, R.; Zhan, J.; Hu, Y. Fabrication of Ce-doped MnO2decorated graphene sheets for fire safety applications of epoxy composites: Flame retardancy, smoke suppression and mechanism. J. Mater. Chem. A 2014, 2, 17341–17351. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, K.; Yang, W.; Xing, W.; Hu, Y.; Gong, X. Surface Modification of Graphene with Layered Molybdenum Disulfide and Their Synergistic Reinforcement on Reducing Fire Hazards of Epoxy Resins. Ind. Eng. Chem. Res. 2013, 52, 17882–17890. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Xing, W.; Yu, B.; Feng, X.; Song, L.; Hu, Y. Self-assembly of Ni–Fe layered double hydroxide/graphene hybrids for reducing fire hazard in epoxy composites. J. Mater. Chem. A 2013, 1, 4383–4390. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wu, T.; Tang, Y.; Xiong, L.; Liu, H.; Zhang, J.; Guo, R.; Zhang, F. Improving Thermal and Flame Retardant Properties of Epoxy Resin with Organic NiFe-Layered Double Hydroxide-Carbon Nanotubes Hybrids. Chin. J. Chem. 2017, 35, 1875–1880. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Groth, K.; Harris, R.; Butler, K.; Shields, J.; Kharchenko, S.; Douglas, J. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymers 2004, 45, 4227–4239. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, W.; Zhang, C.; Liang, R.; Wang, B. Study of fire retardant behavior of carbon nanotube membranes and carbon nanofiber paper in carbon fiber reinforced epoxy composites. Carbon 2010, 48, 1799–1806. [Google Scholar] [CrossRef]
- Kuan, C.-F.; Chen, W.J.; Li, Y.-L.; Chen, C.-H.; Kuan, H.; Chiang, C.-L. Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol–gel method. J. Phys. Chem. Solids 2010, 71, 539–543. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Wen, X.; Jiang, Z.; Wang, Y.; Wang, L.; Zheng, J.; Fu, S.-Y.; Tang, T. Charing polymer wrapped carbon nanotubes for simultaneously improving the flame retardancy and mechanical properties of epoxy resin. Polymers 2011, 52, 4891–4898. [Google Scholar] [CrossRef]
- Chai, G.Q.; Zhu, G.Q.; Gao, Y.; Zhou, J.; Gao, S. Flame Retardancy of Carbon Nanotubes Reinforced Carbon Fiber/Epoxy Resin Composites. Appl. Sci. 2019, 9, 3275. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, N.; Yan, L.; Chu, Z. Functionalized multiwalled carbon nanotubes with monocomponent intumescent flame retardant for reducing the flammability and smoke emission characteristics of epoxy resins. Polym. Adv. Technol. 2018, 29, 3002–3013. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.-J.; Daniel, I.M. Processing of expanded graphite reinforced polymer nanocomposites. Compos. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Hsu, S.-W. Synthesis, characterization and thermal properties of novel epoxy/expandable graphite composites. Polym. Int. 2010, 59, 119–126. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Wang, J.; Zhang, B. Synergistic flame-retardant effect of expandable graphite and phosphorus-containing compounds for epoxy resin: Strong bonding of different carbon residues. Polym. Degrad. Stab. 2016, 128, 89–98. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Hsu, S.-W. Novel epoxy/expandable graphite halogen-free flame retardant composites−preparation, characterization, and properties. J. Polym. Res. 2009, 17, 315–323. [Google Scholar] [CrossRef]
- Vahabi, H.; Saeb, M.R.; Formela, K.; Cuesta, J.-M.L. Flame retardant epoxy/halloysite nanotubes nanocomposite coatings: Exploring low-concentration threshold for flammability compared to expandable graphite as superior fire retardant. Prog. Org. Coatings 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Mamani, A.; Ebrahimi, M.; Ataeefard, M. A study on mechanical, thermal and flame retardant properties of epoxy/expandable graphite composites. Pigment. Resin Technol. 2017, 46, 131–138. [Google Scholar] [CrossRef]
- Gogoi, P.; Boruah, M.; Bora, C.; Dolui, S.K. Jatropha curcas oil based alkyd/epoxy resin/expanded graphite (EG) reinforced bio-composite: Evaluation of the thermal, mechanical and flame retardancy properties. Prog. Org. Coatings 2014, 77, 87–93. [Google Scholar] [CrossRef]
- Yang, P.; Ren, M.; Chen, K.; Liang, Y.; Lü, Q.-F.; Zhang, T. Synthesis of a novel silicon-containing epoxy resin and its effect on flame retardancy, thermal, and mechanical properties of thermosetting resins. Mater. Today Commun. 2019, 19, 186–195. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and Characterization of Layered Silicate-Epoxy Nanocomposites. Chem. Mater. 1994, 6, 1719–1725. [Google Scholar] [CrossRef]
- Zhai, C.; Xin, F.; Chen, Y.; Cai, L.; Qian, L. Flame retardancy of epoxy resin nanocomposite with a novel polymeric nanoflame retardant. Polym. Adv. Technol. 2019, 30, 2833–2845. [Google Scholar] [CrossRef]
- Shih, W.-C.; Ma, C.-C.M. Tetrafunctional aliphatic epoxy I. Synthesis and characterization. J. Appl. Polym. Sci. 1998, 69, 51–58. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer—Layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Zhou, K.; Tang, G.; Gao, R.; Jiang, S. In situ growth of 0D silica nanospheres on 2D molybdenum disulfide nanosheets: Towards reducing fire hazards of epoxy resin. J. Hazard. Mater. 2018, 344, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Song, J.; He, L.; Liang, X.; Ding, H.; Li, Q. Alkoxysilane functionalized polycaprolactone/polysiloxane modified epoxy resin through sol–gel process. Eur. Polym. J. 2008, 44, 940–951. [Google Scholar] [CrossRef]
- Fan, S.; Peng, B.; Yuan, R.; Wu, D.; Wang, X.; Yu, J.; Li, F. A novel Schiff base-containing branched polysiloxane as a self-crosslinking flame retardant for PA6 with low heat release and excellent anti-dripping performance. Compos. Part B Eng. 2020, 183, 107684. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shen, M.-Y.; Kuan, C.-F.; Kuan, H.; Ke, C.-Y.; Chiang, C.-L. Improving Thermal Stability of Polyurethane through the Addition of Hyperbranched Polysiloxane. Polymers 2019, 11, 697. [Google Scholar] [CrossRef]
- Sun, X.; Chen, R.; Gao, X.; Liu, Q.; Liu, J.; Zhang, H.; Yu, J.; Liu, P.; Takahashi, K.; Wang, J. Fabrication of epoxy modified polysiloxane with enhanced mechanical properties for marine antifouling application. Eur. Polym. J. 2019, 117, 77–85. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, S.; Chen, J.; Fan, L. Synthesis and characterization of imide ring and siloxane-containing cycloaliphatic epoxy resins. Eur. Polym. J. 2007, 43, 1470–1479. [Google Scholar] [CrossRef]
- Li, H.-T.; Chuang, H.-R.; Wang, M.-W.; Lin, M.-S. Synthesis, properties and pyrolysis of siloxane- and imide-modified epoxy resin cured with siloxane-containing dianhydride. Polym. Int. 2005, 54, 1416–1421. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.; Feng, H.; Jin, S.; Hao, J. Toughening Effect and Flame-Retardant Behaviors of Phosphaphenanthrene/Phenylsiloxane Bigroup Macromolecules in Epoxy Thermoset. ACS Macromol. 2018, 51, 9992–10002. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.; Chen, Y.; Hao, J. Improving the fracture toughness and flame retardant properties of epoxy thermosets by phosphaphenanthrene/siloxane cluster-like molecules with multiple reactive groups. Compos. Part B Eng. 2019, 178, 107481. [Google Scholar] [CrossRef]
- Macan, J.; Brnardic, I.; Orlic, S.; Ivankovic, H.; Ivankovic, M. Thermal degradation of epoxy–silica organic–inorganic hybrid materials. Polym. Degrad. Stab. 2006, 91, 122–127. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Gilman, J.W.; Butler, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame retardant mechanism of silica gel/silica. Fire Mater. 2000, 24, 277–289. [Google Scholar] [CrossRef]
- Hou, S.; Yang, Y.; Xu, L.; Hou, S.-E. Effects of spherical silica on the properties of an epoxy resin system. J. Appl. Polym. Sci. 2011, 121, 648–653. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Tang, G.; Chen, J.; Huang, Z.-Q.; Hu, Y. Biobased polyelectrolyte multilayer-coated hollow mesoporous silica as a green flame retardant for epoxy resin. J. Hazard. Mater. 2018, 342, 689–697. [Google Scholar] [CrossRef]
- Afzal, A.; Siddiqi, H.M.; Iqbal, N.; Ahmad, Z. The effect of SiO2 filler content and its organic compatibility on thermal stability of epoxy resin. J. Therm. Anal. Calorim. 2012, 111, 247–252. [Google Scholar] [CrossRef]
- Shree, V.; Sen, A. Study of thermal and flame behavior of phosphorus-based silica for epoxy composites. J. Sol-Gel Sci. Technol. 2017, 85, 269–279. [Google Scholar] [CrossRef]
- Vu, C.M.; Nguyen, V.-H.; Bach, Q.-V. Phosphorous-jointed epoxidized soybean oil and rice husk-based silica as the novel additives for improvement mechanical and flame retardant of epoxy resin. J. Fire Sci. 2020, 38, 3–27. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Marciniec, B.; Dutkiewicz, M.; Maciejewski, H. Functionalization of Polyhedral Oligomeric Silsesquioxane (POSS) via Nucleophilic Substitution. Synthesis 2009, 2009, 2019–2024. [Google Scholar] [CrossRef][Green Version]
- Duszczak, J.; Mituła, K.; Januszewski, R.; Żak, P.; Dudziec, B.; Marciniec, B. Highly efficient route for the synthesis of a novel generation of tetraorganofunctional double-decker type of silsesquioxanes. ChemCatChem 2018, 11, 1086–1091. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Song, H.; Han, Y.; Su, H.; Wang, Y.; Wang, Q. Epoxy Resin/POSS Nanocomposites with Toughness and Thermal Stability. J. Photopolym. Sci. Technol. 2017, 30, 25–31. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Liu, F.-Y.; Ma, C.-C.M.; Chou, I.-C.; Riang, L.; Chiang, C.-L.; Yang, J.-C. Syntheses and characterization of novel P/Si polysilsesquioxanes/epoxy nanocomposites. Thermochim. Acta 2008, 473, 7–13. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, W.; He, X.; Yang, R. High-efficiency flame retardency of epoxy resin composites with perfect T8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs). Compos. Sci. Technol. 2016, 127, 8–19. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.; Chang, Y.; Chen, G.; Zeng, B.; Xu, Y.; Luo, W.; Dai, L. Highly transparent and flame-retardant epoxy composites based on a hybrid multi-element containing POSS derivative. RSC Adv. 2017, 7, 46139–46147. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Song, C.F.; Chang, Y.; Chen, G.R.; Xu, Y.T.; Dai, L. Modification of epoxy resin through the self-assembly of a surfactant-like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zeng, Z.; Zhang, Y. Properties of POSS-filled polypropylene: Comparison of physical blending and reactive blending. J. Appl. Polym. Sci. 2008, 110, 3745–3751. [Google Scholar] [CrossRef]
- Bai, H.; Zheng, Y.; Yang, R.; Zhang, A.; Wang, N. Thermal and mechanical properties of liquid-like trisilanol isobutyl-polyhedral oligomeric silsesquioxanes (POSS) derivative/epoxy nanocomposites. Polym. Compos. 2015, 38, 691–698. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, G.; Wang, X. The effect of POSS on the thermal properties of epoxy. Polym. Bull. 2007, 58, 1013–1020. [Google Scholar] [CrossRef]
- Shi, X.; Dai, X.; Cao, Y.; Li, J.; Huo, C.; Wang, X. Degradable poly (lactic acid)/metal–organic framework nanocomposites exhibiting good mechanical, flame retardant, and dielectric properties for the fabrication of disposable electronics. Ind. Eng. Chem. Res. 2017, 56, 3887–3894. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Harris, R.H.; Zhang, X.; Briber, R.M.; Cipriano, B.H.; Raghavan, S.R.; Awad, W.H.; Shields, J.R. Flame retardant mechanism of polyamide 6–clay nanocomposites. Polymers 2004, 45, 881–891. [Google Scholar] [CrossRef]
- Lee, S.K.; Bai, B.C.; Im, J.S.; In, S.J.; Lee, Y.S. Flame retardant epoxy complex produced by addition of montmorillonite and carbon nanotube. J. Ind. Eng. Chem. 2010, 16, 891–895. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Martino, L.; Guigo, N.; Van Berkel, J.; Sbirrazzuoli, N. Influence of organically modified montmorillonite and sepiolite clays on the physical properties of bio-based poly(ethylene 2,5-furandicarboxylate). Compos. Part B Eng. 2017, 110, 96–105. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Yang, W.; Niu, L.; Zhao, J.; Ma, B.; Kang, C. Influence of montmorillonite on the properties of halogen–antimony flame retardant polypropylene composites. Polym. Compos. 2018, 40, 1930–1938. [Google Scholar] [CrossRef]
- Yan, W.; Yu, J.; Zhang, M.; Wang, T.; Li, W.; Qin, S.; Long, L. Enhanced flame retardancy of epoxy resin containing a phenethyl-bridged DOPO derivative/montmorillonite compound. J. Fire Sci. 2017, 36, 47–62. [Google Scholar] [CrossRef]
- Im, J.S.; Lee, S.K.; In, S.J.; Lee, Y.S. Improved flame retardant properties of epoxy resin by fluorinated MMT/MWCNT additives. J. Anal. Appl. Pyrolysis 2010, 89, 225–232. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Yi, D.; Yang, R. Flame retardancy of ammonium polyphosphate–montmorillonite nanocompounds on epoxy resin. J. Fire Sci. 2016, 34, 212–225. [Google Scholar] [CrossRef]
- Unlu, S.M.; Dogan, S.D.; Dogan, M. Comparative study of boron compounds and aluminum trihydroxide as flame retardant additives in epoxy resin. Polym. Adv. Technol. 2014, 25, 769–776. [Google Scholar] [CrossRef]
- Hornsby, P.R. The application of magnesium hydroxide as a fire retardant and smoke-suppressing additive for polymers. Fire Mater. 1994, 18, 269–276. [Google Scholar] [CrossRef]
- Longzhen, Q.; Jianping, L.; Rongcai, X.; Baojun, Q. Structural Characteristics and Flame-Retardant properties of nanosized magnesium HydroXide. J. Semiconduct. 2016, 24, 81–83. [Google Scholar]
- Zhao, Y.; Jiao, Q.; Li, C.; Liang, J. Catalytic synthesis of carbon nanostructures using layered double hydroxides as catalyst precursors. Carbon 2007, 45, 2159–2163. [Google Scholar] [CrossRef]
- Li, F.; Tan, Q.; Evans, D.G.; Duan, X. Synthesis of carbon nanotubes using a novel catalyst derived from hydrotalcite-like Co?Al layered double hydroxide precursor. Catal. Lett. 2005, 99, 151–156. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Song, L.; Yuen, R.K.K.; Yuen, K.K.R. Investigation of Thermal and Combustion Properties for Intumescent Flame-Retardant Ethylene–Viny Acetate Composites Containing Ferrous Disulfide. Ind. Eng. Chem. Res. 2012, 51, 15082–15088. [Google Scholar] [CrossRef]
- Zhang, P.; Song, L.; Lu, H.; Wang, J.; Hu, Y. The influence of expanded graphite on thermal properties for paraffin/high density polyethylene/chlorinated paraffin/antimony trioxide as a flame retardant phase change material. Energy Convers. Manag. 2010, 51, 2733–2737. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Yang, Y.; Ma, J.; Yang, J.; Yin, Q. Preparation of metal-phosphorus hybridized nanomaterials and the action of metal centers on the flame retardancy of epoxy resin. J. Appl. Polym. Sci. 2017, 134, 45445. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhao, H.-B.; Liu, J.; Wang, D.-Y.; Song, Y.-P.; Wang, Y.-Z. Metal compound-enhanced flame retardancy of intumescent epoxy resins containing ammonium polyphosphate. Polym. Degrad. Stab. 2009, 94, 625–631. [Google Scholar] [CrossRef]
- Müller, P.; Schartel, B. Melamine poly(metal phosphates) as flame retardant in epoxy resin: Performance, modes of action, and synergy. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]

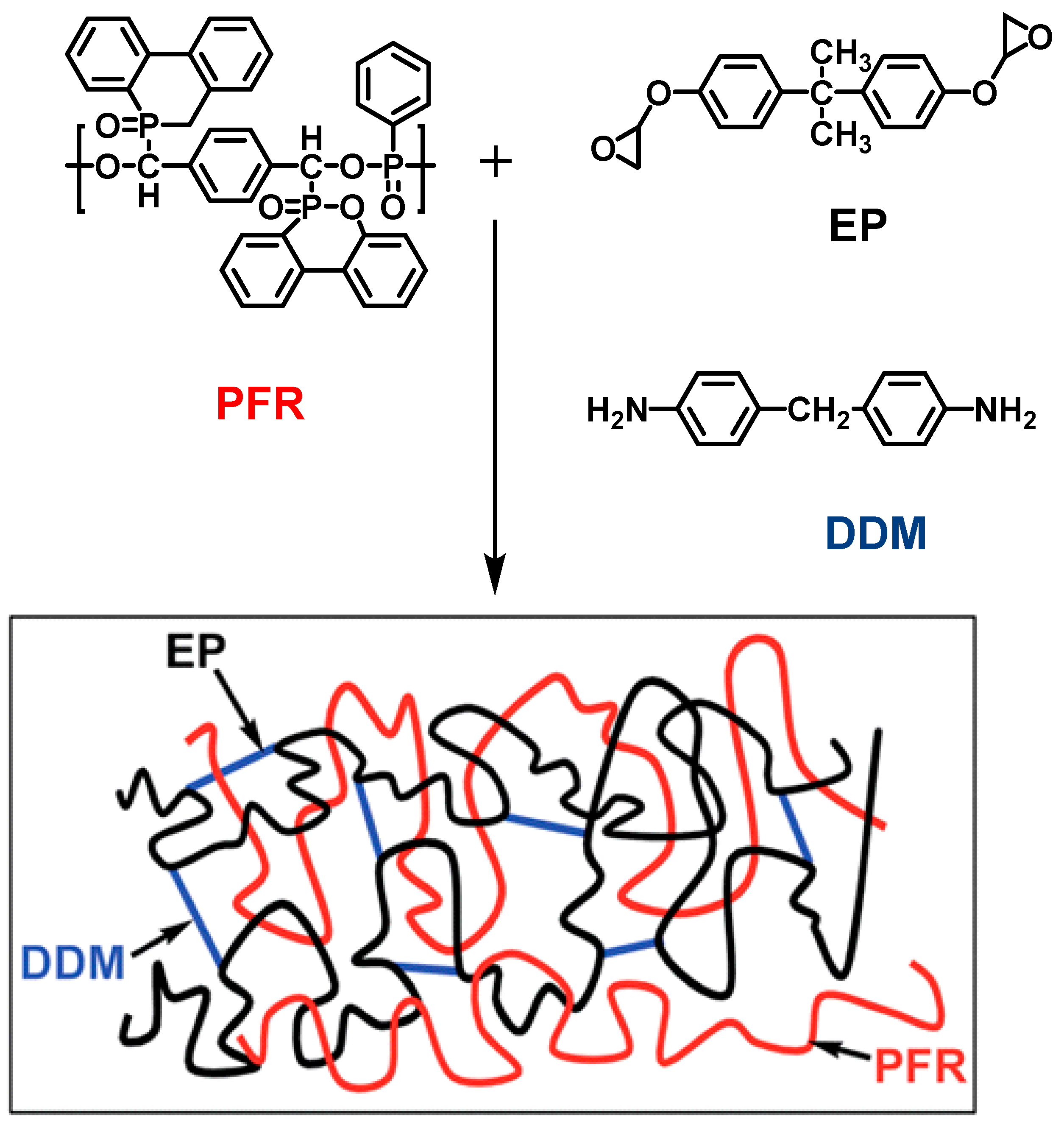
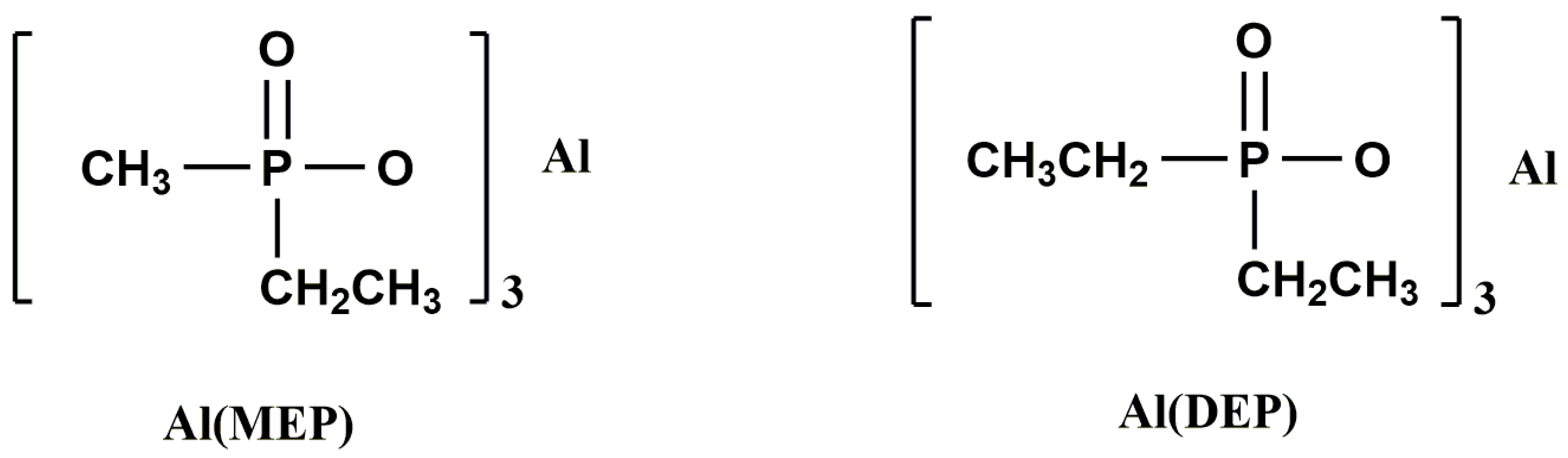
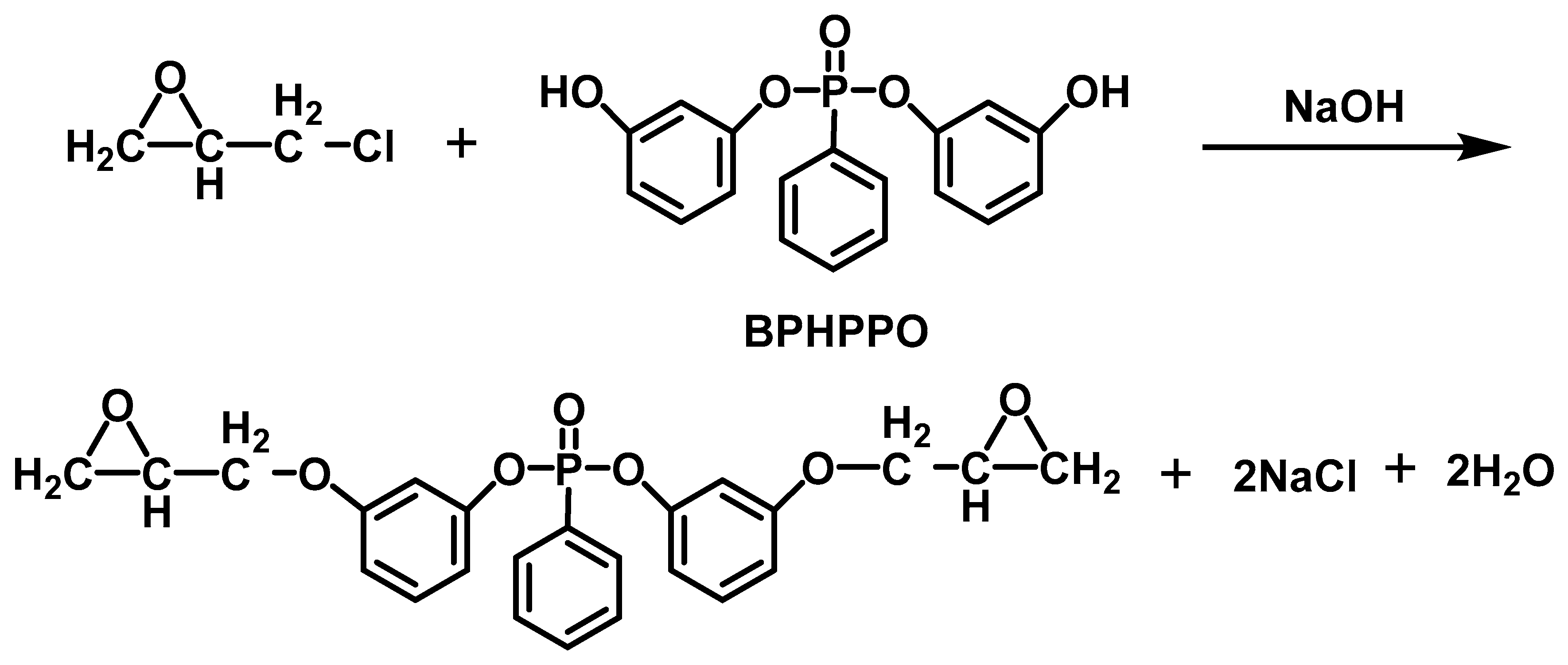
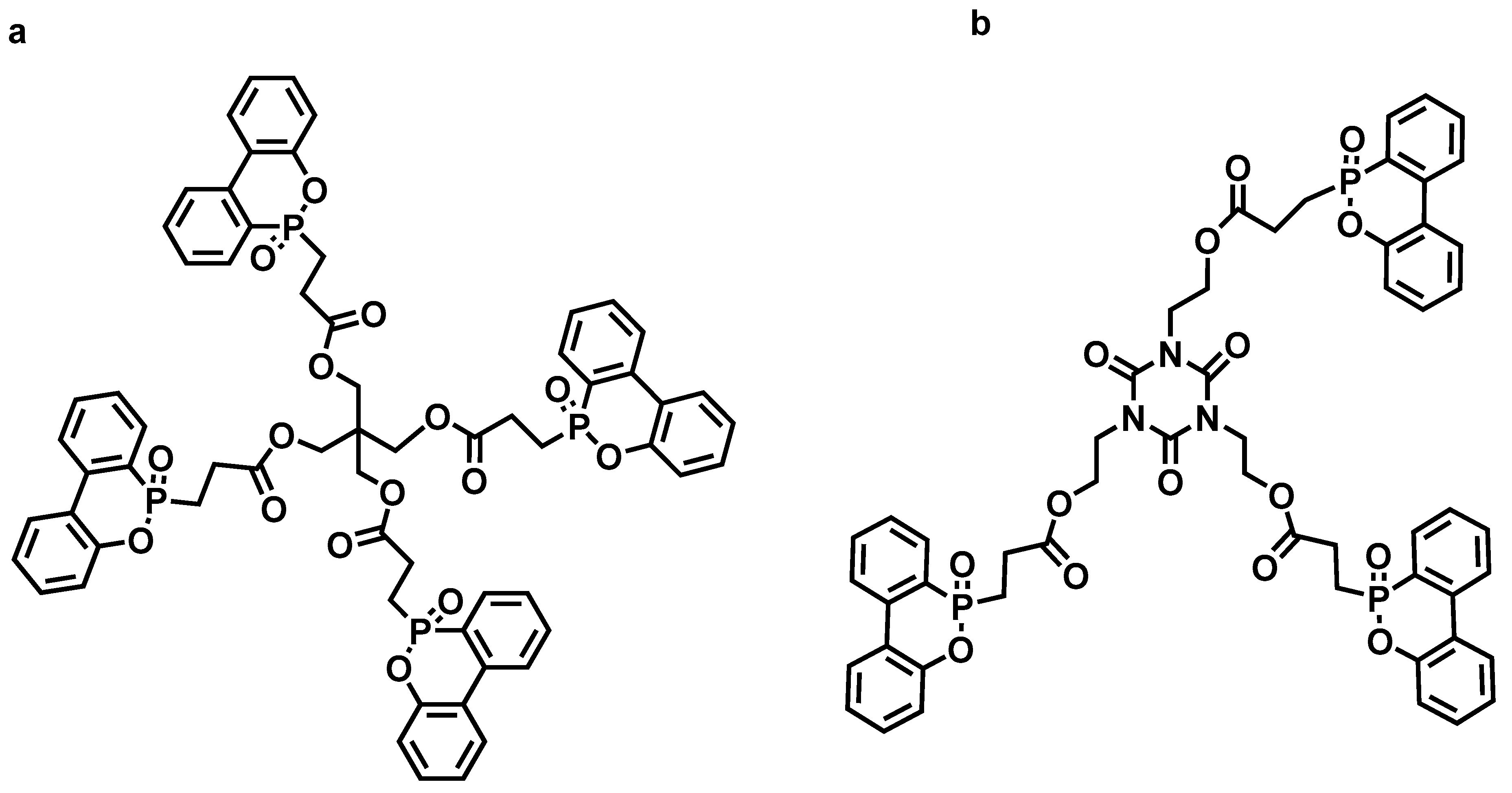


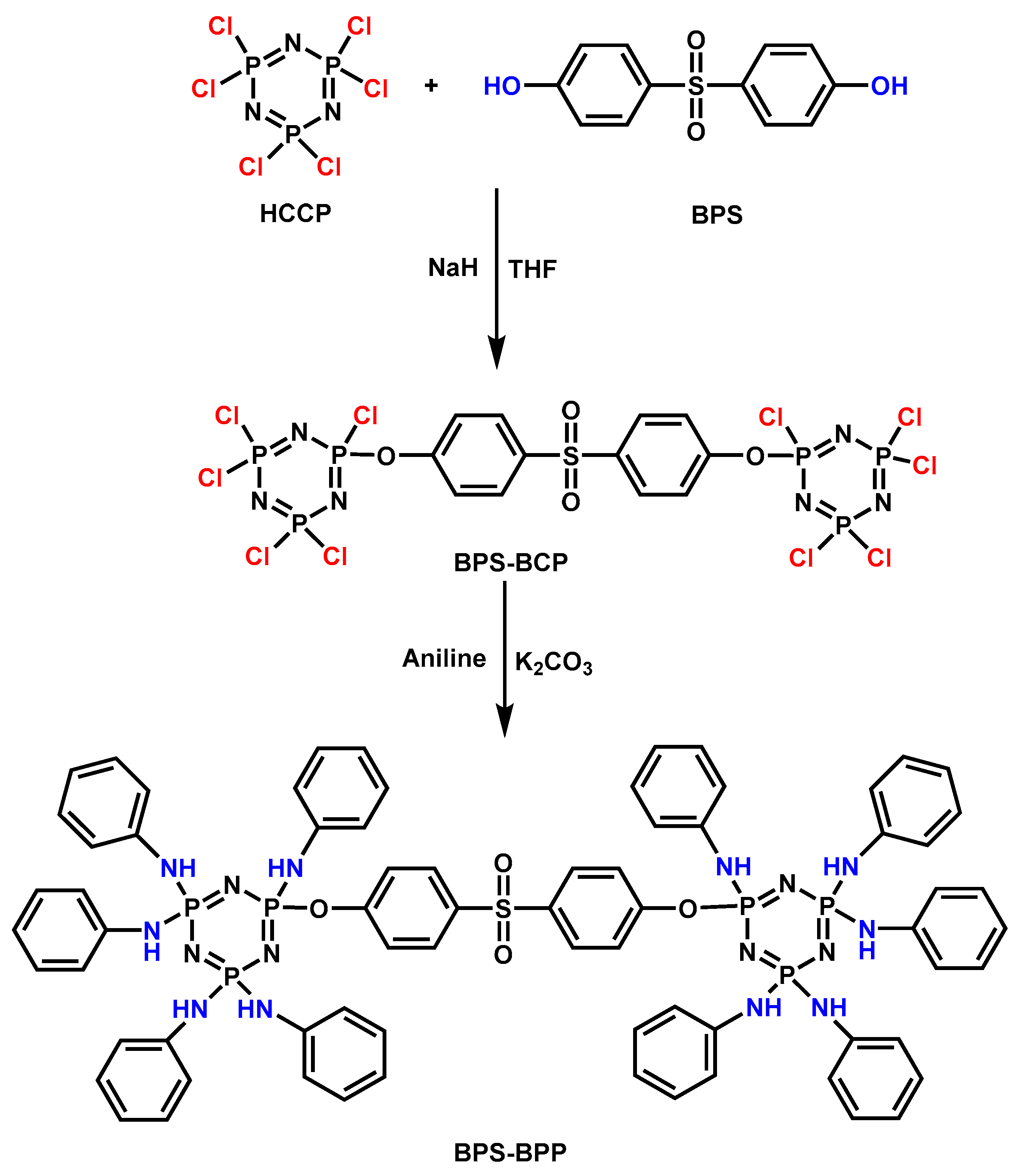



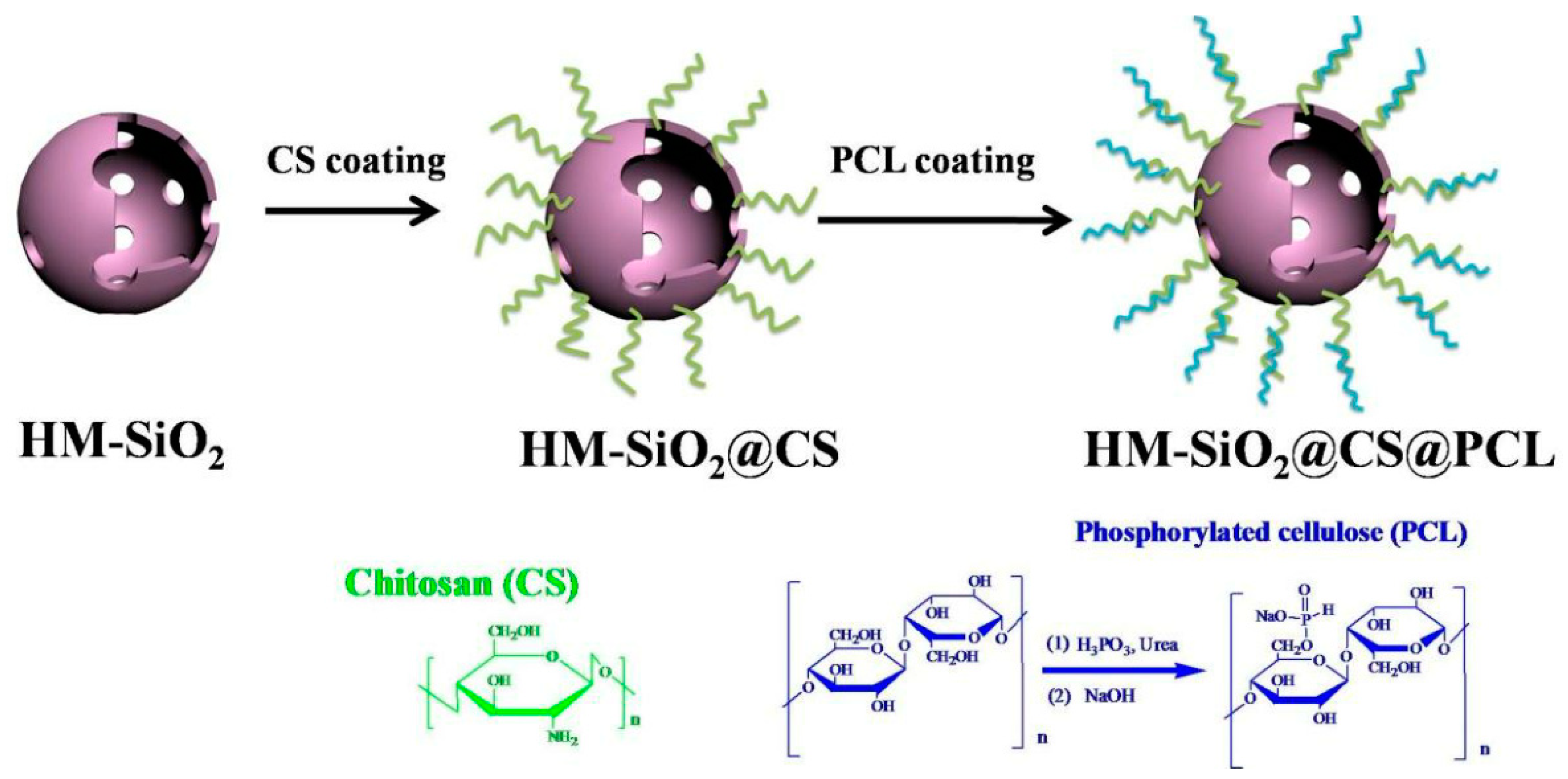


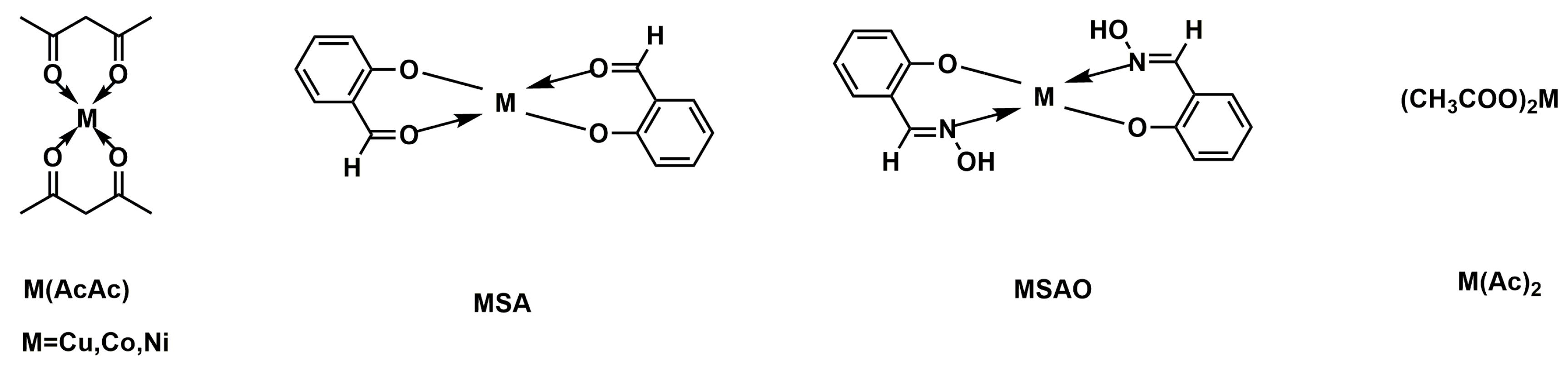
| Sample | E51 (wt.%) | PA650 (wt.%) | MAPP (wt.%) | Cu2O (wt.%) | UL-94 | LOI (%) |
|---|---|---|---|---|---|---|
| Neat EP | 55.56 | 44.44 | NR | 19.0 | ||
| EP/20%MAPP | 44.44 | 35.56 | 20.00 | NR | 31.0 | |
| EP/20%Cu2O | 44.44 | 35.56 | 20.00 | NR | 21.5 | |
| EP/16%MAPP/4%Cu2O | 44.44 | 35.56 | 16.00 | 4.00 | V-0 | 33.5 |
| EP/18%MAPP/2%Cu2O | 44.44 | 35.56 | 18.00 | 2.00 | V-0 | 35.0 |
| EP/18.67%MAPP/1.33%Cu2O | 44.44 | 35.56 | 18.67 | 1.33 | V-0 | 33.5 |
| EP/19%MAPP/1%Cu2O | 44.44 | 35.56 | 19.00 | 1.00 | V-0 | 32.5 |
| EP/13.5%MAPP/1.5%Cu2O | 47.22 | 37.78 | 13.50 | 1.50 | V-0 | 30.0 |
| EP/9%MAPP/1%Cu2O | 50.00 | 40.00 | 9.00 | 1.00 | V-0 | 28.0 |
| EP/8.1%MAPP/0.9%Cu2O | 50.56 | 40.44 | 8.10 | 0.90 | V-1 | 24.5 |
| EPs and Incorporated Phosphorus Flame Retardant | wt.% | LOI (%) | UL-94 | pk-HRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|
| EP/PEI | 1.2 | 23 | NR | 1074 | 45 | [56] |
| EP/PEI-APP | 10 | 26 | V-1 | 280 | 16 | [56] |
| 15 | 29.5 | V-0 | 281 | 11 | [56] | |
| EP/4,4-diaminodiphenyl methan (DDM) | 0(P) | 24 | – | – | – | [57] |
| EP/PA-I | 4.16(P) | 34 | – | – | – | [57] |
| EP/PA-II | 5.1(P) | 35 | – | – | – | [57] |
| EP/MAPP/Cu2O | 0/0 | 19 | NR | 761 | 112 | [59] |
| 20/0 | 31 | NR | 391 | 53 | [59] | |
| 18/2 | 35 | V-0 | 312 | 61 | [59] | |
| EP/HPE | 0 | 23 | – | 1250 ± 10 | – | [63] |
| 33 | 27.5 | – | 491.8 | – | [63] | |
| 66 | 29.3 | – | 391 ± 5 | – | [63] | |
| 100 | 32 | – | 285 ± 5 | – | [63] | |
| EP/hb-FRs | 0 | 18.7 | HB | – | – | [64] |
| EP/hb-polyphosphoramide | 10 | 23.3 | HB | – | – | [64] |
| EP/hb-polyphosphordiamidate | 10 | 22.6 | HB | – | – | [64] |
| EP/hb-polyphosphoramidate | 10 | 22.5 | HB75 | – | – | [64] |
| EP/hb-polyphosphate | 10 | 22.1 | HB | – | – | [64] |
| EP/ITA-HBP | 0 | 26.4 | NR | 678.7 | 157.9 | [66] |
| 3.8 | 36.4 | V-0 | 618.6 | 135.7 | [66] | |
| 7.35 | 37.4 | V-0 | 564.5 | 135.3 | [66] | |
| 10.64 | 41.6 | V-0 | 534 | 125.9 | [66] | |
| 13.7 | 42 | V-0 | 468 | 110.2 | [66] | |
| EP/PFR | 0 | 26.4 | NR | 275.5 | 56.8 | [67] |
| 7.36 | 34.3 | V-1 | 231.7 | 64.8 | [67] | |
| 14.8 | 40 | V-0 | 164.4 | 57.4 | [67] | |
| 22.28 | 42.2 | V-0 | 150.8 | 46.4 | [67] | |
| EP/Al (MEP) | 0 | 20.2 | NR | – | – | [70] |
| 15 | 32.2 | V-0 | – | – | [70] | |
| 20 | 36.4 | V-0 | – | – | [70] | |
| EP/Al (DEP) | 15 | 29.8 | V-0 | – | – | [70] |
| 20 | 35.5 | V-0 | – | – | [70] | |
| EP/BPHPPO | 0 | 22.5 | – | – | – | [71] |
| 7.79(P) | 34 | – | – | – | [71] | |
| EP | 0 | 25 | HB | 1719 | 74.2 | [81] |
| EP/DOPP | 19.6 | 37.9 | V-1 | 1191 | 44.8 | [81] |
| EP/DOPI | 23.1 | 34.2 | V-0 | 869 | 41.5 | [81] |
| EP-CF | 0 | 33.2 | HB | 347 | 26.2 | [81] |
| EP-CF/DOPP | 5.9 | 45.3 | V-0 | 248 | 19.9 | [81] |
| EP-CF/DOPI | 6.9 | 47.7 | V-0 | 247 | 20 | [81] |
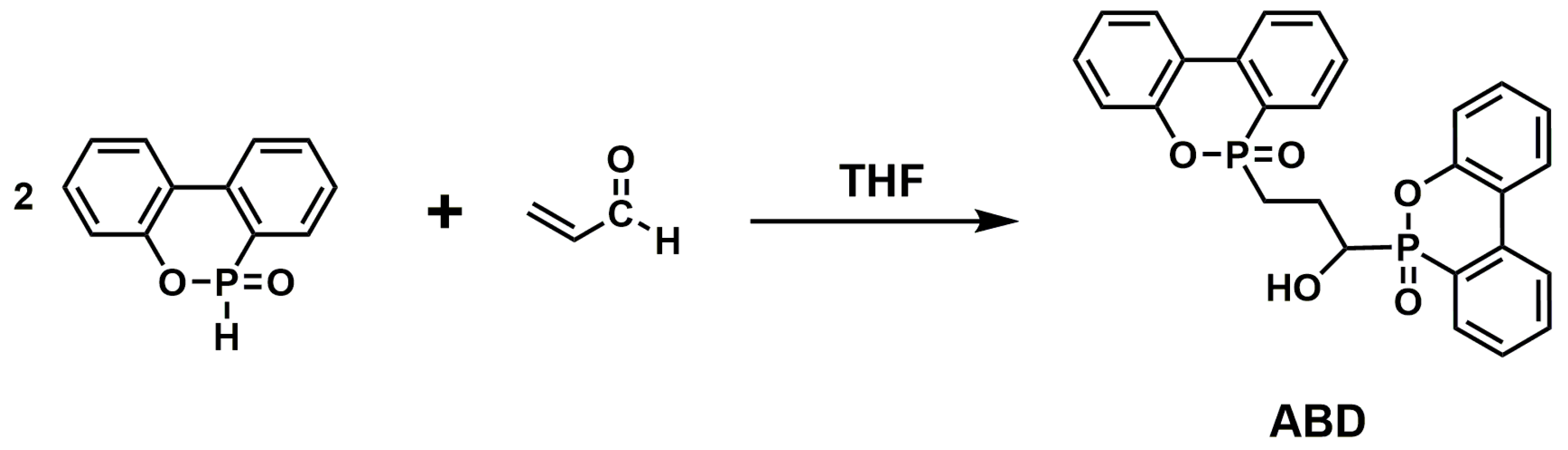
| EP/ABD | 0 | 24.7 | NR | 1420 | 143.6 | [51] |
| 2 | 32.2 | V-1 | – | – | [51] | |
| 3 | 36.2 | V-0 | 1043 | 101.5 | [51] | |
| 4 | 39.1 | V-0 | 933 | 94.3 | [51] | |
| EP/BDM | 0 | 32.5 | V-1 | 825 | 71 | [82] |
| 5 | 34 | V-0 | 812 | 68 | [82] | |
| 10 | 34.6 | V-0 | 755 | 65 | [82] | |
| 20 | 35 | V-0 | 683 | 60 | [82] | |
| 30 | 35.5 | V-0 | 615 | 58 | [82] | |
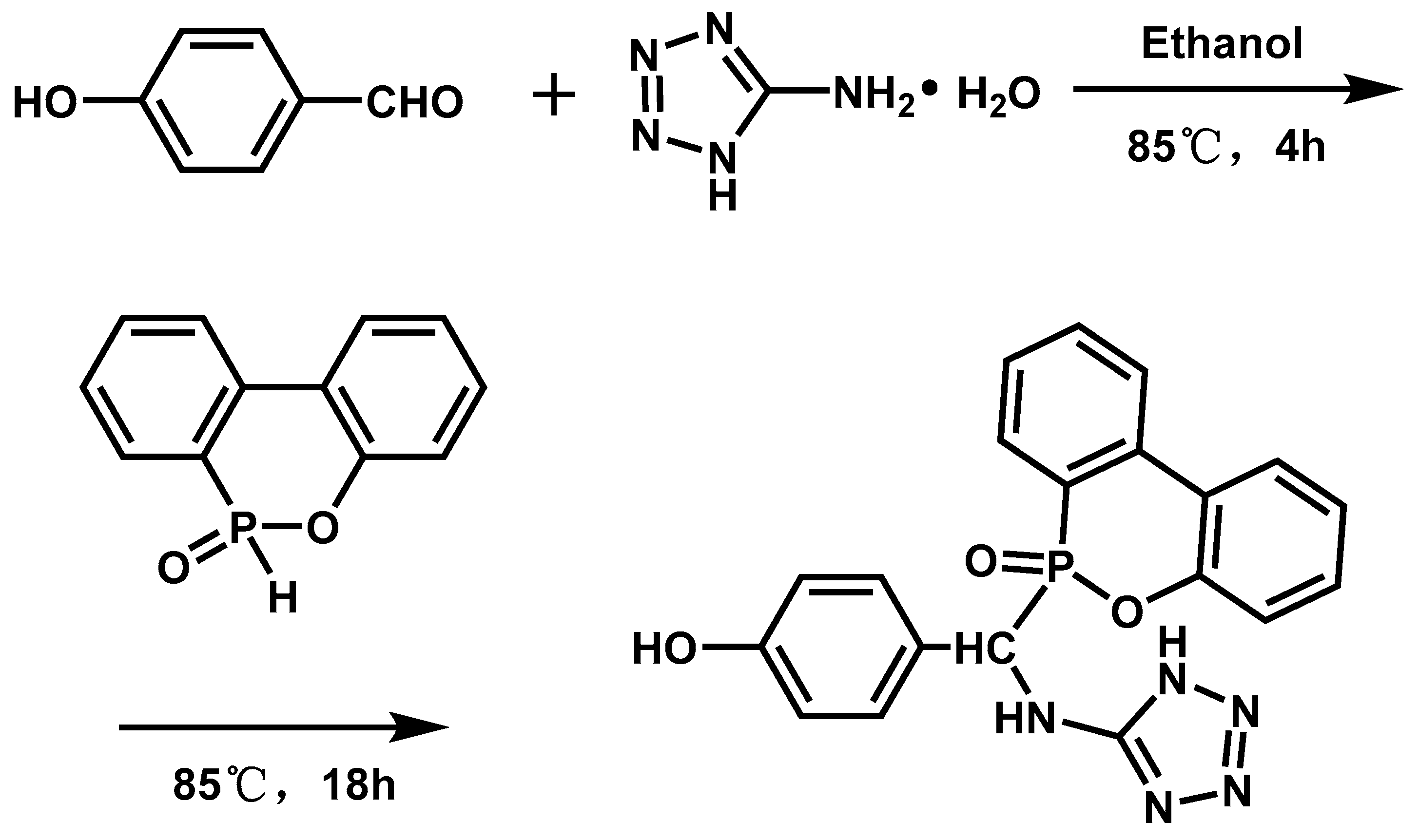
| EP/ATZ | 0 | 25.7 | NR | 654.3 | 100.3 | [83] |
| 3 | 31.2 | V-1 | – | – | [83] | |
| 6 | 33.7 | V-0 | 482.5 | 83.9 | [83] | |
| 9 | 35.9 | V-0 | – | - | [83] | |
| EP/DHBAP | 0 | 25.8 | NR | 1063.1 | 76.1 | [84] |
| 5 | 32.4 | V-1 | – | – | [84] | |
| 8 | 34 | V-0 | 783.3 | 59.9 | [84] | |
| EP/DHBAP | 10 | 34.3 | V-0 | – | – | [84] |
| EP/DOPO-POSS | 0 | 25 | NR | 855 | 112 | [92] |
| 1.5 | 29 | V-1 | – | – | [92] | |
| 2.5 | 30.2 | V-1 | 969 | 103 | [92] | |
| 3.5 | 29.1 | V-1 | – | – | [92] | |
| 5 | 28.5 | NR | 588 | 92 | [92] | |
| 10 | 23 | NR | 483 | 85 | [92] | |
| EP/ZrPP/POSS | 0/0 | 23 | NR | 675 | – | [93] |
| 0/5 | 27.6 | V-2 | 426 | – | [93] | |
| 1/4 | 30.3 | V-1 | 438 | – | [93] | |
| 3/2 | 29.7 | V-1 | 461 | – | [93] | |
| 5/0 | 28.4 | V-2 | 469 | – | [93] | |
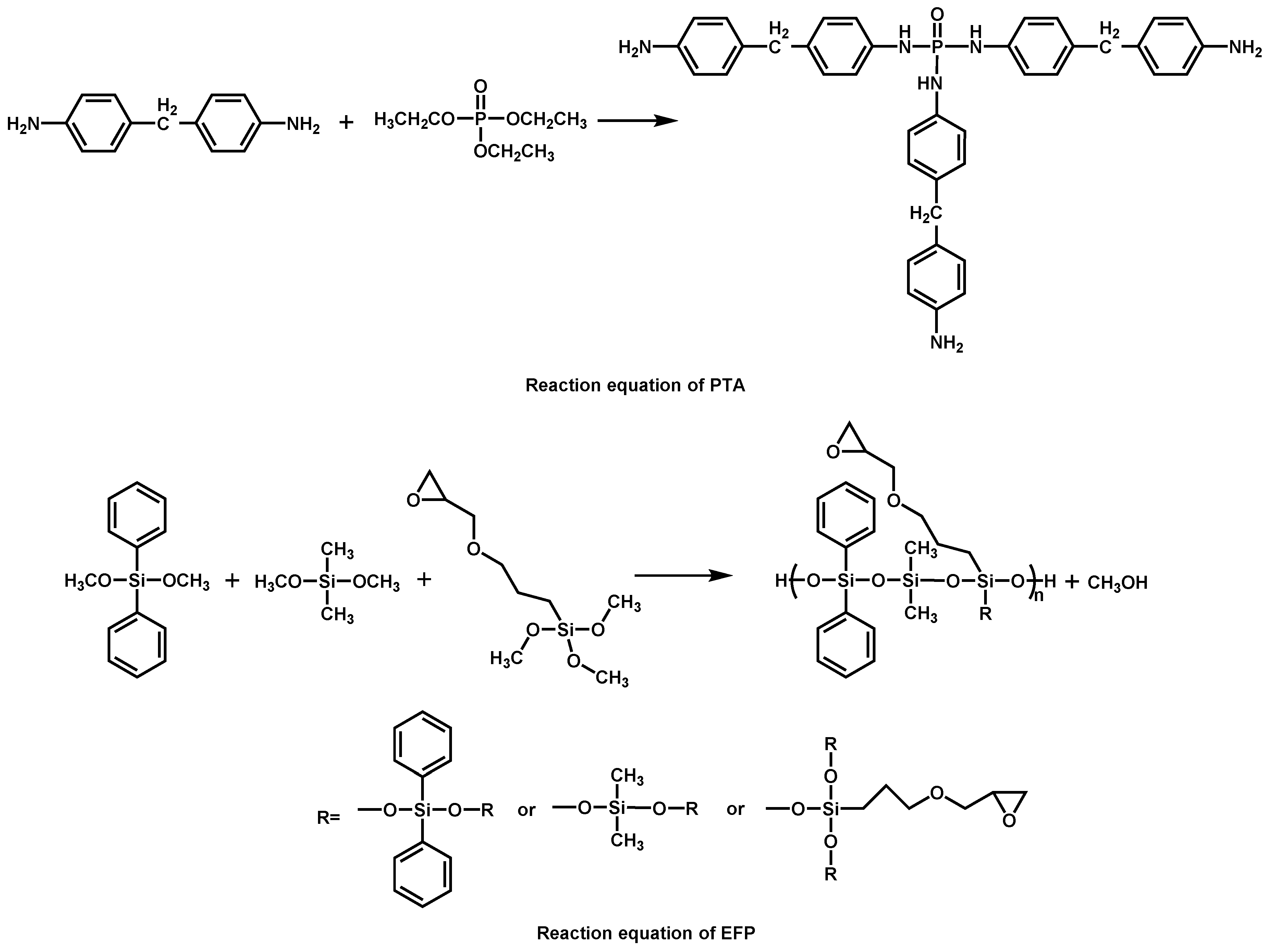
| EP/PTA/EFP | 0/0 | 20.7 | NR | – | – | [87] |
| 10/0 | 23.5 | NR | – | – | [87] | |
| 0/10 | 24.8 | NR | – | – | [87] | |
| 5/5 | 30.2 | V-1 | – | – | [87] | |
| EP/SPDS/SPDM | 0/0 | 20.2 | – | 1650 | 213 | [94] |
| 5.5/0 | 28.9 | – | 1378 | 203 | [94] | |
| 0/5.5 | 25.1 | – | – | – | [94] | |
| 5.2/5.2 | 30.8 | – | 1122 | 207 | [94] | |
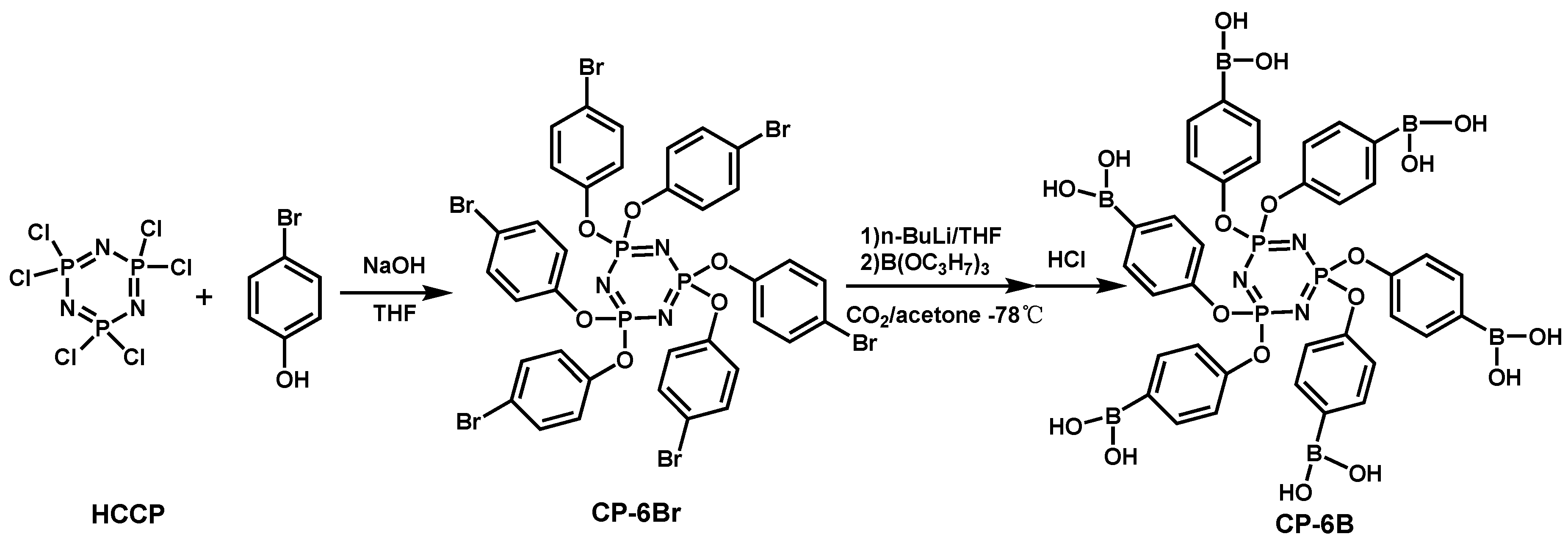
| EP/CP-6B | 0 | 22.8 | NR | 1026 | 83 | [99] |
| 1 | 29.6 | V-1 | 709 | 78 | [99] | |
| 3 | 30.8 | V-0 | 599 | 74 | [99] | |
| 5 | 31.4 | V-0 | 446 | 58 | [99] | |
| 7 | 32.3 | V-0 | 359 | 54 | [99] | |
| 10 | 28.6 | V-0 | 471 | 60 | [99] | |
| CL-CTPN-EP/Dicyandiamide | – | 32.4 | V-0 | – | – | [100] |
| CL-CTPN-EP/DDM | – | 31.6 | V-0 | – | – | [100] |
| CL-CTPN-EP/novolak | – | 30.2 | V-0 | – | – | [100] |
| EP/BPS-BPP | 0 | 21.5 | NR | 1000 | 89 | [103] |
| 3 | 27.5 | NR | – | – | [103] | |
| 6 | 28.7 | V-1 | – | – | [103] | |
| 9 | 29.7 | V-1 | 537 | 76 | [103] | |
| 12 | 28.3 | V-1 | – | – | [103] | |
| EP/CTP-DOPO | 0 | 21.7 | NR | 619.8 | 77.6 | [104] |
| 9.7 | 34.3 | V-1 | – | – | [104] | |
| 10.6 | 36.6 | V-0 | 282.6 | 51.7 | [104] | |
| 11.7 | 38.5 | V-0 | – | – | [104] | |
| 12.6 | 39.8 | V-0 | – | – | [104] | |
| EP/HPCTP/OGPOSS | 0/0 | – | NR | 1321 | 157 | [105] |
| 15/0 | – | V-0 | 1026 | 145 | [105] | |
| 10/5 | – | V-0 | 707 | 123 | [105] | |
| 7.5/7.5 | – | V-0 | 581 | 110 | [105] | |
| 5/10 | – | V-0 | 560 | 105 | [105] | |
| 0/15 | – | NR | 513 | 82 | [105] |
| EPs and Incorporated Carbon-Based Materials | wt.% | LOI (%) | UL-94 | pk-HRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|
| EP | 2 | – | – | 1653 | 129.9 | [114] |
| EP/GNS | 2 | – | – | 1156 | 107.8 | [114] |
| EP/Ce-MnO2 | 2 | – | – | 920 | 96.7 | [114] |
| EP/Ce-MnO2-GNS | 2 | – | – | 765 | 83.8 | [114] |
| EP | 2 | – | – | 1348 | 87.1 | [115] |
| EP/MoS2 | 2 | – | – | 1076 | 75.7 | [115] |
| EP/GNS | 2 | – | – | 965 | 70.1 | [115] |
| EP/MoS2/GNS | 2 | – | – | 730 | 65.1 | [115] |
| EP | 2 | – | – | 1730 | 113.1 | [116] |
| EP/GNS | 2 | – | – | 980 | 65.1 | [116] |
| EP/Ni-Fe LDH | 2 | – | – | 1070 | 58.9 | [116] |
| EP/Ni-Fe LDH/GNS | 2 | – | – | 678 | 44.2 | [116] |
| CF-EP | 0 | – | – | 568 | 23.2 | [120] |
| CF-EP/SWCNT-buckypaper | 1 | – | – | 526 | 24.5 | [120] |
| CF-EP/MWCNT-buckypaper | 1.3 | – | – | 258 | 13.2 | [120] |
| CF-EP/CNF paper | 1.5 | – | – | 508 | 24.8 | [120] |
| EP/VETS-CNT | 0 | 22 | V-1 | – | – | [121] |
| 1 | 23 | V-1 | – | – | [121] | |
| 3 | 25 | V-0 | – | – | [121] | |
| 5 | 26 | V-0 | – | – | [121] | |
| 7 | 27 | V-0 | – | – | [121] | |
| 9 | 29 | V-0 | – | – | [121] | |
| EP | 0 | 21.5 | NR | 900 | – | [122] |
| EP/Melamine | 8 | 22.4 | NR | 750 | – | [122] |
| EP/Mo-PR/Melamine | 2/0 | 24 | NR | 543 | – | [122] |
| 1/8 | 28 | V-2 | – | – | [122] | |
| 2/8 | 29.5 | V-0 | 579 | – | [122] | |
| EP/CNT-PR/Melamine | 1/8 | 27.7 | V-2 | 527 | – | [122] |
| 3/8 | 28.6 | V-2 | 535 | – | [122] | |
| 5/8 | 29.5 | V-0 | 468 | – | [122] | |
| EP/CF/CNT | 0/0 | – | – | 971.7 | 98.8 | [123] |
| 0.5/0 | – | – | 792.7 | 92.5 | [123] | |
| 0.7/0 | – | – | 722.6 | 88.2 | [123] | |
| 1/0 | – | – | 840.2 | 88.9 | [123] | |
| 1.5/0 | – | – | 793.3 | 101.7 | [123] | |
| 0.5/0.5 | – | – | 648.1 | 75 | [123] | |
| 0.7/0.7 | – | – | 635 | 80.3 | [123] | |
| 1/0.5 | – | – | 701.7 | 99.3 | [123] | |
| EP/PPMS-CNT | 0 | 19.3 | HB | 1334.6 | 100.1 | [124] |
| 5 | 21.5 | HB | 1013.4 | 93.7 | [124] | |
| 10 | 22.6 | V-2 | 680.7 | 90.7 | [124] | |
| 15 | 24.5 | V-2 | 444.6 | 77.6 | [124] | |
| epoxy- aliphatic amine system/EG | 0 | 18.7 | – | – | – | [130] |
| 1 | 21.3 | – | – | – | [130] | |
| 2 | 25 | – | – | – | [130] | |
| 3 | 28 | – | – | – | [130] | |
| 4 | 30 | – | – | – | [130] | |
| Jatropha curcas oil-based alkyd-EP/EG | 0 | 18 | – | – | – | [131] |
| 0.5 | 21 | – | – | – | [131] | |
| 1.5 | 24 | – | – | – | [131] | |
| 2.5 | 29 | – | – | – | [131] | |
| 4 | 35 | – | – | – | [131] | |
| 5 | 41 | – | – | – | [131] |
| EPs and Incorporated Silicon Flame Retardants | wt.% | LOI (%) | UL-94 | pk-HRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|
| EP | 0 | 26.4 | NR | 1420 | 144 | [144] |
| EP/DDSi-1 | 4 | 32.4 | V-1 | 1115 | 105 | [144] |
| 6 | 34.1 | V-1 | 907 | 101 | [144] | |
| 8 | 35.9 | V-1 | 743 | 95 | [144] | |
| EP/DDSi-2 | 8 | 34.8 | V-0 | 779 | 98 | [144] |
| EP/DDSi-5 | 8 | 33 | V-0 | 892 | 95 | [144] |
| EP | 0 | 26.4 | NR | 1420 | 144 | [145] |
| EP/DDSi-1 | 4 | 32.4 | V-1 | 1115 | 105 | [145] |
| EP/TriDSi | 4 | 33.4 | V-1 | – | – | [145] |
| EP/TetraDSi | 4 | 34.6 | V-1 | – | – | [145] |
| EP/DDSi-1 | 6 | 34.1 | V-1 | 907 | 101 | [145] |
| EP/TriDSi | 6 | 35.2 | V-0 | 810 | 90 | [145] |
| EP/TetraDSi | 6 | 36 | V-0 | 776 | 83 | [145] |
| EP | 0 | – | – | 1377.7 | 86.6 | [149] |
| EP/HM-SiO2 | 0.5 | – | – | 1226.4 | 67 | [149] |
| 2 | – | – | 860.6 | 69.8 | [149] | |
| EP/HM-SiO2@CS@PCL | 0.5 | – | – | 791.8 | 67.2 | [149] |
| 2 | – | – | 676.3 | 86.3 | [149] | |
| EP | 0 | 20.6 | NR | 811.1 | 114.2 | [152] |
| EP/RH-SiO2/DOPO-J-ESO | 20/0 | 30.9 | NR | 520 | 78.8 | [152] |
| 20/5 | 33.2 | V-0 | 482.2 | 52.9 | [152] | |
| 20/10 | 35.8 | V-0 | 436.8 | 41.5 | [152] | |
| 20/15 | 36.9 | V-0 | 425.9 | 36 | [152] | |
| EP /DOPO-J-ESO | 10 | 31.8 | V-0 | 506.2 | 71.5 | [152] |
| EP | 0 | 23 | NR | 893 | 112 | [160] |
| EP/DPP-POSS | 5 | 33.2 | V-0 | 489 | 94.1 | [160] |
| EP/DPOP-POSS | 5 | 29.3 | V-1 | 419 | 87.8 | [160] |
| EP/DOPO-POSS | 5 | 30 | V-1 | 433 | 91.1 | [160] |
| EP/ODMAS | 0 | 25.6 | NR | – | – | [161] |
| 1 | 29.7 | V-1 | – | – | [161] | |
| 5 | 35.5 | V-0 | – | – | [161] | |
| 10 | 36.5 | V-0 | – | – | [161] | |
| 15 | 37.1 | V-0 | – | – | [161] | |
| EP/POSS-bisDOPO | 0 | 25.4 | – | – | – | [162] |
| 1 | 29.3 | – | – | – | [162] | |
| 5 | 31.7 | – | – | – | [162] | |
| 10 | 33.2 | – | – | – | [162] | |
| 20 | 34.5 | – | – | – | [162] | |
| EP/DOPO | 5 | 29.7 | – | – | – | [162] |
| EP/POSS | 5 | 26.9 | – | – | – | [162] |
| EP/DOPO + POSS | 5 | 29.2 | – | – | – | [162] |
| EPs and Incorporated Nanocomposites | wt.% | LOI (%) | UL-94 | pk-HRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|
| EP | 0 | 21.8 | NR | 781 | 107 | [172] |
| EP/DiDOPO | 1 | 24.1 | V-2 | – | – | [172] |
| 3 | 32.7 | V-0 | – | – | [172] | |
| 7 | 35.7 | V-0 | 491 | 80 | [172] | |
| EP/OMMT | 1 | 22.4 | NR | – | – | [172] |
| 3 | 23.7 | NR | – | – | [172] | |
| 7 | 23.7 | NR | 576 | 98 | [172] | |
| EP/DiDOPO/OMMT | 0.5/0.5 | 23.2 | NR | – | – | [172] |
| 1.5/1.5 | 27.1 | V-0 | – | – | [172] | |
| 3.5/3.5 | 32.2 | V-0 | 369 | 95 | [172] | |
| EP | 0 | 21 | – | – | – | [173] |
| EP/MMT/MWCNT | 1.5/0.1 | 26.4 | – | – | – | [173] |
| EP/Fluorinated MMT | 1.5 | 25.8 | – | – | – | [173] |
| EP/Fluorinated MWCNT | 0.1 | 23 | – | – | – | [173] |
| EP/Fluorinated MMT/Fluorinated MWMMT | 1.5/0.1 | 31 | – | – | – | [173] |
| EP | 0 | 23 | NR | 860 | 112 | [174] |
| EP/APP | 10 | 25 | NR | 458 | 62 | [174] |
| EP/APP + MMT | 10 | 28 | V-0 | 524 | 50 | [174] |
| EP/APP-MMT | 10 | 30 | V-0 | 393 | 34 | [174] |
| EPs and Incorporated Metal-Containing Compounds | wt.% | LOI (%) | UL-94 | pk-HRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|
| EP | 0 | 22.2 | – | – | – | [182] |
| EP/APHNR | 7.8 | 29.8 | – | – | – | [182] |
| EP/APHNSH | 7.8 | 26.4 | – | – | – | [182] |
| EP/FPHNR | 7.8 | 27.4 | – | – | – | [182] |
| EP/FPHNSH | 7.8 | 29.8 | – | – | – | [182] |
| EP/ZPHNR | 7.8 | 22.2 | – | – | – | [182] |
| EP/ZPHNSH | 7.8 | 25.2 | – | – | – | [182] |
| EP | 0 | 19.6 | NR | 939 | 179 | [183] |
| EP/APP | 5 | 27.1 | V-0 | 283 | 111 | [183] |
| EP/APP-CoSA | 4.97/0.03 | 28 | V-0 | – | – | [183] |
| 4.92/0.08 | 28.4 | V-0 | – | – | [183] | |
| 4.83/0.17 | 29.4 | V-0 | 310 | 95 | [183] | |
| 4.75/0.25 | 28.4 | V-0 | – | – | [183] | |
| 4.67/0.33 | 28.7 | V-0 | – | – | [183] | |
| EP | 0 | – | HB | 1068 | 75.8 | [184] |
| EP/MPAlP | 20 | – | HB | 540 | 60 | [184] |
| EP/MPZnP | 20 | – | HB | 312 | 60 | [184] |
| EP/MPMgP | 20 | – | V-1 | 298 | 57.3 | [184] |
| EP/MPZnP + MPP | 10/10 | – | V-1 | 207 | 51.1 | [184] |
| 6.7/13.3 | – | V-0 | 211 | 32.5 | [184] | |
| EP/MPZnP + AlPi-Et | 10/10 | – | HB | 405 | 51.2 | [184] |
| 6.7/13.3 | – | V-1 | 435 | 53.8 | [184] | |
| EP/MPZnP + DOPAc-Bu | 10/10 | – | V-1 | 329 | 57.6 | [184] |
| 6.7/13.3 | – | HB | 412 | 52.1 | [184] | |
| EP/MPZnP + AlO (OH) | 10/10 | – | HB | 438 | 57.2 | [184] |
| 6.7/13.3 | – | HB | 575 | 57.9 | [184] | |
| EP/MPZnP + SiO2 | 10/10 | – | HB | 525 | 62.4 | [184] |
| 6.7/13.3 | – | HB | 681 | 65.6 | [184] | |
| EP/MPP | 20 | – | V-0 | 244 | 26.6 | [184] |
| EP/AlPi-Et | 20 | – | V-0 | 492 | 55.8 | [184] |
| EP/DOPAc-Bu | 20 | – | HB | 624 | 50.2 | [184] |
| EP/AlO (OH) | 20 | – | HB | 870 | 65.5 | [184] |
| EP/SiO2 | 20 | – | HB | 907 | 57.6 | [184] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. https://doi.org/10.3390/ma13092145
Liu Q, Wang D, Li Z, Li Z, Peng X, Liu C, Zhang Y, Zheng P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials. 2020; 13(9):2145. https://doi.org/10.3390/ma13092145
Chicago/Turabian StyleLiu, Quanyi, Donghui Wang, Zekun Li, Zhifa Li, Xiaoliang Peng, Chuanbang Liu, Yu Zhang, and Penglun Zheng. 2020. "Recent Developments in the Flame-Retardant System of Epoxy Resin" Materials 13, no. 9: 2145. https://doi.org/10.3390/ma13092145
APA StyleLiu, Q., Wang, D., Li, Z., Li, Z., Peng, X., Liu, C., Zhang, Y., & Zheng, P. (2020). Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials, 13(9), 2145. https://doi.org/10.3390/ma13092145





