Thermally Sprayed Coatings: Novel Surface Engineering Strategy Towards Icephobic Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods
3. Results and Discussion
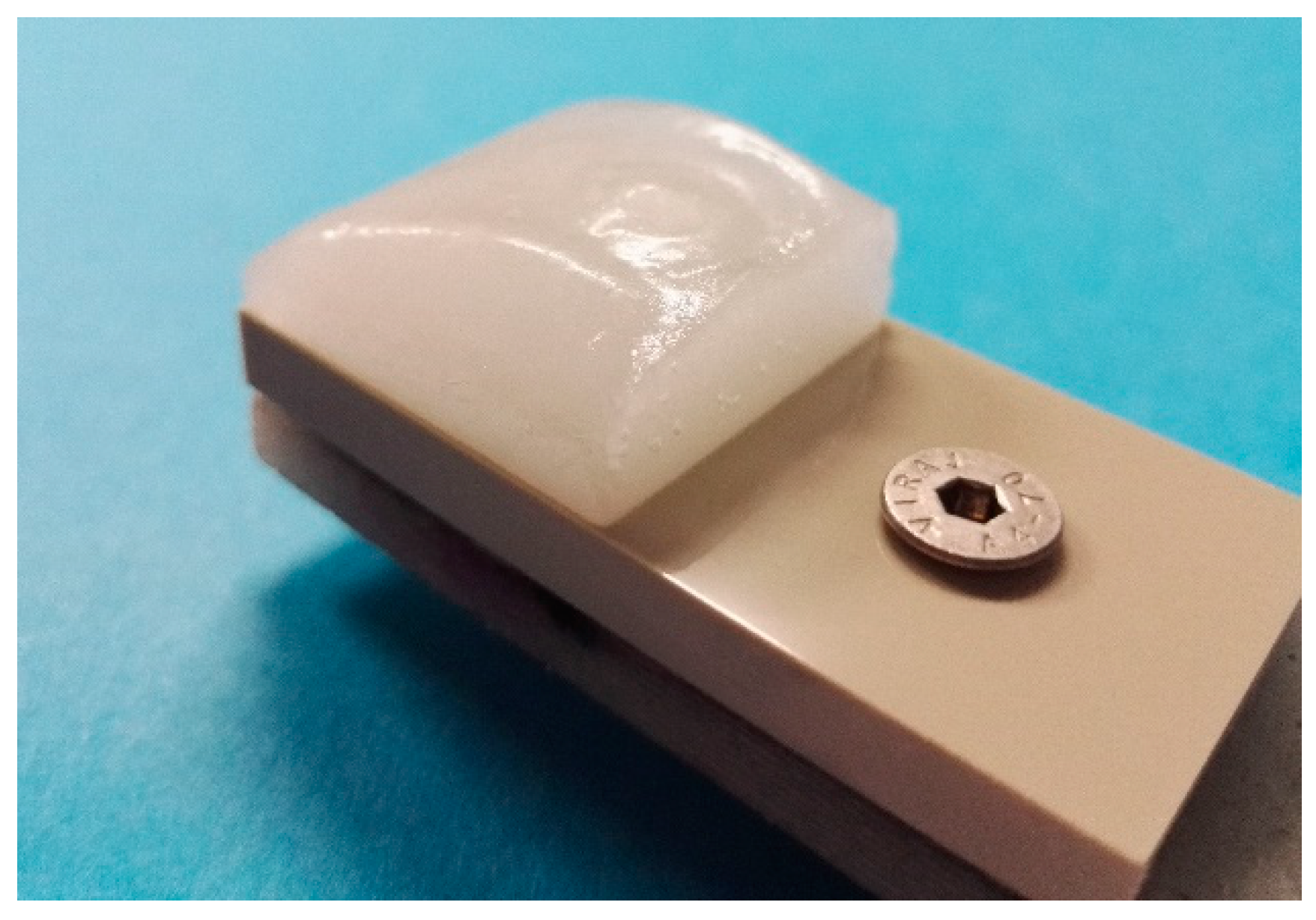
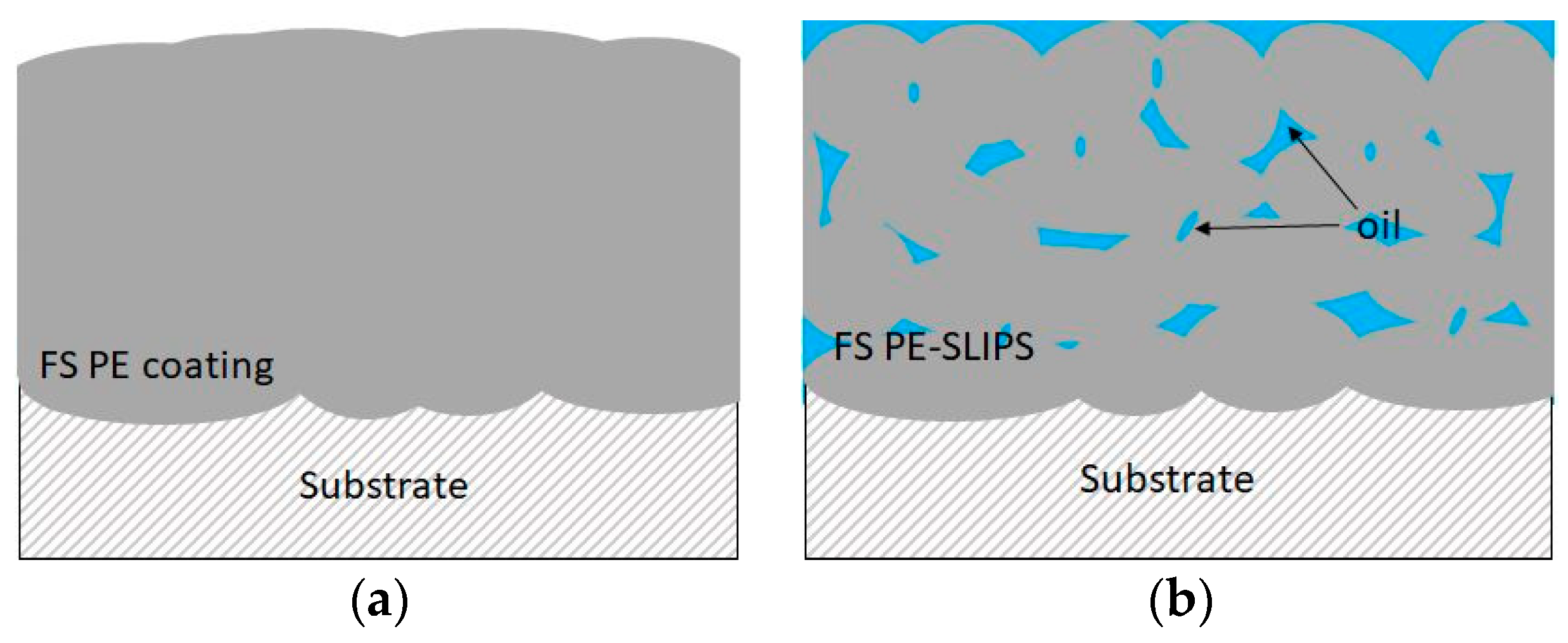
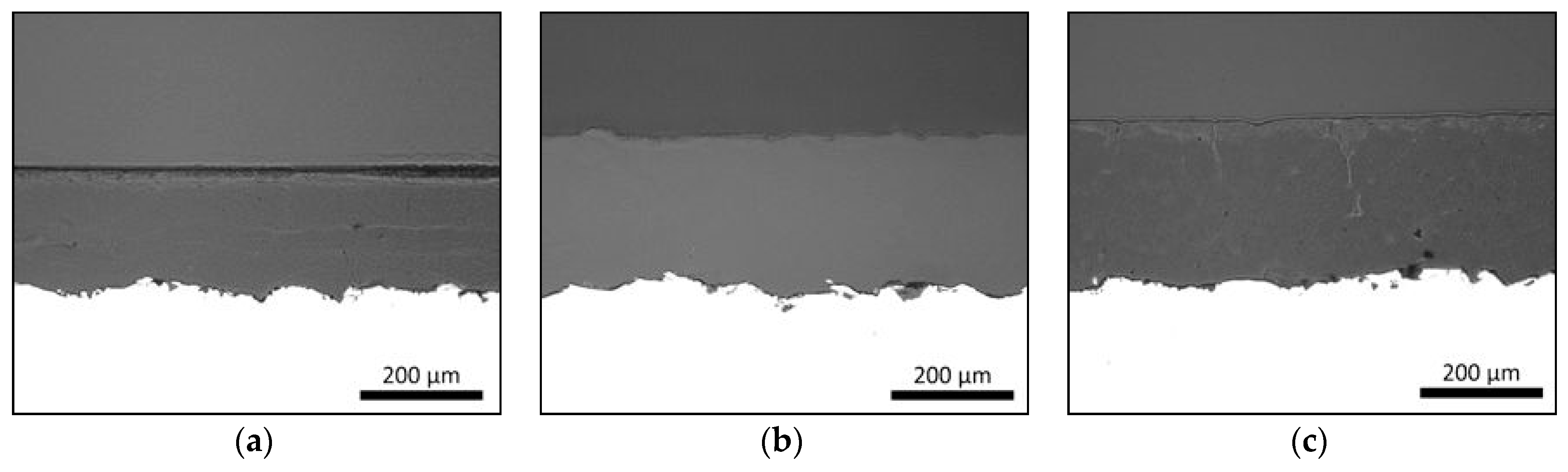
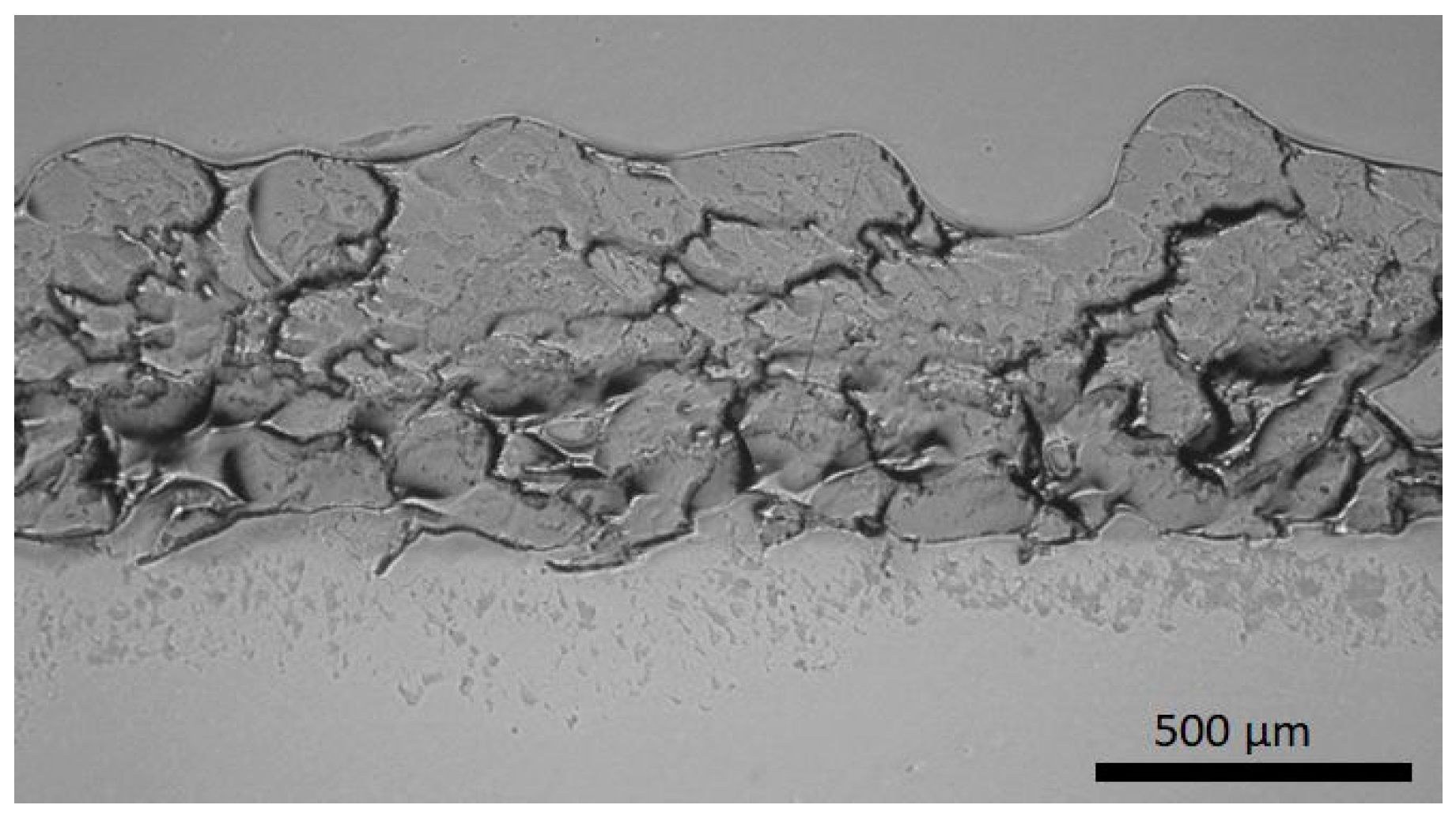
3.1. Coating Structures
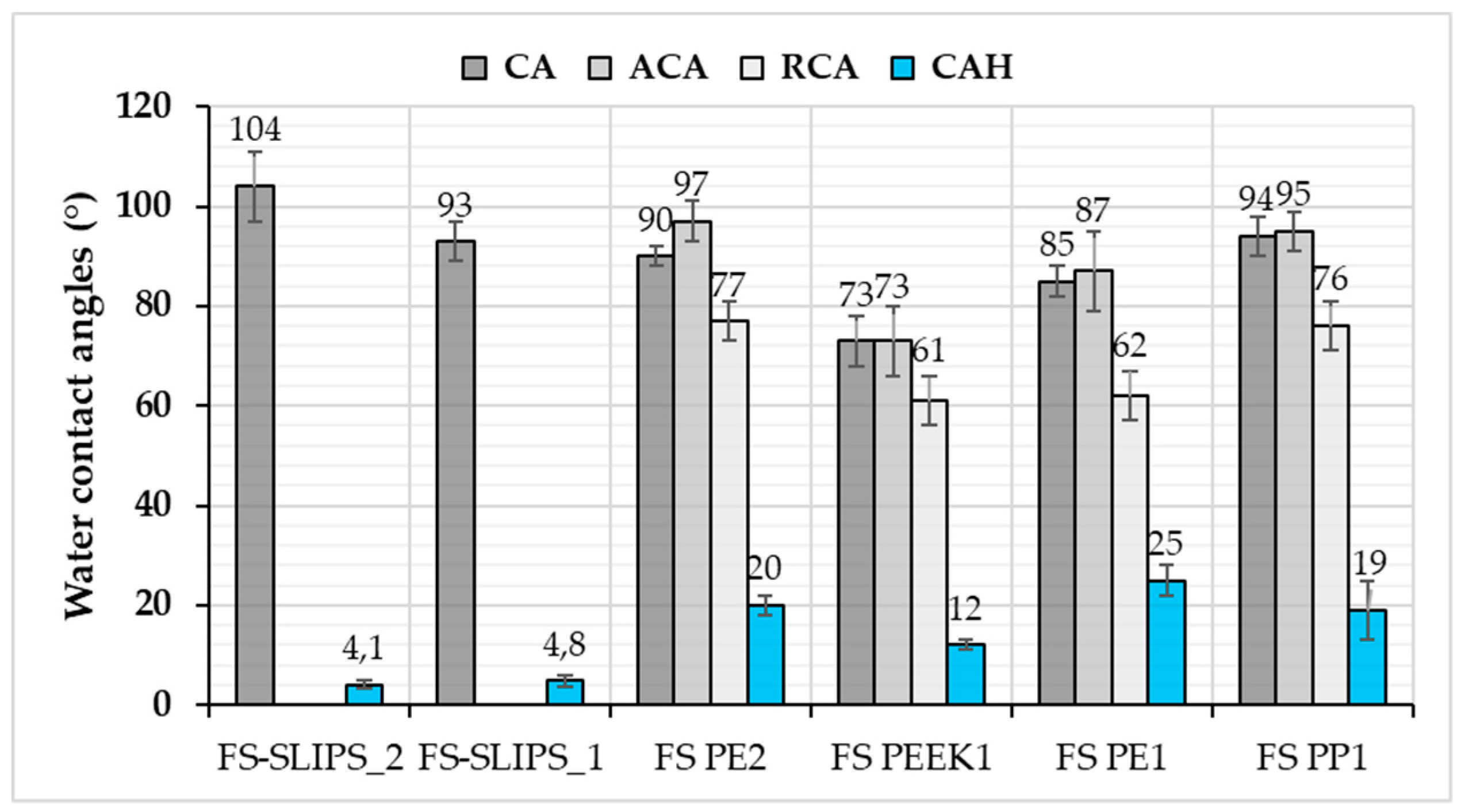
3.2. Wettability of the Surfaces
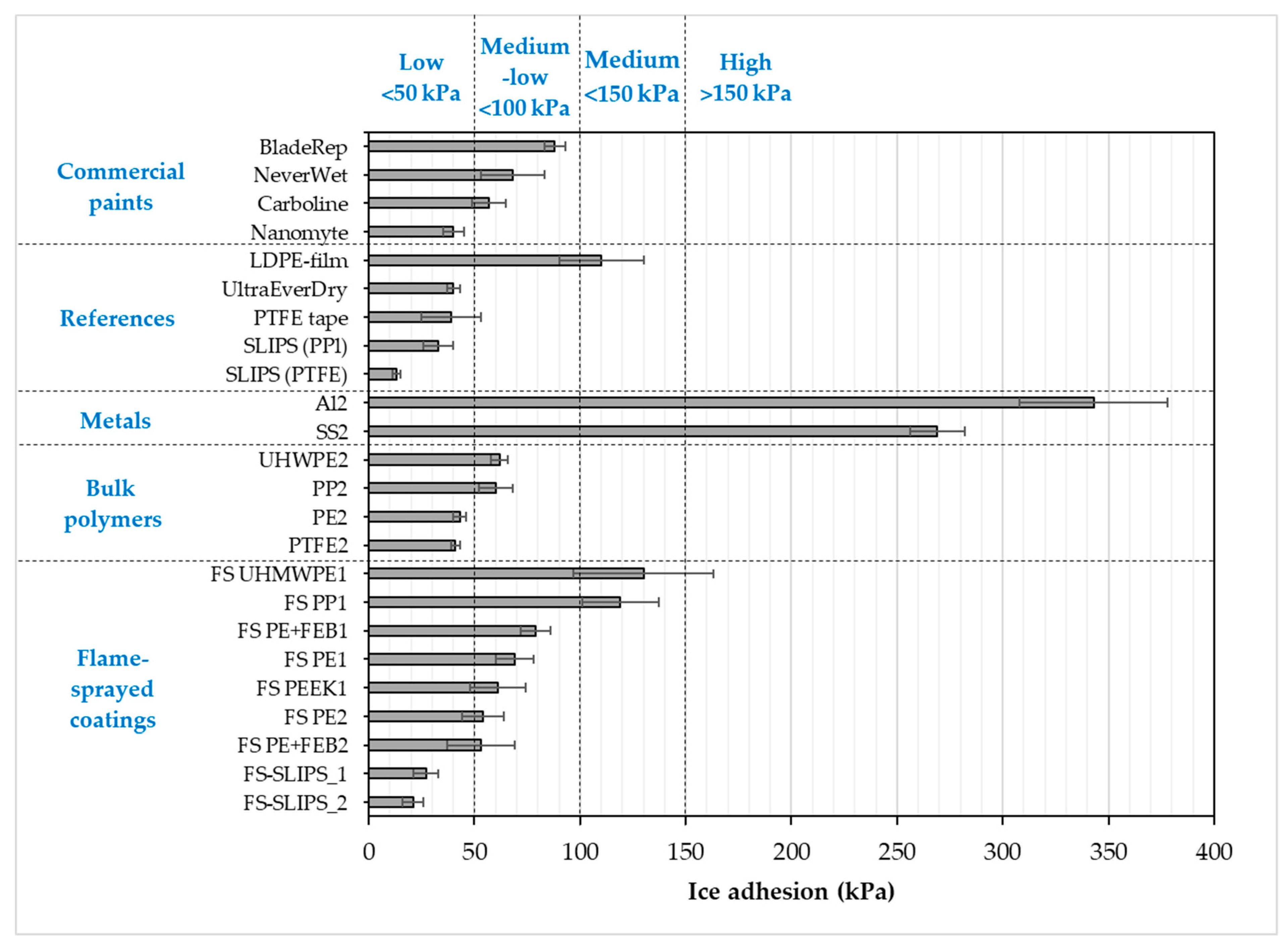
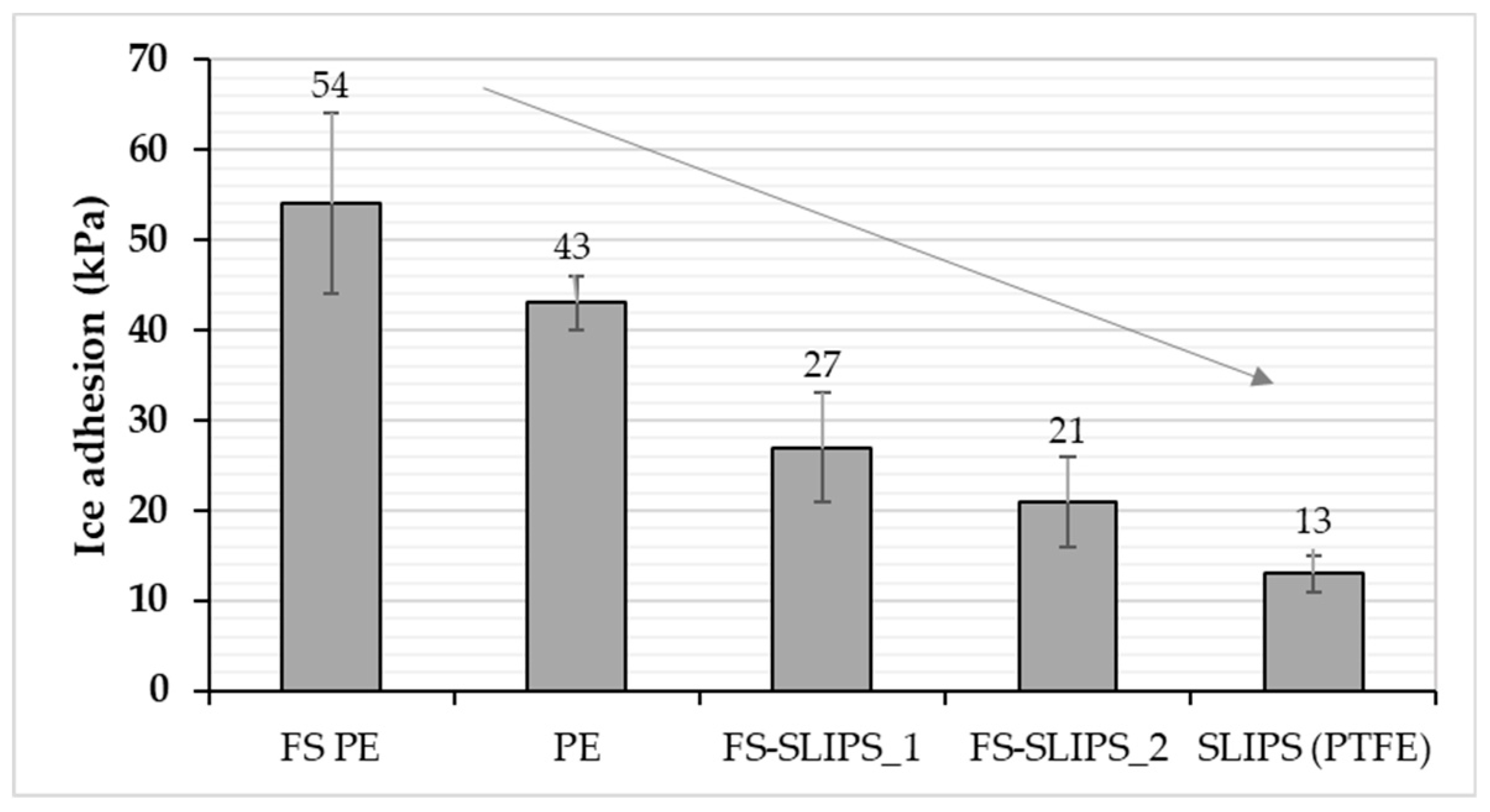
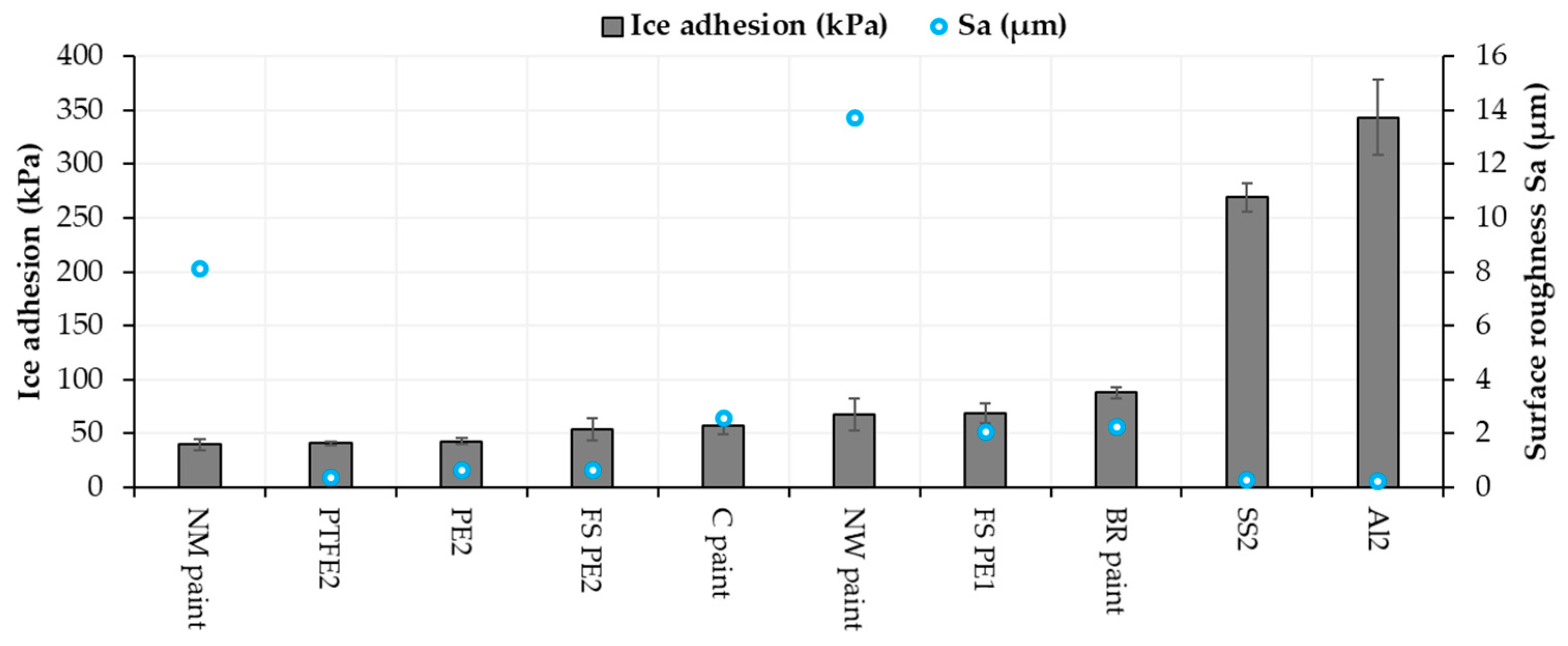
3.3. Ice Adhesion of the Surfaces
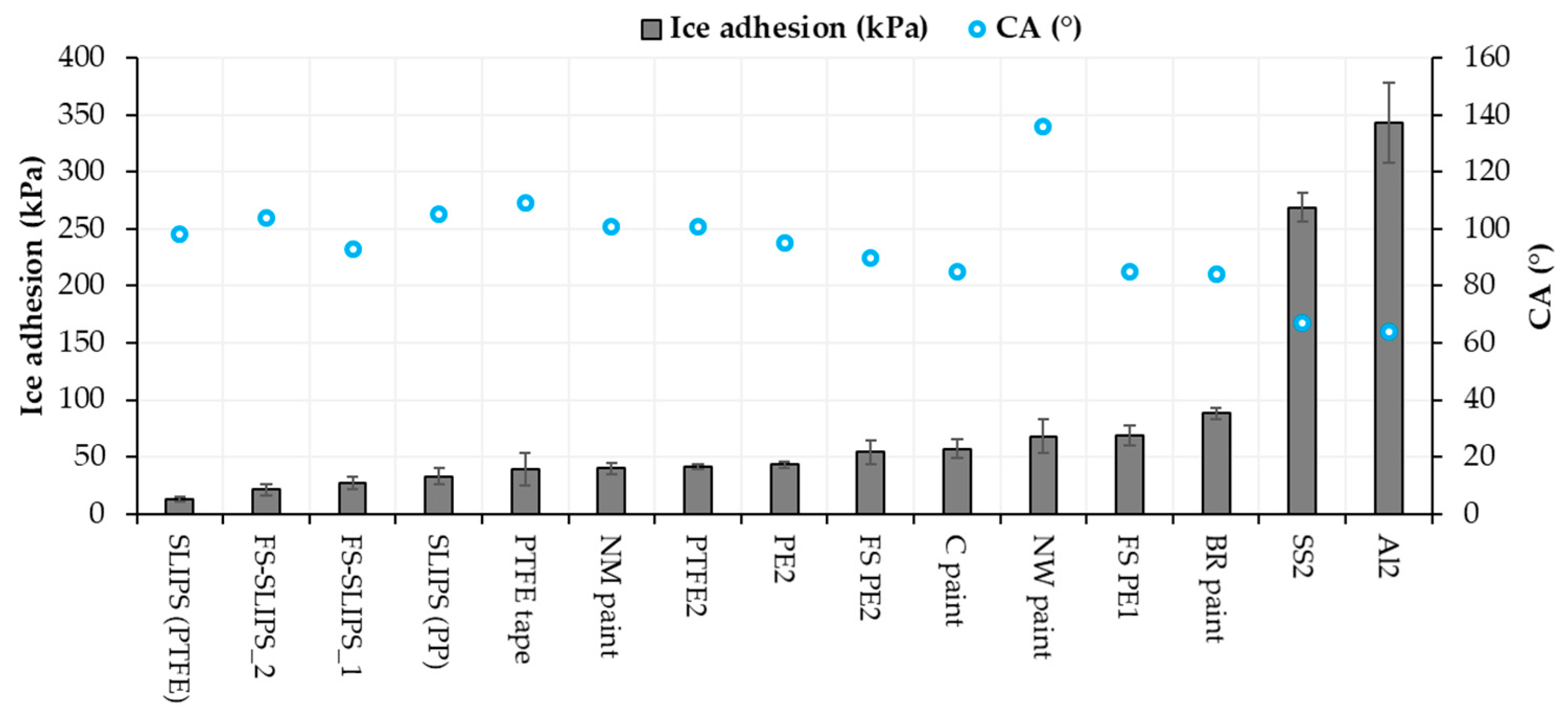
3.4. Comparison between Icephobicity and Surface Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008; 626p. [Google Scholar] [CrossRef]
- Davis, J.R. Handbook of Thermal Spray Technology; ASM International: Novelty, OH, USA, 2004; 338p. [Google Scholar]
- Petrovicova, E.; Schadler, L.S. Thermal spraying of polymers. Int. Mater. Rev. 2002, 47, 169–190. [Google Scholar] [CrossRef]
- Leivo, E.; Wilenius, T.; Kinos, T.; Vuoristo, P.; Mäntylä, T. Properties of Thermally Sprayed Fluoropolymer PVDF, ECTFE, PFA and FEP Coatings. Prog. Org. Coat. 2004, 49, 69–73. [Google Scholar] [CrossRef]
- Winkler, R.; Bultmann, R.F.; Hartmann, S.; Jerz, A. Thermal Spraying of Polymers: Spraying Processes, Materials and New Trends. In Proceedings of the Thermal Spray, Advancing the Science and Applying the Technology, Orlando, FL, USA, 5–8 May 2003; Moreau, C., Marple, B., Eds.; ASM International: Novelty, OH, USA, 2003; pp. 1635–1638. [Google Scholar]
- Ryerson, C. Ice protection of offshore platforms. Cold Reg. Sci. Technol. 2011, 65, 97–110. [Google Scholar] [CrossRef]
- Moore, G. A climatology of vessel icing for the subpolar North Atlantic Ocean. Int. J. Climatol. 2013, 33, 2495–2507. [Google Scholar] [CrossRef]
- Carriveau, R.; Edrisy, A.; Cadieux, P.; Mailloux, R. Ice Adhesion Issues in Renewable Energy Infrastructure. J. Adhes. Sci. Technol. 2012, 26, 447–461. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Su, Y.; Xu, Z. Aircraft flight characteristics in icing conditions. Prog. Aerosp. Sci. 2015, 74, 62–80. [Google Scholar] [CrossRef]
- Farzaneh, M. Atmospheric Icing of Power Networks; Springer: London, UK, 2008; p. 381. [Google Scholar]
- Makkonen, L.; Lehtonen, P.; Hirviniemi, M. Determining ice loads for tower structure design. Eng. Str. 2014, 74, 229–232. [Google Scholar] [CrossRef]
- Kreder, M.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Lamarre, J.-M.; Marcoux, P.; Perrault, M.; Abbott, R.C.; Legoux, J.G. Performance Analysis and Modeling of Thermally Sprayed Resistive Heaters. J. Therm. Spray Technol. 2013, 22, 947–953. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; McDonald, A. Application of Flame-Sprayed Coatings as Heating Elements for Polymer-Based Composite Structures. J. Therm. Spray Technol. 2015, 24, 1289–1301. [Google Scholar] [CrossRef]
- Laforte, C.; Brassard, J.-D.; Volat, C. Extended Evaluation of icephobic coating regarding their field of application. In Proceedings of the International Workshop on Atmospheric Icing of Structures, IWAIS 2019, Reykjavik, Iceland, 23–28 June 2019; 5p. [Google Scholar]
- Brassard, J.-D.; Laforte, C.; Guerin, F.; Blackburn, C. Icephobicity: Definition and Measurement Regarding Atmospheric Icing. Adv. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing Superhydrophobic Coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J.; et al. Icephobicity of Slippery Liquid Infused Porous Surfaces under Multiple Freeze-Thaw and Ice Accretion-Detachment Cycles. Adv. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Stenroos, C.; Kylmälahti, M.; Apostol, M.; Kiilakoski, J.; Vuoristo, P. Anti-icing Behavior of Thermally Sprayed Polymer Coatings. J. Therm. Spray Technol. 2017, 26, 150–160. [Google Scholar] [CrossRef]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Icephobic Behaviour and Thermal Stability of Flame-Sprayed Polyethylene Coating: The Effect of Process Parameters. J. Therm. Spray Technol. 2020, 29, 241–254. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Kylmälahti, M.; Laakso, J.; Vuoristo, P. Thermally Sprayed Slippery and Icephobic Surfaces. In Proceedings of the International Thermal Spray Conference, ITSC2018, Orlando, FL, USA, 7–10 May 2018; Azarmi, F., Balani, K., Eden, T., Hussain, T., Lau, Y.-C., Li, H., Shinoda, K., Eds.; pp. 380–384. [Google Scholar]
- Lampman, S. Characterization and Failure Analysis of Plastics. In ASM International Handbooks; ASM International: Novelty, OH, USA, 2003; p. 482. [Google Scholar]
- Lima, C.; de Souza, N.; Camargo, F. Study of Wear and Corrosion Performance of Thermal Sprayed Engineering Polymers. Surf. Coat. Technol. 2013, 220, 140–143. [Google Scholar] [CrossRef]
- Vuoristo, P.; Leivo, E.; Turunen, E.; Leino, M.; Järvelä, P.; Mäntylä, T. Evaluation of Thermally Sprayed and Other Polymeric Coatings for Use in Natural Gas Pipeline Components. In Proceedings of the Thermal Spray, Advancing the Science and Applying the Technology, Orlando, FL, USA, 5–8 May 2003; Moreau, C., Marple, B., Eds.; ASM International: Novelty, OH, USA, 2003; pp. 1693–1702. [Google Scholar]
- Gawne, D.T.; Bao, Y.; Zhang, T. Influence of Polymer Composition on The Deposition of UHMWPE Coatings. In Proceedings of the Thermal Spray, Advancing the Science and Applying the Technology, Orlando, FL, USA, 5–8 May 2003; Moreau, C., Marple, B., Eds.; ASM International: Novelty, OH, USA, 2003; pp. 1639–1644. [Google Scholar]
- Juuti, P.; Haapanen, J.; Stenroos, C.; Niemelä-Anttonen, H.; Harra, J.; Koivuluoto, H.; Teisala, H.; Lahti, J.; Tuominen, M.; Kuusipalo, J.; et al. Achieving a slippery, liquid-infused porous surface with anti-icing properties by direct deposition of flame synthesized aerosol nanoparticles on a thermally fragile substrate. Appl. Phys. Lett. 2017, 110, 161603. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Stenroos, C.; Ruohomaa, R.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Research on icing behavior and ice adhesion testing of icephobic surfaces. In Proceedings of the International Workshop on Atmospheric Icing of Structures, IWAIS 2015, Uppsala, Sweden, 28 June–3 July 2015; 6p. [Google Scholar]
- Berndt, C.C.; Otterson, D.; Allan, M.L.; Berndt, C.C.; Otterson, D. Polymer Coatings for Corrosion Protection in Biochemical Treatment of Geothermal Residues. Geotherm. Resour. Counc. Trans. 1998, 22, 425–429. [Google Scholar]
- Sugama, T.; Kawase, R.; Berndt, C.C.; Herman, H. An Evaluation of Methacrylic Acid-Modified Poly(Ethylene) Coatings Applied by Flame Spray Technology. Prog. Org. Coat. 1995, 25, 205–216. [Google Scholar] [CrossRef]
- Chen, X.; Gong, Y.; Suo, X.; Huang, J.; Liu, Y.; Li, H. Construction of Mechanically Durable Superhydrophobic Surfaces by Thermal Spray Deposition and Further Surface Modification. Appl. Surf. Sci. 2015, 356, 639–644. [Google Scholar] [CrossRef]
- Bao, Y.; Gawne, D.T.; Zhang, T. Effect of Feedstock Particle Size on the Heat Transfer Rates and Properties of Thermally Sprayed Polymer Coatings. Trans. Inst. Meter. Finish 1995, 73, 119–124. [Google Scholar] [CrossRef]
- Xu, P.; Coyle, T.W.; Pershin, L.; Mostaghimi, J. Superhydrophobic ceramic coating: Fabrication by solution precursor plasma spray and investigation of wetting behavior. J. Coll. Interface Sci. 2018, 523, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Pugh, M.; Moreau, C.; Dolatabadi, A. Developing hydrophobic and superhydrophobic TiO2 coatings by plasma spraying. Surf. Coat. Technol. 2016, 289, 29–36. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Kiilakoski, J.; Vuoristo, P.; Koivuluoto, H. Icephobic Performance of Different Surface Designs and Materials. In Proceedings of the International Workshop on Atmospheric Icing of Structures, IWAIS 2019, Reykjavik, Iceland, 23–28 June 2019; 5p. [Google Scholar]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of Icephobic Coatings: Screening of Different Coatings and Influence of Roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.; Bobji, M.; Chakradhar, R.; Basu, B. Effect of Wettability and Surface Roughness on Ice-Adhesion Strength of Hydrophilic, Hydrophobic and Superhydrophobic Surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Surmeneva, M.; Nikityuk, P.; Hans, M.; Surmenev, R. Deposition of Ultrathin Nano-Hydroxyapatite Films on Laser Micro-Textured Titanium Surfaces to Prepare a Multiscale Surface Topography for Improved Surface Wettability/Energy. Materials 2016, 9, 862. [Google Scholar] [CrossRef]
- He, Y.; Jiang, C.; Cao, X.; Chen, J.; Tian, W.; Yuan, W. Reducing ice adhesion by hierarchical micro-nano-pillars. Appl. Surf. Sci. 2014, 305, 589–595. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Milionis, A.; Loth, E.; Sokhey, J. Self-lubricating icephobic elastomer coating (SLIC) for ultralow ice adhesion with enhanced durability. Cold Reg. Sci. Technol. 2018, 148, 29–37. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Kumar, D.; Ho, J.W.C.; Kanhere, P.D.; Srikanth, N.; Liu, E.; Wilson, P.; Chen, Z. Development of Sol–Gel Icephobic Coatings: Effect of Surface Roughness and Surface Energy. ACS Appl. Mater. Interfaces 2014, 6, 20685–20692. [Google Scholar] [CrossRef] [PubMed]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal Sprayed Functional and Smart Coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijin, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are Superhydrophobic Surfaces Best for Icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.; Farzaneh, M. How wetting hysteresis influences ice adhesion strength on superhydrophobic surfaces. Langmuir 2009, 16, 8854–8856. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.F.; Lee, H.P.; Lim, S.P. The Variation of Ice Adhesion Strength with Substrate Surface Roughness. Meas. Sci. Technol. 2010, 21, 75701–75709. [Google Scholar] [CrossRef]
- Yang, S.; Xia, Q.; Zhu, L.; Xue, J.; Wang, Q.; Chen, Q. Research on the Icephobic Properties of Fluoropolymer-Based Materials. Appl. Surf. Sci. 2011, 257, 4956–4962. [Google Scholar] [CrossRef]
- Zou, M.; Beckford, S.; Wei, R.; Ellis, C.; Hatton, G.; Miller, M.A. Effects of Surface Roughness and Energy on Ice Adhesion Strength. Appl. Surf. Sci. 2011, 257, 3786–3792. [Google Scholar] [CrossRef]


| Surface Treatment | Material | Form | |
|---|---|---|---|
| Flame-Sprayed Coatings | |||
| FS PE 1 | 1 As-sprayed | Polyethylene | Coating |
| FS PE 2 | 2 Polished | Polyethylene | Coating |
| FS PE+FEB 1 | 1 As-sprayed | Polyethylene + perfluoroethylene propylene | Coating |
| FS PE+FEB 2 | 2 Polished | Polyethylene + perfluoroethylene propylene | Coating |
| FS UHMWPE 1 | 1 As-sprayed | Ultra-high molecular weight polyethylene | Coating |
| FS PEEK 1 | 1 As-sprayed | Polyether ether ketone | Coating |
| FS PP 1 | 1 As-sprayed | Polypropylene | Coating |
| FS-SLIPS_1 | SLIPS | Polyethylene (fine) + silicone oil (50 cSt) | SLIPS |
| FS-SLIPS_2 | SLIPS | Polyethylene (coarse) + silicone oil (50 cSt) | SLIPS |
| Bulk Polymers | |||
| PE 2 | 2 Polished | Polyethylene | Bulk plate |
| UHWPE 2 | 2 Polished | Ultra-high molecular weight polyethylene | Bulk plate |
| PP 2 | 2 Polished | Polypropylene | Bulk plate |
| PTFE 2 | 2 Polished | Polytetrafluoroethylene | Bulk plate |
| Bulk Metals | |||
| Al 2 | 2 Polished | Aluminum | Bulk plate |
| SS 2 | 2 Polished | Stainless Steel | Bulk plate |
| References | |||
| LDPE | As-received | Low-density polyethylene | Film |
| PTFE | As-received | Polytetrafluoroethylene | Tape |
| SLIPS (PTFE) | SLIPS | Polytetrafluoroethylene membrane (0.2 µm) + silicone oil (50 cSt) | SLIPS |
| SLIPS (PP) | SLIPS | Polypropylene membrane (0.2 µm) + silicone oil (50 cSt) | SLIPS |
| Commercial Paints | |||
| BladeRep9 | Painted, BR | Polyurethane | Paint |
| Carboline | Painted, C | Elastomeric | Paint |
| Nanomyte | Painted, NM | Nanocomposite | Paint |
| NeverWet | Painted, NW | Superhydrophobic | Paint |
| UltraEverDry | Sprayed | Superhydrophobic | Film |
| Material or Surface | Ice Adhesion (kPa) | Contact Angle (°) | Adv. Contact Angle (°) | Rec. Contact Angle (°) | CA Hysteresis (°) | Roughness Sa (µm) |
|---|---|---|---|---|---|---|
| Flame-Sprayed Coatings | ||||||
| FS PE 1 | 69 (±9) [21] | 85 (±3) | 87 (±8) | 62 (±5) | 25 (±3) | 2.05 [21] |
| FS PE 2 | 54 (±10) [21] | 90 (±2) | 97 (±4) | 77 (±4) | 20 (±2) | 0.64 [21] |
| FS PE + FEB 1 | 79 (±7) [21] | - | - | - | - | 3.95 |
| FS PE + FEB 2 | 53 (±16) [21] | 75 (±5) | 82 (±5) | 65 (±) | 18 (±0.1) | 0.96 [21] |
| FS UHMWPE 1 | 130 (±33) [21] | 91 [21] | - | - | - | 1.65 [21] |
| FS PEEK 1 | 61 (±13) | 73 (±5) | 73 (±7) | 61 (±5) | 12 (±1) | - |
| FS PP 1 | 119 (±18) | 94 (±4) | 95 (±4) | 76 (±5) | 19 (±6) | - |
| FS-SLIPS_1 | 27 (±6) [23] | 93 (±4) | * | * | 4.8 (±1) * | 3.0 ** [23] |
| FS-SLIPS_2 | 21 (±5) [23] | 104 (±7) | * | * | 4.1 (±0.9) * | 38.8 ** [23] |
| Bulk Polymers | ||||||
| PE 2 | 43 (±3) [36] | 95 (±2) | 100 (±2) | 84 (±2) | 16 (±0.6) | 0.64 |
| UHWPE 2 | 62 (±4) [36] | 84 (±2) | - | - | - | - |
| PP 2 | 60 (±8) [36] | 93 (±2) | - | - | - | - |
| PTFE 2 | 41 (±2) [36] | 101 (±0.4) | 105 (±4) | 78 (±6) | 26 (±2) | 0.38 |
| Bulk Metals | ||||||
| Aluminum 2 | 343 (±35) [21] | 64 (±2) | 74 (±1) | 56 (±3) | 19 (±3) | 0.26 [21] |
| Stainless steel 2 | 269 (±13) [21] | 67 (±1) | 85 (±1) | 63 (±3) | 23 (±4) | 0.23 [21] |
| References | ||||||
| LDPE-film | 110 (±20) [28] | - | ||||
| PTFE tape | 39 (±14) [20] | 109 (±3) | 116 (±1) | 108 (±3) | 8 (±2) | - |
| SLIPS (PTFEmemb + sil.oil) | 13 (±2) [20] | 98 (±2) [20] | * | * | 1 (±0.5) * [20] | - |
| SLIPS (PPmemb + sil.oil) | 33 (±7) [20] | 105 (±2) [20] | * | * | 4 (±2) * [20] | - |
| Commercial Paints/Films | ||||||
| Blade Rep9 | 88 (±5) [21] | 84 (±2) | 91 (±0.6) | 62 (±0.5) | 30 (±0.7) | 2.24 [21] |
| Carboline | 57 (±8) [36] | 85 (±1) | 89 (±3) | 65 (±2) | 24 (±0.4) | 2.55 |
| Nanomyte | 40 (±5) [36] | 101 (±1) | 104 (±2) | 91 (±6) | 13 (±4) | 8.1 |
| NeverWet | 68 (±15) | 136 (±3) | 141 (±1) | 138 (±1) | 3.3 (±0.1) | 13.71 |
| UltraEverDry | 40 (±3) [29] | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koivuluoto, H.; Hartikainen, E.; Niemelä-Anttonen, H. Thermally Sprayed Coatings: Novel Surface Engineering Strategy Towards Icephobic Solutions. Materials 2020, 13, 1434. https://doi.org/10.3390/ma13061434
Koivuluoto H, Hartikainen E, Niemelä-Anttonen H. Thermally Sprayed Coatings: Novel Surface Engineering Strategy Towards Icephobic Solutions. Materials. 2020; 13(6):1434. https://doi.org/10.3390/ma13061434
Chicago/Turabian StyleKoivuluoto, Heli, Enni Hartikainen, and Henna Niemelä-Anttonen. 2020. "Thermally Sprayed Coatings: Novel Surface Engineering Strategy Towards Icephobic Solutions" Materials 13, no. 6: 1434. https://doi.org/10.3390/ma13061434
APA StyleKoivuluoto, H., Hartikainen, E., & Niemelä-Anttonen, H. (2020). Thermally Sprayed Coatings: Novel Surface Engineering Strategy Towards Icephobic Solutions. Materials, 13(6), 1434. https://doi.org/10.3390/ma13061434






