Abstract
Here, nitrogen-doped carbon nanotubes (CNT-N) were synthesized using exfoliated graphitic carbon nitride functionalized with nickel oxides (ex-g-C3N4-NixOy). CNT-N were produced at 900 °C in two steps: (1) ex-g-C3N4-NixOy reduction with hydrogen and (2) ethylene assisted chemical vapor deposition (CVD). The detailed characterization of the produced materials was performed via atomic force microscopy (AFM), transmission electron microscopy (TEM), Raman spectroscopy, X-ray diffraction (XRD) and thermogravimetric analysis (TGA). The possible mechanism of nanotubes formation is also proposed.
1. Introduction
The discovery of diverse nanocarbon allotropes and its nanocomposites has inspired scientists for a range of potential applications. Here, for many years, carbon nanotubes have received huge scientific interest due to their unusual features, such as unique atomic structure, high surface, remarkable mechanical, electrical and thermal properties. The modification of carbon nanotubes (CNTs) by attaching functional groups to the surface and doping with heteroatoms or metal/metal oxide nanoparticles is the most promising strategy with respect to their potential application. For example, the doping of CNTs with nitrogen makes defects that alter the chemical properties of CNTs and creates a path to applications.
It is well known that the properties of solid materials are attributed to surface morphology. Thus, materials have to be modified prior to applications to improve their specific properties [1,2,3,4,5,6]. The surface modification of materials can be done by two main paths: (i) by attaching functional groups [7,8,9] and (ii) by introducing heteroatoms (e.g., nitrogen, boron, phosphorus, sulfur) in the molecular structure of materials [10,11,12]. From all of the heteroatoms, nitrogen is the one of the most investigated because it is sustainable for carbon replacing due to the fact that N and C atoms possess similar atomic radius and are chemically relatively easy to replace [13,14]. Furthermore, nitrogen possesses one electron more than carbon, which is desirable for electrochemical reactions such as the oxygen evolution reaction (OER) [15]. Nitrogen doping may also affect electron conductivity, electron-donor properties and chemical stability of the host material due to the modification of the electronic and crystal structure [16]. Moreover, the incorporation of nitrogen into the structure of molecules modifies their geometric properties, which results in an increase in the number of active sites and improves the interaction between the carbon structure and absorbate [17].
Nitrogen-doped carbon nanotubes have found application in many various fields, such as electrochemistry, energy storage, CO2 adsorption, Li-ion batteries and others [18,19,20,21,22]. In this context, Wu et al. [23] demonstrated that N-doped CNT enhanced the energy storage capacity and improved properties of produced batteries. Ariharan et al. [17] revealed that nitrogen-doped carbon nanotubes showed a higher hydrogen storage capacity of ~ 2 wt% total adsorption, which is higher compared to other nitrogen-enriched carbon nanostructures. Another team, Liu et al. [24], reported that nitrogen-doped carbon nanotubes and graphitic carbon nitride nanocomposites exhibit an excellent ability to photocatalytic hydrogen evolution rate of 1208 μmol g−1h−1. In addition to applications related to electrochemistry, nitrogen-doped carbon nanotubes have been used for production sensors [25] or organic pollutant removal [26].
Various preparation methods have been employed to obtain nitrogen-doped carbon nanotubes. The most widely used method of preparing nitrogen-doped carbon nanotubes is chemical vapor deposition [27,28]. One of the most significant advantages of this method is its simplicity, which is the reason for its widespread use. However, N-doped CNT are obtained by other methods as well, such as in situ synthesis and post nitridation treatment [29], catalytic pyrolysis of dimethylformamide [30] and plasma treatment [31].
It is well known that ethylene plays the most important role during the chemical vapor deposition process. It is the main source of carbon, which in this process is necessary for the growth of carbon nanotubes. During the production of single-walled carbon nanotubes and multi-walled carbon nanotubes, hydrocarbons such as ethylene, acetylene, benzene or methane have been decomposed into single carbon atoms or linear dimers/trimers. This step takes place on the surface of metallic catalysts and results in CNT growth [32,33]. The process usually takes place in the temperature range of 500–1000 °C [34].
In this work, we present a novel route to prepare nitrogen-doped carbon nanotubes (CNT-N) from graphitic carbon nitride functionalized with nickel oxides (ex-g-C3N4-NixOy) by the chemical vapor deposition (CVD) method. We also propose the mechanism of reshaping of the flake-like structure into the tubular form.
2. Details Experimental
2.1. Materials and Procedures
Melamine (C3H6N6, 99.0%) and nickel (II) acetate tetrahydrate (Ni(OCOCH3)2·4H2O, ≥ 99.0%) were purchased from Sigma Aldrich (St. Louis, MI, USA).
2.1.1. Synthesis of Exfoliated Graphitic Carbon Nitride
Bulk graphitic carbon nitride (g-C3N4) was synthesized via thermal polycondensation of melamine. The process was carried out in muffle furnace at 550 °C for 2 h with a heating rate of 2 °C/min in air atmosphere. Next, the obtained yellow product was exfoliated by sonication using Tip-ultra-sonicator (500 W, Sonics VC505, Sonics & Materials Inc., Newtown, USA). Here, a certain amount of the bulk g-C3N4 was mixed with isopropyl alcohol and sonicated for 24 h. The final powdered product was obtained by evaporation of the solvent at 50 °C. The exfoliated sample was denoted as ex-g-C3N4.
2.1.2. Synthesis of Functionalized Exfoliated Graphitic Carbon Nitride with Nickel Oxides
In a typical synthesis of the procedure, ex-g-C3N4 and Ni(OCOCH3)2·4H2O in a weight ratio of 1:1 were mixed in isopropyl alcohol by sonication for 3 h. Then, the obtained dispersion was stirred until the solvent evaporated. Finally, the product was annealed at 440 °C for 10 min. with a heating rate of 6 °C/min in a vacuum.
2.1.3. Synthesis of Nitrogen-doped Carbon Nanotubes
Exfoliated graphitic carbon nitride functionalized with nickel oxides (ex-g-C3N4-NixOy) was placed in the ceramic boat and introduced into the tubular furnace. The furnace was heated under flowing nitrogen up to 900 °C. When the temperature was reached, hydrogen was introduced (100 sccm) for 3 h. Afterward, the hydrogen supply was cut off and ethylene was introduced to the furnace for 10 min. Subsequently, the furnace was cooled down to room temperature. The as-obtained product was denoted as CNT-N.
2.2. Characterization
The morphology of the obtained samples was investigated by transmission electron microscopy with energy dispersive X-ray (EDX) spectroscopy (Tecnai F20-based at 200 kV accelerating voltage, Thermo Fisher Scientific, Waltham, MA, USA) and scanning electron microscopy (TESCAN, VEGA 3, acquired in the 30 kV acceleration voltage, TESCAN, Brno, Czech Republic). Selected area electron diffraction (SAED) patterns were obtained using TEM microscope (Thermo Fisher Scientific, Waltham, MA, USA). The AFM study was performed using an atomic force microscope (Nanoscope V Multimode 8, Bruker AXS, Mannheim, Germany). X-ray diffraction (XRD) patterns were carried out using an Empyrean (Malvern Panalytical, Malvern, UK) diffractometer using CuKα radiation. Thermogravimetric analysis was carried out using TA-Q600 SDT TA Instrument (TA Instrument, New Castle, DE, USA) under the air and argon atmosphere at ramping rate of 10 °C/min from room temperature to 900 °C. Raman spectra were determined on inVia Raman Microscope (RENISHAW, New Mills Wotton-under-Edge, UK) with an excitation wavelength of 785 nm.
3. Results and Discussion
3.1. Microscopic Analysis
The morphologies of g-C3N4, ex-g-C3N4 and ex-g-C3N4-NixOy were studied by transmission electron microscopy. The TEM images of the samples are presented in Figure 1. TEM images of g-C3N4 and ex-g-C3N4 (Figure 1A,B) show a visible difference in the thickness of these two samples. Figure 1C and D exhibit that NixOy nanoparticles were successfully deposited on the flakes of ex-g-C3N4 (ex-g-C3N4-NixOy). The average diameter of NixOy particles is ~32 nm.
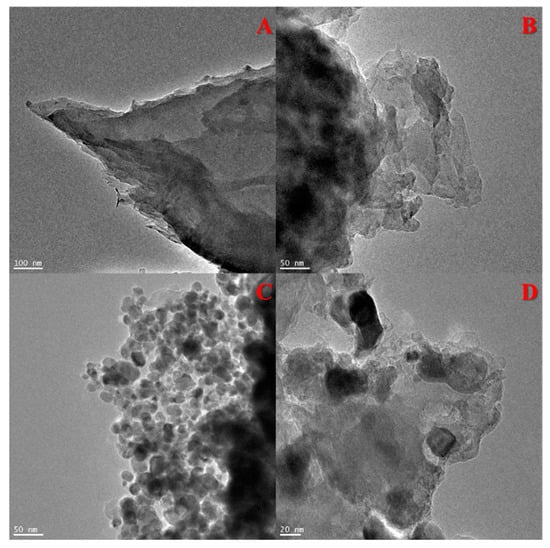
Figure 1.
Transmission electron microscopy (TEM) images of (A) g-C3N4, (B) ex-g-C3N4 and (C,D) ex-g-C3N4-NixOy.
Additionally, the topography of the exfoliated g-C3N4 was also examined via atomic force microscopy (Figure 2). The obtained data indicate that the thickness of ex-g-C3N4 is ~ 4–5 nm, which means that this sample is composed of ~10–13 layers. According to the fact that bulk graphitic carbon nitride is composed of ~100–170 layers, we can conclude that the exfoliation step was efficient. Based on AFM images, the number of ex-g-C3N4 layers is reduced by more than 30 times in comparison to the pristine g-C3N4, which is supported by TEM.
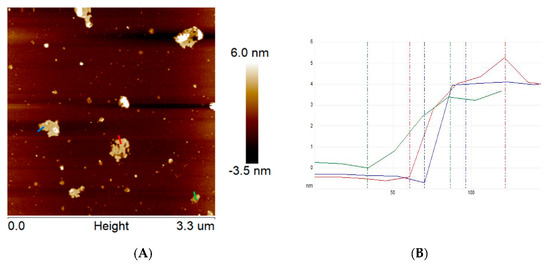
Figure 2.
AFM images of ex-g-C3N4 (A) and corresponding high profile (B).
Figure 3 shows SEM images of g-C3N4, ex-g-C3N4 and the sample produced in the process of hydrogen reduction of ex-g-C3N4-NixOy and next CVD in ethylene in the presence of reduced ex-g-C3N4-NixOy (CNT-N). g-C3N4 and ex-g-C3N4 (Figure 3A,B) exhibited aggregated morphology with irregular multiple-layered structures. The morphology of CNT-N (Figure 3C,D) is quite different from that of g-C3N4 and ex-g-C3N4. This sample is composed of nanotubular structures. Moreover, nickel residues which served as catalysts in nanotube growth were observed inside the tubes (green arrows in Figure 3D). The obtained nanotubes tend to agglomerate into larger clusters. The reference experiment without ethylene was also performed (Figure 4). This confirmed that ethylene is crucial in the process of nitrogen-doped carbon nanotube formation.
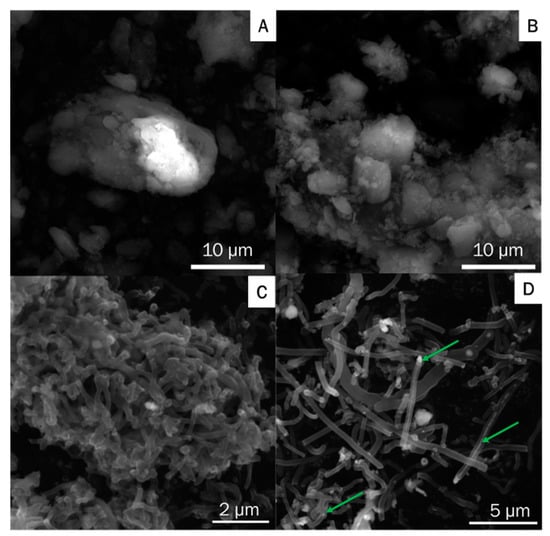
Figure 3.
Scanning electron microscopy (SEM) images of (A) g-C3N4, (B) ex-g-C3N4 and (C,D) CNT-N.
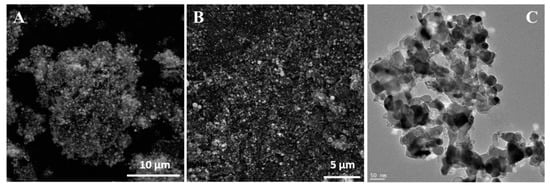
Figure 4.
SEM (A,B) and TEM (C) images of g-C3N4-NixOy treated with hydrogen at 900 °C.
To determine the role of ethylene during tube formation, an additional experiment was performed. In this step, g-C3N4-NixOy was heated to 900 °C and then treated with hydrogen for 3 h. In this case, the sample had not been exposed to ethylene. SEM and TEM images of the obtained product are presented in Figure 4. Here, tubular forms did not evolve. Additionally, metal particles agglomerated into larger clusters. A further experiment with g-C3N4-NixOy treated only with ethylene (no hydrogen step) at 900 °C was conducted (Figure 5). It revealed that the obtained sample contained mostly onion-like particles. Some of these structures are formed on the surface of metal particles. Detailed TEM investigations revealed a few irregular, deformed tubular structures.
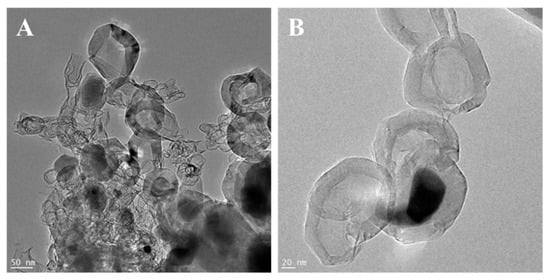
Figure 5.
TEM (A,B) images of g-C3N4-NixOy treated with ethylene at 900 °C.
In order to study the morphology and elemental composition of CNT-N in greater detail, TEM, STEM and EDS analyses were performed (Figure 6). TEM images (Figure 6A,B) confirmed that the nanotubes have a diameter in the range of 200–300 nm. The elemental mapping (Figure 6D,E), carried out for the area marked as a white square on the STEM image (Figure 6C), revealed that carbon and nitrogen are the only elements present in the sample. Moreover, both components are homogeneously distributed in nanotubes. Furthermore, the line scan along the direction denoted by the red line in the STEM image (Figure 6G) was carried out and the obtained profiles are presented in Figure 6F. This analysis additionally confirms the chemical composition of CNT-N nanotubes.
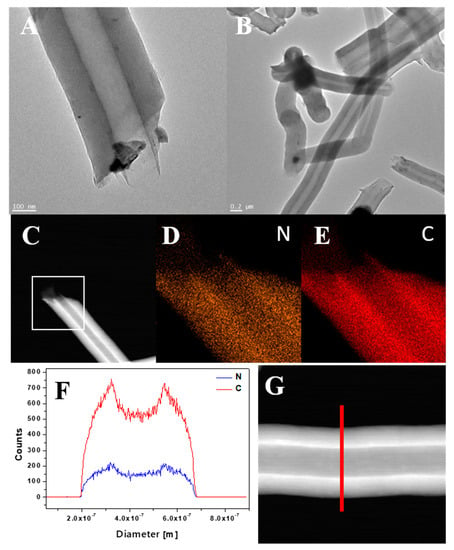
Figure 6.
TEM images of CNT-N (A,B), STEM image of CNT-N (C) with corresponding mapping image of nitrogen (D) and carbon (E), and line profile (F) of CNT_N with corresponding STEM image (G).
Figure 7 presents HR-TEM and SAED images for g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N. For g-C3N4 and ex-g-C3N4 crystal fingers are not observed, which indicates that those products are amorphous, which is confirmed by SEAD analysis [35]. ex-g-C3N4_NixOy and CNT_N exhibit SAED patterns typical for a polycrystalline structure. ex-g-C3N4-NixOy shows a few diffraction rings with discrete reflections corresponding to the different orientations of the single phase. The following rings have been characterized: (002) corresponding to nickel particles, (202) to Ni2O3, (110) to NiO2 and (110) to Ni. The pattern of copper (from TEM grid) can also be observed and is marked with (220). This analysis is in full agreement with the XRD analysis, which proved the presence of different nickel compounds in the sample. CNT-N exhibits three clear rings. The first two rings (111) and (200) are typical for nickel nanoparticles. Similar to the previous sample, the rings from copper can be detected.
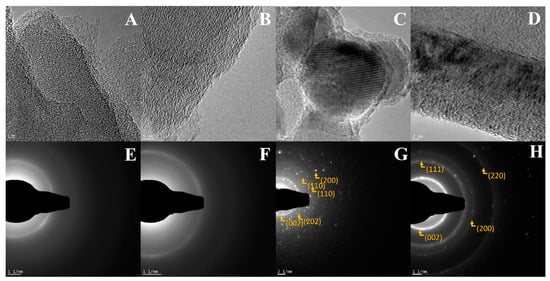
Figure 7.
HR-TEM (A–D) and SAED (E–H) images of g-C3N4 (A,E), ex-g-C3N4 (B,F), ex-g-C3N4-NixOy (C,G) and CNT-N (D,H).
3.2. Spectroscopic, Crystal and Thermogravimetric Studies
XRD patterns of g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N samples are shown in Figure 8. The peaks located at 13.1° and 27.56° can be observed for three samples (g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy) and they are attributed to the presence of graphitic carbon nitride. Peaks at 13.1° and 27.56° correspond to the (100) and (002) crystalline phase, respectively (JCPDS 87–1526). Nickel-functionalized graphitic carbon nitride (ex-g-C3N4-NixOy) exhibits the presence of different forms of a nickel compound. The diffraction peak at 2θ 44.8° and 58.7° is related to the nickel (III) oxide (JCPDS 14–0481), while two peaks at ~39.4° and ~41.8° belong to nickel (II) oxide (JCPDS 85–1977) [36,37]. During the last stage of CNT-N synthesis, nickel oxide is reduced to the metallic form of nickel and catalyzes the growth of nanotubes. This is confirmed by the disappearance of the peaks from NiO and Ni2O3 and the appearance of signals characteristic for the metallic form of nickel located at ~44.5° and ~51.9° (JCPDS 87–0712) [38]. Moreover, the diffraction peak at 26.2° observed for CNT-N is assigned to the (002) plane of graphitic carbon.
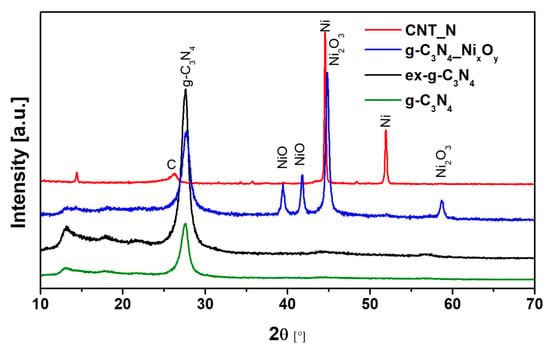
Figure 8.
XRD patterns of g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N.
Thermogravimetric analysis was carried out in order to determine the thermal properties of the samples. Figure 9 represents the TG (a) and DTG (b) curves of g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N heat-treated under air flow. Here, the exfoliation process does not affect the thermal stability of graphitic carbon nitride. For both g-C3N4 and ex-g-C3N4, the degradation process starts at about 500 °C and continues until complete decomposition. Functionalization with nickel oxides (ex-g-C3N4-NixOy) significantly decreased the decomposition temperature to 350 °C. The residue mass after the analysis is ~28.7 wt% and this value is assigned to the amount of nickel compounds in the sample. Two steps can be distinguished on the TGA curve obtained for the CNT-N sample. The first one begins at a temperature of 468 °C and is attributed to the removal of more defected carbon structures. The second one, from a temperature of 565 °C, is related to the degradation of carbon nanotubes. The residual mass is ~24.8 wt% which is the nickel compounds content in the sample.
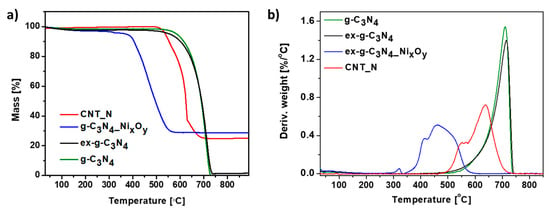
Figure 9.
Thermogravimetric (a) and derivative thermogravimetric curves (b) of g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N conducted in air.
Additionally, TGA analysis performed in argon atmosphere was conducted. Thermogravimetric (TG) (Figure 9a) and derivative thermogravimetric (DTG) (Figure 9b) curves of g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N heat-treated under argon flow are presented in Figure 10. g-C3N4 and ex-g-C3N4 are stable up to ~500 °C, either in air or argon atmosphere (see Figure 9 and Figure 10). This means that both g-C3N4 and ex-g-C3N4 can endure high temperatures, even in an oxidizing atmosphere. The weight loss at above 500 °C is not due to the oxidation by O2, but to the direct thermal decomposition of g-C3N4 itself [39]. Moreover, the residual mass of ~3 wt% is observed in an inert atmosphere. ex-g-C3N4-NixOy exhibited different thermal stability in air and argon atmosphere, which was higher in Ar flow. Furthermore, the residual mass after analysis is ~34 wt% (~24.8 wt% in air). The obtained CNT-N is stable in argon atmosphere.
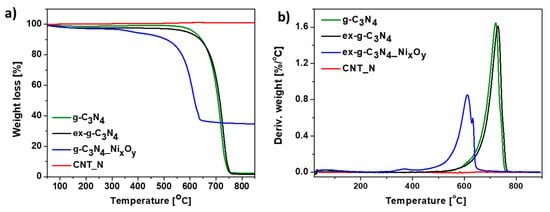
Figure 10.
Thermogravimetric (a) and derivative thermogravimetric (b) curves of g-C3N4, ex-g-C3N4, ex-g-C3N4-NixOy and CNT-N conducted in Ar.
Raman spectroscopy was introduced to provide information about the chemical structures of g-C3N4, ex-g-C3N4 and CNT-N (Figure 11). Raman spectra of g-C3N4 and ex-g-C3N4 contain several bands observed in the range of 700–1630 cm−1 which are attributed to graphitic carbon nitride [40,41,42]. Additionally, the vibrations at 753, 977, 1120, 1156, 1236 and 1314 cm−1 are assigned to the stretching vibration of aromatic C-N heterocycle characteristic to melem. [43,44] The peaks at 700–1000 cm−1 are related to the different types of ring breathing modes of s-triazine. The Raman response of the tubular sample exhibits D (1318 cm−1), G (1580 cm−1) and 2D (2700 cm−1) bands what confirm the formation of graphitic carbon structure in the sample [45,46].
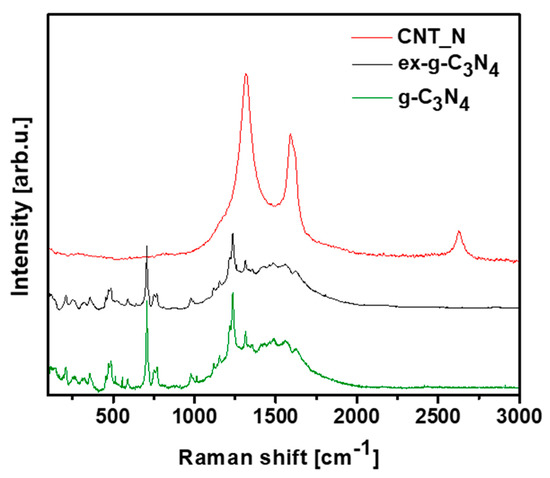
Figure 11.
Raman spectra of g-C3N4, ex-g-C3N4 and CNT-N.
3.3. Mechanism of the CNT-N Formation
All the performed experiments clearly indicate that the metallic nickel serves as an active catalyst for the formation of the nanotubes. To verify this, the additional experiments in which g-C3N4-NixOy were treated with hydrogen or ethylene at 900 °C were performed (see Figure 4 and Figure 5). The tubular forms did not evolve when g-C3N4-NixOy was treated only with H2. Moreover, treating of g-C3N4-NixOy only with ethylene (no hydrogen step) at 900 °C results in the formation of cocktail of carbon onion-like structures or short irregular tube-like structures. Therefore, the key step was the reduction of nickel oxides to metallic nickel in the hydrogen/ethylene flow. This was also confirmed by XRD studies (see Figure 4). In the next step, carbon and nitrogen from g-C3N4 diffused into nickel particles and when the oversaturation was reached these elements started to grow in the form of tubes. The elemental and the structural properties of the tubes were confirmed in EDX analysis and the Raman response of the sample. The whole process of the tubes formation is presented schematically in Figure 12.
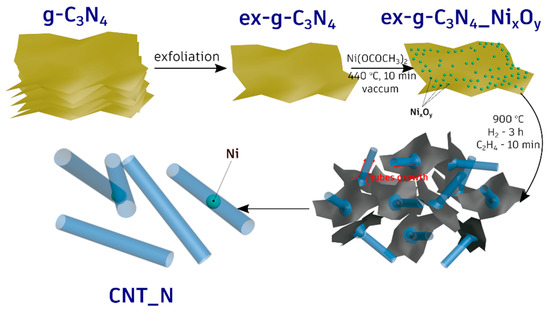
Figure 12.
Schematic diagram of N-doped carbon nanotubes synthesis.
4. Conclusions
In summary, we present a simple and effective method for the synthesis of N-doped carbon nanotubes with a diameter in the range of 200–300 nm. CNT-N were produced in the reaction of chemical vapor deposition in the presence of ethylene flow. Here, exfoliated graphitic carbon nitride functionalized with nickel oxides has been used as a starting material in CNT-N formation. This material plays a double role: (1) nickel was a catalyst in nanotube growth and (2) graphitic carbon nitride served as support for nickel nanoparticles and as carbon/nitrogen source. The results also prove that ethylene plays a crucial role in the formation of tubes. On one side, it carboreduced the nickel oxide (II) to metallic nickel which was an active phase in the growth of the nanotubes, and, on the other hand, it was a source of carbon in the final sample. The obtained N-doped nanotubes may show promising performance in a variety of applications such as catalysis, lithium-ion batteries, supercapacitors and so on.
Author Contributions
Conceptualization, E.M. and B.Z.; Funding acquisition, E.M.; Investigation, K.M. and B.Z.; writing—original draft preparation, K.M., B.Z. and E.M., writing—review and editing, K.M., B.Z. and E.M.; supervision, E.M. and R.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Science Centre, Poland (grant number 2015/19/B/ST8/00648).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Boufi, S.; Celli, A. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Kukulka, W.; Cendrowski, K.; Mijowska, E. Electrochemical performance of MOF-5 derived carbon nanocomposites with 1D, 2D and 3D carbon structures. Electrochem. Acta 2019, 307, 582–594. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, Z.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnS–ZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Chen, C.; Li, H.; Xia, Y.; Zhou, T. MoS2 -CNFs composites to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 129, 178–186. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2019, 219, 499–511. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Li, H.F.; Wu, F.; Wang, C.; Zhang, P.X.; Hu, H.Y.; Xie, N.; Pan, M.; Zeng, Z.; Deng, S.; Wu, M.H.; et al. One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials 2018, 8, 700. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Sun, H.; Suvorova, A.; Saunders, M.; Tade, M.; Wang, S. Heteroatom (N or N-S)-Doping Induced Layered and Honeycomb Microstructures of Porous Carbons for CO2 Capture and Energy Applications. Adv. Funct. Mater. 2016, 26, 8651–8661. [Google Scholar] [CrossRef]
- Liu, J.; Shen, A.; Wei, X.; Zhou, K.; Chen, W.; Chen, F.; Xu, J.; Wang, S.; Dai, L. Ultrathin Wrinkled N Doped Carbon Nanotubes for Noble-Metal Loading and Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 20507–20512. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef] [PubMed]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Hao, J.; Yang, W.; Peng, Z.; Zhang, C.; Huang, Z.; Shi, W. A Nitrogen Doping Method for CoS2 Electrocatalysts with Enhanced Water Oxidation Performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Y.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Controllable Nitrogen Doping of High-Surface-Area Microporous Carbons Synthesized from an Organic–Inorganic Sol–Gel Approach for Li–S Cathodes. ACS Appl. Mater. Interfaces 2015, 7, 21188–21197. [Google Scholar] [CrossRef]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, W. Nitrogen-incorporated carbon nanotube derived from polystyrene and polypyrrole as hydrogen storage material. Int. J. Hydrogen Energy 2018, 43, 5077–5088. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Liu, X.; Chen, J.; Liu, W.; Liu, T.; Sun, M.; Zhao, L.; Ding, F.; Lu, Z.; et al. CuCo2O4/N-Doped CNTs loaded with molecularly imprinted polymer for electrochemical sensor: Preparation, characterization and detection of metronidazole. Biosens. Bioelectron. 2019, 142, 111483. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, J.-S.; Chen, X.-B.; Xiong, H.-M. Heteroatom-doped carbon dots based catalysts for oxygen reduction reactions. J. Colloid Interface Sci. 2019, 537, 716–724. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Li, R.; Tu, K.; Li, J.; Xie, Z.; Lei, J.; Liu, D.; Qu, D. Self-assembled N-doped carbon with a tube-in-tube nanostructure for lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 559, 224–253. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Hsu, W.-L.; Lin, S.-Y.; Juang, R.-S. Enhanced CO2 adsorption on activated carbon fibers grafted with nitrogen-doped carbon nanotubes. Materials 2017, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Adjizian, L.-L.; Leghrib, R.; Koos, A.A.; Suarez-Martinez, I.; Crossley, A.; Wagner, P.; Grobert, N.; Llobet, E.; Ewels, C.P. Boron- and nitrogen-doped multi-wall carbon nanotubes for gas detection. Carbon 2014, 66, 662–673. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, C.; Xie, Z.; Ai, L.; Liu, Y.; Zhou, Q.; Jiang, J.; Sun, H.; Wang, S. Cobalt@nitrogen-doped bamboo-structured carbon nanotube to boost photocatalytic hydrogen evolution on carbon nitride. Appl. Catal. B Environ. 2019, 254, 443–451. [Google Scholar] [CrossRef]
- Liu, Q.; Pu, Z.; Asiri, A.M.; Sun, X. Bamboo-like nitrogen-doped carbon nanotubes toward fluorescence recovery assay for DNA detection. Sens. Actuators B Chem. 2015, 206, 37–42. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Chen, Z. Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 2010, 48, 3057–3065. [Google Scholar] [CrossRef]
- Nxumalo, E.N.; Nyamori, V.O.; Coville, N.J. CVD synthesis of nitrogen doped carbon nanotubes using ferrocene/aniline mixtures. J. Organomet. Chem. 2008, 693, 2942–2948. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2020, 260, 118105. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Golberg, D.; Xu, F. Structure and nitrogen incorporation of carbon nanotubes synthesized by catalytic pyrolysis of dimethylformamide. Carbon 2004, 42, 2625–2633. [Google Scholar] [CrossRef]
- Chetty, R.; Kundu, S.; Xia, W.; Bron, M.; Schuhmann, W.; Chirila, V.; Brandl, W.; Reincecke, T.; Muhler, M. PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim. Acta 2009, 54, 4208–4215. [Google Scholar] [CrossRef]
- Kumar, M. Carbon Nanotube Synthesis and Growth Mechanism. In Carbon Nanotubes-Synthesis, Characterization, Applications; Web of science: Philadelphia, PA, USA, 2011; pp. 147–170. ISBN 978-953-307-497-9. [Google Scholar]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.A.; Abdel-Salam, N.M.; Ghaffar, A. CVD Synthesis, Functionalization and CO2 Adsorption Attributes of Multiwalled Carbon Nanotubes. Processes 2019, 7, 634. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef]
- Day, S.; Bhattacharjee, S.; Chaudhuri, M.G.; Bose, R.S.; Halder, S.; Ghosh, C.K. Synthesis of pure nickel(III) oxide nanoparticles at room temperature for Cr(VI) ion removal. RSC Adv. 2015, 5, 54717–54726. [Google Scholar] [CrossRef]
- Rifaya, M.N.; Theivasanthi, T.; Alagar, M. Chemical Capping Synthesis of Nickel Oxide Nanoparticles and their Characterizations Studies. Nanosci. Nanotechnol. 2012, 5, 134–138. [Google Scholar] [CrossRef]
- Arghavanian, R.; Bostani, B.; Parvini-Ahmadi, N. Characterisation of coelectrodeposited Ni–Al composite coating. Surf. Eng. 2015, 31, 189–193. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Enhanced catalytic activity of potassium-doped graphitic carbon nitride induced by lower valence position. Appl. Catal. B Environ. 2015, 164, 77–81. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, W.J.; Chen, X.J.; Wang, J.; Zhu, Y.F. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780. [Google Scholar] [CrossRef]
- Hu, S.W.; Yang, L.W.; Tian, Y.; Wei, X.L.; Ding, J.W.; Zhong, J.X.; Chu, P.K. Simultaneous nanostructure and heterojunction engineering of graphitic carbon nitride via in situ Ag doping for enhanced photoelectrochemical activity. Appl. Catal. B Environ. 2015, 163, 611–622. [Google Scholar] [CrossRef]
- Liu, S.; Ke, J.; Sun, H.; Liu, J.; Tade, M.O.; Wang, S. Size dependence of uniformed carbon spheres in promoting graphitic carbon nitride toward enhanced photocatalysis. Appl. Catal. B Environ. 2017, 204, 358–364. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.G.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Zinin, P.V.; Ming, L.C.; Sharma, S.K.; Khabashesku, V.N.; Liu, X.; Hong, S.; Endo, S.; Acosta, T. Ultraviolet and near-infrared Raman spectroscopy of graphitic C3N4 phase. Chem. Phys. Lett. 2009, 472, 69–73. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).