Numerical Simulation of the Behavior of Hydrogen Source in a Novel Welding Process to Reduce Diffusible Hydrogen
Abstract
1. Introduction
2. Simulation Model
2.1. Arc Model
2.2. Simplified Evaporation Model of the Hydrogen Source Gas
3. Diffusible Hydrogen Content Measurement
3.1. Preparation of Measurement Samples
3.2. Collection of Diffusible Hydrogen
3.3. Measurement of Amount of Hydrogen
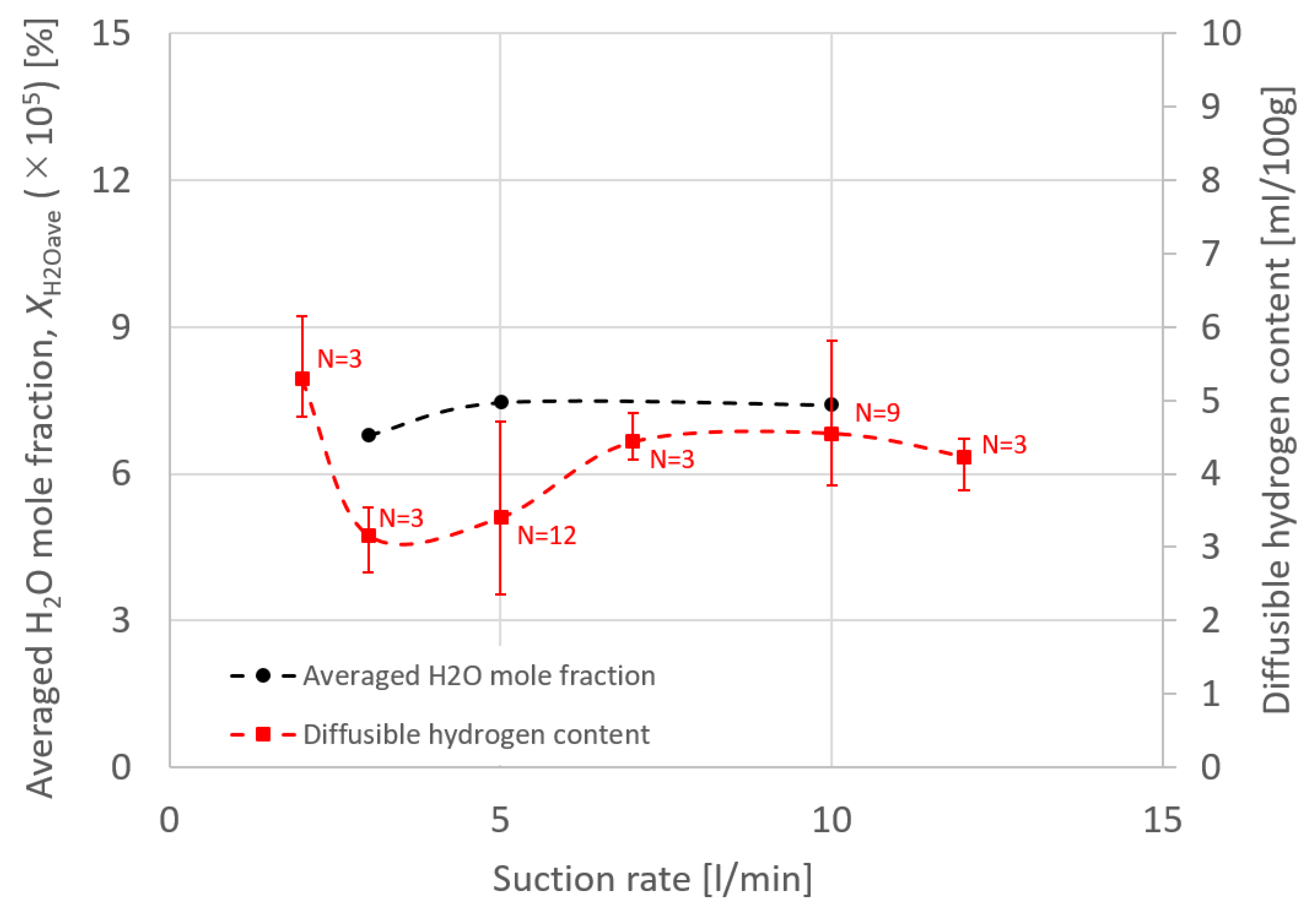
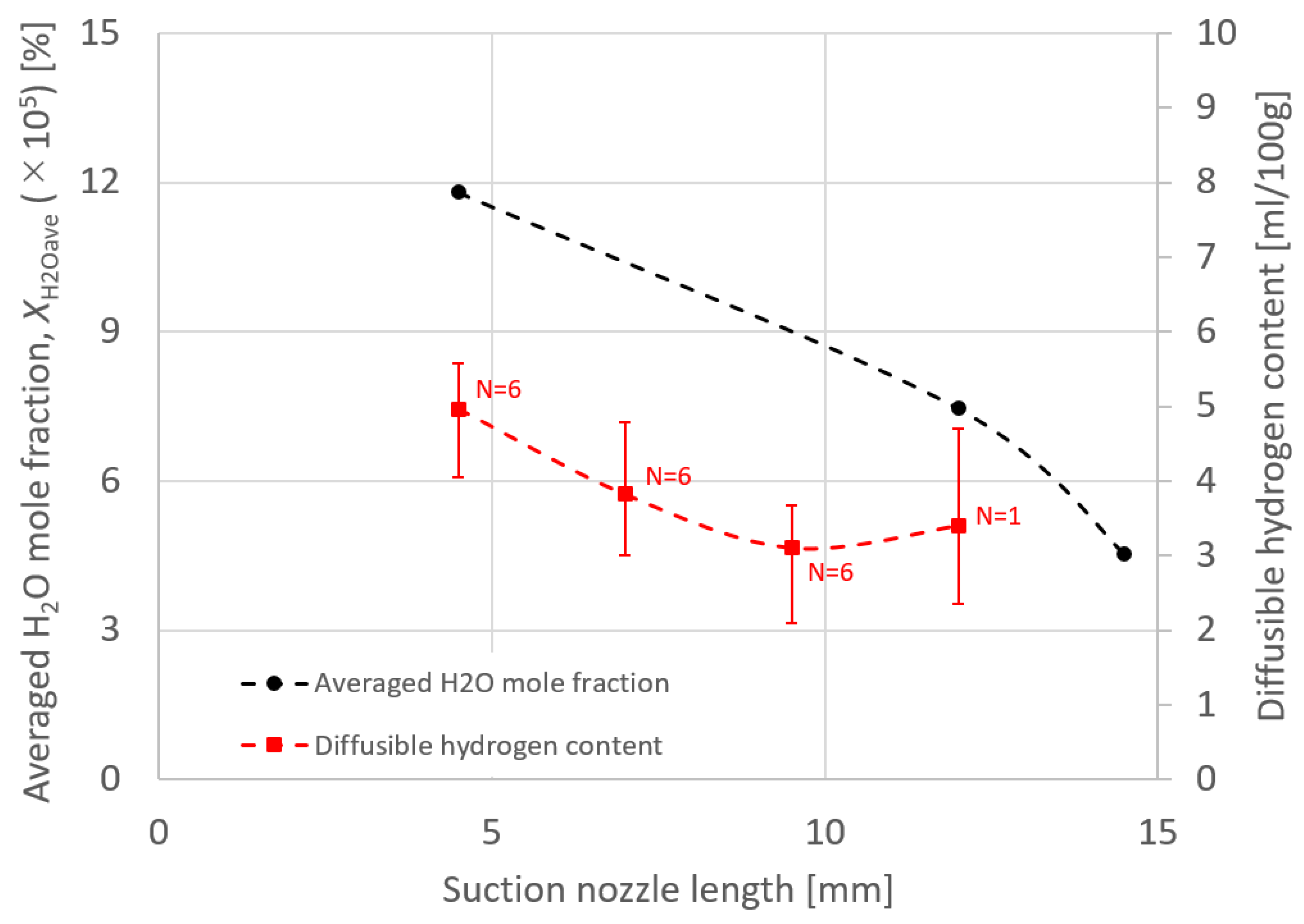
4. Results and Discussion
5. Conclusions
- (1)
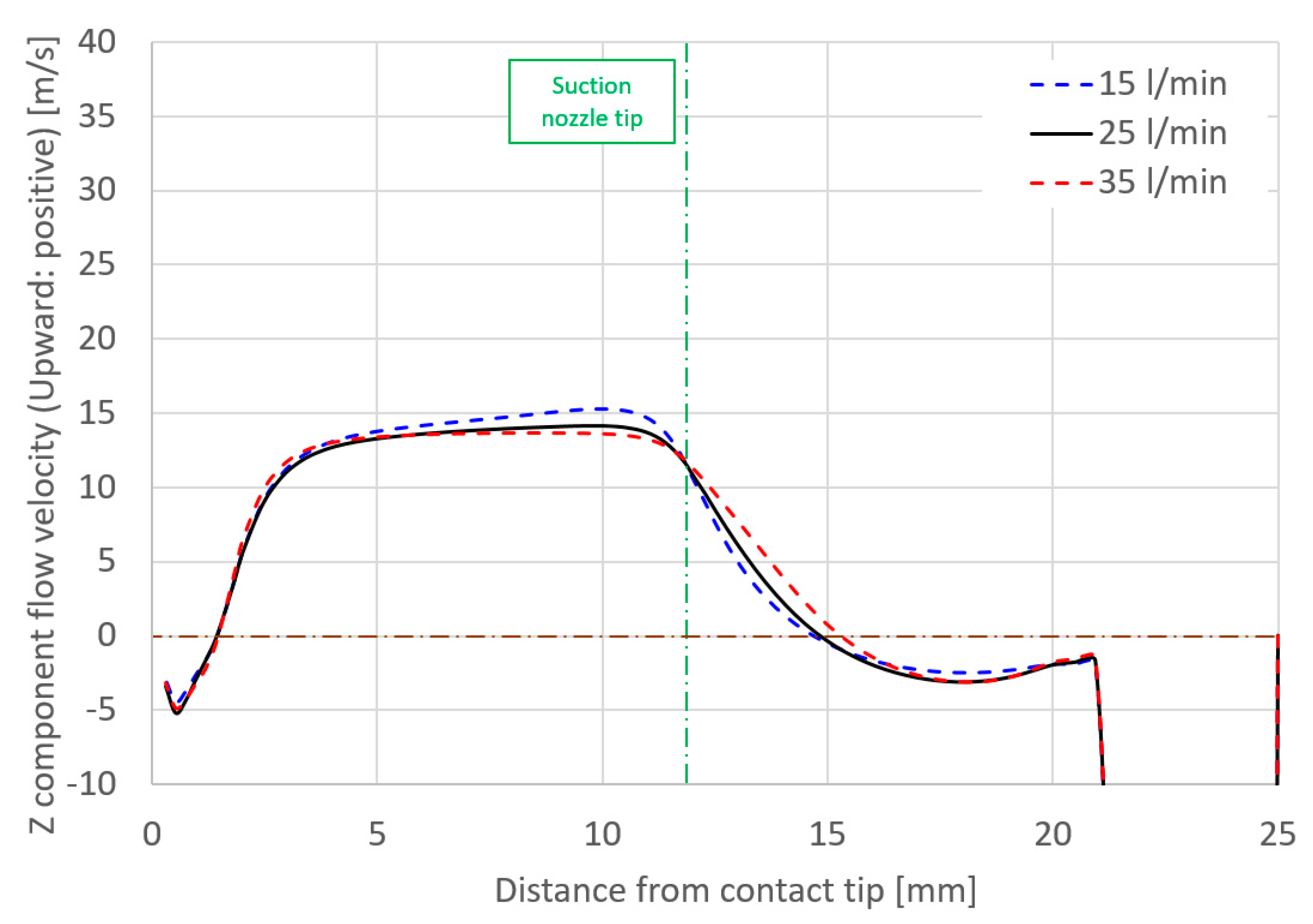
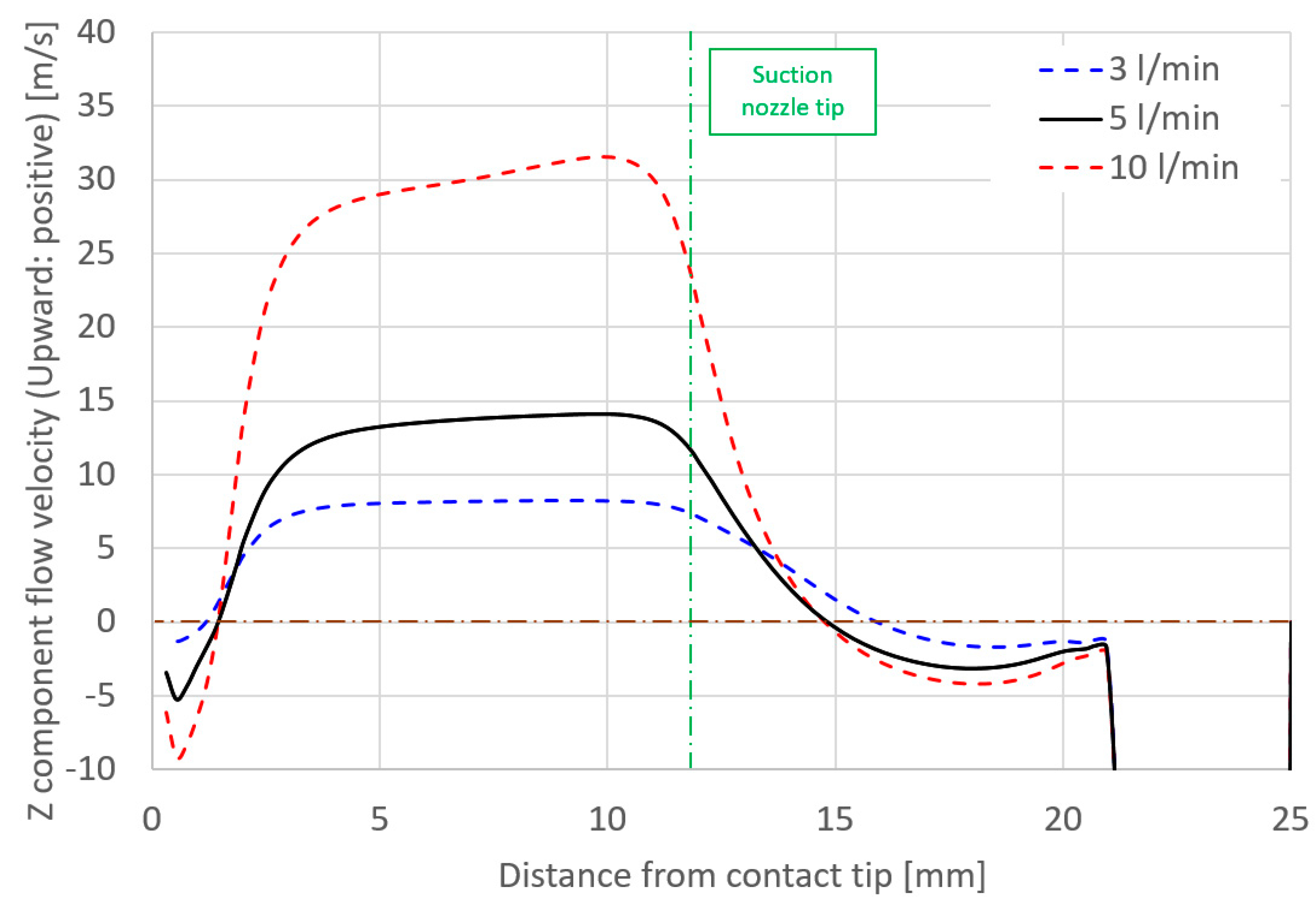
- Some of the shielding gas introduced from a shielding nozzle was drawn inward and branched into an upward flow that was sucked into the suction nozzle and a downward flow to a base metal. This branching height was defined as the suction limit height, which decisively governed the suction efficiency.
- (2)
- The effect of gas suction from the nozzle on the shielding effect of the welding was shown to be small.
- (3)
- In order to reduce the diffusible hydrogen, it was suggested that the suction limit height should be controlled towards below the wire position, where the evaporation rate of the hydrogen source gas peaks through optimization of the suction nozzle design and the torch operating conditions.
- (4)
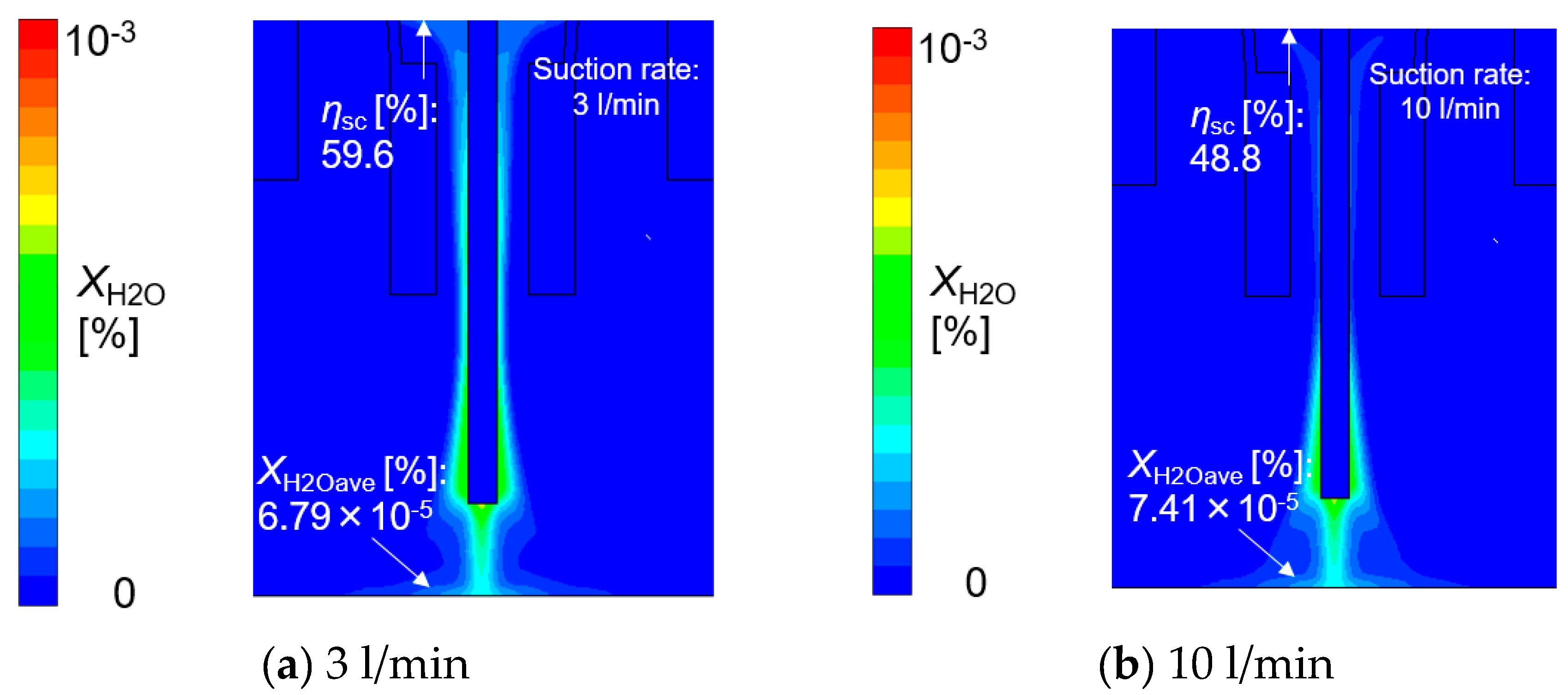
- The relative relationship between shielding gas flow and suction gas flow around the vicinity of the suction nozzle were thought to govern the suction limit height. When the effect of the shielding gas flow was stronger than that of the suction gas, the suction limit height moved to a lower location, leading to a smaller XH2Oave.
- (5)
- This method was suggested to be particularly effective for reducing the diffusible hydrogen content to extend the suction nozzle length. However, further development to prevent spatter attachment to the nozzle is required.
Author Contributions
Funding
Conflicts of Interest
References
- Kasuya, T. Cold Cracking Assessment of Steel by Experiments and Numerical Simulations. J. Jpn. Weld. Soc. 2015, 84, 45–50. [Google Scholar] [CrossRef]
- Yurioka, N.; Kasuya, T. A Chart Method to Determine Necessary Preheat Temperature in Steel Welding. Q. J. Jpn. Weld. Soc. 1995, 13, 347–357. [Google Scholar] [CrossRef]
- Swierczynska, A. Effect of Storage Conditions of Rutile Flux Cored Welding Wires on Properties of Welds. Adv. Mater. Sci. 2019, 19, 46–56. [Google Scholar] [CrossRef]
- Kawabe, N.; Maruyama, T.; Yamazaki, K.; Suzuki, R. Development of low hydrogen welding process for gas shielded arc welding. Weld. World 2016, 60, 383–392. [Google Scholar]
- Mukai, N.; Suzuki, R. Research on the Effects of Welding-wire-related Factors Affecting Diffusible Hydrogen Content. Q. J. Jpn Weld. Soc. 2017, 35, 102–109. [Google Scholar] [CrossRef][Green Version]
- Fydrych, D.; Świerczyńska, A.; Tomków, J. Diffusible hydrogen control in flux cored arc welding process. Key Eng. Mater. 2014, 597, 171–178. [Google Scholar] [CrossRef]
- Godai, T.; Sugino, M. The Hydrogen Sources in Covered Arc Welding. J. Jpn. Weld. Soc. 1976, 45–47, 14–20. [Google Scholar]
- Yamane, K. Low Hydrogen Type Electrode. J. Jpn. Weld. Soc. 1999, 68, 18–24. [Google Scholar] [CrossRef]
- Kasuya, T.; Simura, R.; Totsuka, Y. Annealing of flux cored wires and its effect on diffusible hydrogen content. Prepr. Natl. Meet. Jpn. Weld. Soc. 2009, 84, 311–312. [Google Scholar]
- Lensing, C.A.; Park, Y.D.; Maroef, I.S.; Olson, D.L. Yttrium Hydrogen Trapping to Manage Hydrogen in HSLA Steel Welds. Weld. J. 2004, 83–89, 254s–266s. [Google Scholar]
- Park, Y.D.; Maroef, I.S.; Landau, A.; Olson, D.L. Retained Austenite as a Hydrogen Trap in Steel Welds. Weld. J. 2002, 81–82, 27s–35s. [Google Scholar]
- Mathushita, M.; Liu, S. Hydrogen Control in Steel Weld Metal by Means of Fluoride Additions in Welding Flux. Weld. J. 2000, 80, 295s–303s. [Google Scholar]
- Fiore, S.; Barhorst, S.; Amata, M.; Bundy, J. A new method for reducing diffusible hydrogen in weld metal. IIW Doc. XII-2103-12. 2012. [Google Scholar]
- Mukai, N.; Maruyama, T.; Suzuki, R. Research and Development on the Welding Process for Reducing Diffusible Hydrogen. Q. J. Jpn Weld. Soc. 2018, 36, 86–93. [Google Scholar] [CrossRef][Green Version]
- Mukai, N.; Inoue, Y.; Sasakura, S.; Kinoshita, Y. Prevention of Cold Cracking by the Welding Process for Reducing Diffusible Hydrogen in High-Tensile Thick Plate Welding. Q. J. Jpn Weld. Soc. 2020, 38, 41–51. [Google Scholar] [CrossRef][Green Version]
- Tashiro, S.; Mukai, N.; Inoue, Y.; Murphy, A.B.; Suga, T.; Tanaka, M. Numerical Simulation of Gas Flow in a Novel Torch for Reducing Diffusible Hydrogen. J. Smart Process. 2019, 8, 219–224. [Google Scholar] [CrossRef]
- Tanaka, M.; Lowke, J.J. Predictions of weld pool profiles using plasma physics. J. Phys. D Appl. Phys. 2007, 40, R1–R23. [Google Scholar] [CrossRef]
- Wilke, C.R. A Viscosity Equation for Gas Mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]
- Murphy, A.B.; Arundell, C.J. Transport coefficients of argon, nitrogen, oxygen, argon-nitrogen and argon-oxygen plasmas. Plasma Chem. Plasma Process. 1994, 14, 451–490. [Google Scholar] [CrossRef]
- Murphy, A.B. Transport coefficients of air, argon-air, nitrogen-air and oxygen-air plasmas. Plasma Chem. Plasma Process. 1995, 15, 279–307. [Google Scholar] [CrossRef]
- Murphy, A.B. The effect of metal vapour in arc welding. J. Phys. D Appl. Phys. 2010, 43, 434001. [Google Scholar] [CrossRef]
- Boulos, M.I.; Fauchais, P.; Pfender, E. Thermal Plasmas: Fundamentals and Applications; Springer: New York, NY, USA, 1994; pp. 4–5. [Google Scholar]
- Subcommittee No.2, Technical Committee, Welding Rod Division, Japan Welding Engineering Society Method for Measurement of Hydrogen Evolved Gas Shielded Arc Welds. IIW Doc. II-A-561-82. 1982.
- de Abreu, L.C.; Modenesi, P.J.; Villani-Marques, P. Comparative study of methods for determining the diffusible hydrogen content in welds. Weld. Int. 2009, 9, 26–31. [Google Scholar] [CrossRef]
- Kannengiesser, T.; Tiersch, N. Comparative Study between Hot Extraction Methods and Mercury Method-A National Round Robin Test. Weld. World 2010, 54, R108–R114. [Google Scholar] [CrossRef]
- Padhy, G.K.; Komizo, Y. Diffusible Hydrogen in Steel Weldments. Trans. JWRI 2013, 42, 39–62. [Google Scholar]
- Tashiro, S.; Murphy, A.B.; Tanaka, M. Numerical simulation of fume formation process in GMA welding. Weld. World 2018, 62, 1331–1339. [Google Scholar] [CrossRef]
- Uda, M.; Ohno, S.; Wada, T. Solubility of Nitrogen in Arc- and Levitation-Melted Iron Alloys. J. Jpn. Weld. Soc. 1969, 38, 382–392. [Google Scholar] [CrossRef]
- Kodama, S.; Sugiura, K.; Nakanishi, S.; Tsujimura, Y.; Tanaka, M.; Murphy, A.B. Nitrogen Absorption Phenomenon of GTA Welding with Nitrogen Mixed Shielding Gases. Q. J. Jpn. Weld. Soc. 2013, 31, 41–47. [Google Scholar] [CrossRef]
- Xiong, Z.; Zheng, W.; Tang, L.; Yang, J. Self-Gathering Effect of the Hydrogen Diffusion in Welding Induced by the Solid-State Phase Transformation. Materials 2019, 12, 2897. [Google Scholar] [CrossRef]
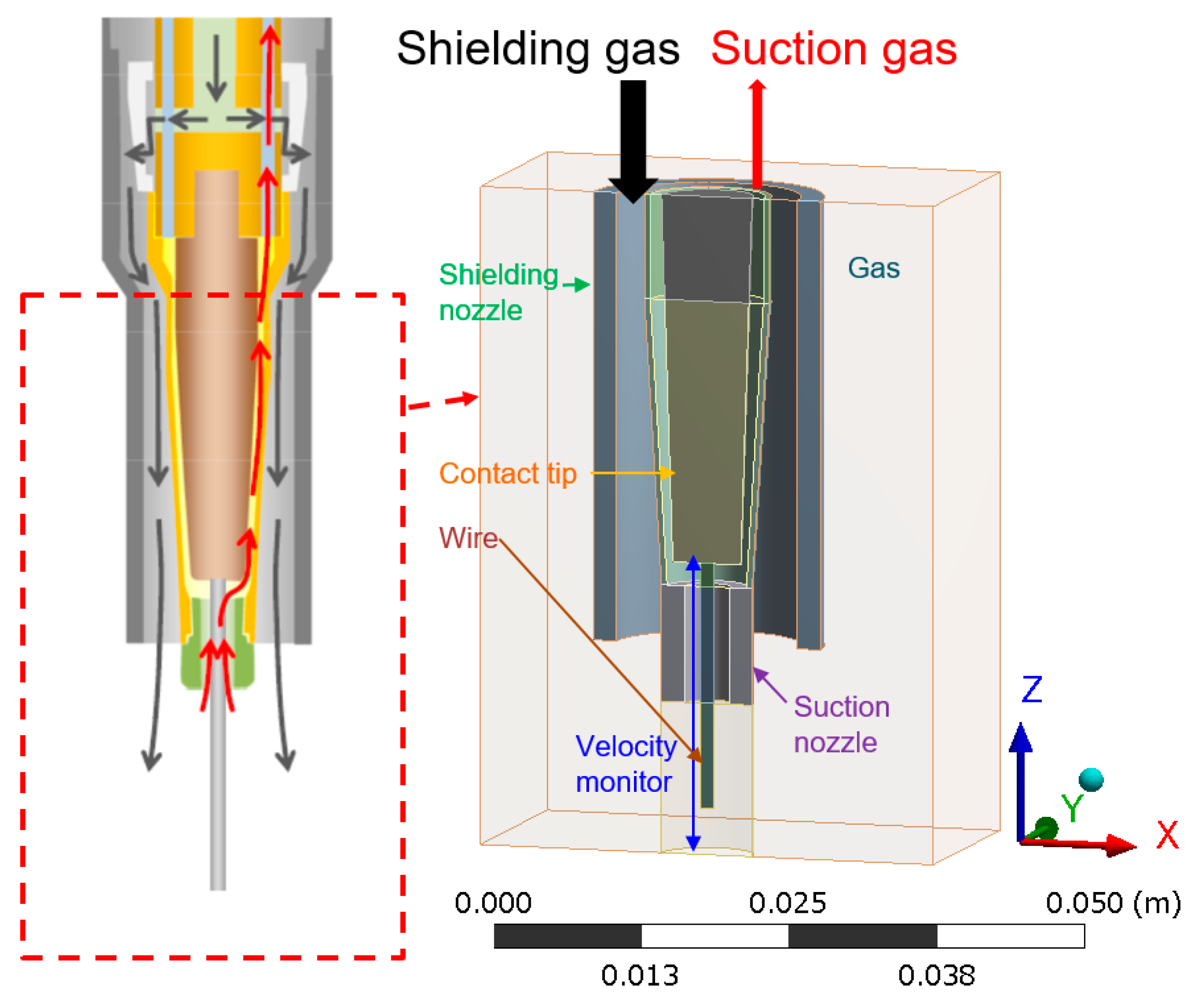











| Region Size | 40.0 mm(X) × 20.0 mm(Y) × 56.5 mm(Z) |
|---|---|
| Shielding gas | CO2 |
| Shielding gas flow rate | 15, 25, or 35 l/min (25 l/min is the standard condition) |
| Suction nozzle length | 4.5, 12, or 14.5 mm (12 mm is the standard condition) |
| Suction nozzle diameter | 4 mm |
| Suction rate | 3, 5, or 10 l/min (5 l/min is the standard condition) |
| Contact tip-to-work distance (CTWD) | 25 mm |
| Arc length | 4 mm |
| Welding current | DC280 A |
| Boundary | Velocity | Mass Fraction of CO2 | Mass Fraction of H2O | Energy | Electric Potential | Vector Potential | |
|---|---|---|---|---|---|---|---|
| Top | Shielding gas inlet | = shield | YCO2 = 1 | YH2O = 0 | 300 K | ∂Φ/∂n = 0 | ∂Ai/∂n = 0 |
| Suction gas outlet | = suction | ∂YCO2/∂n = 0 | ∂YH2O/∂n = 0 | 300 K | ∂Φ/∂n = 0 | ∂Ai/∂n = 0 | |
| Nozzles | 300 K | ∂Φ/∂n = 0 | ∂Ai/∂n = 0 | ||||
| Contact tip | 300 K | σ∂Φ/∂n = jgiven | ∂Ai/∂n = 0 | ||||
| Gas | P = 0 | YCO2 = 0 (inflow) YCO2/∂n = 0 (outflow) | YH2O = 0 (inflow) YH2O/∂n = 0 (outflow) | 300 K | ∂Φ/∂n = 0 | ∂Ai/∂n = 0 | |
| Side | Gas | P = 0 | YCO2 = 0 (inflow) YCO2/∂n = 0 (outflow) | YH2O = 0 (inflow) YH2O/∂n = 0 (outflow) | 300 K | ∂Φ/∂n = 0 | Ai = 0 |
| Bottom | Base metal | = 0 | ∂YCO2/∂n = 0 | ∂YH2O/∂n = 0 | 300 K | Φ = 0 | ∂Ai/∂n = 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashiro, S.; Mukai, N.; Inoue, Y.; Murphy, A.B.; Suga, T.; Tanaka, M. Numerical Simulation of the Behavior of Hydrogen Source in a Novel Welding Process to Reduce Diffusible Hydrogen. Materials 2020, 13, 1619. https://doi.org/10.3390/ma13071619
Tashiro S, Mukai N, Inoue Y, Murphy AB, Suga T, Tanaka M. Numerical Simulation of the Behavior of Hydrogen Source in a Novel Welding Process to Reduce Diffusible Hydrogen. Materials. 2020; 13(7):1619. https://doi.org/10.3390/ma13071619
Chicago/Turabian StyleTashiro, Shinichi, Naoki Mukai, Yoshihide Inoue, Anthony B. Murphy, Tetsuo Suga, and Manabu Tanaka. 2020. "Numerical Simulation of the Behavior of Hydrogen Source in a Novel Welding Process to Reduce Diffusible Hydrogen" Materials 13, no. 7: 1619. https://doi.org/10.3390/ma13071619
APA StyleTashiro, S., Mukai, N., Inoue, Y., Murphy, A. B., Suga, T., & Tanaka, M. (2020). Numerical Simulation of the Behavior of Hydrogen Source in a Novel Welding Process to Reduce Diffusible Hydrogen. Materials, 13(7), 1619. https://doi.org/10.3390/ma13071619






