Reactive Infiltration and Microstructural Characteristics of Sn-V Active Solder Alloys on Porous Graphite
Abstract
1. Introduction
2. Experimental Details
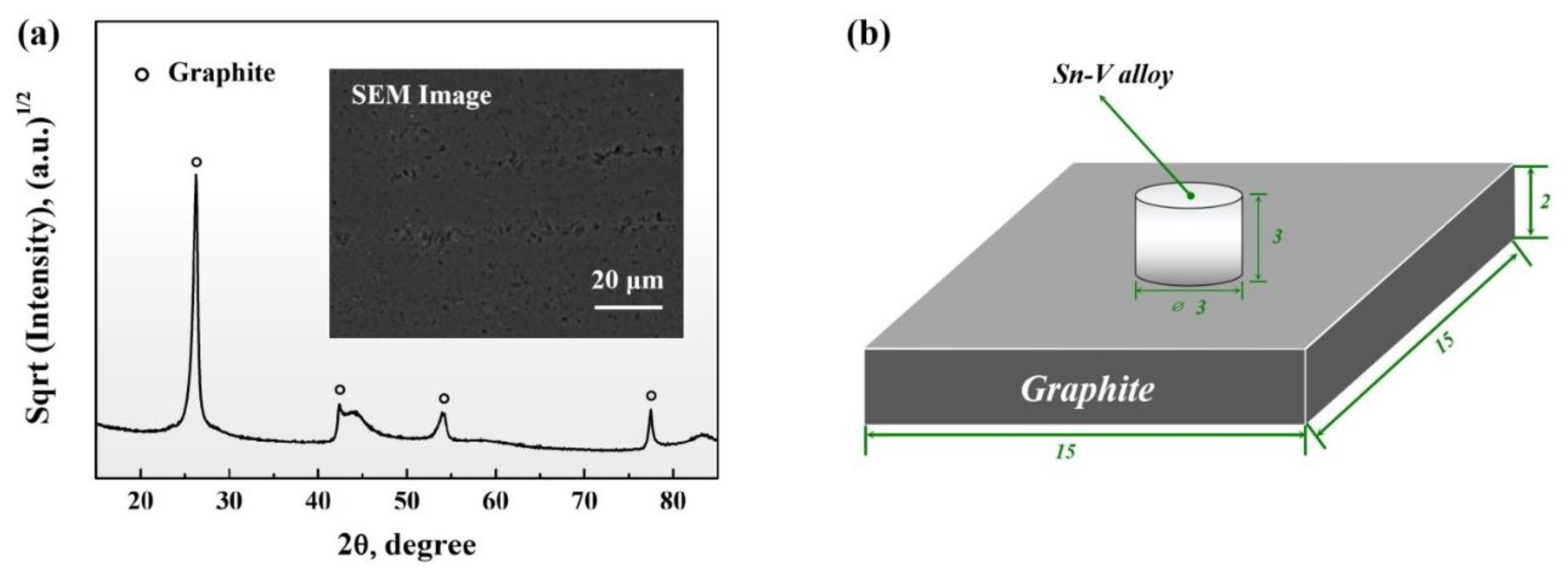
2.1. Materials
2.2. Wetting Experiment
2.3. Microstructural Characterization
3. Results and Discussion
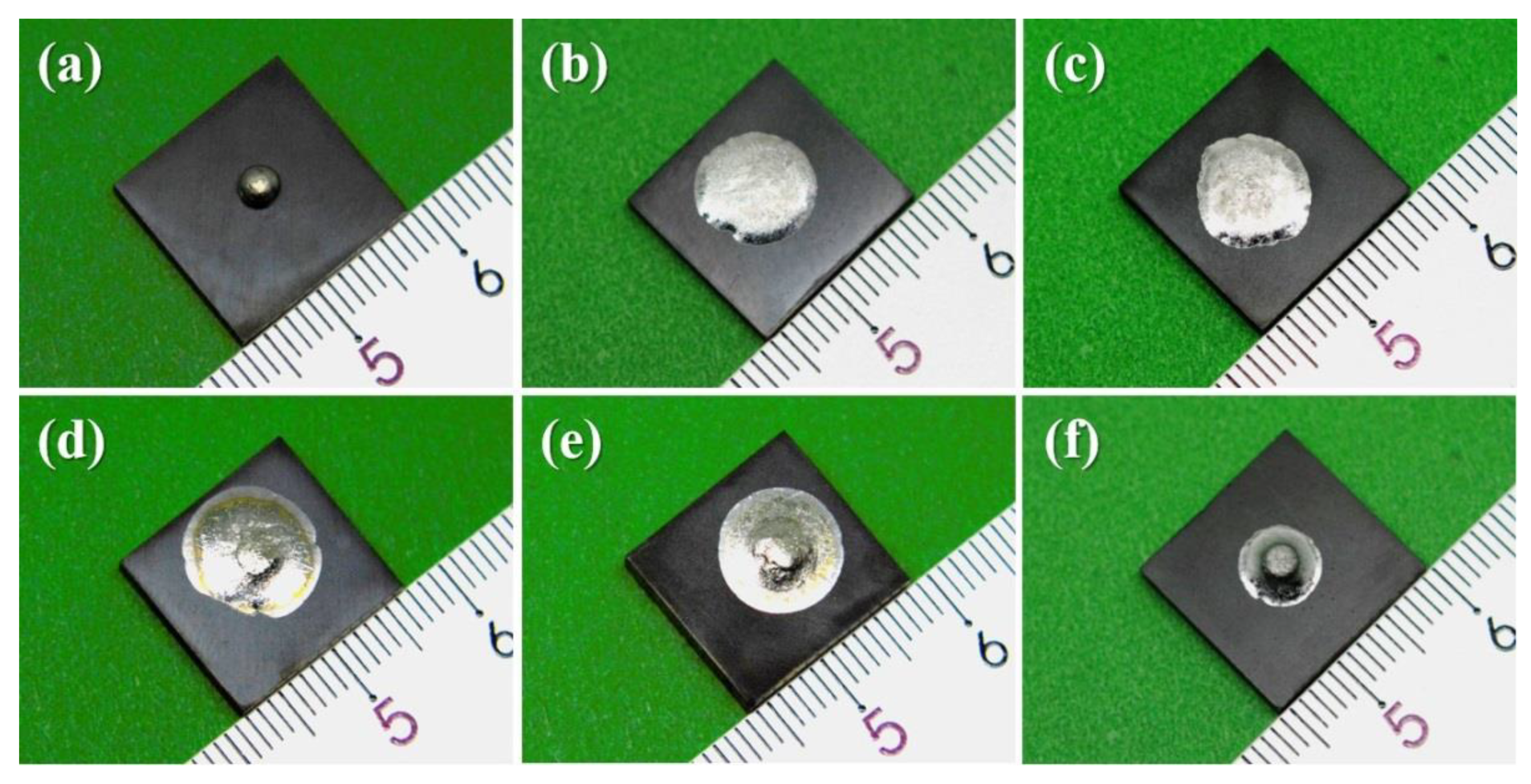
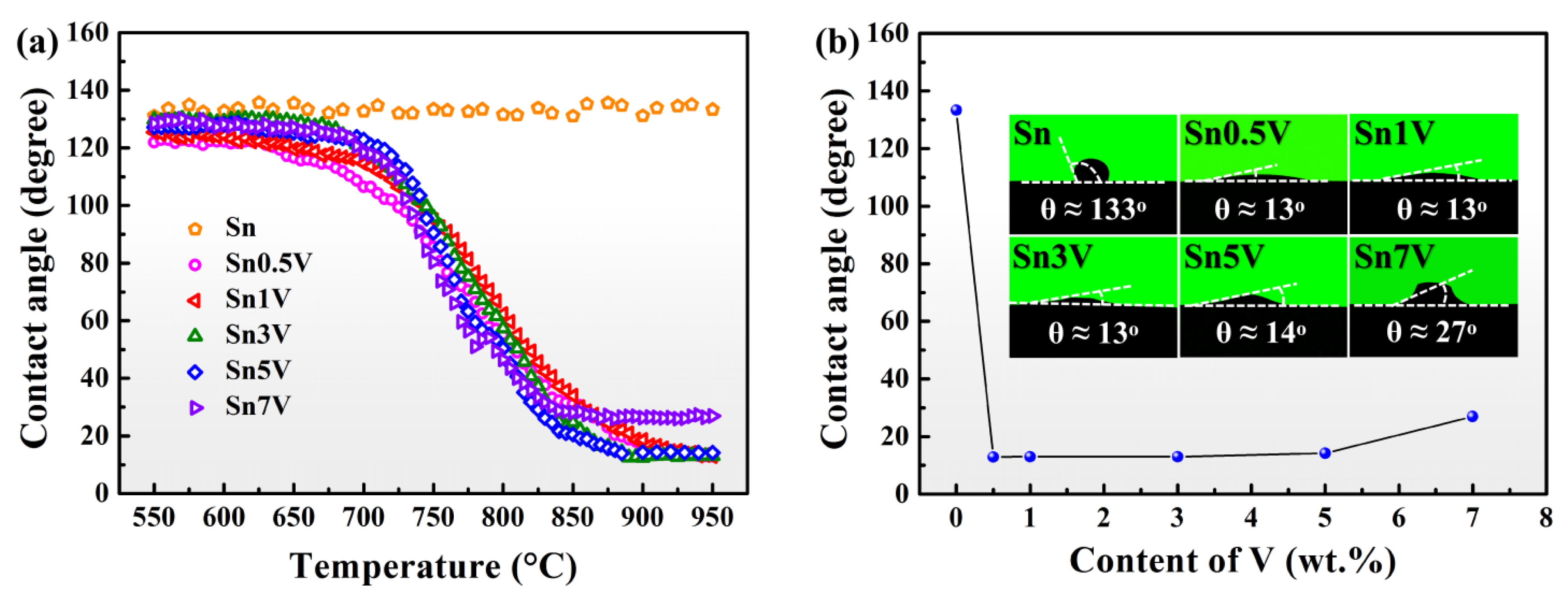
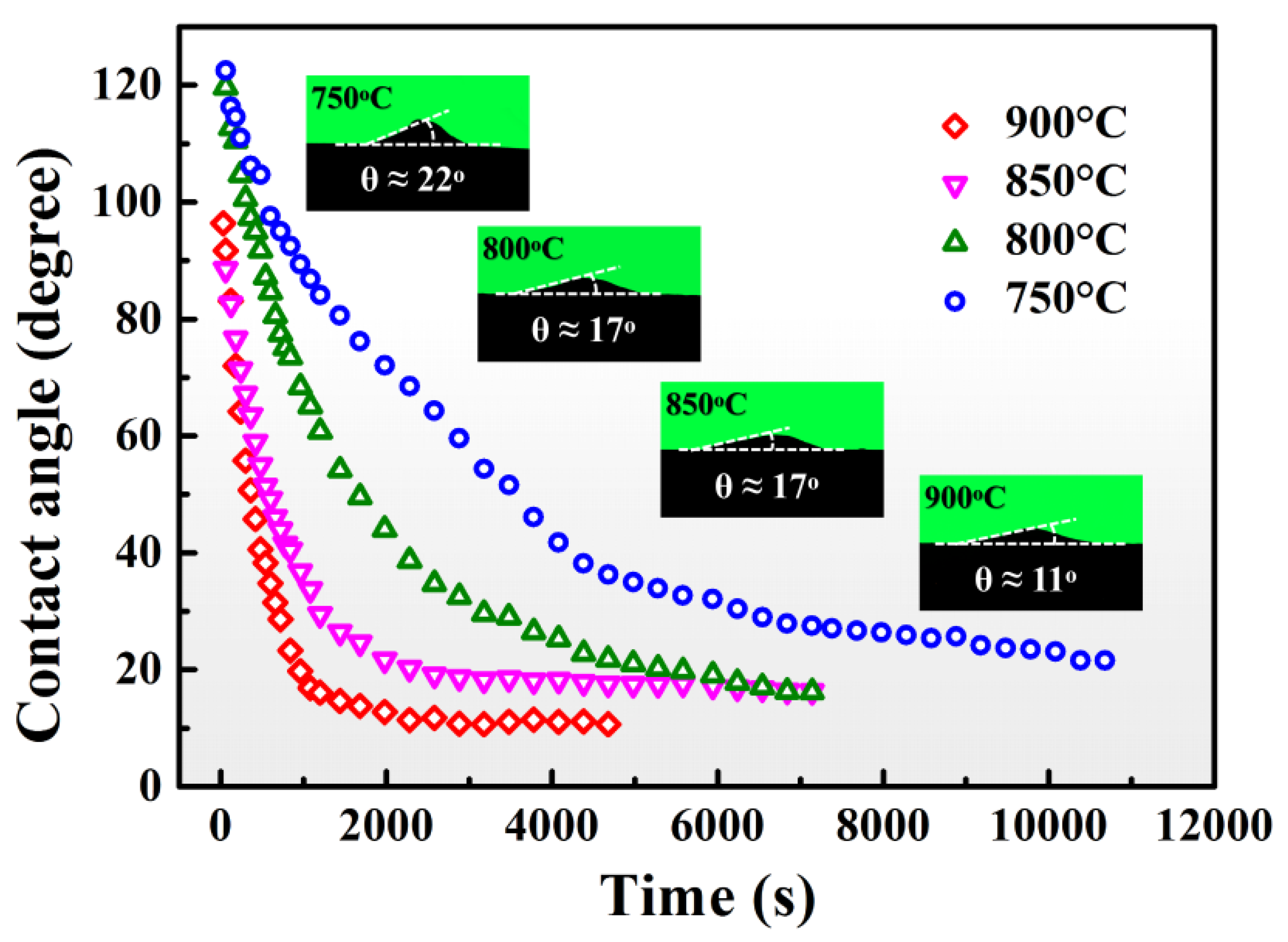
3.1. Spreading Characteristics
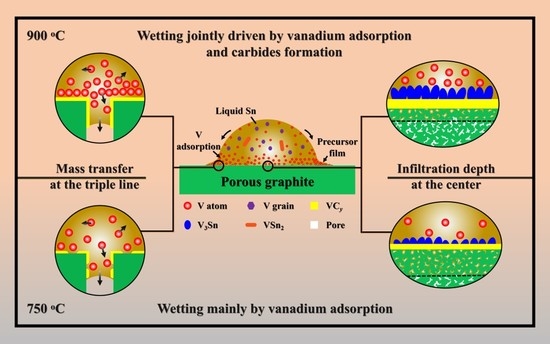
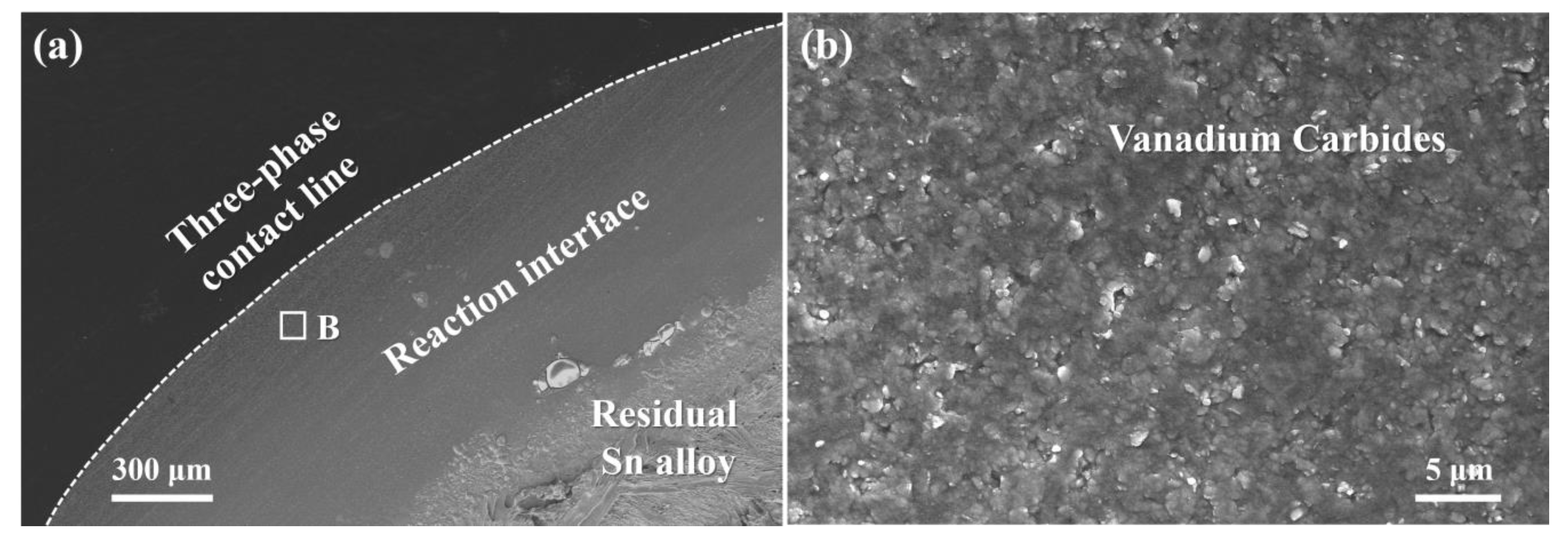
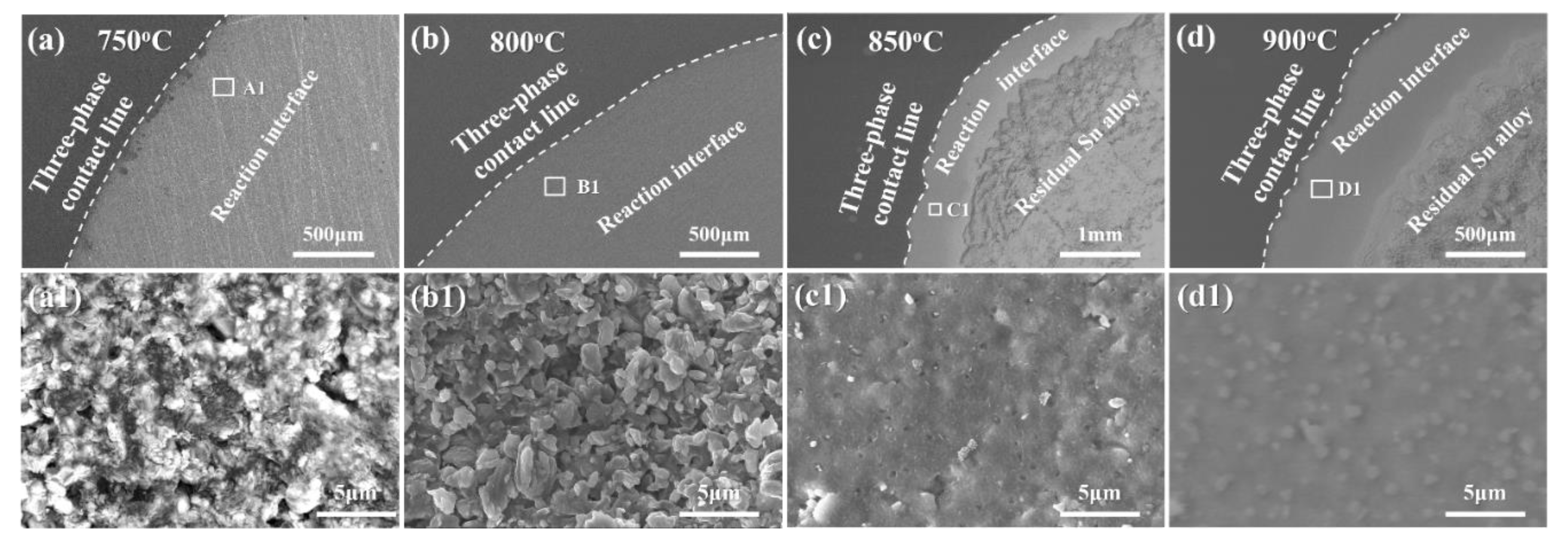
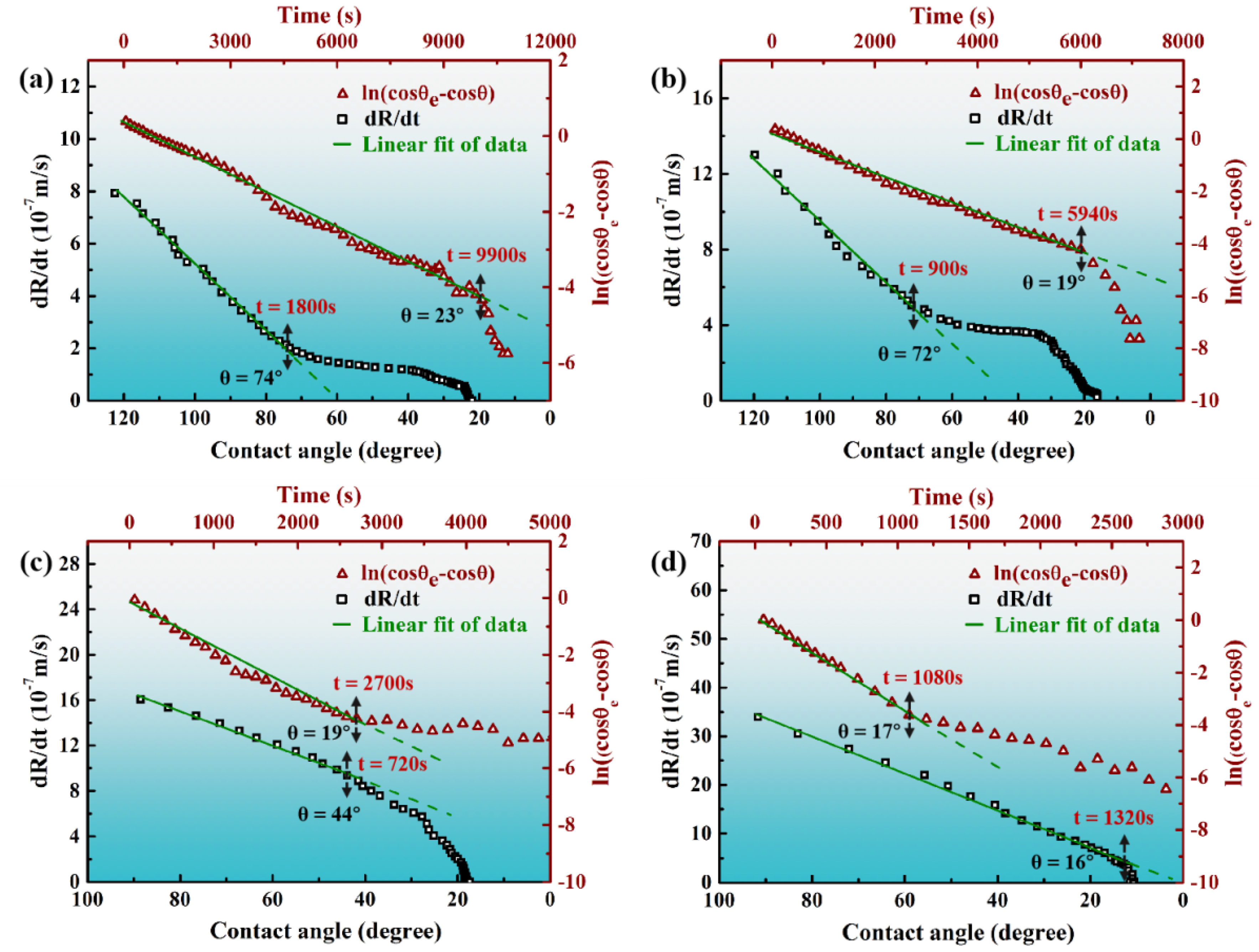
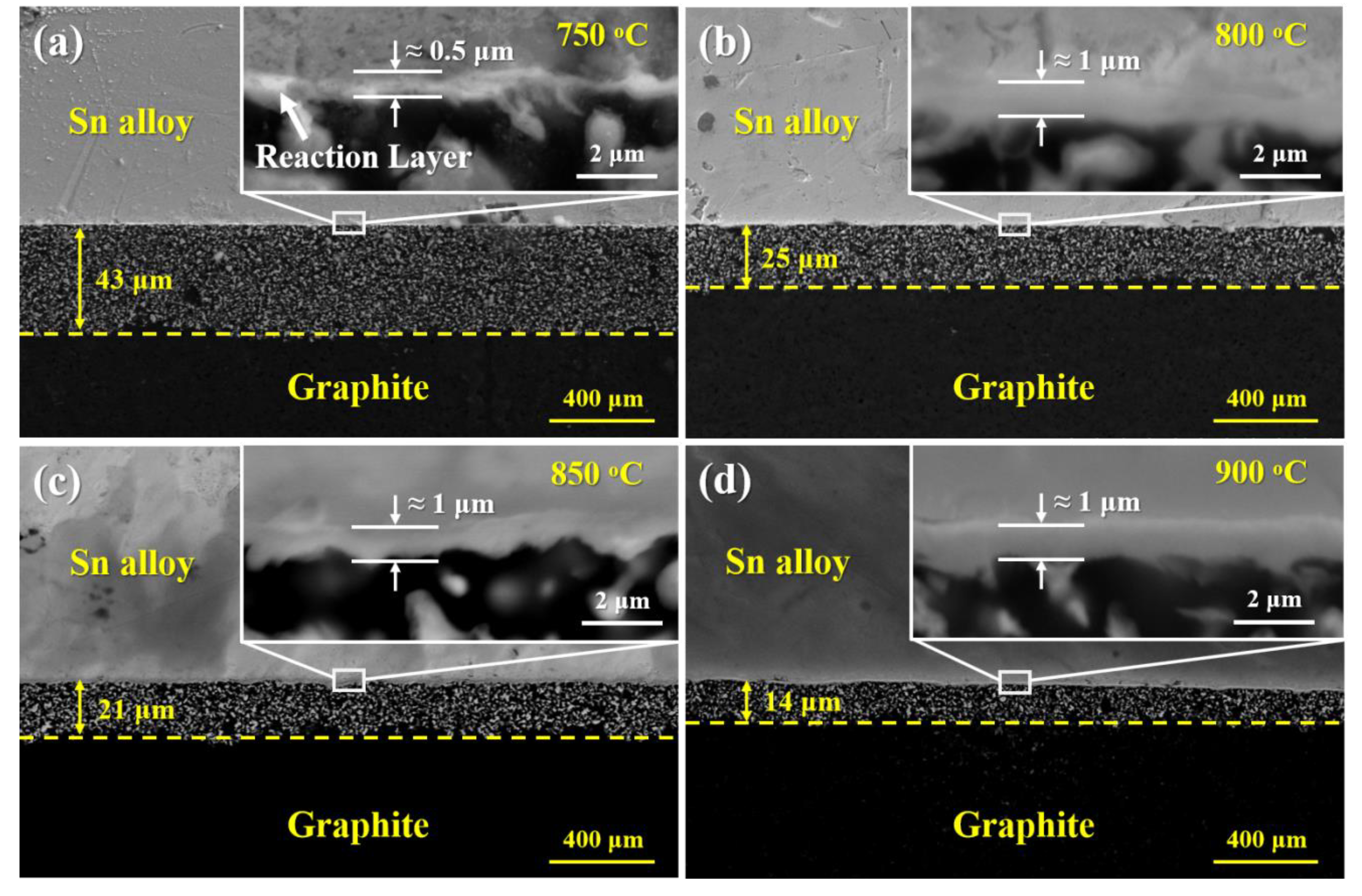
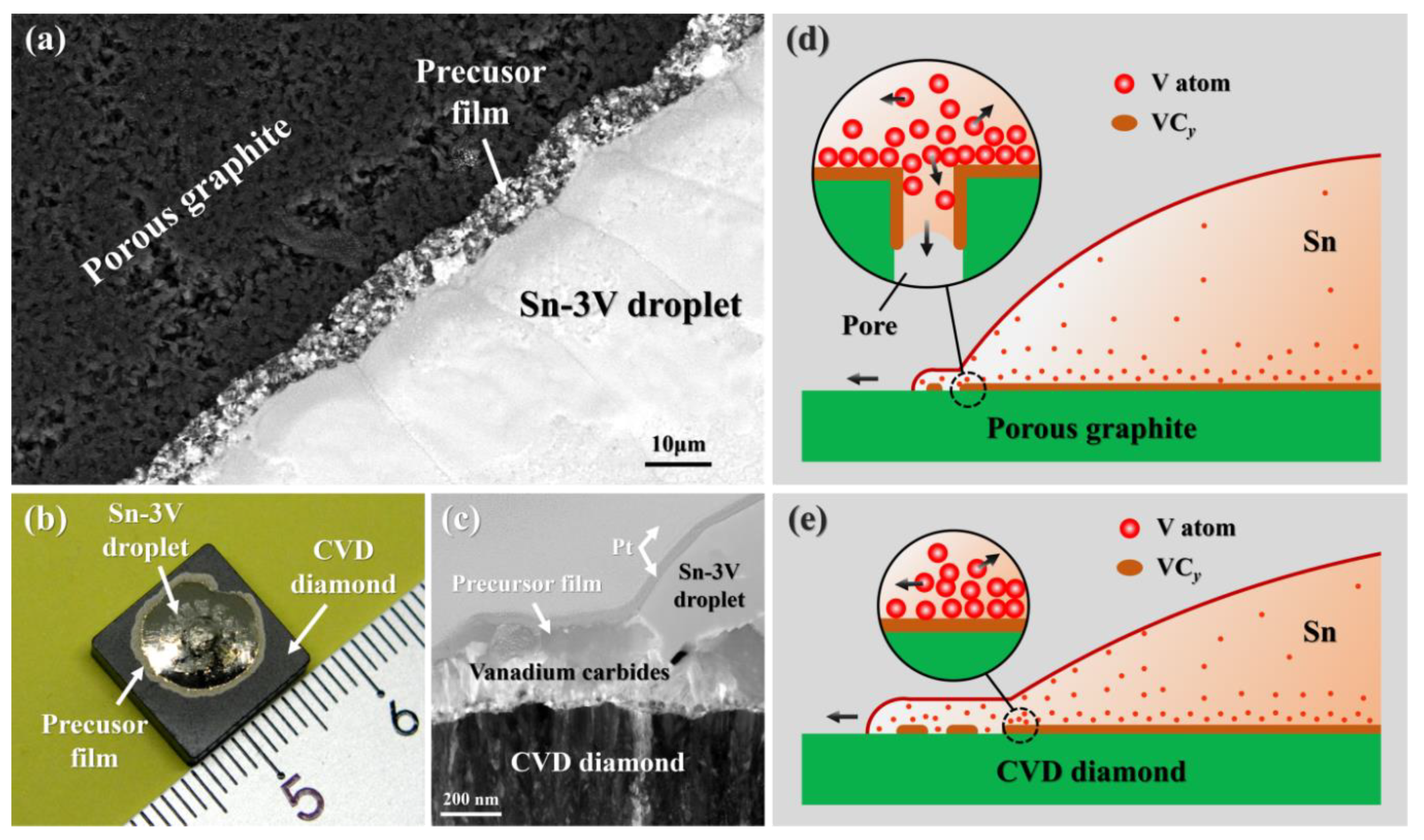
3.2. Reactive Infiltrating
3.3. Effects of Porosity
4. Conclusions
- (1)
- V concentrations have a minor influence on the final apparent contact angles of Sn-V alloys on porous graphite and a trace doping of 0.5 wt.% V obviously improved the wettability of liquid Sn on porous graphite.
- (2)
- Sn-V alloys approximately started to spread on porous graphite at 650 °C and reached the quasi-equilibrium state at 900 °C. The spreading kinetics of Sn-V alloys on porous graphite at 750–900 °C was well described by the classical chemical reaction-controlled model. However, thermodynamic analysis and associated microstructural characterization evidenced that, besides the formation of vanadium carbides, the adsorption of active V element at the three-phase contact line considerably contributed to the spreading and infiltrating of Sn-V alloys on porous graphite.
- (3)
- The formation of continuous phase of vanadium carbides resulted in the closure of pores, and hence stopped the infiltration of Sn-V alloys in porous graphite substrate. Consequently, the infiltration depth of Sn-V alloys in porous graphite decreased by the accelerated carbides formation at increased wetting temperature.
- (4)
- The difference in mass transfer at the three-phase contact line was accountable for the difference in wetting behaviors between porous graphite and CVD diamond. The presence of pores in graphite substrate impeded the stacking of active V atoms at the wetting three-phase contact line, which was responsible for the difference in the wettability of Sn-V alloy on porous graphite and polycrystalline CVD diamond.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, K.; Jia, C.; Guo, H.; Li, W. On the thermal conductivity of Cu–Zr/diamond composites. Mater. Des. 2013, 45, 36–42. [Google Scholar] [CrossRef]
- Sung, J.C.; Sung, M. The brazing of diamond. Int. J. Refract. Met. Hard Mater. 2009, 27, 382–393. [Google Scholar] [CrossRef]
- Casalegno, V.; Salvo, M.; Ferraris, M. Surface modification of carbon/carbon composites to improve their wettability by copper. Carbon 2012, 50, 2296–2306. [Google Scholar] [CrossRef]
- Xiong, H.-P.; Chen, B.; Pan, Y.; Zhao, H.-S.; Ye, L. Joining of Cf/SiC composite with a Cu–Au–Pd–V brazing filler and interfacial reactions. J. Eur. Ceram. Soc. 2014, 34, 1481–1486. [Google Scholar] [CrossRef]
- Morscher, G.N.; Shpargel, T.P.; Asthana, R. Active metal brazing of titanium to high-conductivity carbon-based sandwich structures. Mater. Sci. Eng. A 2008, 498, 31–36. [Google Scholar]
- Zhang, J.; Wang, T.; Liu, C.; He, Y. Effect of brazing temperature on microstructure and mechanical properties of graphite/copper joints. Mater. Sci. Eng. A 2014, 594, 26–31. [Google Scholar] [CrossRef]
- Park, J.-W.; Mendez, P.; Eagar, T. Strain energy distribution in ceramic-to-metal joints. Acta Mater. 2002, 50, 883–899. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhou, Z.; Ge, C. Brazing of doped graphite to Cu using stress relief interlayers. J. Mater. Process. Technol. 2009, 209, 2662–2670. [Google Scholar] [CrossRef]
- Qin, Y.; Feng, J. Active brazing carbon/carbon composite to TC4 with Cu and Mo composite interlayers. Mater. Sci. Eng. A 2009, 525, 181–185. [Google Scholar] [CrossRef]
- Song, X.; Li, H.; Zeng, X.; Zhang, L. Brazing of C/C composites to Ti6Al4V using graphene nanoplatelets reinforced TiCuZrNi brazing alloy. Mater. Lett. 2016, 18, 232–235. [Google Scholar] [CrossRef]
- Lin, T.; Yang, M.; He, P.; Huang, C.; Pan, F.; Huang, Y. Effect of in situ synthesized TiB whisker on microstructure and mechanical properties of carbon–carbon composite and TiBw/Ti–6Al–4V composite joint. Mater. Des. 2011, 32, 4553–4558. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Li, M.; Lin, J.; Ma, Q.; Zhang, A.; Zhong, Z.; Qi, J.; Feng, J. Three-dimensional graphene-reinforced Cu foam interlayer for brazing C/C composites and Nb. Carbon 2017, 118, 723–730. [Google Scholar] [CrossRef]
- Yu, W.; Liu, S.; Liu, X.; Liu, M.; Shi, W. Interface reaction in ultrasonic vibration-assisted brazing of aluminum to graphite using Sn–Ag–Ti solder foil. J. Mater. Process. Technol. 2015, 221, 285–290. [Google Scholar] [CrossRef]
- Tsao, L.C.; Hsieh, M.J.; Chen, T.Y.; Cheng, S.Y.; Chen, C.W. Active soldering of aluminum-graphite composite to aluminum using Sn3.5Ag4Ti0.5Cu active filler. Int. J. Mater. Res. 2016, 107, 860–866. [Google Scholar] [CrossRef]
- Mortimer, D.A.; Nicholas, M. The wetting of carbon by copper and copper alloys. J. Mater. Sci. 1970, 5, 149–155. [Google Scholar] [CrossRef]
- Devincent, S.M.; Michal, G.M. Reaction layer formation at the graphite/copper-chromium alloy interface. Metall. Trans. A 1993, 24, 53–60. [Google Scholar] [CrossRef]
- Yang, L.; Shen, P.; Lin, Q.; Qiu, F.; Jiang, Q. Wetting of porous graphite by Cu–Ti alloys at 1373K. Mater. Chem. Phys. 2010, 124, 499–503. [Google Scholar] [CrossRef]
- Mao, W.; Yamaki, T.; Miyoshi, N.; Shinozaki, N.; Ogawa, T. Wettability of Cu-Ti Alloys on Graphite in Different Placement States of Copper and Titanium at 1373 K (1100 °C). Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 2262–2272. [Google Scholar] [CrossRef]
- Yang, L.; Shen, P.; Lin, Q.; Qiu, F.; Jiang, Q. Effect of Cr on the wetting in Cu/graphite system. Appl. Surf. Sci. 2011, 257, 6276–6281. [Google Scholar] [CrossRef]
- Fu, W.; Hu, S.; Song, X.; Zhao, Y.; Bian, H.; Jin, C. Wetting Behaviors and Interfacial Characteristics of Sn0.3Ag0.7Cu Alloys Containing Ti or Cr on Graphite. Metall. Mater. Trans. A 2018, 29, 5823–5832. [Google Scholar] [CrossRef]
- Fu, W.; Hu, S.P.; Song, X.G.; Li, J.X.; Cao, J.; Feng, J.C.; Wang, G.D. Wettability and bonding of graphite by Sn0.3Ag0.7Cu-Ti alloys. Carbon 2017, 121, 536–543. [Google Scholar] [CrossRef]
- Chen, J.; Liao, X.; Lin, Q.; Mu, D.; Huang, H.; Xu, X.; Huang, H. Reactive wetting of binary SnCr alloy on polycrystalline chemical vapour deposited diamond at relatively low temperatures. Diam. Relat. Mater. 2019, 92, 92–99. [Google Scholar] [CrossRef]
- Liao, X.; Mu, D.; Fu, W.; Huang, H.; Huang, H. Low-temperature wetting mechanisms of polycrystalline chemical vapour deposition (CVD) diamond by Sn-Ti solder alloys. Mater. Des. 2019, 182, 108039. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Lin, Q.; Wu, Y.; Mu, D. Interfacial microstructures and mechanical integrity of synthetic diamond brazed by a low-temperature Cu-Sn-Cr filler alloy. Diam. Relat. Mater. 2019, 97, 107440. [Google Scholar] [CrossRef]
- Xiong, H.-P.; Chen, B.; Mao, W.; Li, X.-H. Joining of Cf/SiC Composite With Pd-Co-V Brazing Filler. Weld. World 2012, 56, 76–80. [Google Scholar] [CrossRef]
- Yamazaki, T.; Suzumura, A. Reaction products at brazed interface between Ag–Cu–V filler metal and diamond (111). J. Mater. Sci. 2006, 41, 6409–6416. [Google Scholar] [CrossRef]
- Liao, X.; He, Q.; Lin, Q.; Mu, D.; Huang, H.; Huang, H. Reactive Wetting of Sn-V Solder Alloys on Polycrystalline CVD Diamond. Appl. Surf. Sci. 2019, 504, 144508. [Google Scholar] [CrossRef]
- Mu, D.; Feng, K.; Lin, Q.; Huang, H. Low-temperature wetting of sapphire using Sn–Ti active solder alloys. Ceram. Int. 2019, 45, 22175–22182. [Google Scholar] [CrossRef]
- Gremillard, L.; Saiz, E.; Radmilovic, V.R.; Tomsia, A.P. Role of titanium on the reactive spreading of lead-free solders on alumina. J. Mater. Res. 2006, 21, 3222–3233. [Google Scholar] [CrossRef]
- Eustathopoulos, N. Progress in understanding and modeling reactive wetting of metals on ceramics. Curr. Opin. Solid State Mater. Sci. 2005, 9, 152–160. [Google Scholar] [CrossRef]
- Saiz, E.; Tomsia, A.P. Kinetics of high-temperature spreading. Curr. Opin. Solid State Mater. Sci. 2005, 9, 167–173. [Google Scholar] [CrossRef]
- Mortensen, A.; Drevet, B.; Eustathopoulos, N. Kinetics of diffusion-limited spreading of sessile drops in reactive wetting. Scr. Mater. 1997, 36, 645–651. [Google Scholar] [CrossRef]
- Dezellus, O.; Hodaj, F.; Eustathopoulos, N. Chemical reaction-limited spreading: The triple line velocity versus contact angle relation. Acta Mater. 2002, 50, 4741–4753. [Google Scholar] [CrossRef]
- Sui, R.; Ju, C.; Zhong, W.; Lin, Q. Improved wetting of Al2O3 by molten Sn with Ti addition at 973–1273 K. J. Alloys Compd. 2018, 739, 616–622. [Google Scholar] [CrossRef]
- Connors, K.A. Chemical Kinetics: The Study of Reaction Rates in Solution; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1990. [Google Scholar]
- Bougiouri, V.; Voytovych, R.; Dezellus, O.; Eustathopoulos, N. Wetting and reactivity in Ni-Si/C system: Experiments versus model predictions. J. Mater. Sci. 2007, 42, 2016–2023. [Google Scholar] [CrossRef]
- Lin, Q.; Cao, R. Characteristics of spreading dynamics for adsorption wetting at high temperatures. Comput. Mater. Sci. 2015, 99, 29–32. [Google Scholar] [CrossRef]
- Bougiouri, V.; Voytovych, R.; Rojo-Calderon, N.; Narciso, J.; Eustathopoulos, N. The role of the chemical reaction in the infiltration of porous carbon by NiSi alloys. Scr. Mater. 2006, 54, 1875–1878. [Google Scholar] [CrossRef]
- Sobczak, N.; Sobczak, J.; Rohatgi, P.K.; Ksiazek, M.; Radziwill, W.; Morgiel, J. Interaction between Ti or Cr containing copper alloys and porous graphite substrate. In Proceedings of the International Conference High Temp. Capillarity, Cracow, Poland, 29 June–2 July 1997; pp. 145–152. [Google Scholar]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Singh, M.; Behrendt, D.R. Reactive melt infiltration of silicon-molybdenum alloys into microporous carbon preforms. Mater. Sci. Eng. A 1995, 194, 193–200. [Google Scholar] [CrossRef]
- Voytovych, R.; Bougiouri, V.; Calderon, N.R.; Narciso, J.; Eustathopoulos, N. Reactive infiltration of porous graphite by NiSi alloys. Acta Mater. 2008, 56, 2237–2246. [Google Scholar] [CrossRef]
- Dezellus, O.; Eustathopoulos, N. Fundamental issues of reactive wetting by liquid metals. J. Mater. Sci. 2010, 45, 4256–4264. [Google Scholar] [CrossRef]
- Shen, P.; Zheng, X.H.; Lin, Q.L.; Zhang, D.; Jiang, Q.C. Wetting of Polycrystalline α-Al2O3 by Molten Zr55Cu30Al10Ni5 metallic glass alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2009, 40, 444–449. [Google Scholar] [CrossRef]
- Lin, Q.; Qiu, F.; Sui, R. Characteristics of precursor film in the wetting of Zr-based alloys on ZrC substrate at 1253 K. Thin Solid Films. 2014, 558, 231–236. [Google Scholar] [CrossRef]
- Xian, A.P. Precursor film of tin-based active solder wetting on ceramics. J. Mater. Sci. 1993, 28, 1019–1030. [Google Scholar] [CrossRef]
- Bormashenko, E. Apparent contact angles for reactive wetting of smooth, rough, and heterogeneous surfaces calculated from the variational principles. J. Colloid Interface Sci. 2019, 537, 597–603. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liao, X.; Lin, Q.; Mu, D.; Lu, J.; Huang, H.; Huang, H. Reactive Infiltration and Microstructural Characteristics of Sn-V Active Solder Alloys on Porous Graphite. Materials 2020, 13, 1532. https://doi.org/10.3390/ma13071532
Zhang Y, Liao X, Lin Q, Mu D, Lu J, Huang H, Huang H. Reactive Infiltration and Microstructural Characteristics of Sn-V Active Solder Alloys on Porous Graphite. Materials. 2020; 13(7):1532. https://doi.org/10.3390/ma13071532
Chicago/Turabian StyleZhang, Yubin, Xinjiang Liao, Qiaoli Lin, Dekui Mu, Jing Lu, Hui Huang, and Han Huang. 2020. "Reactive Infiltration and Microstructural Characteristics of Sn-V Active Solder Alloys on Porous Graphite" Materials 13, no. 7: 1532. https://doi.org/10.3390/ma13071532
APA StyleZhang, Y., Liao, X., Lin, Q., Mu, D., Lu, J., Huang, H., & Huang, H. (2020). Reactive Infiltration and Microstructural Characteristics of Sn-V Active Solder Alloys on Porous Graphite. Materials, 13(7), 1532. https://doi.org/10.3390/ma13071532