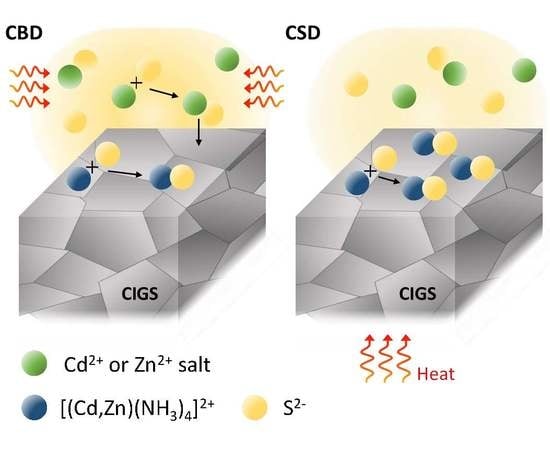
A Comparative Study of (Cd,Zn)S Buffer Layers for Cu(In,Ga)Se2 Solar Panels Fabricated by Chemical Bath and Surface Deposition Methods
Abstract
Share and Cite
Bae, D. A Comparative Study of (Cd,Zn)S Buffer Layers for Cu(In,Ga)Se2 Solar Panels Fabricated by Chemical Bath and Surface Deposition Methods. Materials 2020, 13, 1622. https://doi.org/10.3390/ma13071622
Bae D. A Comparative Study of (Cd,Zn)S Buffer Layers for Cu(In,Ga)Se2 Solar Panels Fabricated by Chemical Bath and Surface Deposition Methods. Materials. 2020; 13(7):1622. https://doi.org/10.3390/ma13071622
Chicago/Turabian StyleBae, Dowon. 2020. "A Comparative Study of (Cd,Zn)S Buffer Layers for Cu(In,Ga)Se2 Solar Panels Fabricated by Chemical Bath and Surface Deposition Methods" Materials 13, no. 7: 1622. https://doi.org/10.3390/ma13071622
APA StyleBae, D. (2020). A Comparative Study of (Cd,Zn)S Buffer Layers for Cu(In,Ga)Se2 Solar Panels Fabricated by Chemical Bath and Surface Deposition Methods. Materials, 13(7), 1622. https://doi.org/10.3390/ma13071622