Effects of Accelerators and Retarders in Early Strength Development of Concrete Based on Low-Temperature-Cured Ordinary Portland and Calcium Sulfoaluminate Cement Blends
Abstract
1. Introduction
2. Experimental Work
2.1. Materials
2.2. Experimental Outline and Mixture Proportions
2.3. Test Methods
2.3.1. Mortar Test
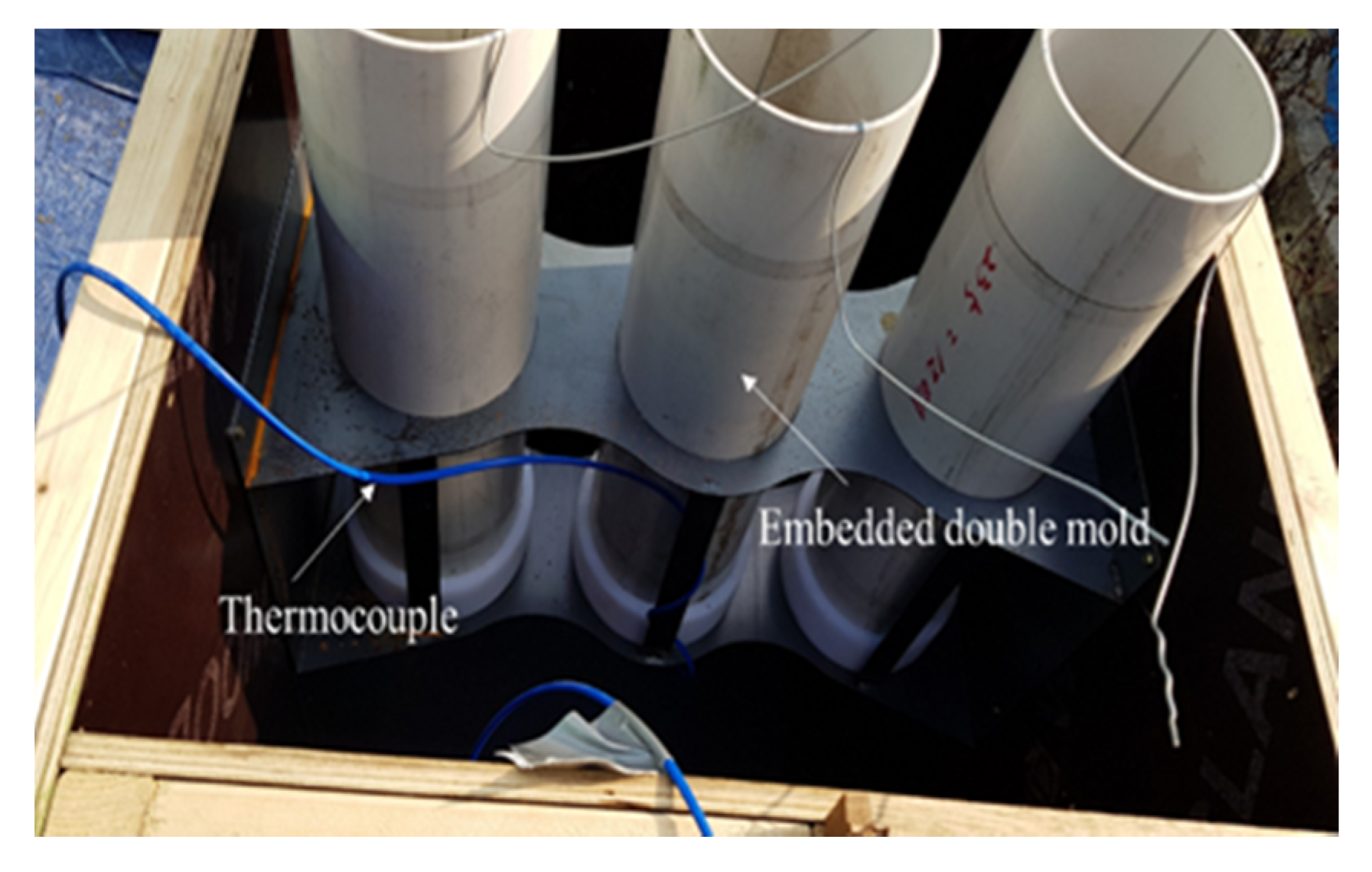
2.3.2. Application for Concrete
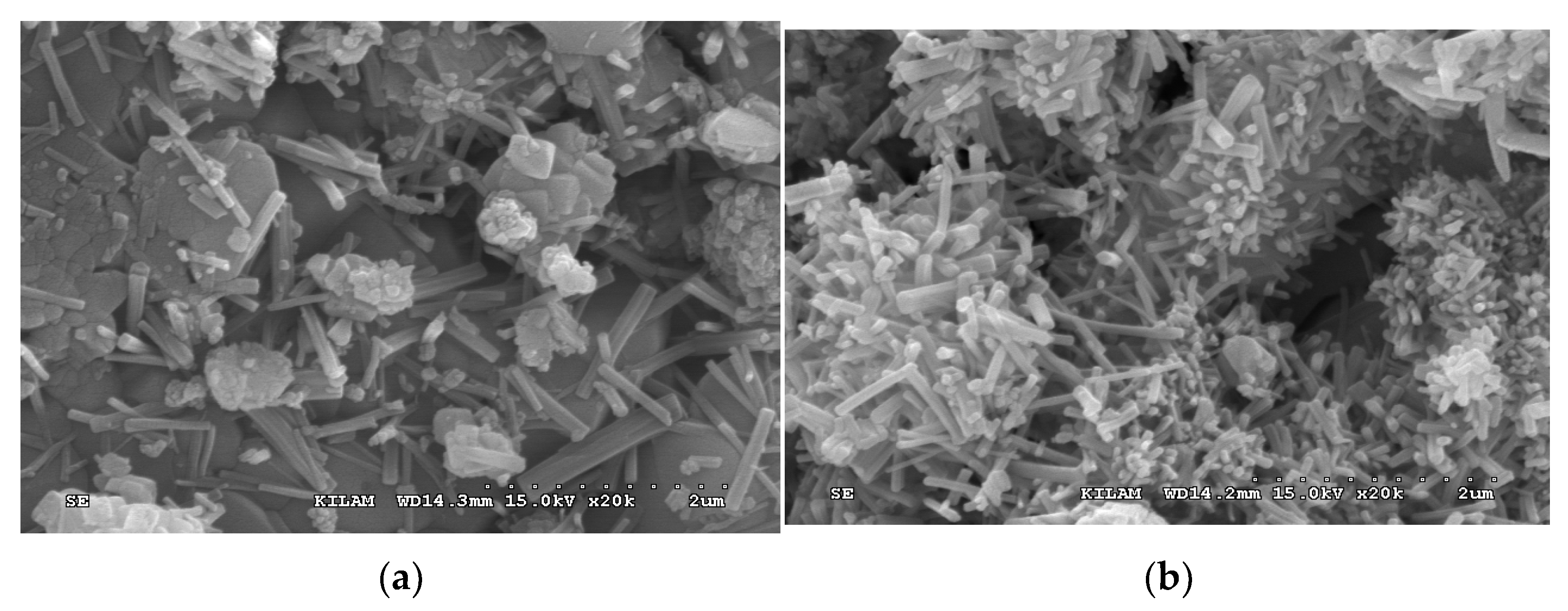
3. Results and Discussion
3.1. Properties of the Mortar
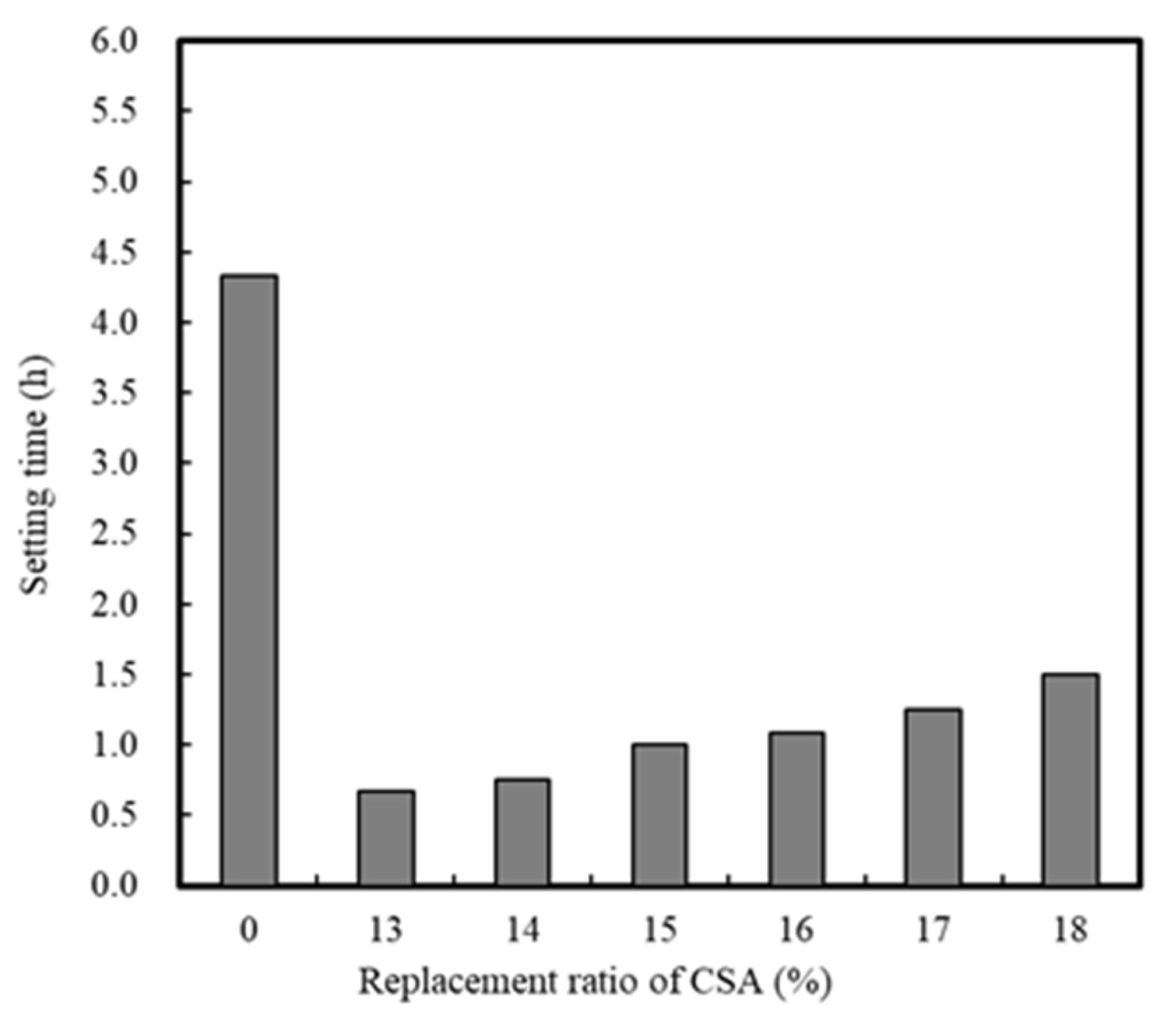
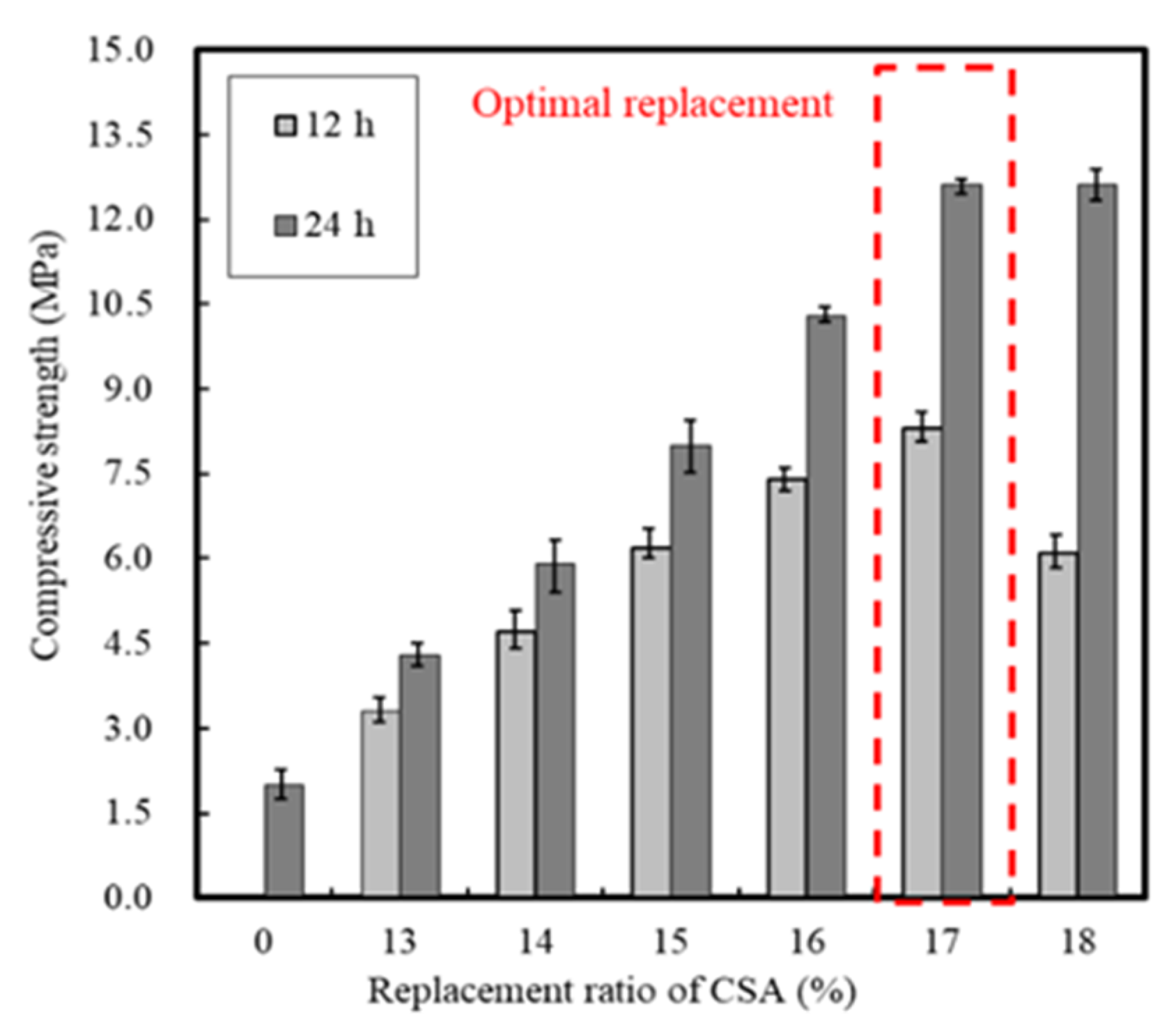
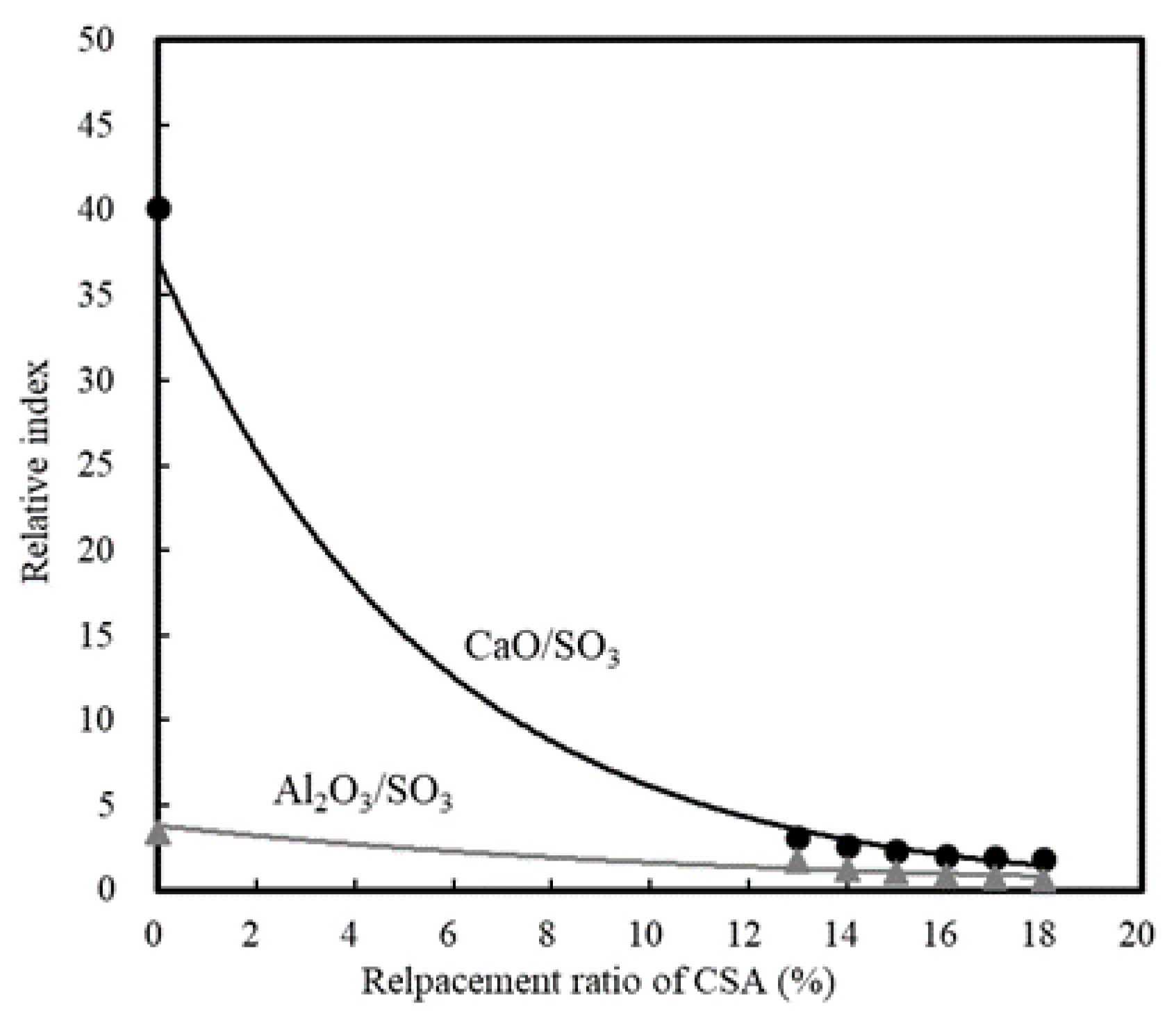
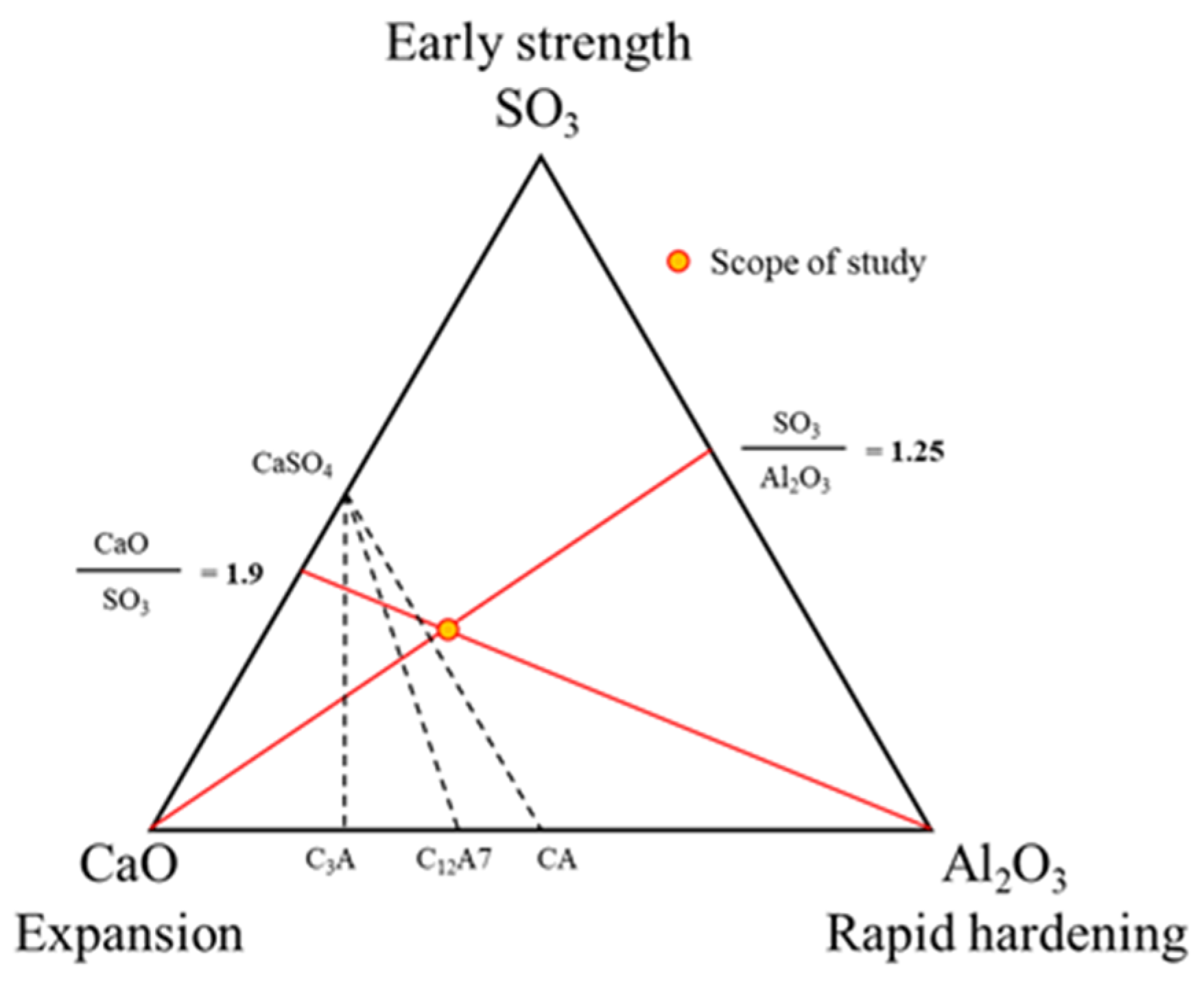
3.1.1. Effect of CSA Replacement
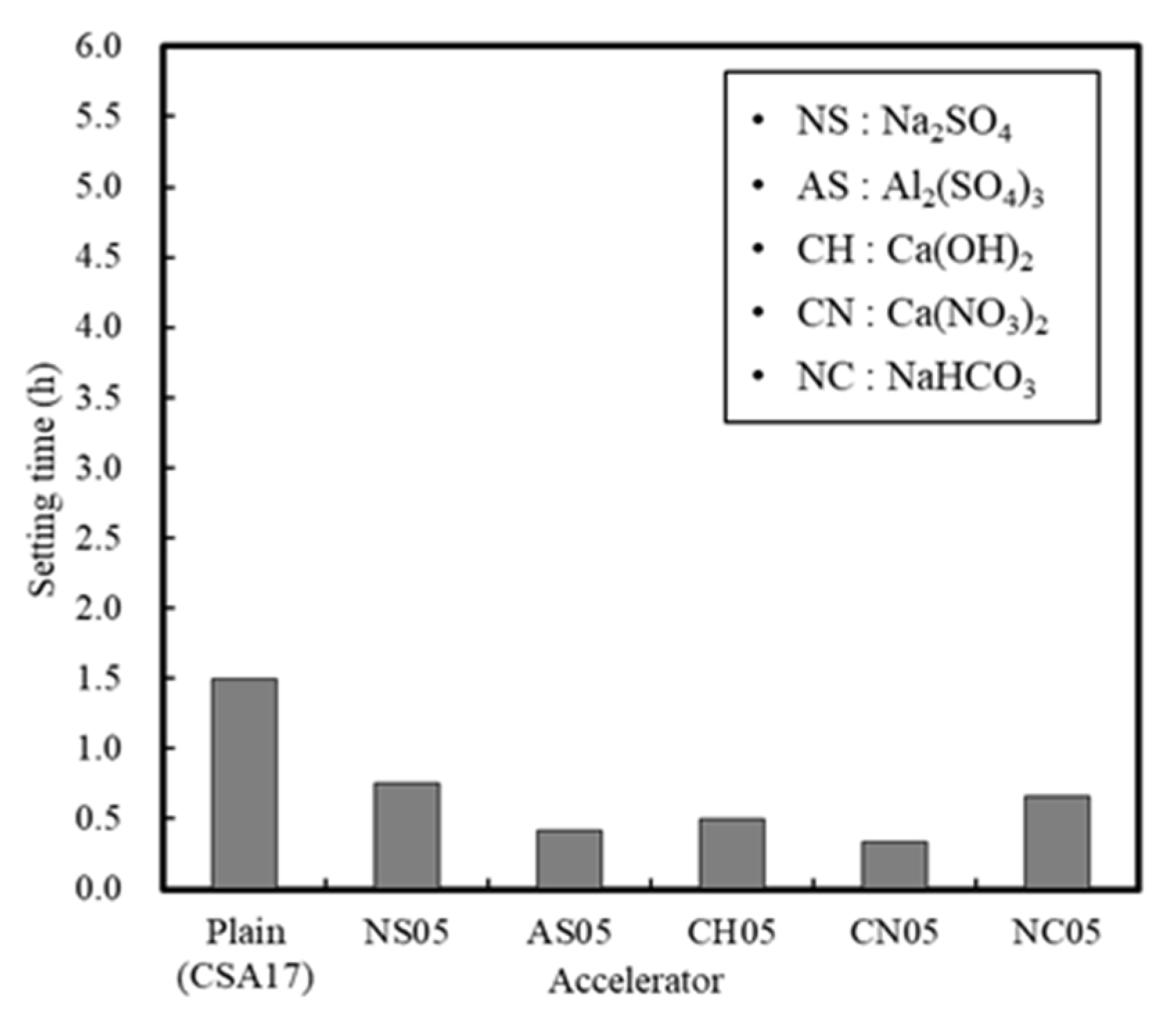
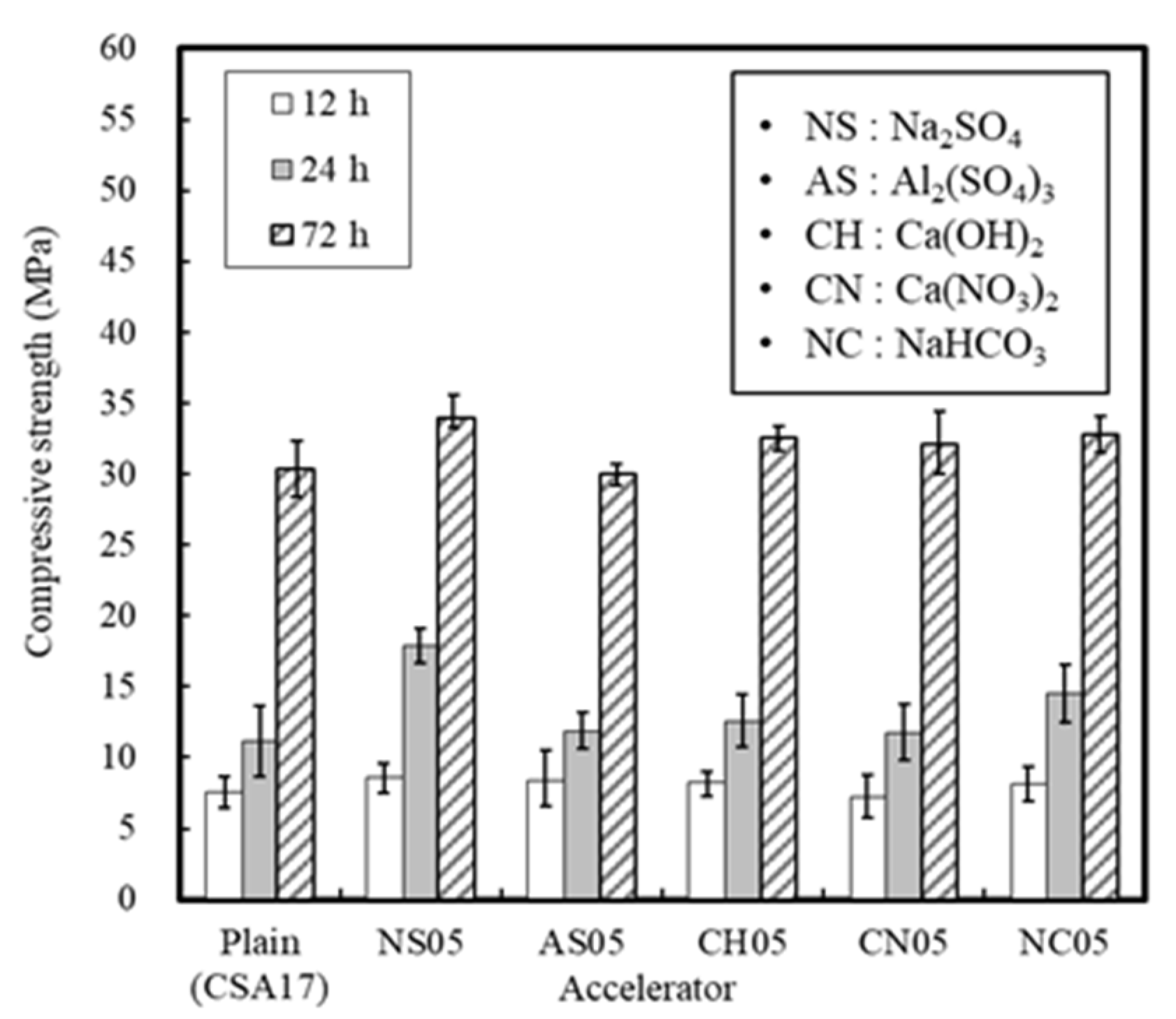
3.1.2. Effect of the Accelerator
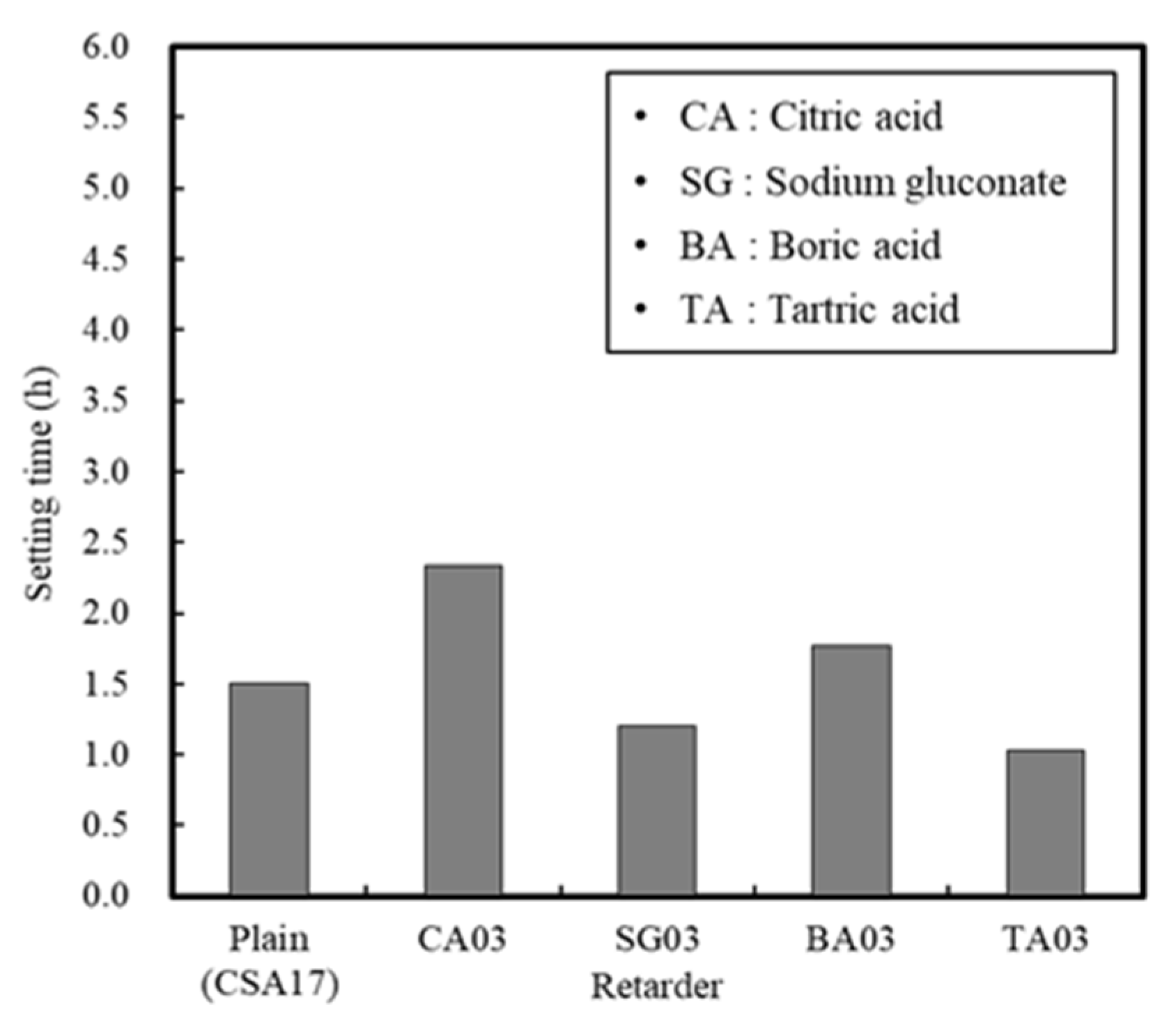
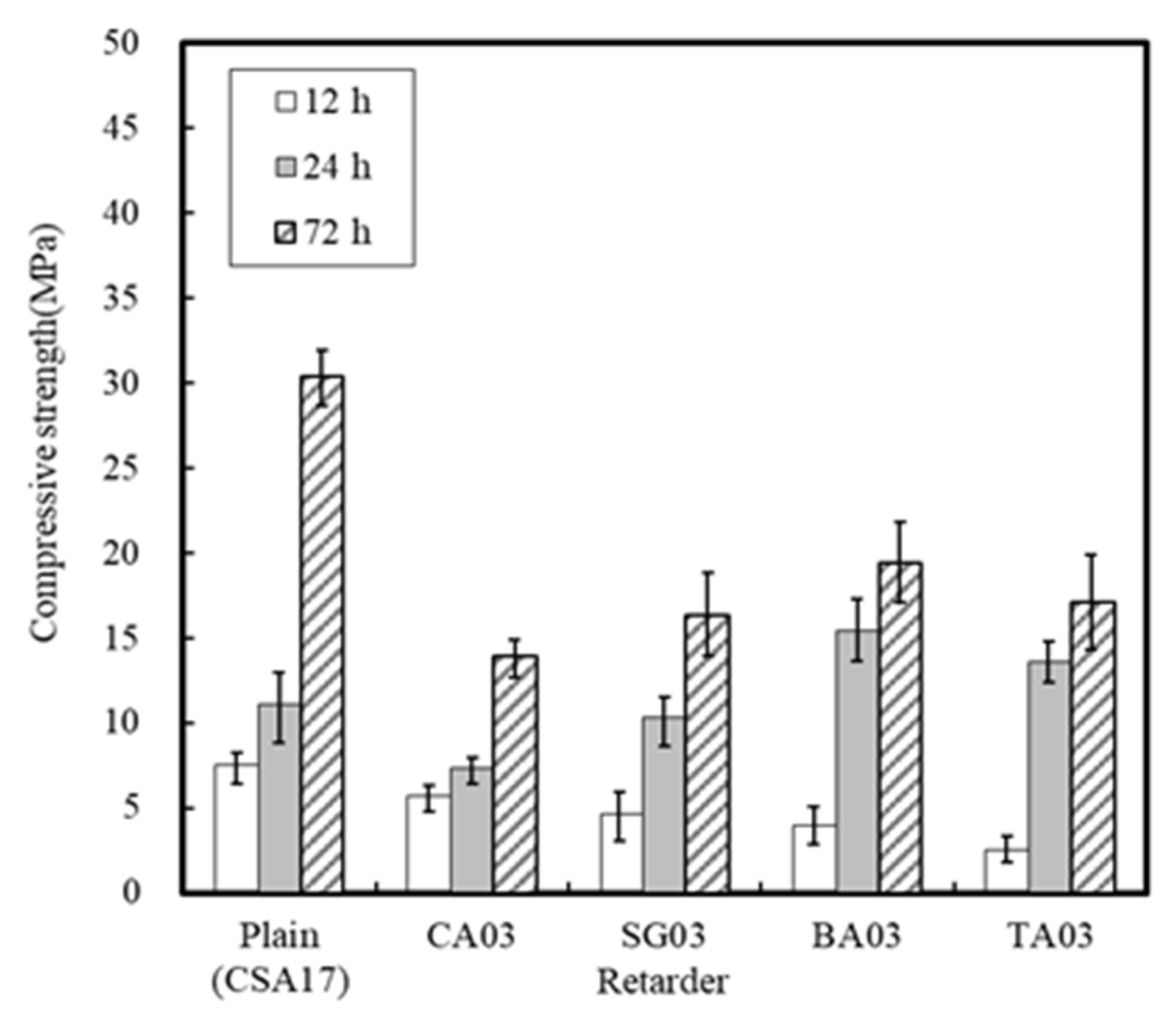
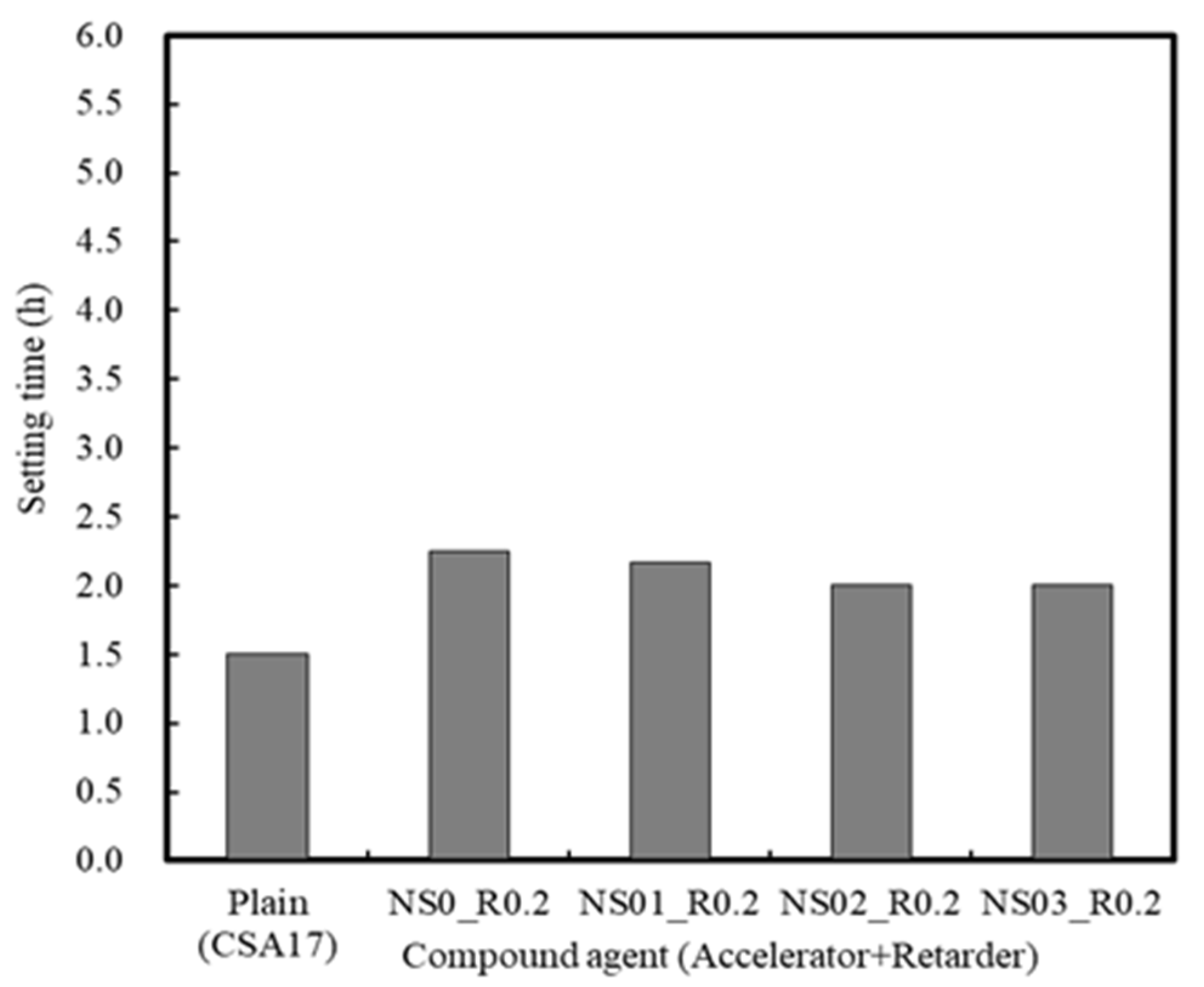
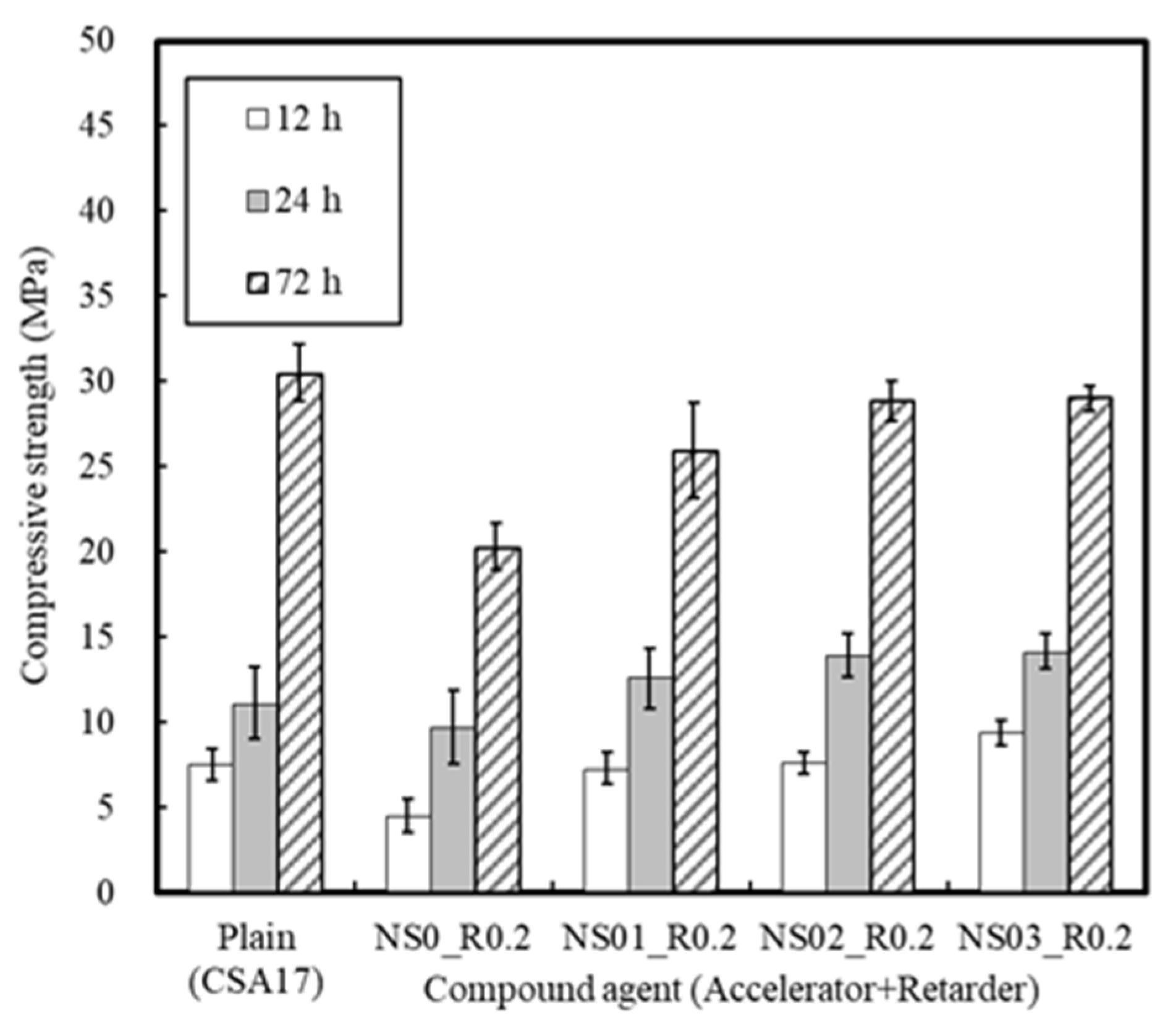
3.1.3. Effect of the Retarder and Accelerator
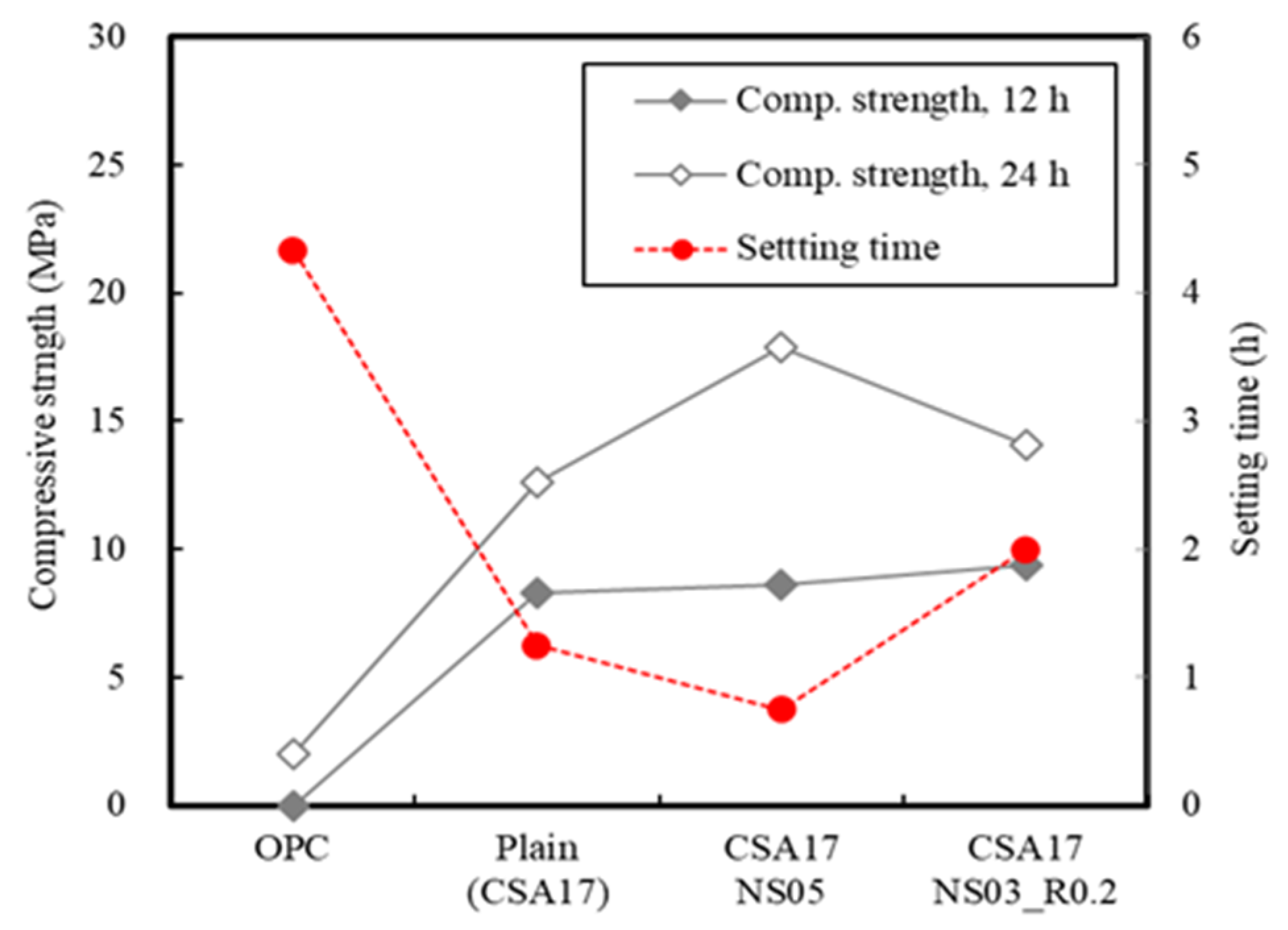
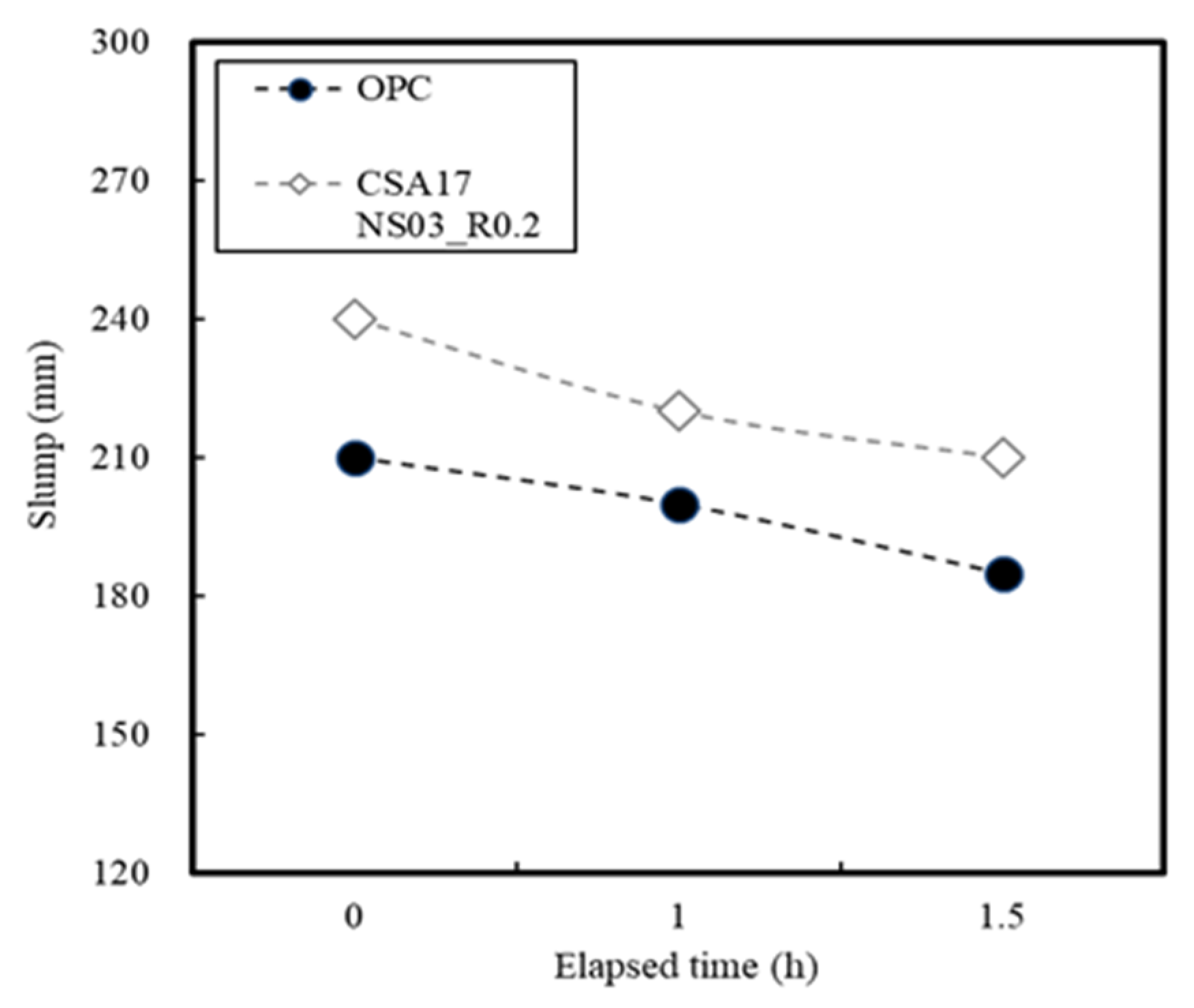
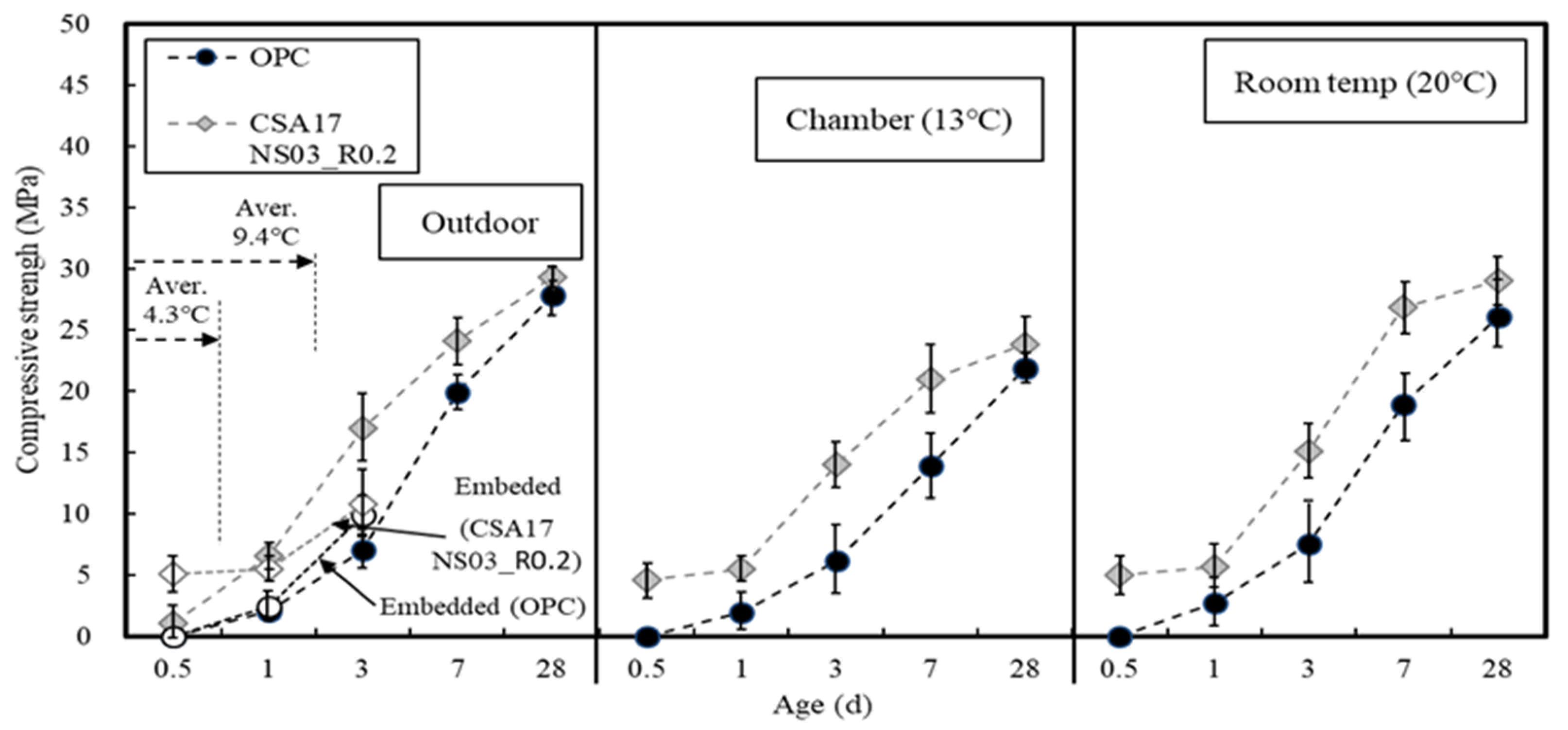
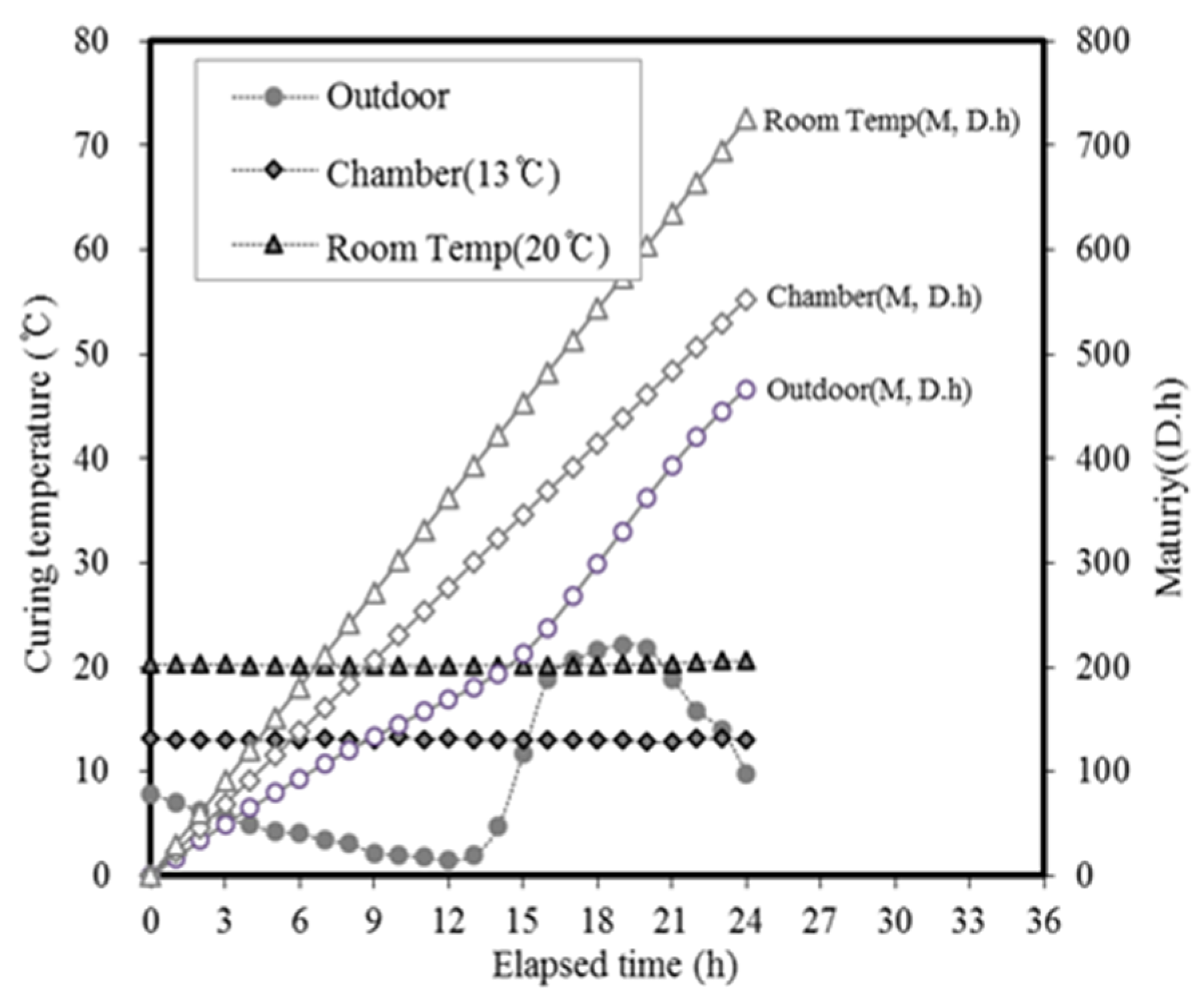
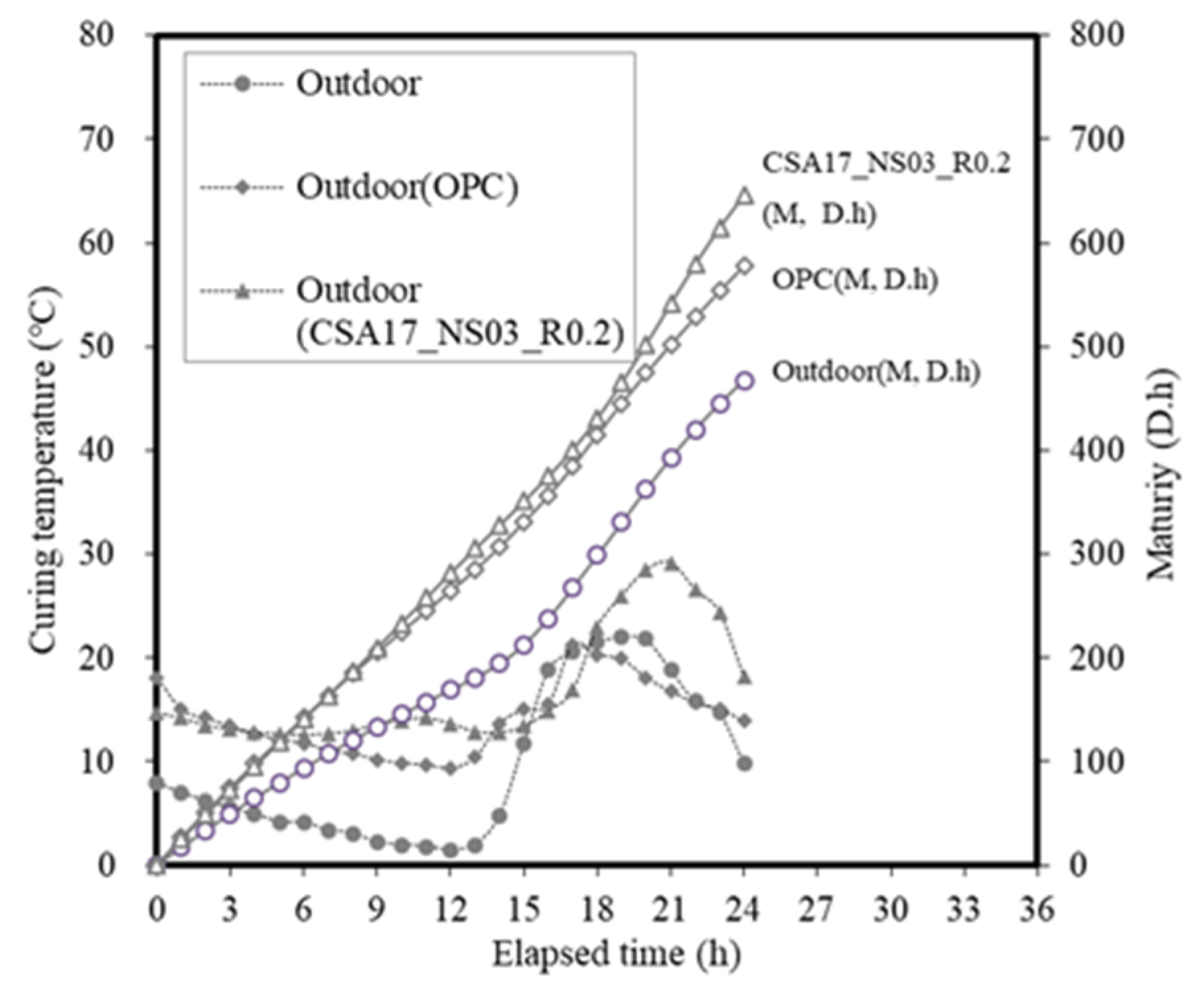
3.2. Concrete Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gartner, E.M.; Young, J.F.; Damidot, D.A.; Jawed, I. Composition of cement phases. In Structure and Performance of Cements, 2nd ed.; Bensted, J., Barnes, P., Eds.; CRC Press: London, UK, 2002; pp. 57–113. [Google Scholar]
- Dachtar, J. Calcium Sulfoaluminate Cement as Binder for Structural Concrete. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2004. [Google Scholar]
- Barnes, P.; Bensted, J. Structure and Performance of Cements, 2nd ed.; CRC Press: London, UK, 2002. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Juilland, P.; Gallucci, E.; Flatt, R.; Scrivener, K. Dissolution theory applied to the induction period in alite hydration. Cem. Concr. Res. 2010, 40, 831–844. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Zhang, L.; Su, M.; Wang, Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Mehta, P.K. Investigations on energy-saving cements. World Cement Technol. 1980, 11, 166–177. [Google Scholar]
- Odler, I. Special Inorganic Cements; CRC Press: London, UK, 2003. [Google Scholar]
- Juenger, M.; Winnefeld, F.; Provis, J.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Popescu, C.; Muntean, M.; Sharp, J. Industrial trial production of low energy belite cement. Cem. Concr. Compos. 2003, 25, 689–693. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to low CO2 cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Haha, M.B. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Xu, L. Hydration evolution and compressive strength of calcium sulphoaluminate cement constantly cured over the temperature range of 0 to 800 °C. Cem. Concr Res. 2017, 100, 203–213. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Adams, M.P.; Ideker, J.H. Influence of aggregate type on conversion and strength in calcium aluminate cement concrete. Cem. Concr. Res. 2017, 100, 284–296. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Kang, H.; Chun, S.; Moon, J. The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars. Materials 2019, 12, 1072. [Google Scholar] [CrossRef]
- Gwon, S.; Jang, S.; Shin, M. Combined Effects of Set Retarders and Polymer Powder on the Properties of Calcium Sulfoaluminate Blended Cement Systems. Materials 2018, 11, 825. [Google Scholar] [CrossRef]
- Glasser, F.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Pera, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2003, 33, 371–676. [Google Scholar] [CrossRef]
- Mehta, P.K.; Klein, A. Formation of ettringite by hydration of a system containing an anhydrous calcium sulfoaluminate. J. Am. Ceram. Soc. 1965, 48, 435–436. [Google Scholar] [CrossRef]
- Li, P.; Ma, Z.; Zhang, Z.; Li, X.; Lu, X.; Hou, P.; Du, P. Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement. Materials 2019, 12, 3131. [Google Scholar] [CrossRef]
- Clark, B.A.; Brown, P.W. The formation of calcium sulfoaluminate hydrate compounds Part I. Cem. Concr. Res. 1999, 29, 1943–1948. [Google Scholar] [CrossRef]
- Sahu, S.; Havlica, J.; Tomková, V.; Majling, J. Hydration behaviour of sulphoaluminate belite cement in the presence of various calcium sulphates. Thermochim. Acta 1991, 175, 45–52. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. ZKG Int. 2009, 12, 42–53. [Google Scholar]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calci-um sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
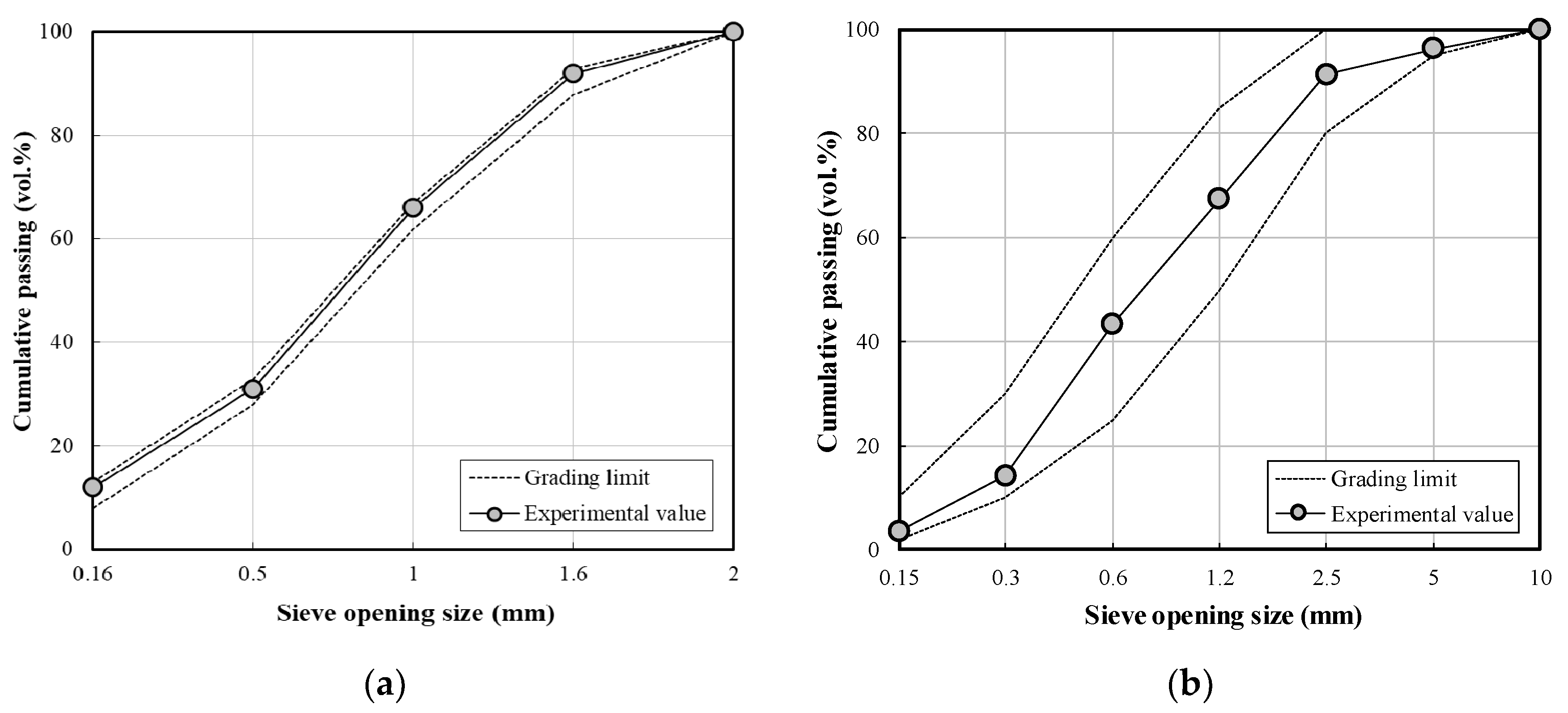
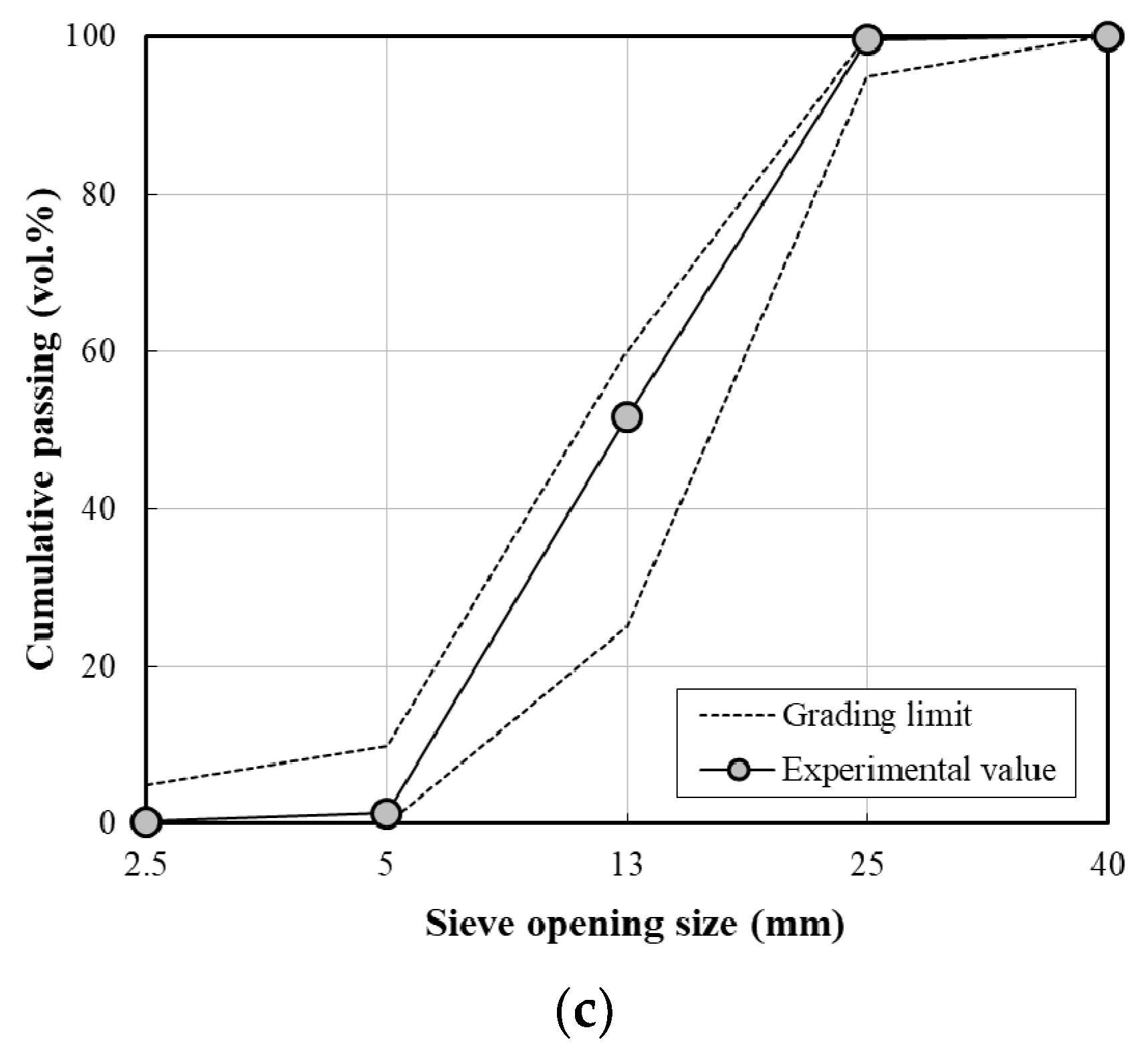
- ASTM C136/C136M-19, Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2019; pp. 1–5.
- ASTM C778, Standard Specification for Standard Sand. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2017; pp. 1–3.
- ASTM C1437, Standard Test Method for Flow of Hydraulic Cement Mortar. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2015; pp. 1–2.
- ASTM C403/C403M, Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2016; pp. 1–7.
- ASTM C109/C109M REV A, Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2017; pp. 1–10.
- ASTM C143/C143M REV A, Standard Test Method for Slump of Hydraulic-Cement Concrete. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2015; pp. 1–4.
- ASTM C231/C231M-17a, Standard Test Method for Air Content of Freshly Mixed Concrete by the Pressure Method. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2017; pp. 1–10.
- ASTM C873/C873M, Standard Test Method for Compressive Strength of Concrete Cylinders Cast in Place in Cylindrical Molds. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2015; pp. 1–4.
- ASTM C39/C39M, Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2018; pp. 1–8.
- Wang, Y.; Yu, L.; Wang, J.; Guan, X. Effects of Aluminum Sulfate and Quicklime/Fluorgypsum Ratio on the Properties of Calcium Sulfoaluminate (CSA) Cement-Based Double Liquid Grouting Materials. Materials 2019, 12, 1222. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T. Influences of Chemical Composition and Fineness on the Development of Concrete Strength by Curing Conditions. Materials 2019, 12, 4061. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T. Effects of High CaO Fly Ash and Sulfate Activator as a Finer Binder for Cementless Grouting Material. Materials 2019, 12, 3664. [Google Scholar] [CrossRef]
- Aggoun, S.; Cheikh-Zouaoui, M.; Chikh, N.; Duval, R. Effect of some admixtures on the setting time and strength evolution of cement pastes at early ages. Constr. Build. Mater. 2008, 22, 106–110. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- ASTM C1074, Standard Practice for Estimating Concrete Strength by the Maturity Method. In American Society of Testing and Materials; ASTM: West Conshohocken, PA, USA, 2019; pp. 1–10.

| Materials | Chemical Compositions (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | TiO2 | SO3 | LOI | |
| OPC (1) | 60.2 | 21.6 | 5.15 | 3.30 | 2.30 | 0.99 | 0.53 | - | 1.50 | 2.41 |
| GGBS (2) | 44.9 | 35.4 | 13.0 | 0.47 | 5.01 | 0.37 | - | - | 1.31 | 0.69 |
| FA (3) | 4.00 | 57.9 | 20.5 | 6.80 | 1.38 | 1.18 | 0.89 | 0.13 | - | 4.60 |
| CSA (4) | 45.51 | 4.91 | 22.36 | 1.74 | 1.57 | 0.17 | 0.43 | - | 22.63 | 1.90 |
| Material | Property | |
|---|---|---|
| OPC | ASTM Type I Ordinary Portland cement Density: 3150 kg/m3, fineness: 330 m2/kg | |
| FA | Fly Ash Density: 2140 kg/m3, fineness: 396 m2/kg | |
| GGBS | Ground granulated blast-furnace Slag Density: 2860 kg/m3, fineness: 430 m2/kg | |
| CSA | Calcium sulphoaluminate Density: 2890 kg/m3, fineness: 466 m2/kg | |
| Fine aggregate | S1 | ISO Standard sand, Size: 2 mm Fineness modulus: 2.99, density: 2620 kg/m3, SiO2: 99% |
| S2 | Sea sand, Size: 5 mm Fineness modulus: 2.01, density: 2600 kg/m3, absorption: 0.79% | |
| S3 | Crushed sand, Size: 5 mm Fineness modulus: 3.29, density: 2570 kg/m3, absorption: 0.87% | |
| Coarse aggregate | Crushed granitic aggregate, Size: 25 mm Fineness modulus: 6.94, density: 2600 kg/m3, absorption: 0.76% | |
| Chemical admixture | Polycarboxylic acid group, density: 1260 kg/m3 | |
| Accelerator material | NS | Na2SO4, density: 3350 kg/m3, solubility: 13.9 g/100 mL (20 °C) |
| AS | Al2(SO4)3, density: 2672 kg/m3, solubility: 36.4 g/100 mL (20 °C) | |
| CH | Ca(OH)2, density: 2211 kg/m3, solubility: 17.3 g/100 mL (20 °C) | |
| CN | Ca(NO3)2, density: 2504 kg/m3, solubility: 51.4 g/100 mL (20 °C) | |
| NC | NaHCO3, density: 2200 kg/m3, solubility: 9.6 g/100 mL (20 °C) | |
| Retarder | CA: Citric acid, SG: Sodium gluconate, BA: Boric acid, TA: Tartaric acid | |
| Series | Type | Factor | CSA Rate of OPC (%) | Curing Temperature (°C) | Chemical Admixture | Evaluation Item |
|---|---|---|---|---|---|---|
| Ⅰ | Mortar | Replacement ratio of CSA | 0, 13, 14, 15, 16, 17, 18 | 20 | - | Setting time (h) Compressive strength -12 h, 24 h |
| Accelerator | 17 | 20 | NS, AS, CH, CN, NC (B × 0.5%) | Setting time (h) Compressive strength -12 h, 24 h, 72 h | ||
| Retarder | 17 | 20 | CA, SG, BA, TA (B × 0.2%) | |||
| Accelerator + Retarder | 17 | 20 | NS (B × 1%, 2%, 3%) CA (B × 0.2%) | |||
| Ⅱ | Concrete | Application (Batch plant + Mock up member) | 17 | Outdoor Air Chamber (13) Room temp(20) | AD+ NS + CA (B × 3.2) | Slump Compressive strength-12 h, 24 h, 72 h 7 D, 28 D-Mock up member: 12 h, 24 h, 72 h |
| Series | W/C (%) | C:S (1) | Cement (g) | Water (g) | AD (2) (B ×%) |
|---|---|---|---|---|---|
| Ⅰ (Mortar) | 50 | 1:3 | 450 | 225 | 0.7 |
| Series | W/B (%) | S/a (%) | Unit Weight (kg/m3) | AD (3) (B ×%) | AC (B ×%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | C (1) | CSA | GGBS | FA | S (2) | G | ||||||
| Ⅱ (Concrete) | Plain | 53.0 | 49.0 | 175 | 215 | - | 66 | 50 | 880 | 916 | 0.7 | - |
| CA17 | 53.0 | 49.0 | 175 | 274 | 56 | - | - | 899 | 901 | - | 3.1 | |
| Series | Evaluation Item | Test Method | Size (mm) |
|---|---|---|---|
| Ⅰ. Mortar test | Setting time (h) | ASTM C403/C403M [33] | - |
| Compressive strength (MPa) | ASTM C109/C109M [34] | 40 × 40 × 160 | |
| Ⅱ. Concrete test | Slump (mm) | ASTM C143 [35] | - |
| Air content (%) | ASTM C231 [36] | - | |
| Compressive strength (MPa) | ASTM C873 [37] | Ø100 × 200 | |
| ASTM C39 [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Lee, J.; Choi, H. Effects of Accelerators and Retarders in Early Strength Development of Concrete Based on Low-Temperature-Cured Ordinary Portland and Calcium Sulfoaluminate Cement Blends. Materials 2020, 13, 1505. https://doi.org/10.3390/ma13071505
Lee T, Lee J, Choi H. Effects of Accelerators and Retarders in Early Strength Development of Concrete Based on Low-Temperature-Cured Ordinary Portland and Calcium Sulfoaluminate Cement Blends. Materials. 2020; 13(7):1505. https://doi.org/10.3390/ma13071505
Chicago/Turabian StyleLee, Taegyu, Jaehyun Lee, and Hyeonggil Choi. 2020. "Effects of Accelerators and Retarders in Early Strength Development of Concrete Based on Low-Temperature-Cured Ordinary Portland and Calcium Sulfoaluminate Cement Blends" Materials 13, no. 7: 1505. https://doi.org/10.3390/ma13071505
APA StyleLee, T., Lee, J., & Choi, H. (2020). Effects of Accelerators and Retarders in Early Strength Development of Concrete Based on Low-Temperature-Cured Ordinary Portland and Calcium Sulfoaluminate Cement Blends. Materials, 13(7), 1505. https://doi.org/10.3390/ma13071505







