Thermal Conductivity Characterization of Thermal Grease Containing Copper Nanopowder
Abstract
1. Introduction
2. Experiment Equipment and Method
2.1. Experiment Preparation
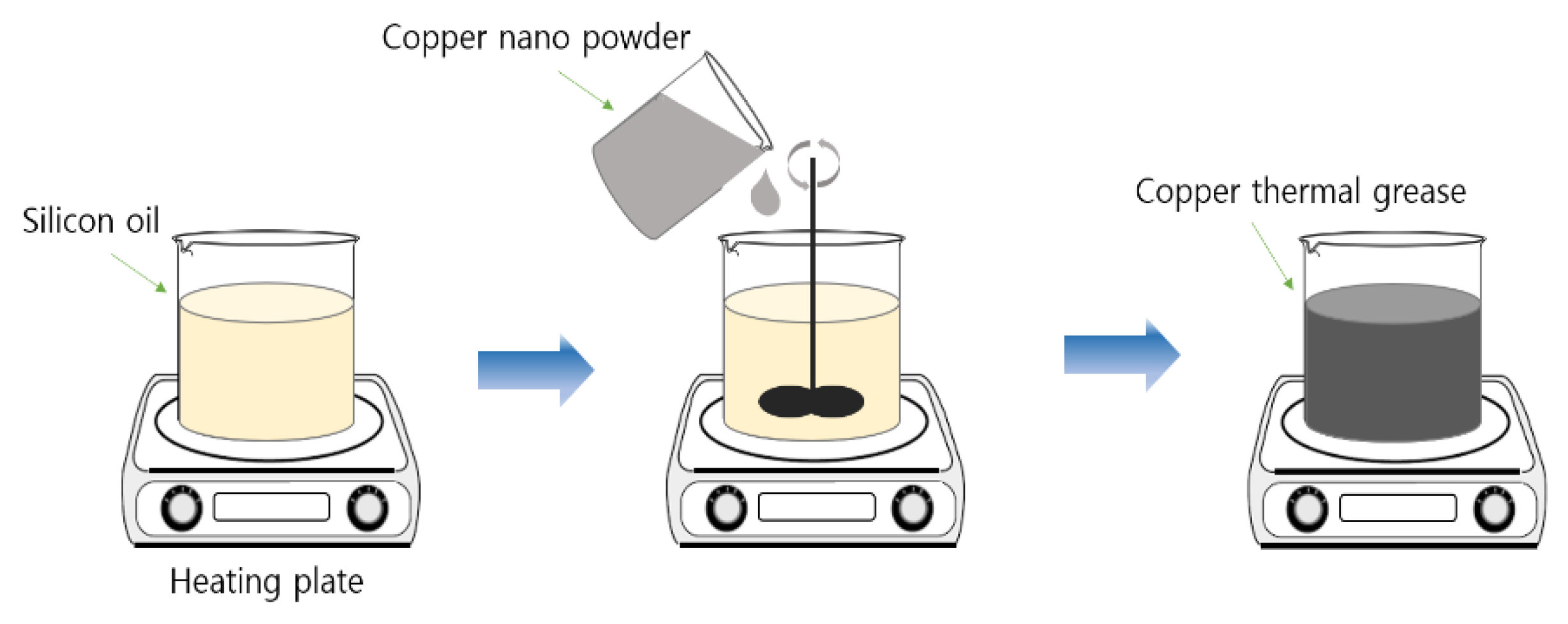
2.2. Nano Thermal Grease Manufactured
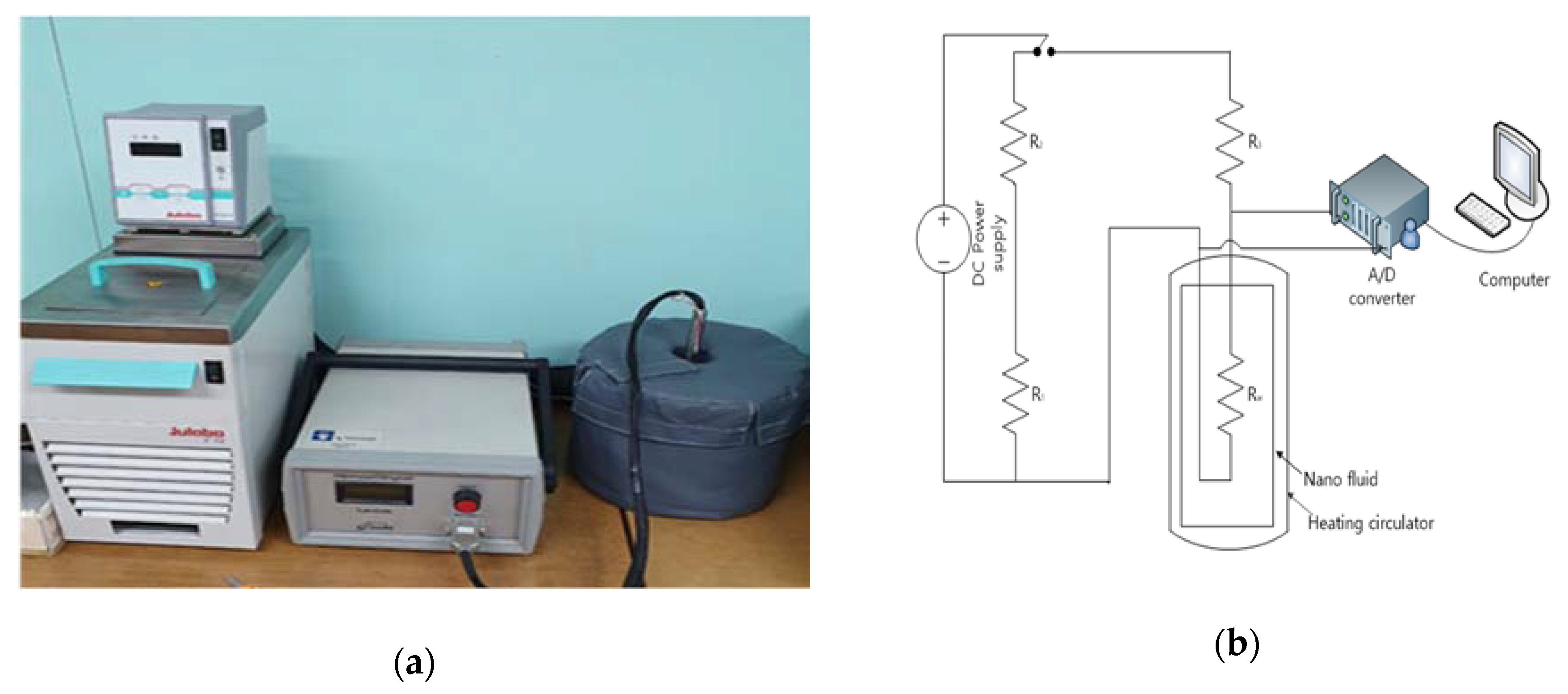
2.3. Measuring Equipment
3. Results
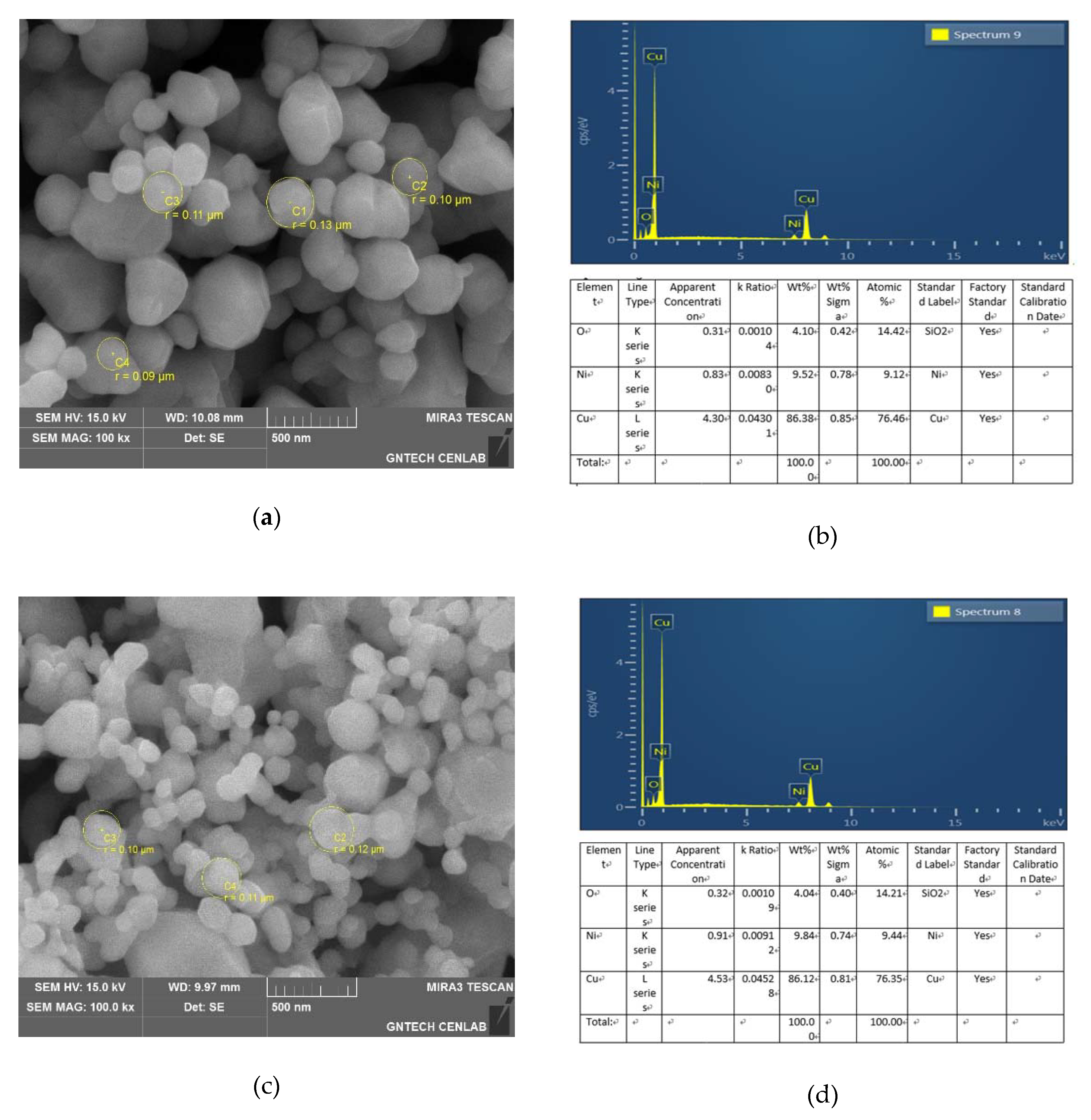
3.1. Copper Nano Powder Manufacturing Result
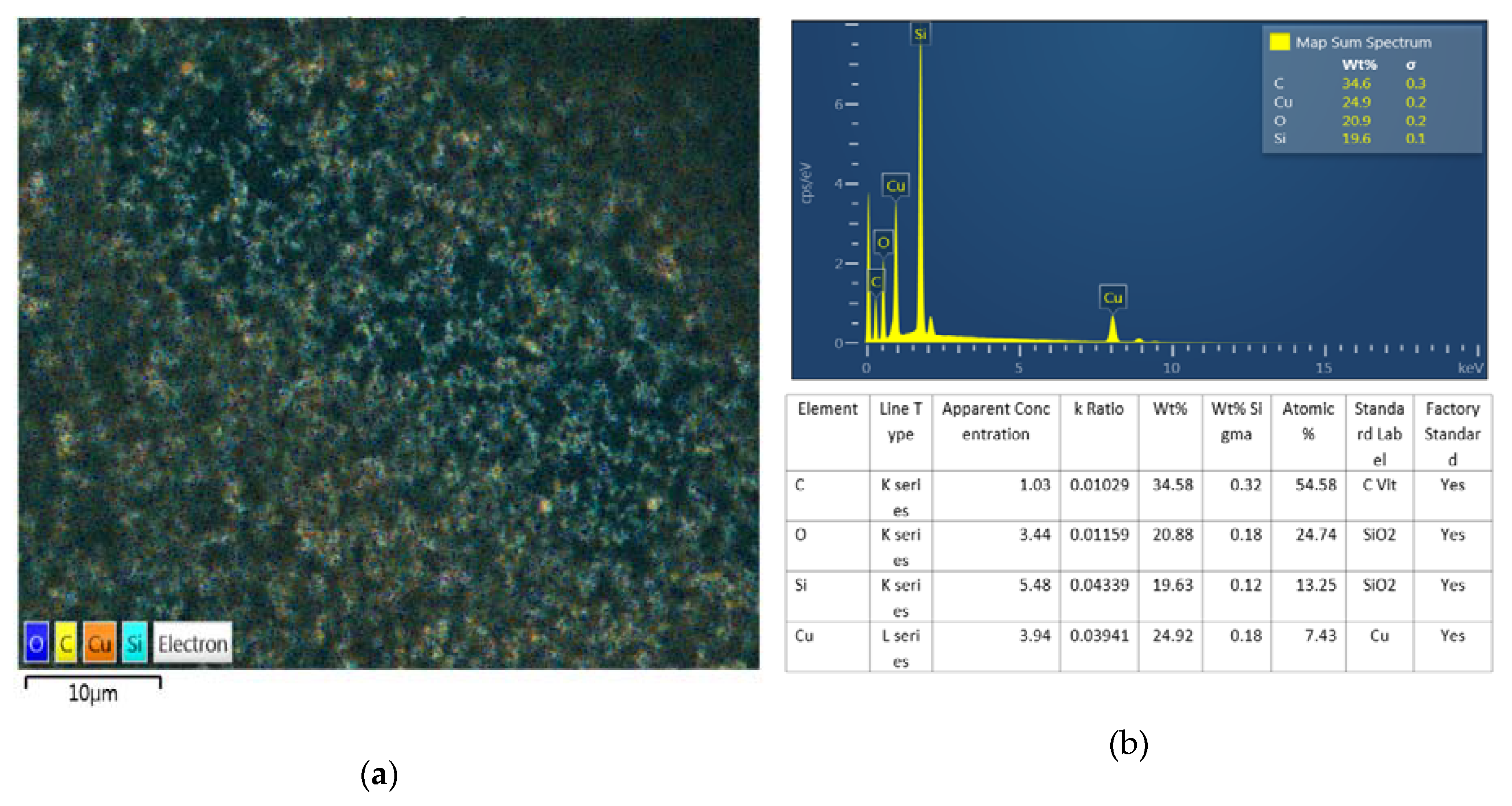
3.2. Thermal Grease Manufacturing Result
3.3. Thermal Conductivity Characteristics of Manufactured Thermal Grease
3.4. Thermal Conductivity of Thermal Grease
4. Conclusions
- As a result of observation by FE-SEM, it can be seen that the prepared nano-powder has a uniform spherical shape with an average size of 100 nm in radius. In addition, it was confirmed that the particle size volume ratio was 85:15.
- As a result of BEI observation after nano thermal grease production, it was confirmed that the nano powders were not agglomerated but evenly distributed.
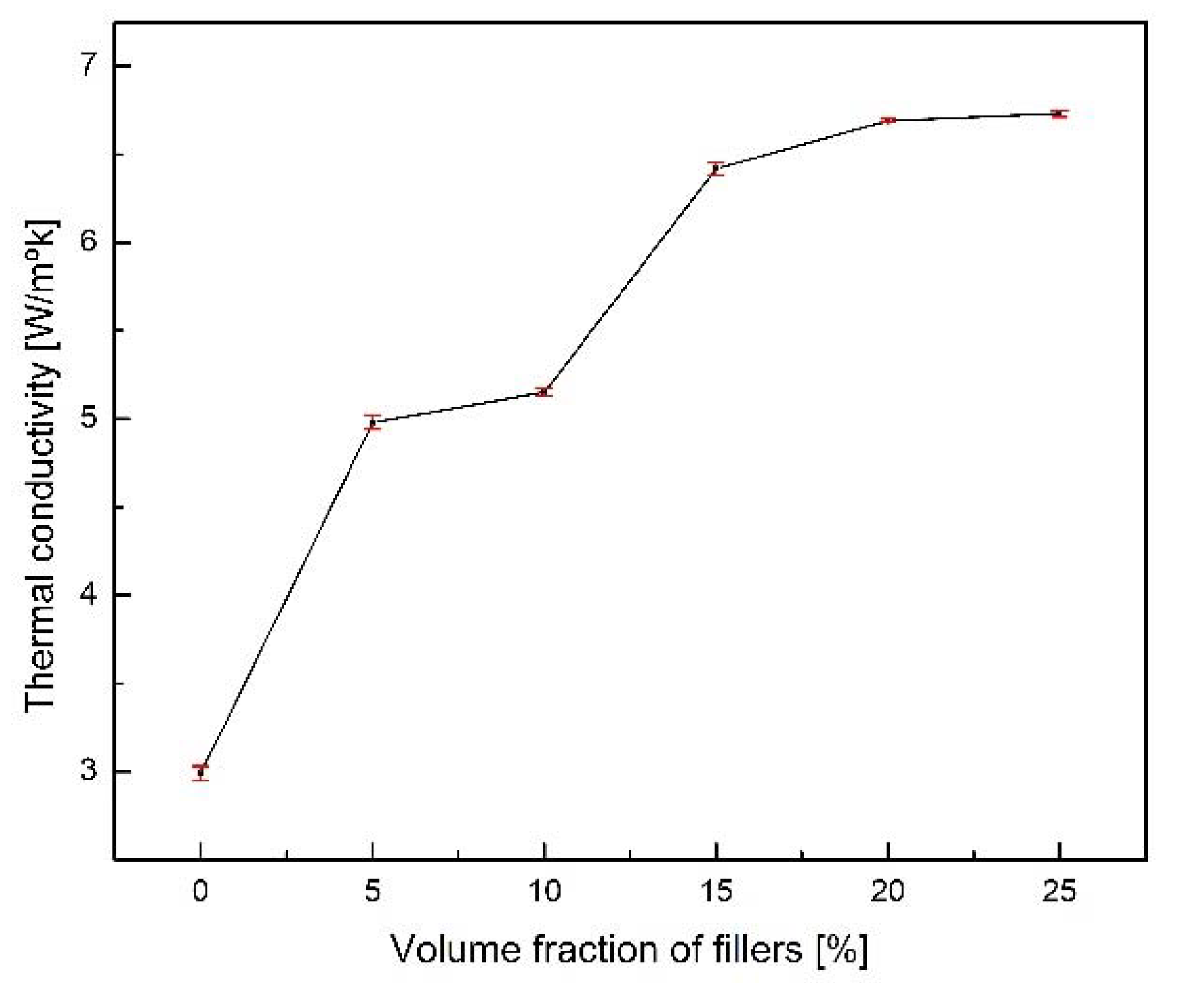
- Up to 15 vol. %, the upward trend of thermal conductivity increases steadily, while after 20 vol. %, the increase trend decreases. In addition, the increase rate of thermal conductivity from 0 to 5 vol. % and 10 to 15 vol. % is the largest.
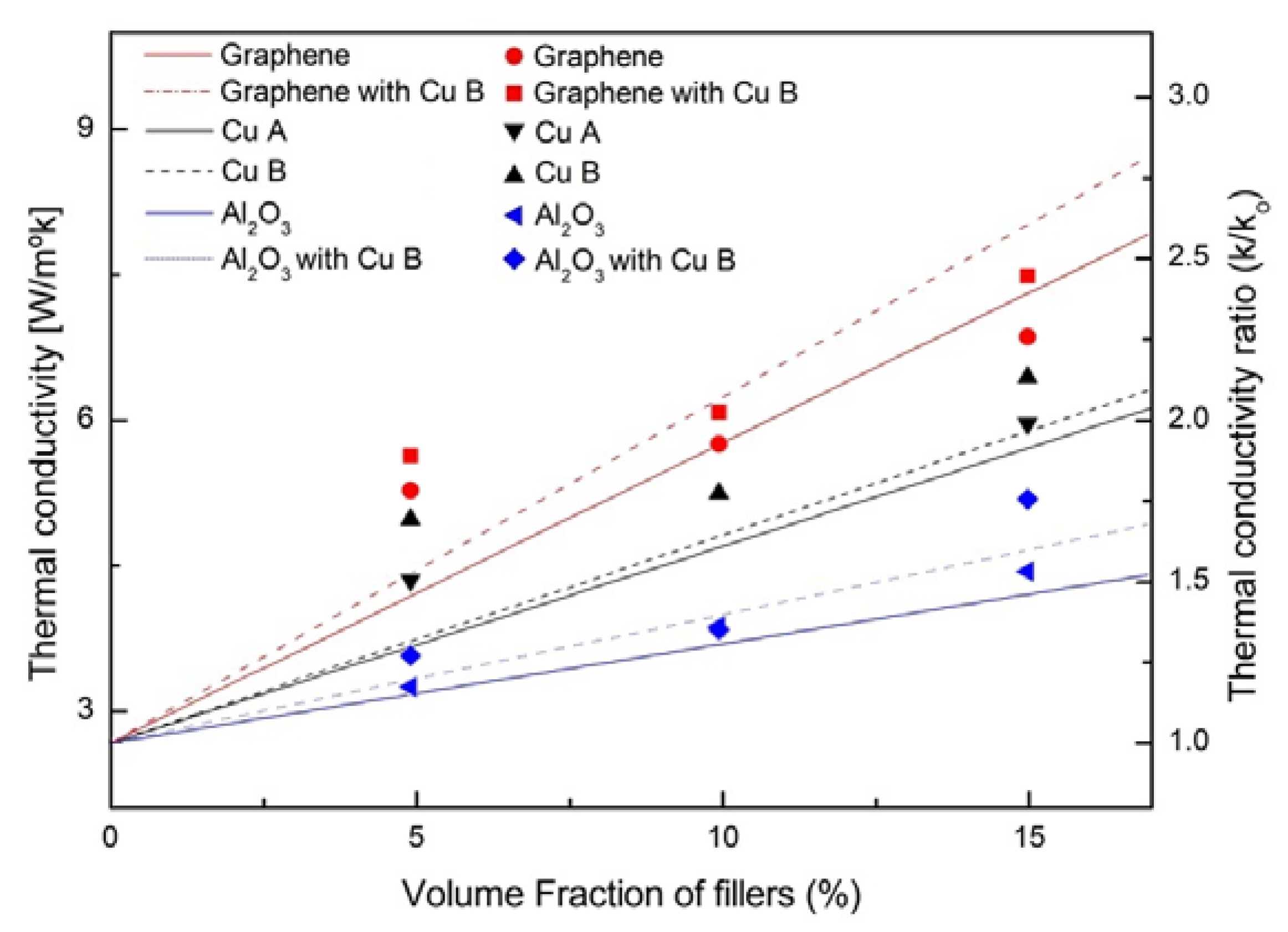
- There is no significant difference in thermal conductivity between copper powder A and copper powder B, but there is a slight difference in thermal conductivity when other additives are added.
Author Contributions
Funding
Conflicts of Interest
References
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Sarvar, F.; Whalley, D.C.; Conway, P.P. Thermal interface materials—A review of the state of the art. In Proceedings of the 2006 1st Electronic Systemintegration Technology Conference, IEEE, Dresden, Germany, 5 September 2006; Volume 2, pp. 1292–1302. [Google Scholar]
- Razeeb, K.M.; Dalton, E.; Cross, G.L.W.; Robinson, A.J. Present and future thermal interface materials for electronic devices. Int. Mater. Rev. 2018, 63, 1–21. [Google Scholar] [CrossRef]
- Sartre, V.; Lallemand, M. Enhancement of thermal contact conductance for electronic systems. Appl. Therm. Eng. 2001, 21, 221–235. [Google Scholar] [CrossRef]
- Prasher, R. Thermal interface materials: Historical perspective, status, and future directions. Proc. IEEE 2006, 94, 1571–1586. [Google Scholar] [CrossRef]
- Shahil, K.M.; Balandin, A.A. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Chung, D.D.L. Thermal interface materials. J. Mater. Eng. Perform. 2001, 10, 56–59. [Google Scholar] [CrossRef]
- Kreupl, F.; Graham, A.P.; Duesberg, G.S.; Steinhögl, W.; Liebau, M.; Unger, E.; Hönlein, W. Carbon nanotubes in interconnect applications. Microelectron. Eng. 2002, 64, 399–408. [Google Scholar] [CrossRef]
- Leong, C.K.; Chung, D.D.L. Carbon black dispersions as thermal pastes that surpass solder in providing high thermal contact conductance. Carbon 2003, 41, 2459–2469. [Google Scholar] [CrossRef]
- Shaikh, S.; Lafdi, K.; Silverman, E. The effect of a CNT interface on the thermal resistance of contacting surfaces. Carbon 2007, 45, 695–703. [Google Scholar] [CrossRef]
- Gaier, J.R.; YoderVandenberg, Y.; Berkebile, S.; Stueben, H.; Balagadde, F. The electrical and thermal conductivity of woven pristine and intercalated graphite fiber-polymer composites. Carbon 2003, 41, 2187–2193. [Google Scholar] [CrossRef]
- Viswanath, R.; Wakharkar, V.; Watwe, A.; Lebonheur, V. Thermal performance challenges from silicone to systems. Intel. Technol. J. 2000, 3, 1–16. [Google Scholar]
- Gwinn, J.P.; Webb, R.L. Performance and testing of thermal interface materials. Microelectron. J. 2003, 34, 215–222. [Google Scholar] [CrossRef]
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N.; Goh, T.J. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Chen, H.; Wei, H.; Chen, M.; Meng, F.; Li, H.; Li, Q. Enhancing the effectiveness of silicone thermal grease by the addition of functionalized carbon nanotubes. Appl. Surf. Sci. 2013, 283, 525–531. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L.; Mroz, C. Thermally conducting aluminum nitride polymer-matrix composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1749–1757. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, D.; Wang, C.; An, Q.; Qi, S. Effect of filler size distribution on the mechanical and physical properties of alumina-filled silicone rubber. Polym. Eng. Sci. 2008, 48, 1381–1388. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; An, Q.; Ou, H. Thermal properties of heat conductive silicone rubber filled with hybrid fillers. J. Compos. Mater. 2008, 42, 173–187. [Google Scholar] [CrossRef]
- Kemaloglu, S.; Ozkoc, G.; Aytac, A. Properties of thermally conductive micro and nano size boron nitride reinforced silicon rubber composites. Thermochim. Acta 2010, 499, 40–47. [Google Scholar] [CrossRef]
- Becker, G.; Lee, C.; Lin, Z. Thermal conductivity in advanced chips: Emerging generation of thermal greases offers advantages. Adv. Packag. 2005, 14, 14. [Google Scholar]
- Lin, W.; Zhang, R.; Moon, K.S.; Wong, C.P. Molecular phonon couplers at carbon nanotube/substrate interface to enhance interfacial thermal transport. Carbon 2010, 48, 107–113. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, L.; Zhu, Z.; Zhao, J.; Zhang, Z. Graphene based silicone thermal greases. Phys. Lett. A 2014, 378, 207–211. [Google Scholar] [CrossRef]
- Lee, G.H.; Park, J.H.; Rhee, C.K.; Kim, W.W. Fabrication of Al nano powders by pulsed wire evaporation (PWE) method. J. Ind. Eng. Chem. 2003, 9, 71–75. [Google Scholar]
- Yılmaz, F.; Lee, D.J.; Song, J.W.; Hong, H.S.; Son, H.T.; Yoon, J.S.; Hong, S.J. Fabrication of cobalt nano-particles by pulsed wire evaporation method in nitrogen atmosphere. Powder Technol. 2013, 235, 1047–1052. [Google Scholar] [CrossRef]
- Kim, H.S.; Yilmaz, F.; Dharmaiah, P.; Lee, D.J.; Lee, T.H.; Hong, S.J. Characterization of Cu and Ni Nano-Fluids Synthesized by Pulsed Wire Evaporation Method. Arch. Metall. Mater. 2017, 62, 999–1004. [Google Scholar] [CrossRef]
- Peñas, J.R.V.; Ortiz de Zarate, J.M.; Khayet, M. Measurement of the thermal conductivity of nanofluids by the multicurrent hot-wire method. J. Appl. Phys. 2008, 104, 044314. [Google Scholar] [CrossRef]
- Hwang, J.; Han, T.H. Performance-aware Dynamic Thermal Management by Adaptive Vertical Throttling in 3D Network-on-Chip. J. Inst. Electron. Inf. Eng. 2014, 51, 103–110. [Google Scholar] [CrossRef]
- Cumberland, D.J.; Crawford, R.J. Handbook of Powder Technology. In The Packing of Particles; Elsevier Science: Amsterdam, The Netherlands, 1987; Volume VI. [Google Scholar]
- Yu, W.; Xie, H.; Yin, L.; Zhao, J.; Xia, L.; Chen, L. Exceptionally high thermal conductivity of thermal grease: Synergistic effects of graphene and alumina. In International Journal of Thermal Sciences 91; Elsevier Science: Amsterdam, The Netherlands, 2015; pp. 76–82. [Google Scholar]


| Material | Voltage (V) | Number of Time | Wire Diameter (mm) | Wire Length (mm) |
|---|---|---|---|---|
| Copper | 4500 | 2000 | 0.2 | 32 |
| No. | Previous Thermal Grease (cc) | S/O (cc) | Volume Ratio (Vol. %) | Copper (g) |
|---|---|---|---|---|
| 1 | 100 | 100 | 0 | 0 |
| 2 | - | 95 | 5 | 44.7 |
| 3 | - | 90 | 10 | 89.4 |
| 4 | - | 85 | 15 | 134.1 |
| 5 | - | 80 | 20 | 178.8 |
| 6 | - | 75 | 25 | 223.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Kim, H.; An, J.; Choi, S.; Yang, J.; Jeong, H.; Huh, S. Thermal Conductivity Characterization of Thermal Grease Containing Copper Nanopowder. Materials 2020, 13, 1893. https://doi.org/10.3390/ma13081893
Kang H, Kim H, An J, Choi S, Yang J, Jeong H, Huh S. Thermal Conductivity Characterization of Thermal Grease Containing Copper Nanopowder. Materials. 2020; 13(8):1893. https://doi.org/10.3390/ma13081893
Chicago/Turabian StyleKang, Haneul, Hyunji Kim, Jihye An, Siyeon Choi, Jinho Yang, Hyomin Jeong, and Sunchul Huh. 2020. "Thermal Conductivity Characterization of Thermal Grease Containing Copper Nanopowder" Materials 13, no. 8: 1893. https://doi.org/10.3390/ma13081893
APA StyleKang, H., Kim, H., An, J., Choi, S., Yang, J., Jeong, H., & Huh, S. (2020). Thermal Conductivity Characterization of Thermal Grease Containing Copper Nanopowder. Materials, 13(8), 1893. https://doi.org/10.3390/ma13081893





