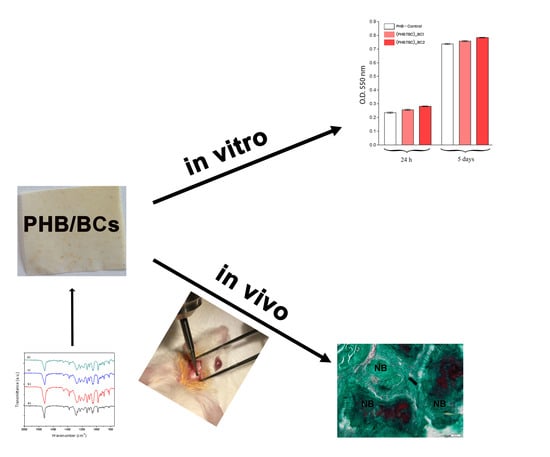
Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Obtaining of Bacterial Cellulose
2.2.2. Preparation of PHB/BC Scaffolds
2.2.3. Characterization of the Polymeric Scaffolds
2.2.4. In Vitro Biocompatibility Evaluation
In Vitro Cell Culture Model
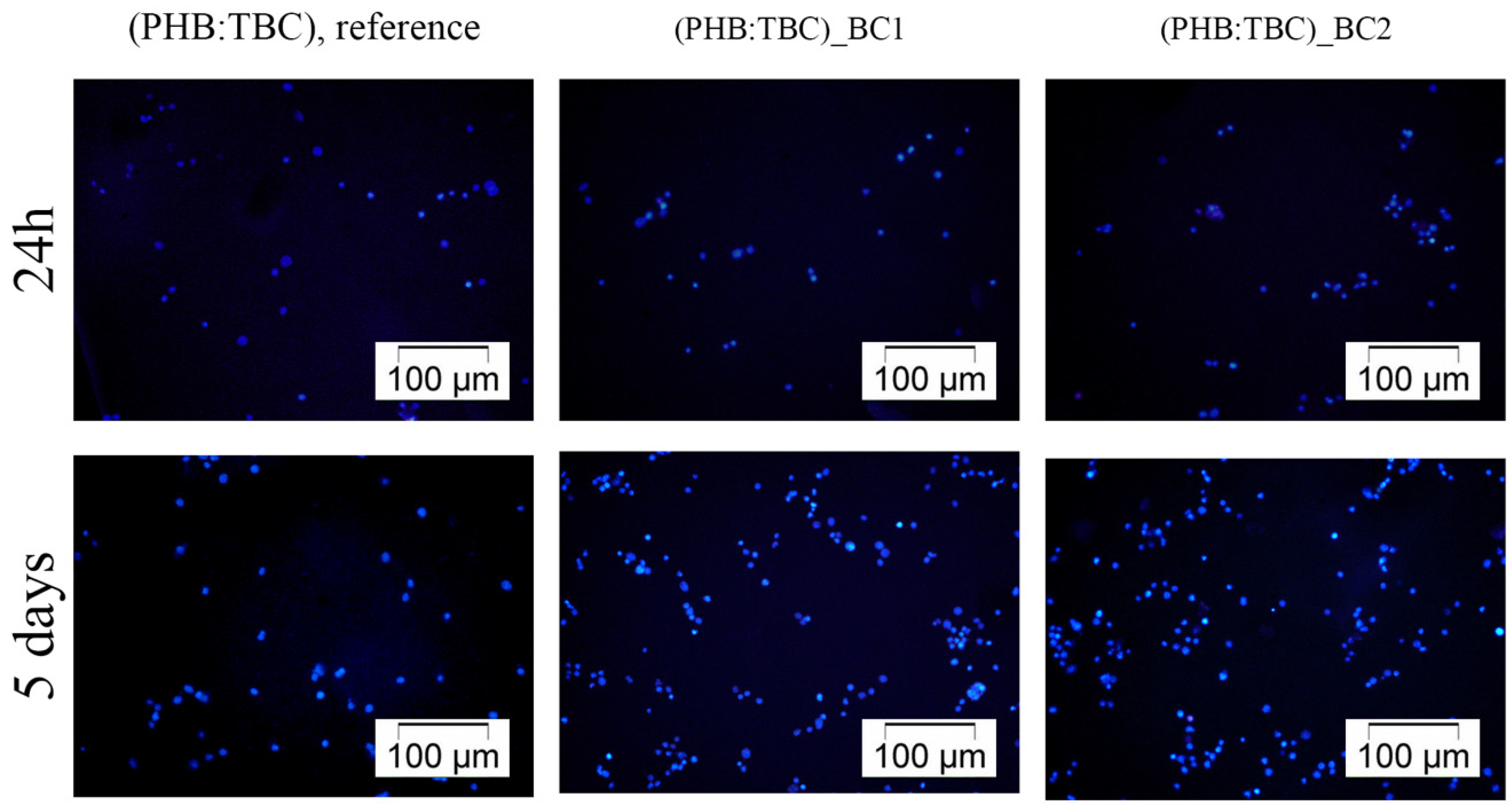
DAPI Staining of the Cell Nuclei
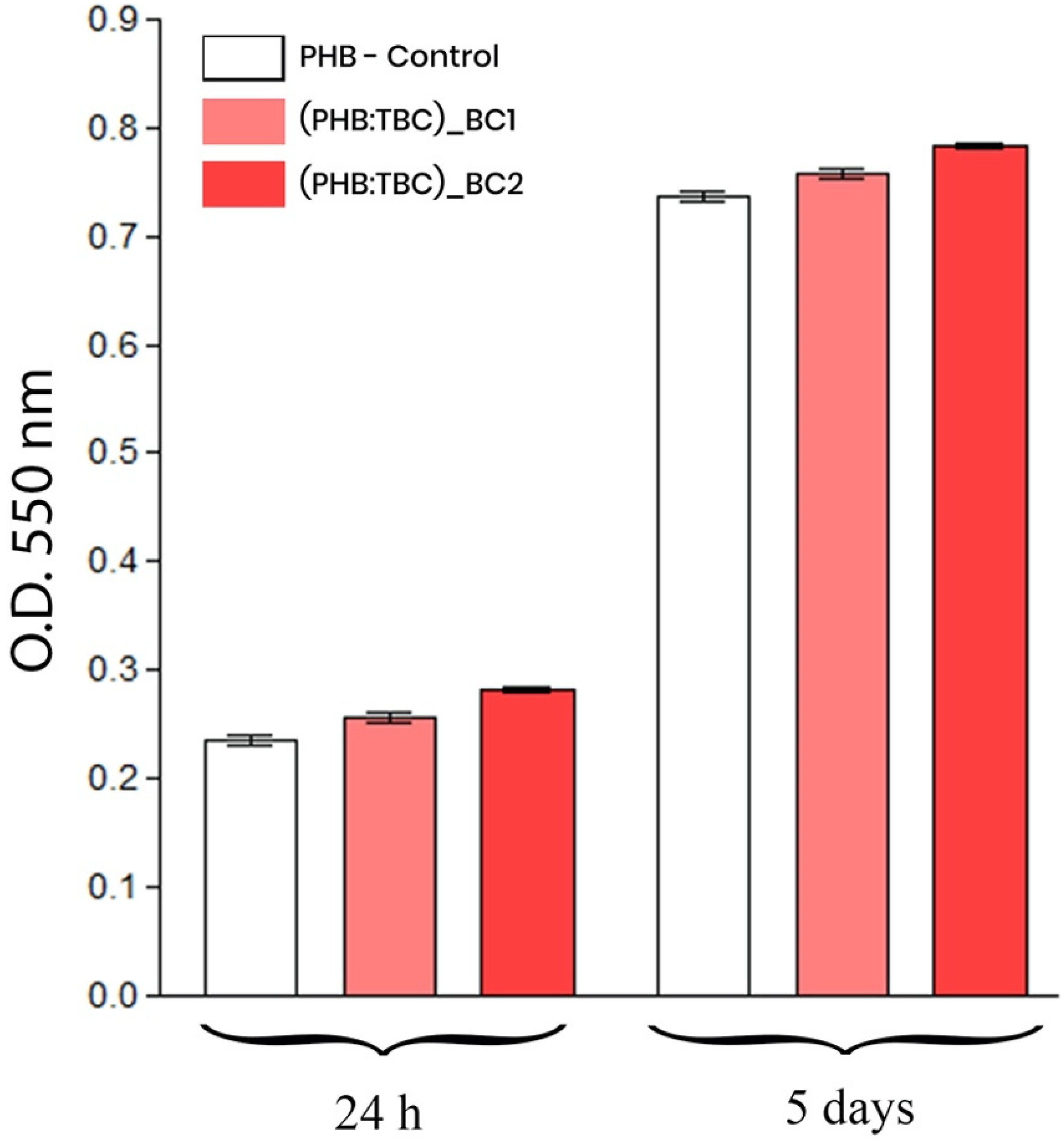
MTT Assay
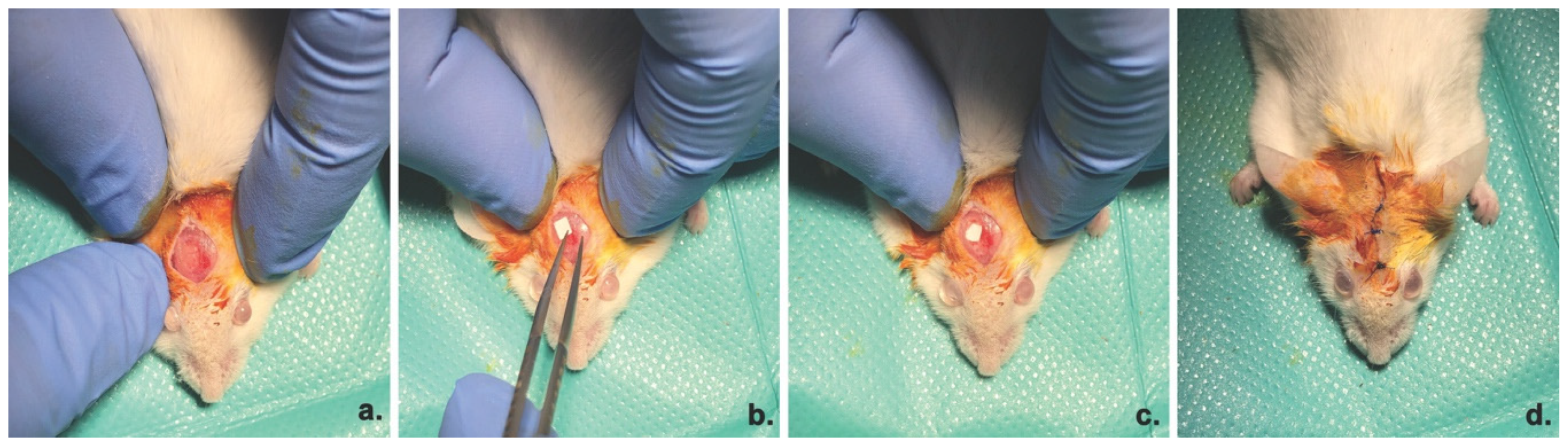
2.2.5. In Vivo Osteogenic Potential Evaluation
Animal model and Surgical Procedure
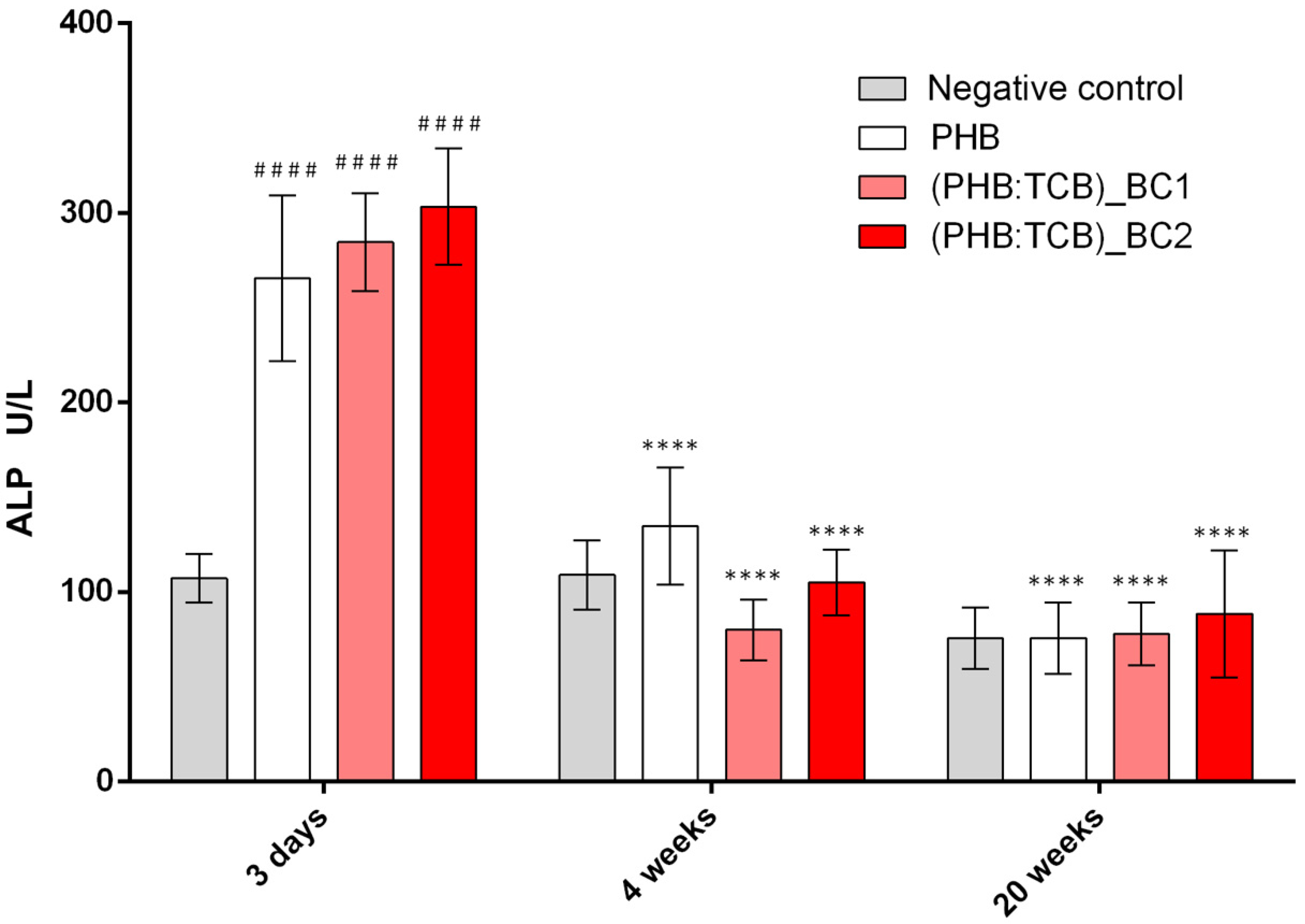
ALP Activity
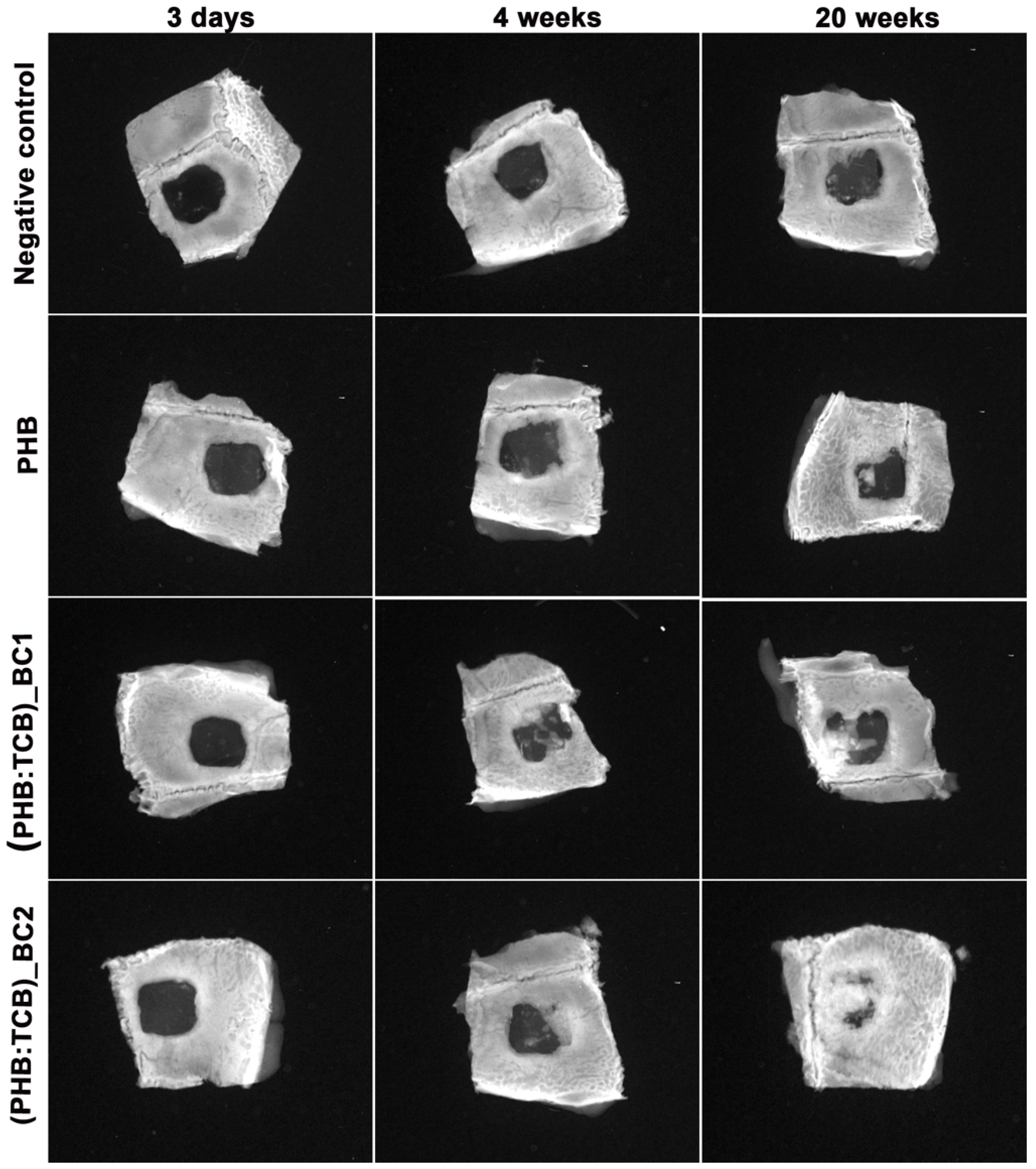
Radiographic Analysis
Histology and Morphometry Analysis
Immunofluorescence
2.2.6. Statistical Analysis
3. Results
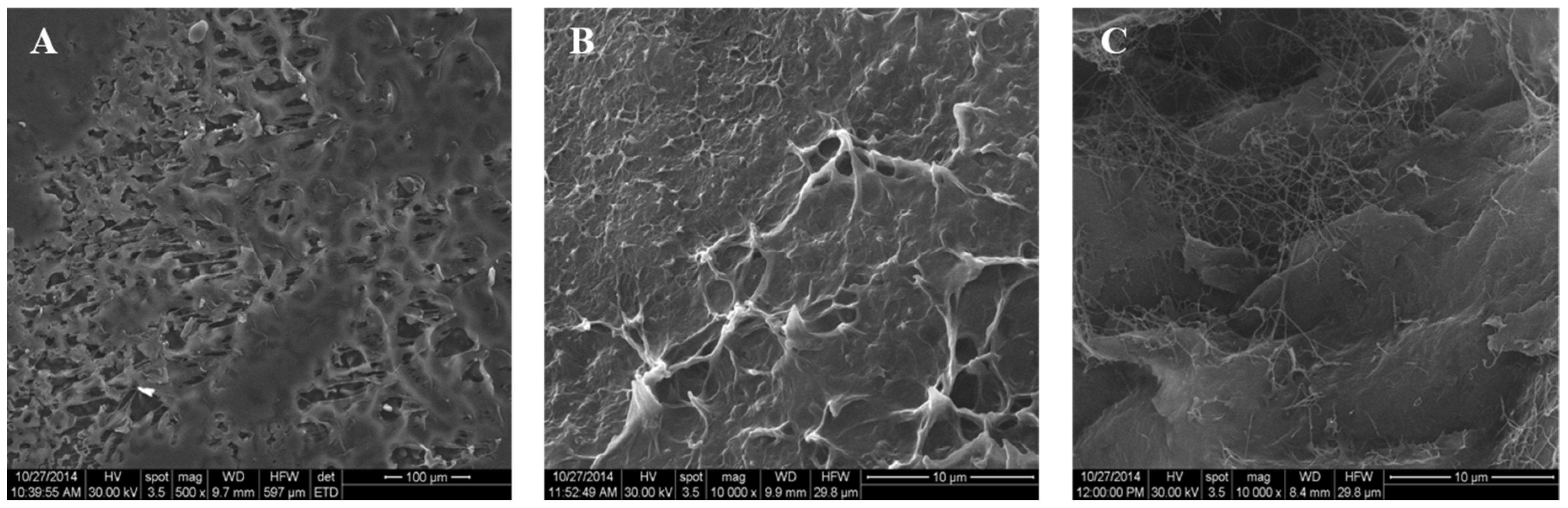
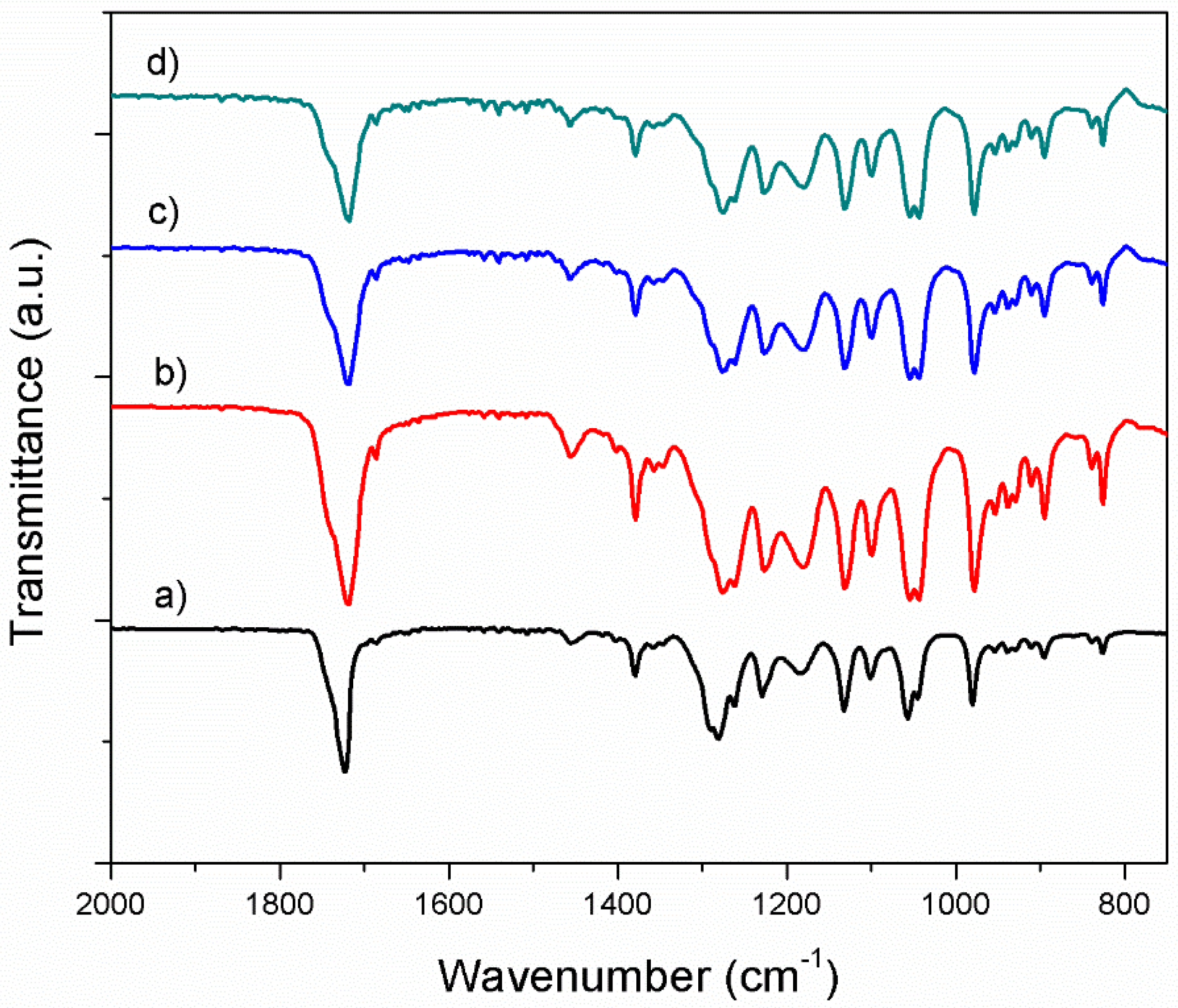
3.1. Characterization of the PHB/BC Scaffolds
3.1.1. Morphological Investigation
3.1.2. Fourier Transform Infrared Spectroscopy in Attenuated Total Reflectance mode (ATR-FTIR) Investigation
3.1.3. Mechanical Evaluation of the Composite Samples
3.2. In Vitro Biocompatibility of PHB/BC Scaffolds
3.2.1. 3T3-L1 Cells Distribution on the Materials Surface
3.2.2. 3T3-L1 Preadipocytes Viability and Proliferation Potential
3.3. In Vivo Osteogenic Potential of BC/PHB Scaffolds
3.3.1. Alkaline Phosphatase (ALP) Activity
3.3.2. X-ray Analysis
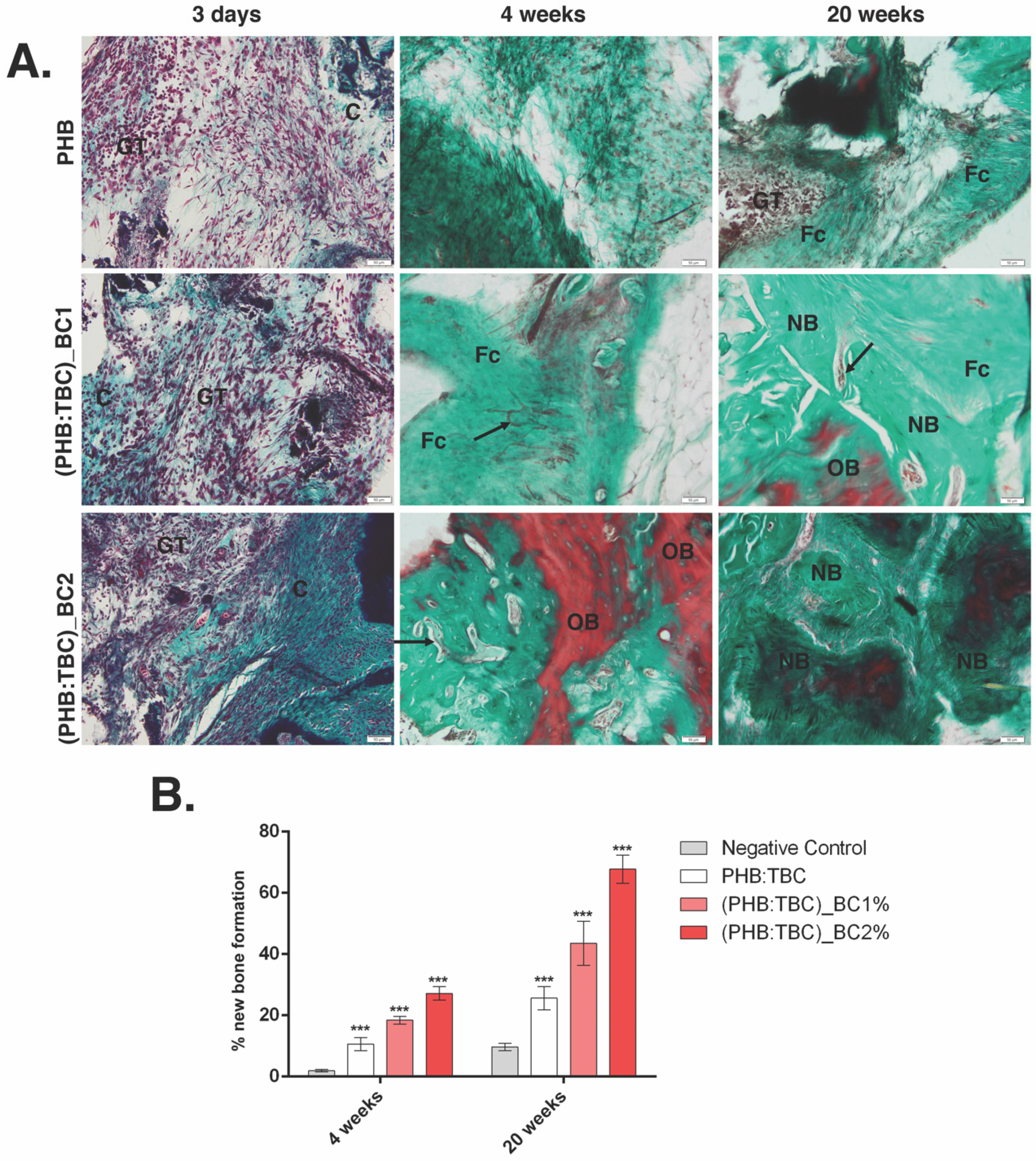
3.3.3. Histology and Histomorphometry Analysis
3.3.4. Osteogenic Differentiation of PHB/BC on Scaffolds
4. Discussion
5. Conclusions
- PHB/BC scaffolds are able to support 3T3-L1 preadipocytes viability and proliferation, with no signs of apparent toxicity and a good biocompatibility;
- BC reinforced PHB had an enhanced effects on osteoblast differentiation in vivo, based on the increased OSX expression and enhanced ALP activity in the first 4 weeks post-implantation;
- The X-ray and histology/histomorphometry analysis of the in vivo data showed that BC reinforced PHB induces new bone formation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Available online: https://www.hindawi.com/journals/ijps/2011/290602/ (accessed on 3 February 2020).
- Oliveira Barud, H.G.; Barud, H.d.S.; Cavicchioli, M.; do Amaral, T.S.; de Oliveira Junior, O.B.; Santos, D.M.; Petersen, A.L.d.O.A.; Celes, F.; Borges, V.M.; de Oliveira, C.I.; et al. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carbohydr. Polym. 2015, 128, 41–51. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Song, W.; Zeng, Q.; Yin, X.; Zhu, L.; Gong, T.; Pan, C. Preparation and anticoagulant properties of heparin-like electrospun membranes from carboxymethyl chitosan and bacterial cellulose sulfate. Int. J. Biol. Macromol. 2018, 120, 1396–1405. [Google Scholar] [CrossRef]
- Luz, E.P.C.G.; Borges, M.d.F.; Andrade, F.K.; Rosa, M.d.F.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Vieira, R.S. Strontium delivery systems based on bacterial cellulose and hydroxyapatite for guided bone regeneration. Cellulose 2018, 25, 6661–6679. [Google Scholar] [CrossRef]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.; Vasile, E.; Galateanu, B.; Bunea, M.-C.; Casarica, A.; Stanescu, P.O. Bacterial Cellulose-polyhydroxyalkanoates Composites. Mater. Plast. 2014, 51, 1–5. [Google Scholar]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Vilela, C.; Silvestre, A.J.D.; Deepa, B.; Resnik, M.; Freire, C.S.R.; Cordeiro, N. Physicochemical surface properties of bacterial cellulose/polymethacrylate nanocomposites: An approach by inverse gas chromatography. Carbohydr. Polym. 2019, 206, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, W.; Khan, T.; Ul-Islam, M.; Khan, R.; Hussain, Z.; Khalid, A.; Wahid, F. Development of modified montmorillonite-bacterial cellulose nanocomposites as a novel substitute for burn skin and tissue regeneration. Carbohydr. Polym. 2019, 206, 548–556. [Google Scholar] [CrossRef]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 917–929. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chun Chen, J.; Chen, G.-Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioactive Materials 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H.; Yang, R.; Wu, Q.; Chen, G.-Q.; Zhang, Z.-M. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ma, X.; Fang, J. Influence of carbon black on the properties of plasticized poly(lactic acid) composites. Polym. Degrad. Stab. 2008, 93, 1044–1052. [Google Scholar] [CrossRef]
- Zembouai, I.; Kaci, M.; Bruzaud, S.; Benhamida, A.; Corre, Y.-M.; Grohens, Y. A study of morphological, thermal, rheological and barrier properties of Poly(3-hydroxybutyrate-Co-3-Hydroxyvalerate)/polylactide blends prepared by melt mixing. Polym. Test. 2013, 32, 842–851. [Google Scholar] [CrossRef]
- Galego, N.; Rozsa, C.; Sánchez, R.; Fung, J.; Vázquez, A.; Santo Tomás, J. Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polym. Test. 2000, 19, 485–492. [Google Scholar] [CrossRef]
- Fu, H.; Doll, B.; McNelis, T.; Hollinger, J.O. Osteoblast differentiation in vitro and in vivo promoted by Osterix. J. Biomed. Mater. Res. A 2007, 83, 770–778. [Google Scholar] [CrossRef]
- Tu, Q.; Valverde, P.; Chen, J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem. Biophys. Res. Commun. 2006, 341, 1257–1265. [Google Scholar] [CrossRef]
- Shamsuria, O.; Fadilah, A.S.; Asiah, A.B.; Rodiah, M.R.; Suzina, A.H.; Samsudin, A.R. In vitro cytotoxicity evaluation of biomaterials on human osteoblast cells CRL-1543; hydroxyapatite, natural coral and polyhydroxybutarate. Med. J. Malays. 2004, 59 (Suppl. SB), 174–175. [Google Scholar]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Cool, S.M.; Kenny, B.; Wu, A.; Nurcombe, V.; Trau, M.; Cassady, A.I.; Grøndahl, L. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composite biomaterials for bone tissue regeneration: In vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J. Biomed. Mater. Res. A 2007, 82, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jia, S.; Jia, Y.; Yang, H. The influence of fermentation conditions and post-treatment methods on porosity of bacterial cellulose membrane. World J. Microbiol. Biotechnol. 2009, 26, 125. [Google Scholar] [CrossRef]
- Wu, H.-L.; Bremner, D.H.; Wang, H.-J.; Wu, J.-Z.; Li, H.-Y.; Wu, J.-R.; Niu, S.-W.; Zhu, L.-M. Fabrication and investigation of a biocompatible microfilament with high mechanical performance based on regenerated bacterial cellulose and bacterial cellulose. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.M.F.; Yassuda, D.H.; Sader, M.S.; Fernandes, G.V.O.; Soares, G.D.A.; Granjeiro, J.M. Osteogenic effect of tricalcium phosphate substituted by magnesium associated with Genderm® membrane in rat calvarial defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, K.B.; Cuenin, M.F.; Peacock, M.E.; Billman, M.A.; Swiec, G.D.; Buxton, T.B.; Singh, B.B.; McPherson, J.C. Effect of a hydroxyapatite tricalcium phosphate alloplast on osseous repair in the rat calvarium. J. Periodontol. 2006, 77, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lim, Y.-M.; Jeong, S.I.; An, S.-J.; Kang, S.-S.; Jeong, C.-M.; Huh, J.-B. The effect of bacterial cellulose membrane compared with collagen membrane on guided bone regeneration. J. Adv. Prosthodont. 2015, 7, 484–495. [Google Scholar] [CrossRef]
- Lee, Y.-J.; An, S.-J.; Bae, E.-B.; Gwon, H.-J.; Park, J.-S.; Jeong, S.I.; Jeon, Y.-C.; Lee, S.-H.; Lim, Y.-M.; Huh, J.-B. The Effect of Thickness of Resorbable Bacterial Cellulose Membrane on Guided Bone Regeneration. Materials 2017, 10, 320. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Martínez, H.; Brackmann, C.; Enejder, A.; Gatenholm, P. Mechanical stimulation of fibroblasts in micro-channeled bacterial cellulose scaffolds enhances production of oriented collagen fibers. J. Biomed. Mater. Res. A 2012, 100, 948–957. [Google Scholar] [CrossRef]
- Rambo, C.R.; Recouvreux, D.O.S.; Carminatti, C.A.; Pitlovanciv, A.K.; Antônio, R.V.; Porto, L.M. Template assisted synthesis of porous nanofibrous cellulose membranes for tissue engineering. Mater. Sci. Eng. C 2008, 28, 549–554. [Google Scholar] [CrossRef]
- Baek, W.; Lee, M.; Jung, J.W.; Kim, S.; Akiyama, H.; de Crombrugghe, B.; Kim, J. Positive Regulation of Adult Bone Formation by Osteoblast-Specific Transcription Factor Osterix. J. Bone Miner. Res. 2009, 24, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J. Orthop. Surg. Res. 2010, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jeong, H.M.; Jin, Y.-H.; Li, H.; Yeo, C.-Y.; Lee, K.-Y. Akt phosphorylates and regulates the osteogenic activity of Osterix. Biochem. Biophys. Res. Commun. 2011, 411, 637–641. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Xie, W.; Zeng, D.; Cai, D.; Wang, H.; Zhou, C.; Wang, J.; Li, L. Alkaline phosphatase enzyme-induced biomineralization of chitosan scaffolds with enhanced osteogenesis for bone tissue engineering. Chem. Eng. J. 2019, 371, 618–630. [Google Scholar] [CrossRef]

| Sample | PHB (wt.%) | TBC (wt.%) | BC (wt.%) | NaCl (wt.%) |
|---|---|---|---|---|
| Neat PHB | 100 | − | − | − |
| PHB:TBC | 80 | 20 | − | − |
| (PHB:TBC)_BC1 | 76.8 | 19.2 | 1 | 3 |
| (PHB:TBC)_BC2 | 76 | 19 | 2 | 3 |
| Sample | A1380 cm−1/A1185 cm−1 |
|---|---|
| Neat PHB | 1.08 |
| PHB:TBC | 0.89 |
| (PHB:TBC)_BC1 | 0.75 |
| (PHB:TBC)_BC2 | 0.59 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Young Modulus (MPa) |
|---|---|---|---|
| PHB:TBC | 9 ± 0.7 | 3 ± 0.2 | 1250 ± 98 |
| PHB:TBC_BC1 | 12 ± 0.9 | 1.9 ± 0.4 | 1310 ± 75 |
| PHB:TBC_BC2 | 15 ± 1.0 | 1.5 ± 0.3 | 1400 ± 101 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.-C.; et al. Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials 2020, 13, 1433. https://doi.org/10.3390/ma13061433
Codreanu A, Balta C, Herman H, Cotoraci C, Mihali CV, Zurbau N, Zaharia C, Rapa M, Stanescu P, Radu I-C, et al. Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials. 2020; 13(6):1433. https://doi.org/10.3390/ma13061433
Chicago/Turabian StyleCodreanu, Ada, Cornel Balta, Hildegard Herman, Coralia Cotoraci, Ciprian Valentin Mihali, Nicoleta Zurbau, Catalin Zaharia, Maria Rapa, Paul Stanescu, Ionut-Cristian Radu, and et al. 2020. "Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice" Materials 13, no. 6: 1433. https://doi.org/10.3390/ma13061433
APA StyleCodreanu, A., Balta, C., Herman, H., Cotoraci, C., Mihali, C. V., Zurbau, N., Zaharia, C., Rapa, M., Stanescu, P., Radu, I.-C., Vasile, E., Lupu, G., Galateanu, B., & Hermenean, A. (2020). Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials, 13(6), 1433. https://doi.org/10.3390/ma13061433