Life Cycle Assessment of the Sustainability of Enhancing the Photodegradation Activity of TiO2 with Metal-Doping
Abstract
1. Introduction
2. Materials and Methods
2.1. Scope and System Boundaries
2.2. Synthesis Routes
2.3. Life Cycle Inventory Data
- Isopropanol {GLO}
- Sodium hydroxide, without water, in 50% solution state {GLO};
- Cobalt {GLO};
- Nickel sulfate {GLO};
- Manganese sulfate {GLO};
- Iron (III) chloride, without water, in 40% solution state {GLO};
- Water, deionized, from tap water;
- Electricity, medium voltage {PT}.
2.4. Environmental Impact Assessment
2.5. Sensitivity Analysis
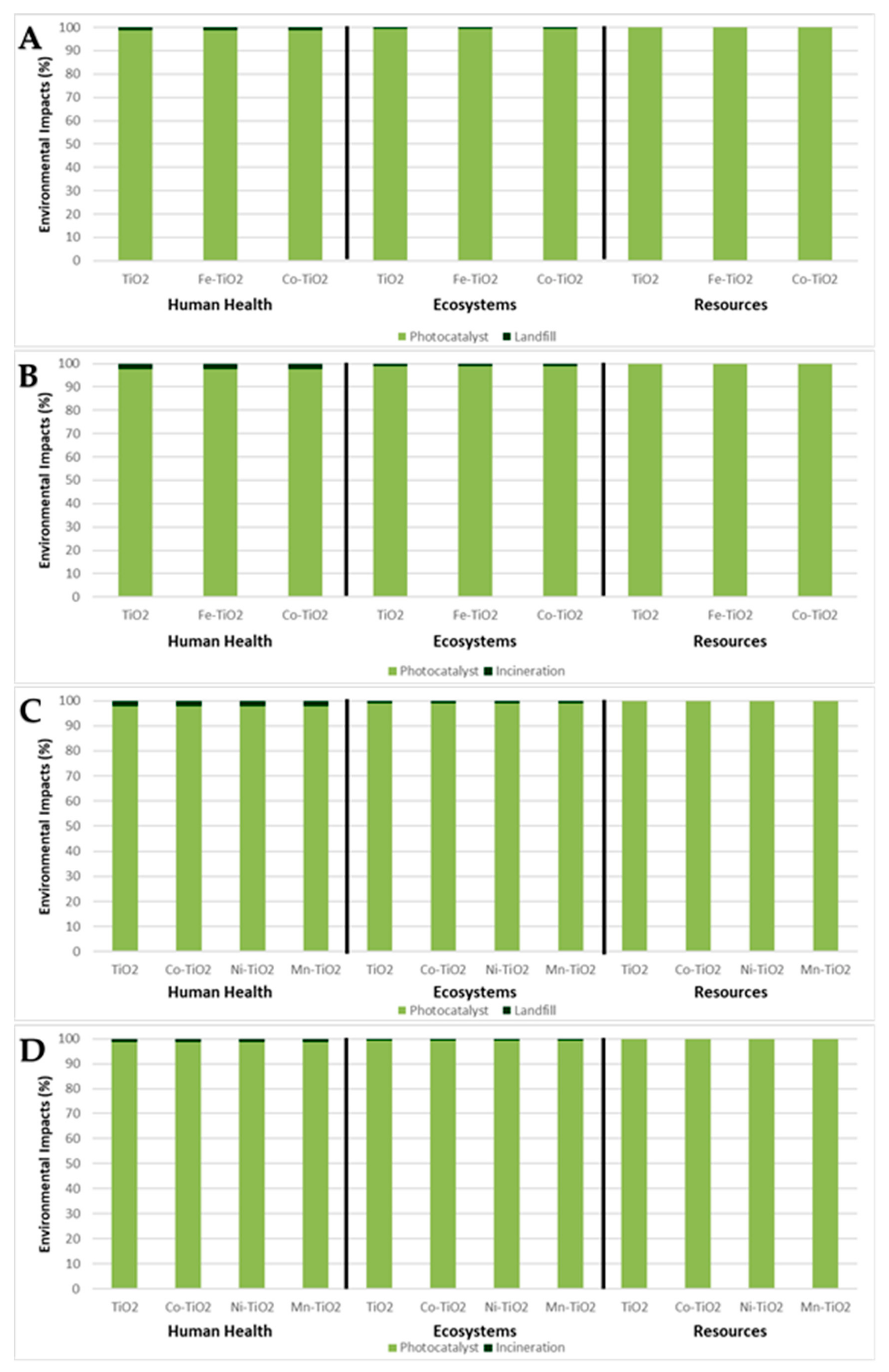
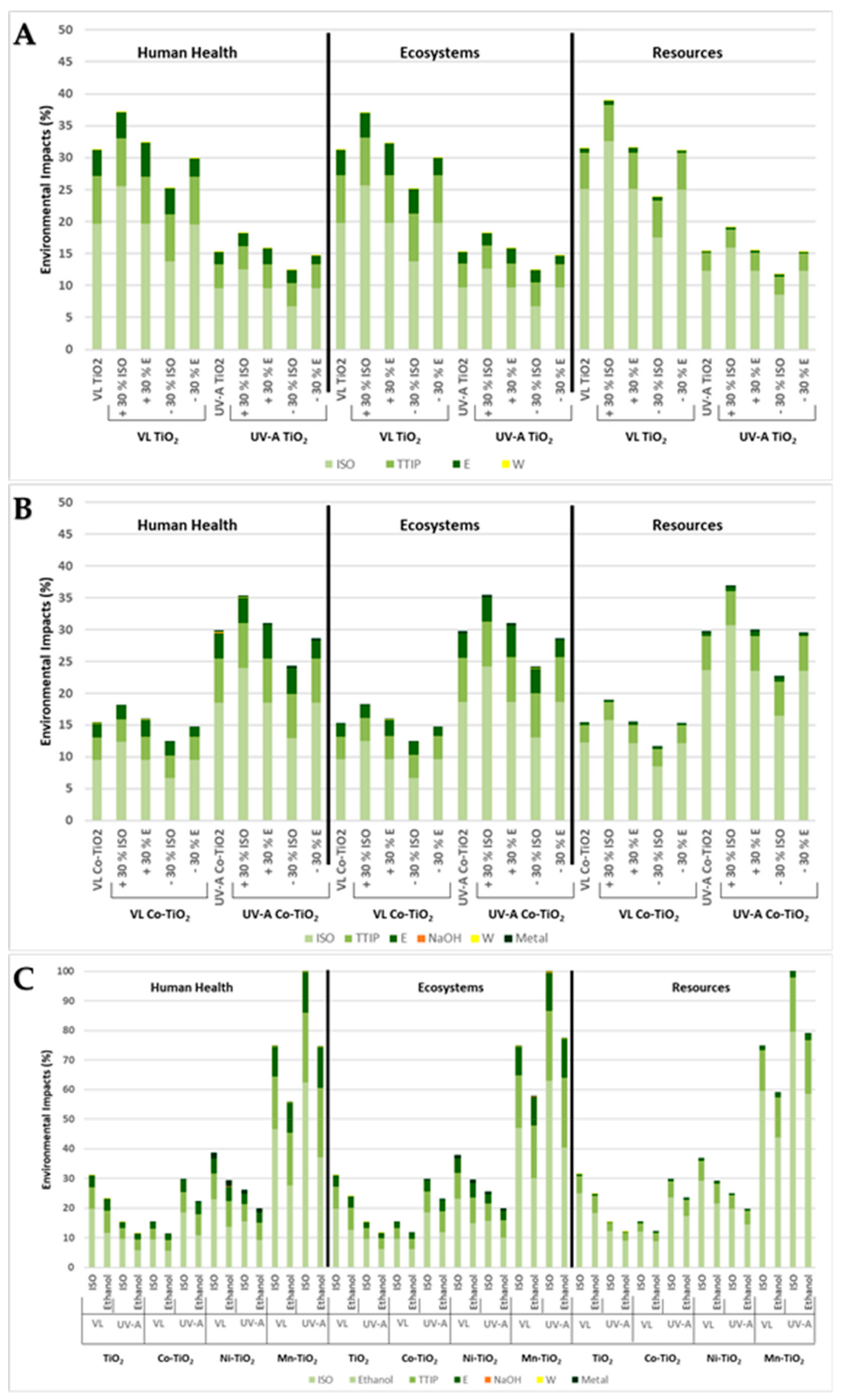
3. Results
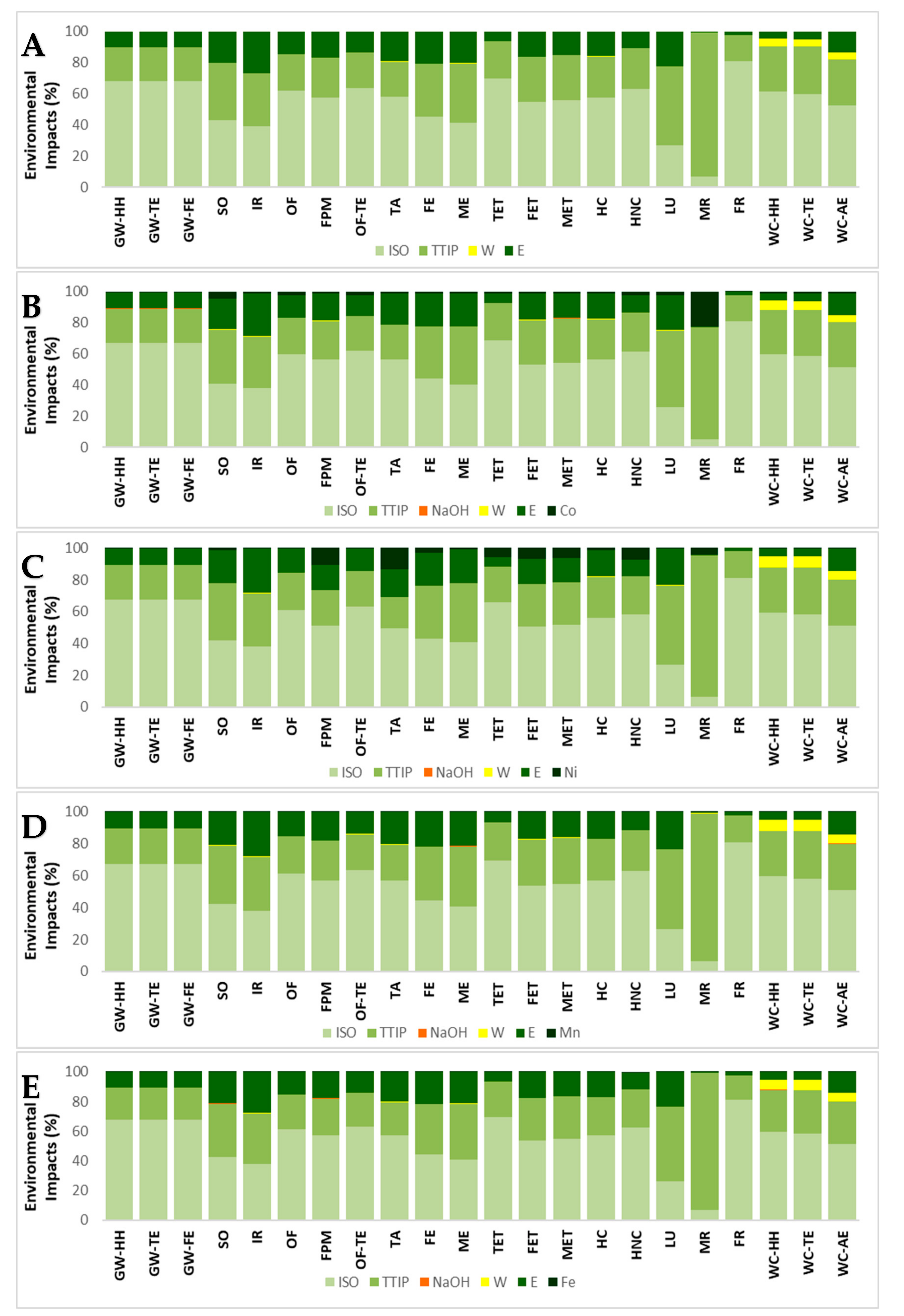
3.1. Synthesis Comparison by Weight
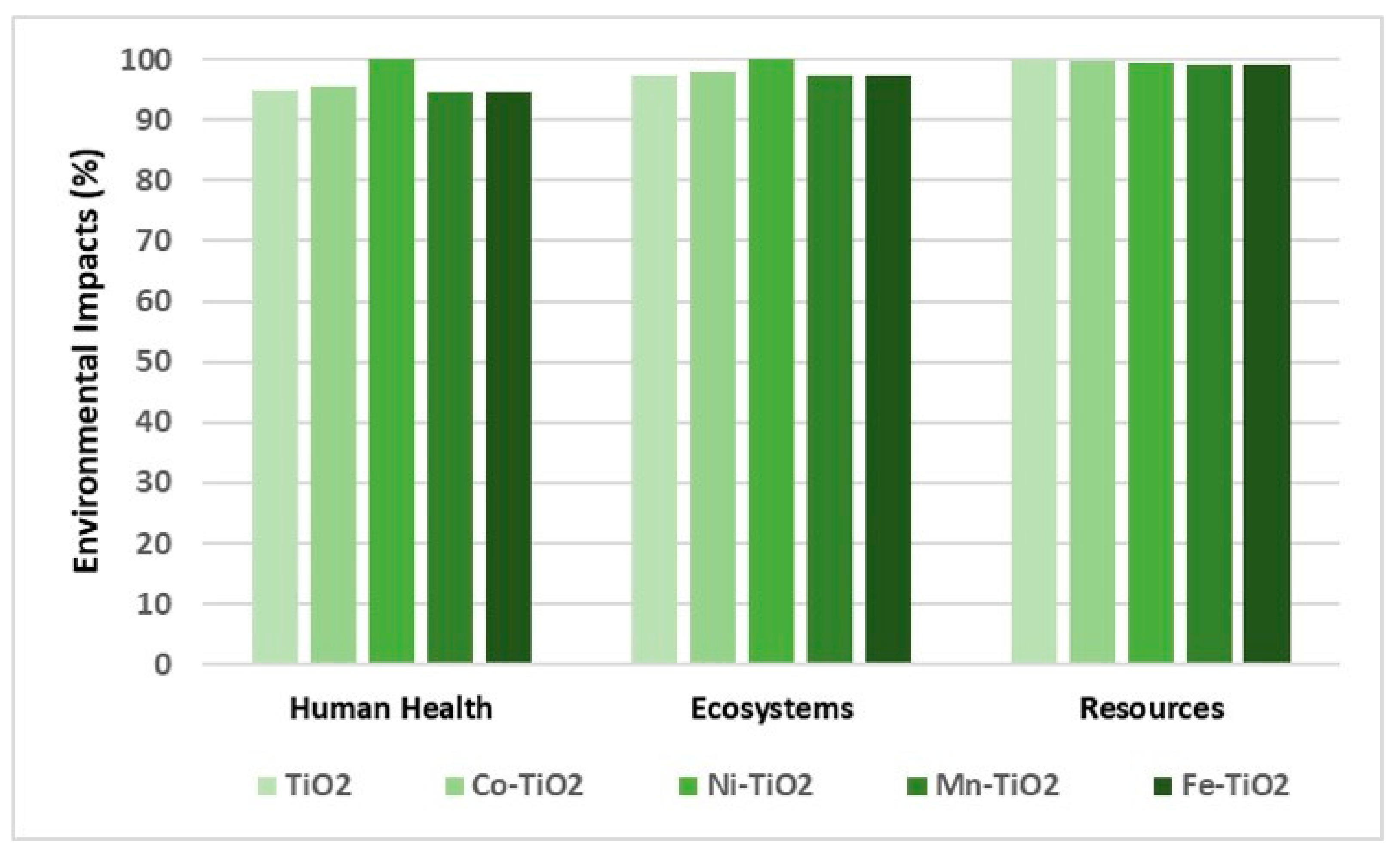
3.2. Synthesis Comparison by Photocatalytic Activity
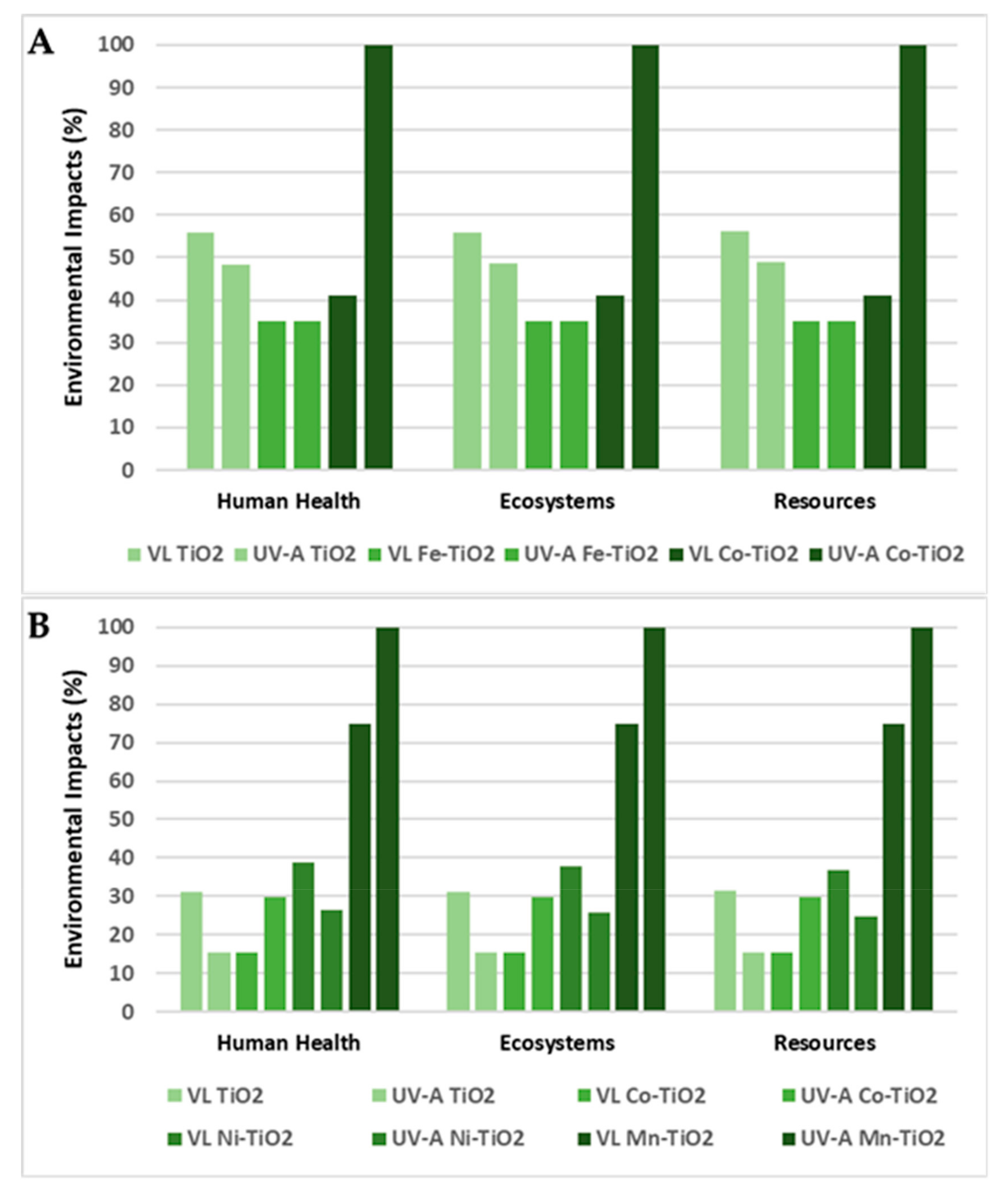
3.3. Sensitivity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gnanasekaran, L.; Hemamalini, R.; Saravanan, R.; Ravichandran, K.; Gracia, F.; Gupta, V. Intermediate state created by dopant ions (Mn, Co and Zr) into TiO2 nanoparticles for degradation of dyes under visible light. J. Mol. Liq. 2016, 223, 652–659. [Google Scholar] [CrossRef]
- Rizzi, V.; Prasetyanto, E.A.; Chen, P.; Gubitosa, J.; Fini, P.; Agostiano, A.; De Cola, L.; Cosma, P. Amino grafted MCM-41 as highly efficient and reversible ecofriendly adsorbent material for the Direct Blue removal from wastewater. J. Mol. Liq. 2019, 273, 435–446. [Google Scholar] [CrossRef]
- Rizzi, V.; Romanazzi, F.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Petrella, A.; Cosma, P. Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and Their Mixture from Wastewater. Biomolecules 2019, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- El Mragui, A.; Daou, I.; Zegaoui, O. Influence of the preparation method and ZnO/(ZnO + TiO2) weight ratio on the physicochemical and photocatalytic properties of ZnO-TiO2 nanomaterials. Catal. Today 2018, 312–322, 41–51. [Google Scholar] [CrossRef]
- El Mragui, A.; Zegaoui, O.; Daou, I. Synthesis, characterization and photocatalytic properties under visible light of doped and co-doped TiO2-based nanoparticles. Mater. Today: Proc. 2019, 13, 857–865. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Grubb, G.F.; Bakshi, B.R. Life Cycle of Titanium Dioxide Nanoparticle Production, Impact of Emissions and Use of Resources. J. Ind. Ecol. 2010, 15, 81–95. [Google Scholar] [CrossRef]
- Joshi, M.M.; Labhsetwar, N.K.; Mangrulkar, P.A.; Tijare, S.N.; Kamble, S.P.; Rayalu, S.S. Visible light induced photoreduction of methyl orange by N-doped mesoporous titania. Appl. Catal. A 2009, 357, 26–33. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Band Gap implications on Nano-TiO2 surface modification with ascorbic acid for visible light-active polypropylene coated photocatalyst. Nanomaterials 2018, 8, 599–613. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, S.; Wang, L.; Xiao, H. Black NiO-TiO2 nanorods for sola photocatalysis: Recognition of electronic structure and reaction mechanism. Appl. Catal. B 2018, 224, 705–714. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Wang, E.; Yang, W.; Cao, Y. Unique surface chemical species on Indium doped TiO2 and their effect on the visible light photocatalytic activity. J. Phys. Chem. C 2009, 113, 20912–20917. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, J.; Chen, M.; Shen, K.; Zhang, D.; Zhang, J.; Zhang, G.; Liu, Q.; Cao, Y. Boosted visible-light photodegradation of methylene blue by V and Co Co-doped TiO2. Materials 2018, 11, 1946–1958. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Chen, Y. Structure and photocatalytic properties of Mn-doped TiO2 loaded on wood-based activated carbon fiber composites. Materials 2017, 10, 631–641. [Google Scholar]
- El Mragui, A.; Zegaoui, O.; Daou, I.; Esteves da Silva, J.C.G. Preparation, characterization, and photocatalytic activity under UV and visible light of Co, Mn, and Ni mono-doped and (P,Mo) and (P,W) co-doped TiO2 nanoparticles: A comparative study. Environ. Sci. Pollut. Res. 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- El Mragui, A.; Logvina, Y.; Pinto da Silva, L.; Zegaoui, O.; Esteves da Silva, J.C.G. Synthesis of Fe- and Co-Doped TiO2 with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874–3888. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Oliveira, J.A.B.P.; Santos, S.M.; Gil, M.V.; Otero, M.; Calisto, V.; Estves, V.I. Production of highly efficient activated carbons from industrial wastes for the removal of pharmaceuticals from water—A full factorial design. J. Hazard. Mater. 2019, 370, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, M.J.; Zimmerman, J.B.; Anastas, P.T. Toward Green Nano, E-factor analysis of several nanomaterial syntheses. J. Ind. Ecol. 2008, 12, 316–328. [Google Scholar]
- Pourzahedi, L.; Eckelman, M.J. Comparative life cycle assessment of silver nanoparticle synthesis routes. Environ. Sci. 2015, 2, 361–369. [Google Scholar] [CrossRef]
- Bafana, A.; Kumar, S.V.; Temizel-Sekeryan, S.; Dahoumane, S.A.; Haselbach, L.; Jeffryes, C.S. Evaluating microwave-synthesized silver nanoparticles from silver nitrate with life cycle assessment techniques. Sci. Total Environ. 2018, 636, 936–943. [Google Scholar] [CrossRef]
- Yubing, P.; Feng, T.; Adam, P.; Laratte, B.; Ionescu, R.E. Fate and characterization factors of nanoparticles in seventeen subcontinental freshwaters: A case study on copper nanoparticles. Environ. Sci. Technol. 2016, 50, 9370–9379. [Google Scholar]
- Upadhyayula, V.K.K.; Meyer, D.E.; Curran, M.A.; Gonzalez, M.A. Life cycle assessment as a tool to enhance the environmental performance of carbon nanotube products: A review. J. Clean. Prod. 2012, 26, 37–47. [Google Scholar] [CrossRef]
- Feijoo, S.; González-García, S.; Moldes-Diz, Y.; Vazquez-Vasquez, C.; Feijoo, G.; Moreira, M.T. Comparative life cycle assessment of diferente synthesis routes of magnetic nanoparticles. J. Clean. Prod. 2017, 143, 528–538. [Google Scholar] [CrossRef]
- Ramos, A.; Teixeira, C.A.; Rouboa, A. Assessment study of an advanced gasification strategy at low temperature for syngas generation. Int. J. Hydrog. Energy 2018, 43, 10155–10166. [Google Scholar] [CrossRef]
- Ramos, A.; Teixeira, C.A.; Rouboa, A. Environmental analysis of waste-to-energy—A portuguese case study. Energies 2018, 11, 548–574. [Google Scholar] [CrossRef]
- Pini, M.; González, E.I.C.; Neri, P.; Siligardi, C.; Ferrari, A.M. Assessment of Environmental Performance of TiO2 Nanoparticles Coated Self-Cleaning Float Glass. Coatings 2017, 7, 8. [Google Scholar] [CrossRef]
- Christé, S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of the environmental impact and efficiency of N-doping strategies in the synthesis of carbon dots. Materials 2020, 13, 504–518. [Google Scholar] [CrossRef]
- Sendão, R.; Martínez de Yuso, M.; Algarra, M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J. Clean. Prod. 2020, 254, 120080–120090. [Google Scholar] [CrossRef]
- Tsang, M.; Philippot, G.; Aymonier, C.; Sonnemann, G. Anticipatory Life-cycle assessment of supercritical fluid synthesis of barium strontium titanate nanoparticles. Electron. Suppl. Mater. (Esi) Green Chem. 2016, 18, 4924–4933. [Google Scholar] [CrossRef]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; Zelm, R. ReCiPe2016: A harmonized life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle. Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Kazemi, A.; Bahramifar, N.; Heydari, A.; Olsen, S.I. Life cycle assessment of nanoadsorbents at early stage technological development. J. Clean. Prod. 2018, 174, 527–537. [Google Scholar] [CrossRef]
- Pianosi, F.; Beven, K.; Freer, J.; Hall, J.W.; Rougier, J.; Stephenson, D.B.; Wagener, T. Sensitivity analysis of environmental models: A systematic review with practical workflow. Environ. Model. Softw. 2016, 79, 214–232. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Z.; Hicks, A.L. Life Cycle Impact of Titanium Dioxide Nanoparticles Synthesis through Physical, Chemical, and Biological Routes. Environ. Sci. Technol. 2019, 53, 4078–4087. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]

| Photocatalyst | CBZ | MO | ||
|---|---|---|---|---|
| VL | UV-A | VL | UV-A | |
| Fe–TiO2 | 1.00 | 1.00 | - | - |
| Undoped TiO2 | 1.59 | 1.38 | 2.04 | 1.00 |
| Co–TiO2 | 1.16 | 2.83 | 1.00 | 1.94 |
| Ni–TiO2 | - | - | 2.41 | 1.63 |
| Mn–TiO2 | - | - | 4.90 | 6.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, S.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Life Cycle Assessment of the Sustainability of Enhancing the Photodegradation Activity of TiO2 with Metal-Doping. Materials 2020, 13, 1487. https://doi.org/10.3390/ma13071487
Fernandes S, Esteves da Silva JCG, Pinto da Silva L. Life Cycle Assessment of the Sustainability of Enhancing the Photodegradation Activity of TiO2 with Metal-Doping. Materials. 2020; 13(7):1487. https://doi.org/10.3390/ma13071487
Chicago/Turabian StyleFernandes, Sónia, Joaquim C.G. Esteves da Silva, and Luís Pinto da Silva. 2020. "Life Cycle Assessment of the Sustainability of Enhancing the Photodegradation Activity of TiO2 with Metal-Doping" Materials 13, no. 7: 1487. https://doi.org/10.3390/ma13071487
APA StyleFernandes, S., Esteves da Silva, J. C. G., & Pinto da Silva, L. (2020). Life Cycle Assessment of the Sustainability of Enhancing the Photodegradation Activity of TiO2 with Metal-Doping. Materials, 13(7), 1487. https://doi.org/10.3390/ma13071487







