Surface Modification of Lightweight Mortars by Nanopolymers to Improve Their Water-Repellency and Durability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples
2.3. Methods
- (a)
- polar component of SFE:
- (b)
- dispersion component of SFE:where: is the SFE of diiodomethane, is the dispersive component of diiodomethane SFE, is the polar component of diiodomethane SFE, is the SFE of distilled water diiodomethane, is the polar component of water SFE, is the contact angle (CA) of diiodomethane and is the CA of water.
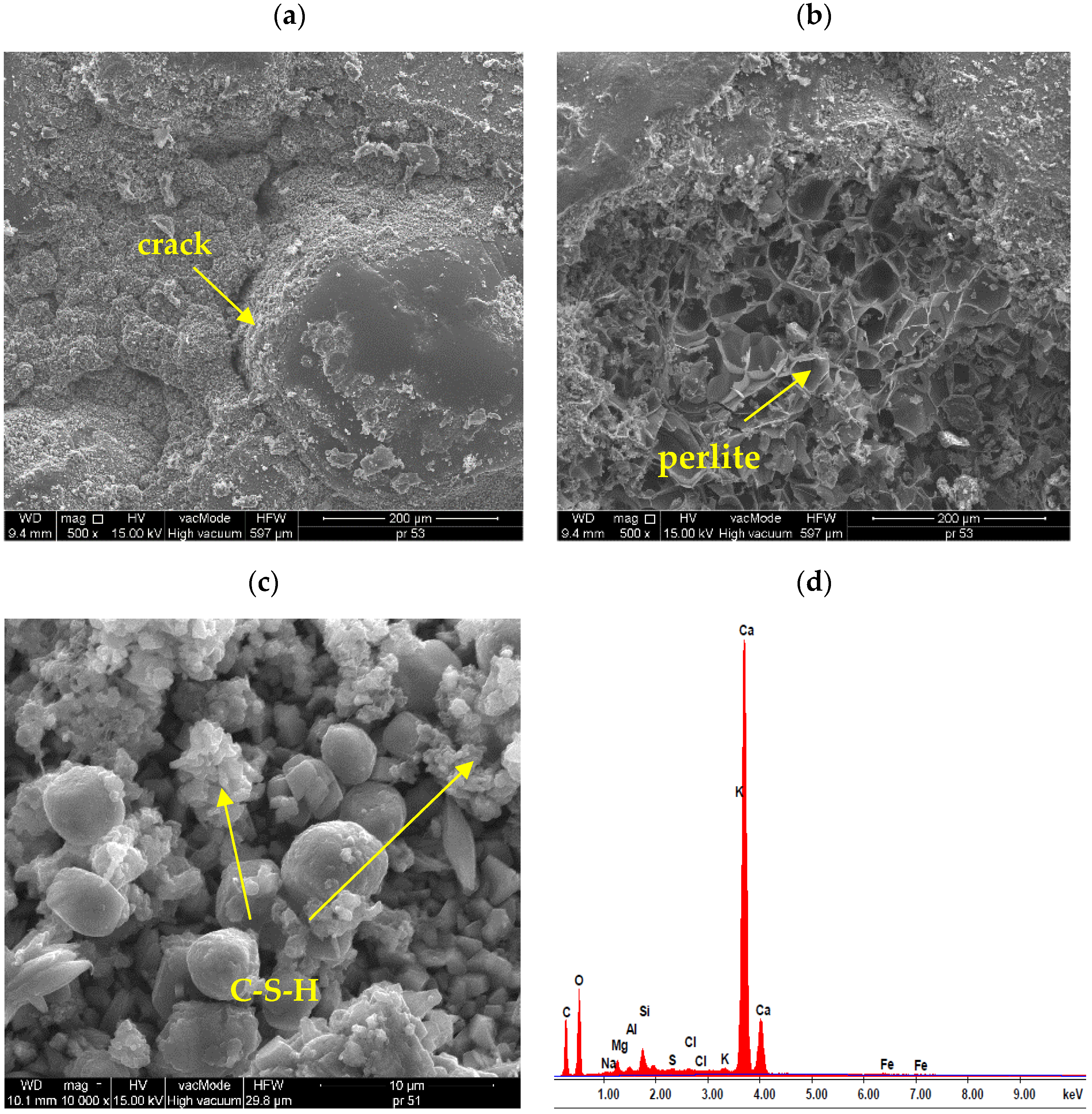
3. Results and Discussion
4. Conclusions
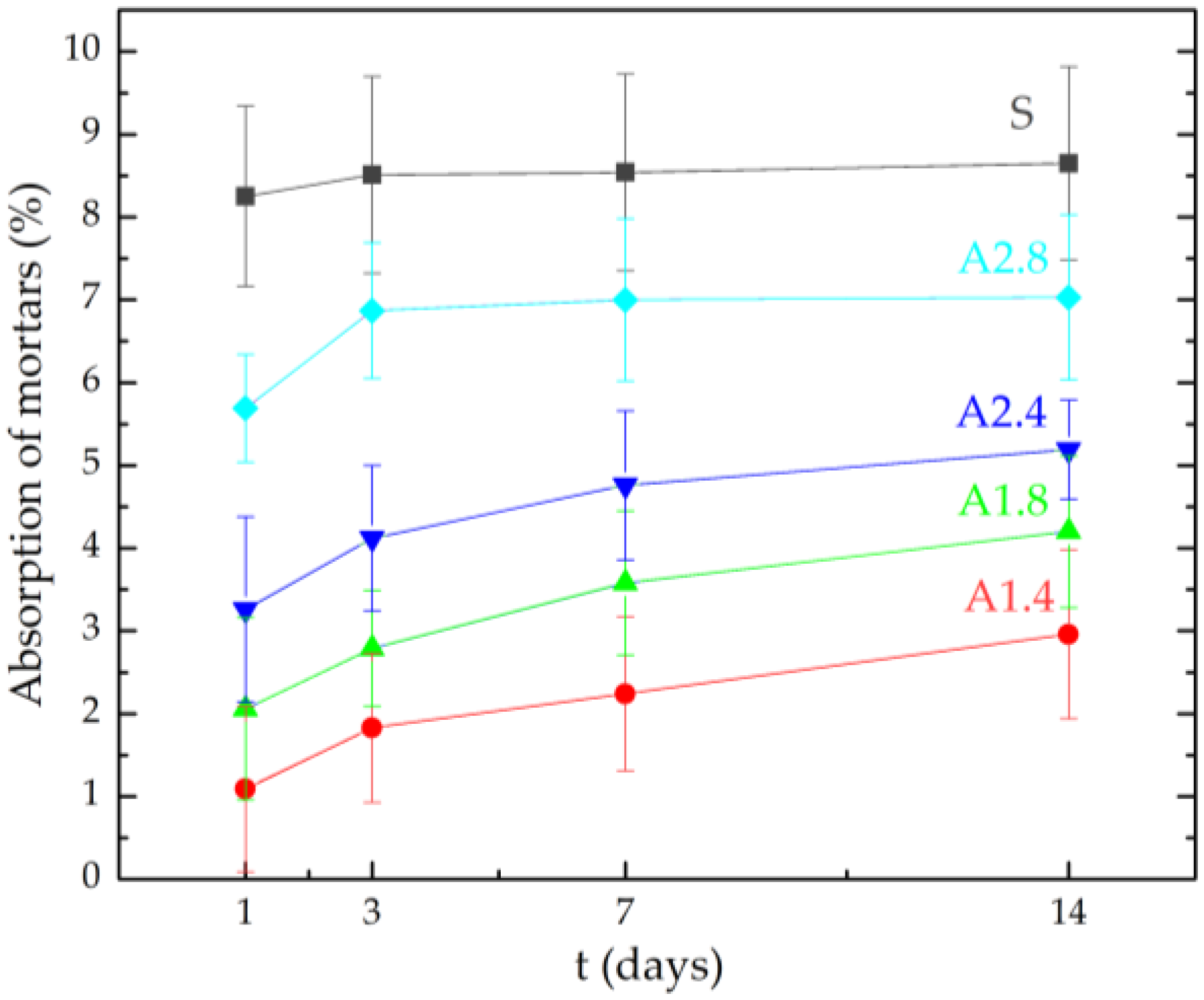
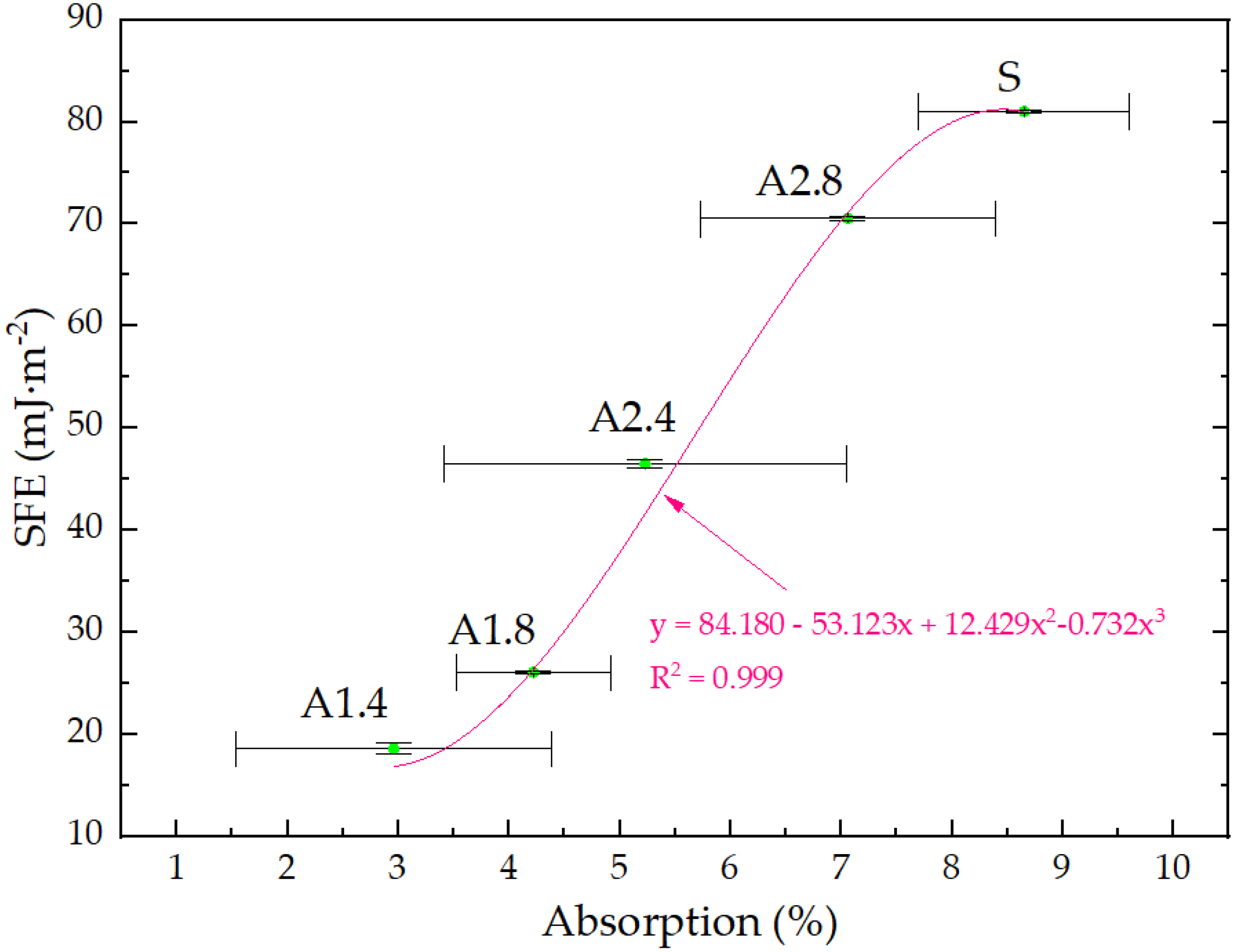
- Surface hydrophobization of light mortars with perlite effectively reduced the absorbability of the samples. The best results were obtained on agent A1.4. After the first day of testing, water absorption decreased by 7.5 times and after 14 days 2.9 times compared to reference mortars S.
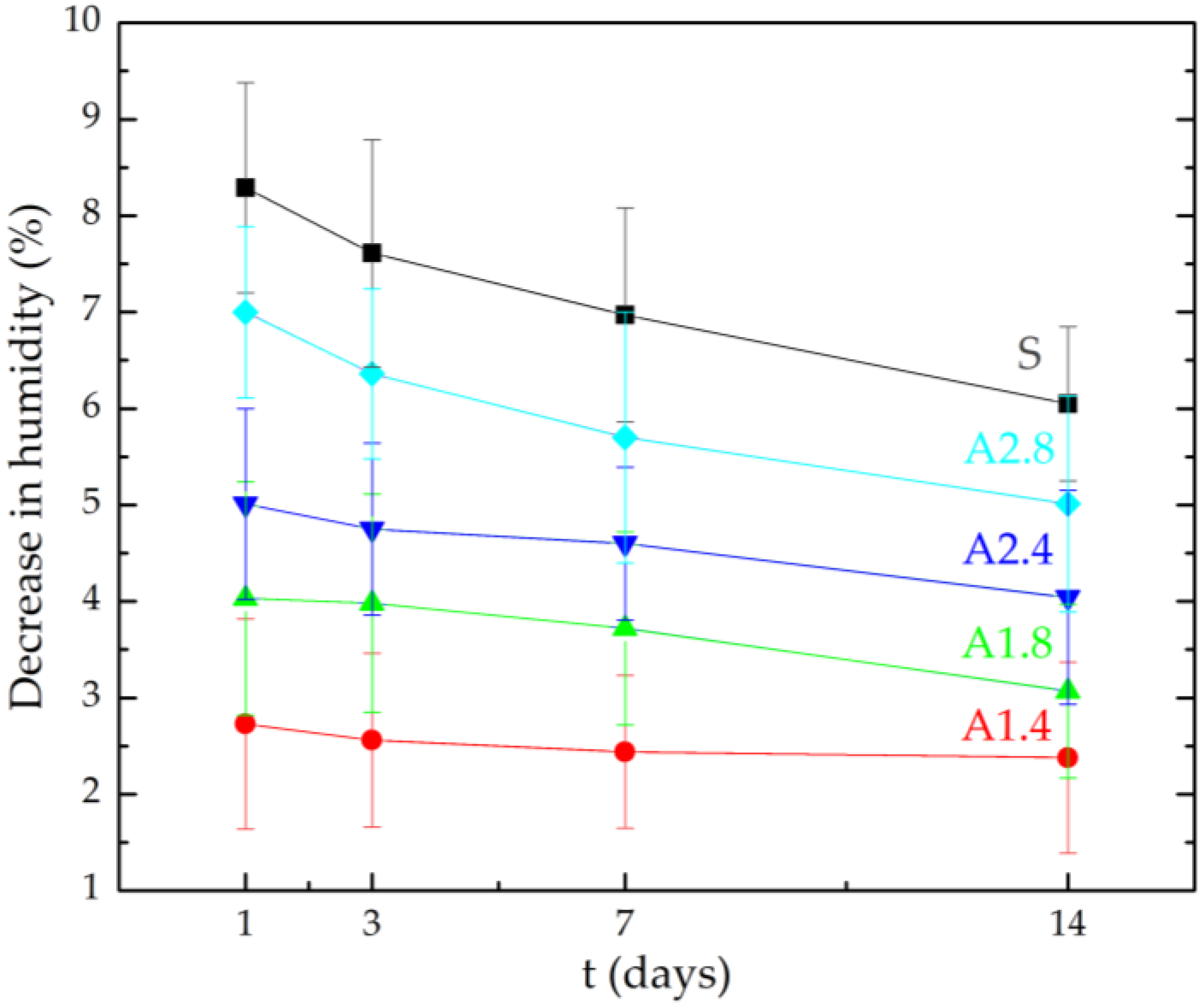
- Samples A1.4 were characterized by the highest tightness, in which a decrease in water vapour diffusion capacity by 61% in comparison to standard samples was observed.
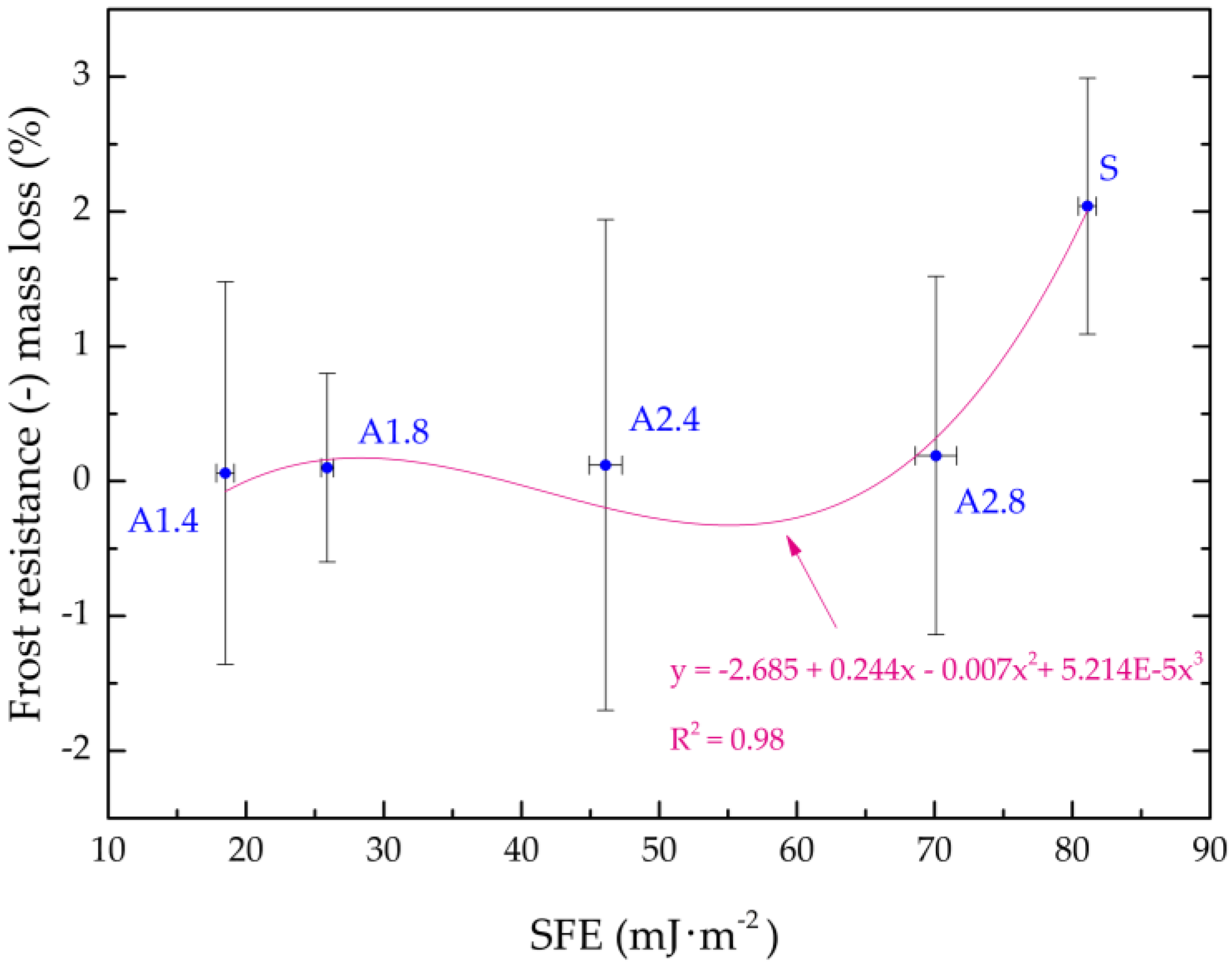
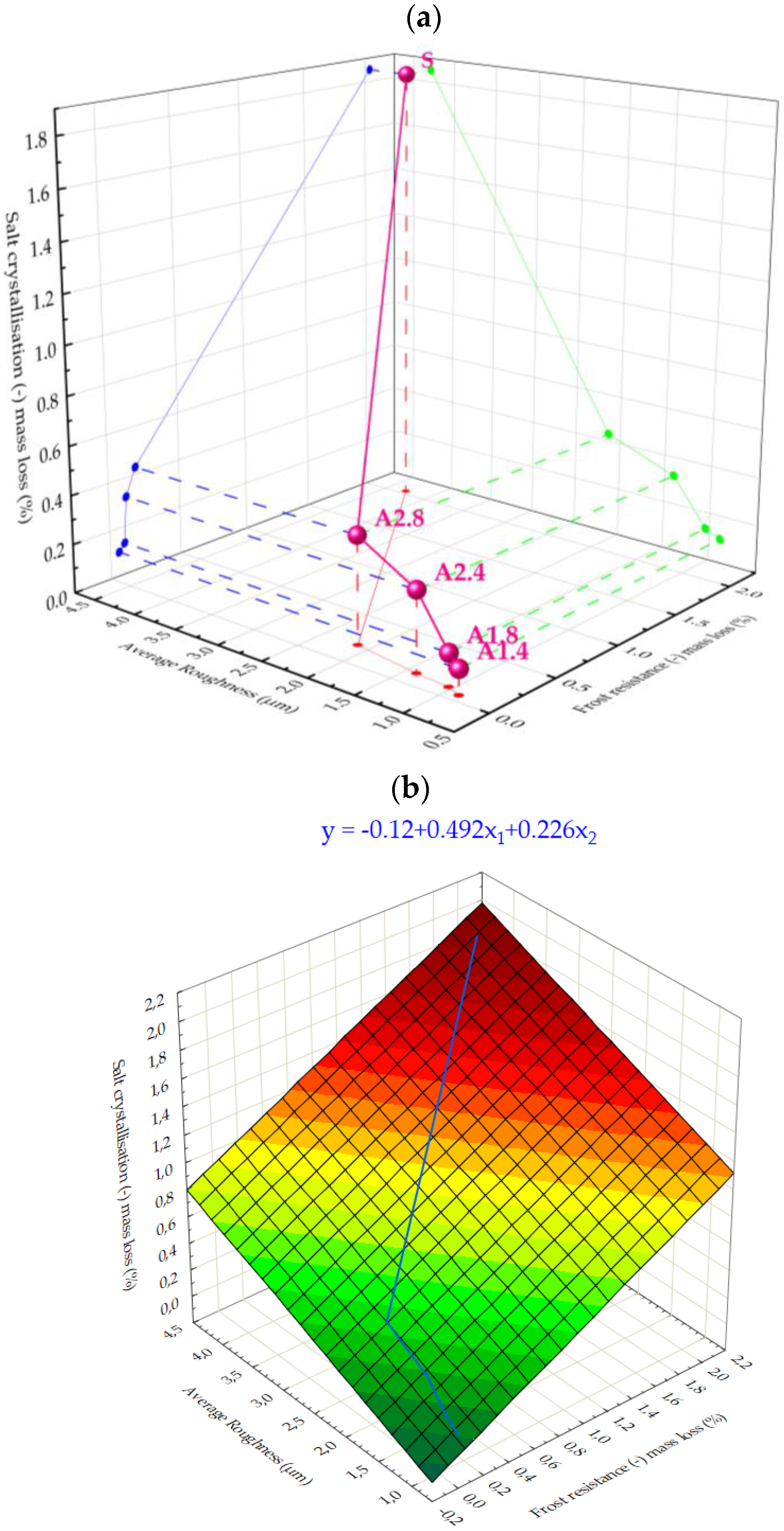
- All mortars proved to be frost resistant. A decrease in weight loss was observed from 2.04% in standard samples, to 0.06–0.19% in hydrophobized mortars.
- Hydrophobized A1 and A2 mortars showed resistance to sulphate crystallisation. A decrease in weight loss after salt crystallisation from 1.85% in S samples to 0.1–0.43% in hydrophobized samples was observed.
- Samples covered with propyl silicates (A2) proved to be wettable (CA < 90°), while samples A1 showed hydrophobic properties. The highest CA equal to 107.5° was obtained in mortars A1.4. This is an almost 9-fold increase compared to standard samples S.
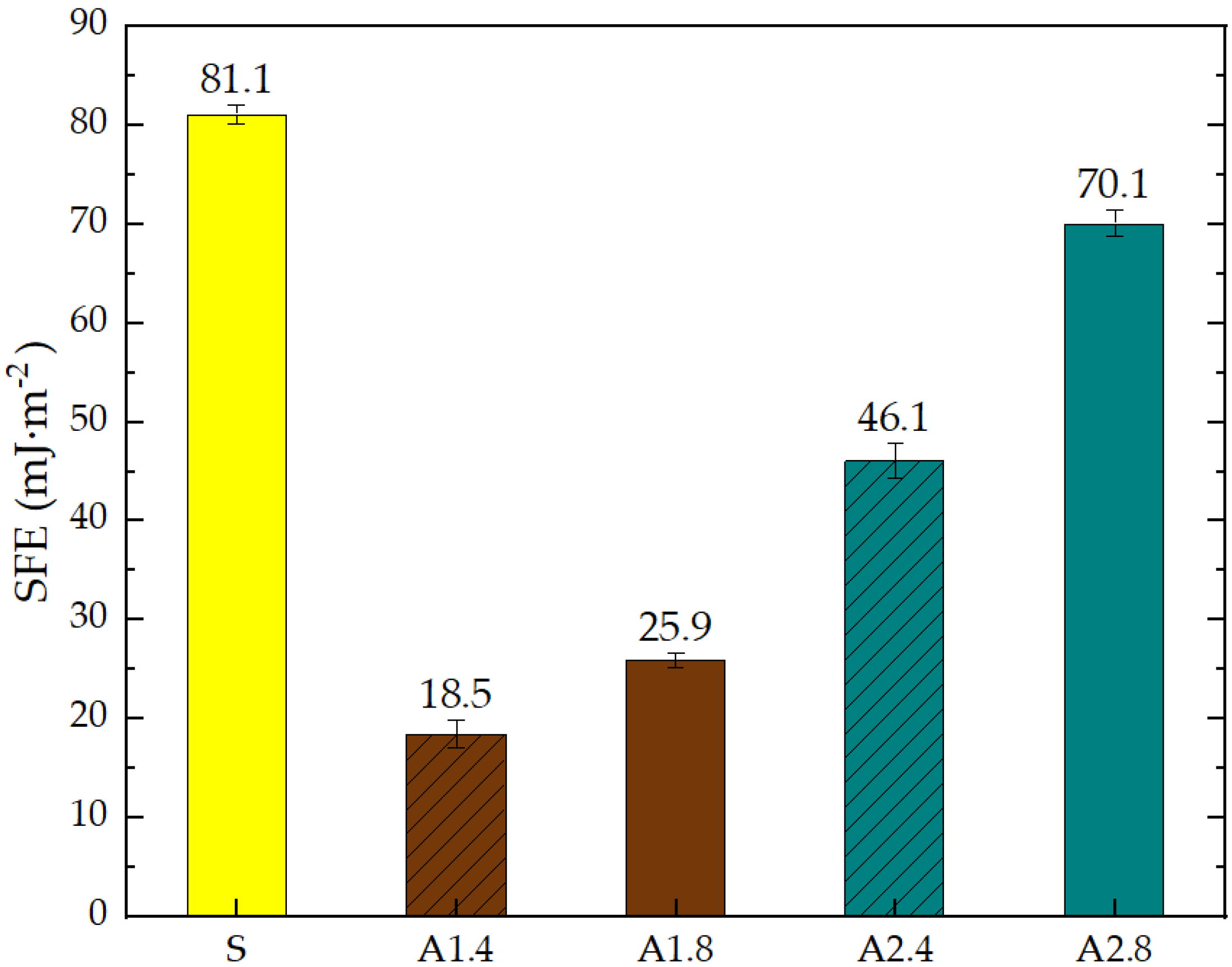
- Mortars A1.4 showed the smallest total SFE, equal to 18.5 mJ∙m−2, which is 77% lower than standard S mortars.
- The smoothest surface area was obtained in mortars A1.4, where Ra decreased 4.85 times. The study showed that the higher the roughness of the mortar, the higher the absorbency and lower the frost resistance.
- The preparation based on silane (A1) proved to be more effective in improving all properties of hydrophobized mortars. Studies have shown greater effectiveness of higher concentration preparations, diluted in water in the ratio 1:4.
Author Contributions
Funding
Conflicts of Interest
References
- Corinaldesi, V.; Mazzoli, A.; Siddique, R. Characterization of lightweight mortars containing wood processing by-products waste. Constr. Build. Mater. 2016, 123, 281–289. [Google Scholar] [CrossRef]
- Tamanna, K.; Raman, S.N.; Jamil, M.; Hamid, R. Utilization of wood waste ash in construction technology: A review. Constr. Build. Mater. 2020, 237, 117654. [Google Scholar] [CrossRef]
- Seghir, N.T.; Mellas, M.; Sadowski, Ł.; Krolicka, A.; Żak, A.; Ostrowski, K. The Utilization of Waste Marble Dust as a Cement Replacement in Air-Cured Mortar. Sustainability 2019, 11, 2215. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Siddique, R.; Klimek, B.; Franus, M. The use of zeolite, lightweight aggregate and boiler slag in restoration renders. Constr. Build. Mater. 2017, 142, 162–174. [Google Scholar] [CrossRef]
- De-Carvalho, R.; Teixeira-Dias, F.; Varum, H. Cyclic behaviour of a lightweight mortar with cork granulate composite. Compos. Struct. 2013, 95, 748–755. [Google Scholar] [CrossRef]
- Ferrándiz-Mas, V.; Bond, T.; García-Alcocel, E.; Cheeseman, C.R. Lightweight mortars containing expanded polystyrene and paper sludge ash. Constr. Build. Mater. 2014, 61, 285–292. [Google Scholar] [CrossRef]
- Kramar, D.; Bindiganavile, V. Impact response of lightweight mortars containing expanded perlite. Cem. Concr. Compos. 2013, 37, 205–214. [Google Scholar] [CrossRef]
- Lanzón, M.; García-Ruiz, P.A. Lightweight cement mortars: Advantages and inconveniences of expanded perlite and its influence on fresh and hardened state and durability. Constr. Build. Mater. 2008, 22, 1798–1806. [Google Scholar] [CrossRef]
- Gadea, J.; Rodríguez, A.; Campos, P.L.; Garabito, J.; Calderón, V. Lightweight mortar made with recycled polyurethane foam. Cem. Concr. Compos. 2010, 32, 672–677. [Google Scholar] [CrossRef]
- Arroyo, R.; Horgnies, M.; Junco, C.; Rodríguez, A.; Calderón, V. Lightweight structural eco-mortars made with polyurethane wastes and non-Ionic surfactants. Constr. Build. Mater. 2019, 197, 157–163. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Saccani, A.; Sandrolini, F. New polymer mortars containing polymeric wastes. Part 1. Microstructure and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2000, 31, 97–106. [Google Scholar] [CrossRef]
- Degirmenci, N.; Yilmaz, A. Use of pumice fine aggregate as an alternative to standard sand in production of lightweight cement mortar. Indian J. Eng. Mater. Sci. 2011, 18, 61–68. [Google Scholar]
- Gong, J.; Duan, Z.; Sun, K.; Xiao, M. Waterproof properties of thermal insulation mortar containing vitrified microsphere. Constr. Build. Mater. 2016, 123, 274–280. [Google Scholar] [CrossRef]
- Łukowski, P. Polymer-Cement Composites Containing Waste Perlite Powder. Materials 2016, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Setyowati, E. Eco-building Material of Styrofoam Waste and Sugar Industry Fly-ash based on Nano-technology. Procedia Environ. Sci. 2014, 20, 245–253. [Google Scholar] [CrossRef]
- Khmiri, A.; Chaabouni, M.; Samet, B. Chemical behaviour of ground waste glass when used as partial cement replacement in mortars. Constr. Build. Mater. 2013, 44, 74–80. [Google Scholar] [CrossRef]
- Stefanidou, M.; Ioanna-Maria, M.; Myrta, G.-M. Influence of aerogel as aggregate in the properties of cement mortars. IOP Conf. Ser. Earth Environ. Sci. 2020, 410, 012117. [Google Scholar] [CrossRef]
- Koksal, F.; Mutluay, E.; Gencel, O. Characteristics of isolation mortars produced with expanded vermiculite and waste expanded polystyrene. Constr. Build. Mater. 2020, 236, 117789. [Google Scholar] [CrossRef]
- Contrafatto, L.; Lazzaro Danzuso, C.; Gazzo, S.; Greco, L. Physical, mechanical and thermal properties of lightweight insulating mortar with recycled Etna volcanic aggregates. Constr. Build. Mater. 2020, 240, 117917. [Google Scholar] [CrossRef]
- Saghrouni, Z.; Baillis, D.; Naouar, N.; Blal, N.; Jemni, A. Thermal Properties of New Insulating Juncus Maritimus Fibrous Mortar Composites/Experimental Results and Analytical Laws. Appl. Sci. 2019, 9, 981. [Google Scholar] [CrossRef]
- Aspasia, K.; Maria, S. Advances in clay-based mortars for protection against water penetration. Ceram. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Stratoura, M.; Iaz, D.R.; Badogiannis, E. Chloride Penetration in Lightweight Aggregate Mortars Incorporating Supplementary Cementing Materials. Adv. Civ. Eng. 2018, 2018, 9759167. [Google Scholar] [CrossRef]
- Giosuè, C.; Mobili, A.; Yu, Q.L.; Brouwers, H.J.H.; Ruello, M.L.; Tittarelli, F. Properties of multifunctional lightweight mortars containing zeolite and natural fibers. J. Sustain. Cem. Mater. 2019, 8, 214–227. [Google Scholar] [CrossRef]
- Shahbazi, R.; Korayem, A.H.; Razmjou, A.; Duan, W.H.; Wang, C.M.; Justnes, H. Integrally hydrophobic cementitious composites made with waste amorphous carbon powder. Constr. Build. Mater. 2020, 233, 117238. [Google Scholar] [CrossRef]
- Giosuè, C.; Mobili, A.; Toscano, G.; Ruello, M.L.; Tittarelli, F. Effect of Biomass Waste Materials as Unconventional Aggregates in Multifunctional Mortars for Indoor Application. Procedia Eng. 2016, 161, 655–659. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.; Rogkala, A.; Stamatis, P.; Tsikouras, B.; Papoulis, D.; Lampropoulou, P.; Hatzipanagiotou, K. The Influence of Alteration of Aggregates on the Quality of the Concrete: A Case Study from Serpentinites and Andesites from Central Macedonia (North Greece). Geosciences 2018, 8, 115. [Google Scholar] [CrossRef]
- Gonilho Pereira, C.; Castro-Gomes, J.; Pereira de Oliveira, L. Influence of natural coarse aggregate size, mineralogy and water content on the permeability of structural concrete. Constr. Build. Mater. 2009, 23, 602–608. [Google Scholar] [CrossRef]
- Piasta, W.; Góra, J.; Turkiewicz, T. Properties and durability of coarse igneous rock aggregates and concretes. Constr. Build. Mater. 2016, 126, 119–129. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Du, Y.; Jiang, J.; Sun, W. The effect of superhydrophobic nano-silica particles on the transport and mechanical properties of hardened cement pastes. Constr. Build. Mater. 2018, 182, 620–628. [Google Scholar] [CrossRef]
- Husni, H.; Nazari, M.R.; Yee, H.M.; Rohim, R.; Yusuff, A.; MohdAriff, M.A.; Ahmad, N.N.R.; Leo, C.P.; Junaidi, M.U.M. Superhydrophobic rice husk ash coating on concrete. Constr. Build. Mater. 2017, 144, 385–391. [Google Scholar] [CrossRef]
- Flores-Vivian, I.; Hejazi, V.; Kozhukhova, M.I.; Nosonovsky, M.; Sobolev, K. Self-assembling particle-siloxane coatings for superhydrophobic concrete. ACS Appl. Mater. Interfaces 2013, 5, 13284–13294. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Łagód, G. Effect of surface moisture on the effectiveness of the hydrophobisation of mortars with pumice aggregate. AIP Conf. Proc. 2018, 1988, 020023. [Google Scholar] [CrossRef]
- Frattolillo, A.; Giovinco, G.; Mascolo, M.C.; Vitale, A. Effects of hydrophobic treatment on thermophysical properties of lightweight mortars. Exp. Therm. Fluid Sci. 2005, 30, 27–35. [Google Scholar] [CrossRef]
- Novak, V.; Zach, J. The effect of hydrophobization on the properties of mortar mixtures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 385, 012040. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Li, W. Effect of PDMS on the waterproofing performance and corrosion resistance of cement mortar. Appl. Surf. Sci. 2020, 507, 145016. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Duda, S.; Garbacz, M.; Łagód, G. Hydrophobisation of mortars containing waste polyurethane foam. MATEC Web Conf. 2018, 163, 04006. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Siddique, R.; Łagód, G. Properties of hydrophobized lightweight mortars with expanded cork. Constr. Build. Mater. 2017, 155, 15–25. [Google Scholar] [CrossRef]
- Izarra, I.; Cubillo, J.; Serrano, A.; Rodriguez, J.F.; Carmona, M. A hydrophobic release agent containing SiO2-CH3 submicron-sized particles for waterproofing mortar structures. Constr. Build. Mater. 2019, 199, 30–39. [Google Scholar] [CrossRef]
- Pan, S.; Guo, R.; Björnmalm, M.; Richardson, J.J.; Li, L.; Peng, C.; Bertleff-Zieschang, N.; Xu, W.; Jiang, J.; Caruso, F. Coatings super-repellent to ultralow surface tension liquids. Nat. Mater. 2018, 17, 1040–1047. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent Flame-Retardant and Self-Healing Superhydrophobic Coatings on Cotton Fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef]
- Li, R.; Hou, P.; Xie, N.; Ye, Z.; Cheng, X.; Shah, S.P. Design of SiO2/PMHS hybrid nanocomposite for surface treatment of cement-based materials. Cem. Concr. Compos. 2018, 87, 89–97. [Google Scholar] [CrossRef]
- Ramachandran, R.; Sobolev, K.; Nosonovsky, M. Dynamics of Droplet Impact on Hydrophobic/Icephobic Concrete with the Potential for Superhydrophobicity. Langmuir 2015, 31, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Fic, S.; Barnat-Hunek, D. The Effectiveness of Hydrophobisation of Porous Building Materials by Using the Polymers and Nanopolymers Solutions. Int. J. Mater. Sci. Eng. 2014, 2, 93–98. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Smarzewski, P. Increased water repellence of ceramic buildings by hydrophobisation using high concentration of organic solvents. Energy Build. 2015, 103, 249–260. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 197-1:2012. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements; CEN: Brussels, Belgium, 2012. [Google Scholar]
- Polish Committee for Standardization. PN-B-19707:2013-10. Cement. Special Cement. Composition, Requirements and Compliance Criteria; PKN: Warsaw, Poland, 2013. [Google Scholar]
- Barnat-Hunek, D. Surface Free Energy as a Factor Affecting Hydrophobisation Effectiveness in Protection of Building Construction; Publishing House Lublin University of Technology: Lublin, Poland, 2016. [Google Scholar]
- European Committee for Standardization. EN 196-7:2009. Methods of Testing Cement—Part 7: Methods of Taking and Preparing Samples of Cement; CEN: Brussels, Belgium, 2009. [Google Scholar]
- Protectosil®—Water Repellents—Protectosil®—Your Partner for Building Protection. Available online: https://www.protectosil.com/product/protectosil/en/products/water-repellents/ (accessed on 19 February 2020).
- European Committee for Standardization. EN 1936:2010. Natural Stone Test Methods—Determination of Real Density and Apparent Density and of Total and Open Porosity; CEN: Brussels, Belgium, 2010. [Google Scholar]
- European Committee for Standardization. EN 1015-10:2001. Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar; CEN: Brussels, Belgium, 2001. [Google Scholar]
- European Committee for Standardization. EN 13755:2008. Natural Stone Test Methods—Determination of Water Absorption at Atmospheric Pressure; CEN: Brussels, Belgium, 2008. [Google Scholar]
- European Committee for Standardization. EN 1015-11:2001. Methods of Test for Mortar for Masonary—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar; CEN: Brussels, Belgium, 2001. [Google Scholar]
- Polish Committee for Standardization. PN-B-12012:2007. Methods of Test for Masonry units—Determination of Freeze/Thaw Resistance of Clay Masonry Units; PKN: Warsaw, Poland, 2007. [Google Scholar]
- European Committee for Standardization. EN 12370:2001. Natural Stone Test Methods—Determination of Resistance to Salt Crystallisation; CEN: Brussels, Belgium, 2001. [Google Scholar]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen-Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Smarzewski, P. Influence of hydrophobisation on surface free energy of hybrid fiber reinforced ultra-high performance concrete. Constr. Build. Mater. 2016, 102, 367–377. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Grzegorczyk-Frańczak, M.; Szymańska-Chargot, M.; Łagód, G. Effect of Eco-Friendly Cellulose Nanocrystals on Physical Properties of Cement Mortars. Polymers 2019, 11, 2088. [Google Scholar] [CrossRef]
- British Committee for Standardization. BS EN ISO 4287:1998+A1:2009. Geometrical Product Specification (GPS). Surface Texture: Profile Method. Terms, Definitions and Surface Texture Parameters; BSI: London, UK, 2009. [Google Scholar]
- Błaszczyński, T.; Osesek, M.; Gwozdowski, B.; Ilski, M. Performance of Hydrophobisation Techniques in Case of Reinforced Concrete Structures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 22059. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Kou, S.C.; Poon, C.S.; Dai, J.G.; Li, Q.Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Maaloufa, Y.; Mounir, S.; Khabbazi, A.; Kettar, J.; Khaldoun, A. Thermal Characterization of Materials based on Clay and Granular: Cork or Expanded Perlite. Energy Procedia 2015, 74, 1150–1161. [Google Scholar] [CrossRef]
- Oktay, H.; Yumrutaş, R.; Akpolat, A. Mechanical and thermophysical properties of lightweight aggregate concretes. Constr. Build. Mater. 2015, 96, 217–225. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Góra, J.; Andrzejuk, W.; Łagód, G. The Microstructure-Mechanical Properties of Hybrid Fibres-Reinforced Self-Compacting Lightweight Concrete with Perlite Aggregate. Materials 2018, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. A synopsis about perlite as building material—A best practice guide for Civil Engineer. Constr. Build. Mater. 2016, 121, 338–353. [Google Scholar] [CrossRef]
- Darweesh, H.H.M. Utilization of Perlite Rock in Blended Cement-Part I: Physicomechanical Properties. Direct Res. J. Chem. Mater. Sci. 2014, 2, 1–12. [Google Scholar]
- Fodil, D.; Mohamed, M. Compressive strength and corrosion evaluation of concretes containing pozzolana and perlite immersed in aggressive environments. Constr. Build. Mater. 2018, 179, 25–34. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M.A. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Widomski, M.K. Effect of Hydrophobisation on Durability Related Properties of Concrete with Expanded Perlite. In Proceedings of the 5th International Congress on Technology—Engineering, Kuala Lumpur, Malaysia, 1–2 February 2018. [Google Scholar]
- Liu, Z.; Hansen, W. Effect of hydrophobic surface treatment on freeze-thaw durability of concrete. Cem. Concr. Compos. 2016, 69, 49–60. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Soliman, A.M.; Nehdi, M.L. Effect of surface treatment on durability of concrete exposed to physical sulfate attack. Constr. Build. Mater. 2014, 73, 674–681. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Liu, J.; Hou, D.; Geng, Y.; Chen, X.; Gao, Y.; Jin, Z.; Yin, B. Protective Mechanism of Silane on Concrete upon Marine Exposure. Coatings 2019, 9, 558. [Google Scholar] [CrossRef]
- Klein, N.S.; Bachmann, J.; Aguado, A.; Toralles-Carbonari, B. Evaluation of the wettability of mortar component granular materials through contact angle measurements. Cem. Concr. Res. 2012, 42, 1611–1620. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, M. Using surface free energy method to study the cohesion and adhesion of asphalt mastic. Constr. Build. Mater. 2013, 47, 254–260. [Google Scholar] [CrossRef]
- Stefanidou, M.; Matziaris, K.; Karagiannis, G. Hydrophobization by Means of Nanotechnology on Greek Sandstones Used as Building Facades. Geosciences 2013, 3, 30–45. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Luo, H.; Miao, J. Research on protection of the architectural glazed ceramics in the Palace Museum, Beijing. J. Cult. Herit. 2010, 11, 279–287. [Google Scholar] [CrossRef]
- Najduchowska, M.; Pichniarczyk, P. Effect of hydrophobic compounds on the properties of cement and gypsum mortars. Cem. Lime Concr. 2010, 15, 141–148. [Google Scholar]
- Barnat-Hunek, D.; Łagód, G.; Fic, S.; Jarosz-Hadam, M. Effect of polysiloxanes on roughness and durability of basalt fibres-reinforced cement mortar. Polymers 2018, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Courard, L.; Michel, F.; Perkowicz, S.; Garbacz, A. Effects of limestone fillers on surface free energy and electrical conductivity of the interstitial solution of cement mixes. Cem. Concr. Compos. 2014, 45, 111–116. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Sassani, A.; Sundararajan, S.; Taylor, P.C. Superhydrophobic coatings on Portland cement concrete surfaces. Constr. Build. Mater. 2017, 141, 393–401. [Google Scholar] [CrossRef]
- She, W.; Wang, X.; Miao, C.; Zhang, Q.; Zhang, Y.; Yang, J.; Hong, J. Biomimetic superhydrophobic surface of concrete: Topographic and chemical modification assembly by direct spray. Constr. Build. Mater. 2018, 181, 347–357. [Google Scholar] [CrossRef]
- Lazauskas, A.; Baltrusaitis, J.; Grigaliūnas, V.; Jucius, D.; Guobienė, A.; Prosyčevas, I.; Narmontas, P. Characterization of plasma polymerized hexamethyldisiloxane films prepared by arc discharge. Plasma Chem. Plasma Process. 2014, 34, 271–285. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Zhevnenko, S.N.; Emel’yanenko, A.M.; Gol’Dshtein, R.V.; Epifanov, V.P. Adhesive strength of the contact of ice with a superhydrophobic coating. Dokl. Chem. 2013, 448, 71–75. [Google Scholar] [CrossRef]
- Palou, M.T.; Bagel, L.; Zivica, V.; Kuliffayova, M.; Kozankova, J. Influence of hydrothermal curing regimes on the hydration of fiber-reinforced cement composites. J. Therm. Anal. Calorim. 2013, 113, 219–229. [Google Scholar] [CrossRef]
| Components | Unit | S | A1.4 | A1.8 | A2.4 | A2.8 |
|---|---|---|---|---|---|---|
| Portland cement CEM I 42.5R | (kg∙m−3) | 562 | 562 | 562 | 562 | 562 |
| Sand 0–2.0 mm | (kg∙m−3) | 1194 | 1194 | 1194 | 1194 | 1194 |
| Perlite 0.5–2.0 mm | (kg∙m−3) | 16.15 | 16.15 | 16.15 | 16.15 | 16.15 |
| Water | (kg∙m−3) | 252.9 | 252.9 | 252.9 | 252.9 | 252.9 |
| Superplasticizer | (kg∙m−3) | 2.2485 | 2.2485 | 2.2485 | 2.2485 | 2.2485 |
| Dilution of hydrophobic agent | - | - | 1:4 | 1:8 | 1:4 | 1:8 |
| Specific Surface (cm2∙g−1) | Initial Setting Time (min) | End Setting Time (min) | Specific Gravity (g∙cm−3) | Water Demand (%) | Compressive Strength After 2 Days (MPa) | Compressive Strength After 28 Days (MPa) |
|---|---|---|---|---|---|---|
| 3.426 | 146 | 190 | 3.09 | 27.6 | 28.8 | 54.1 |
| Bulk Density (kg∙m−3) | Porosity (%) | Water Absorptivity (%) | Thermal Conductivity (W∙(m2∙K)−1) | Compressive Strength (N∙mm−2) |
|---|---|---|---|---|
| 90 | 33 | 52 | 0.049 | 3.3 |
| Symbol of the Sample | Average Absorption (%) | |||
|---|---|---|---|---|
| t (days) | ||||
| 1 | 3 | 7 | 14 | |
| S | 8.25 | 8.51 | 8.54 | 8.65 |
| A1.4 | 1.09 | 1.83 | 2.24 | 2.96 |
| A1.8 | 2.06 | 2.79 | 3.58 | 4.20 |
| A2.4 | 3.26 | 4.12 | 4.76 | 5.19 |
| A2.8 | 5.69 | 6.87 | 7.00 | 7.03 |
| Symbol of the Sample | Average Humidity (%) | |||
|---|---|---|---|---|
| t (days) | ||||
| 1 | 3 | 7 | 14 | |
| S | 8.29 | 7.61 | 6.97 | 6.05 |
| A1.4 | 2.73 | 2.56 | 2.44 | 2.38 |
| A1.8 | 4.03 | 3.98 | 3.72 | 3.07 |
| A2.4 | 5.01 | 4.75 | 4.60 | 4.04 |
| A2.8 | 7.00 | 6.36 | 5.70 | 5.01 |
| Durability/ Physical Properties | Unit | S | A1.4 | A1.8 | A2.4 | A2.8 |
|---|---|---|---|---|---|---|
| Apparent density | (g∙mm−3) | 1.70 | - | - | - | - |
| Specific density | (g∙mm−3) | 2.55 | - | - | - | - |
| Porosity | (%) | 14.34 | - | - | - | - |
| Total porosity | (%) | 33.80 | - | - | - | - |
| Flexural strength | (N∙mm−2) | 5.6 | - | - | - | - |
| Compressive strength | (N∙mm−2) | 37.3 | - | - | - | - |
| Frost resistance (-) mass loss | (%) | 2.04 | 0.06 | 0.10 | 0.12 | 0.19 |
| Salt crystallisation (-) mass loss | (%) | 1.85 | 0.10 | 0.13 | 0.32 | 0.43 |
| Type of Mortar | Contact Angle | |
|---|---|---|
| Water θw (°) | Diiodomethane θd (°) | |
| S |  | 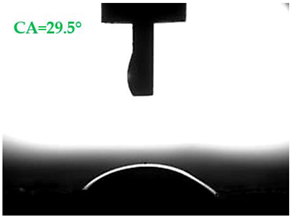 |
| A1.4 | 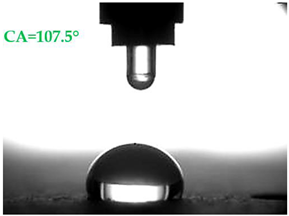 | 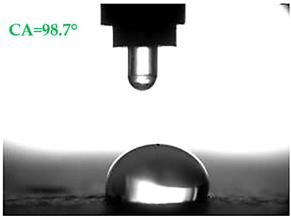 |
| A1.8 | 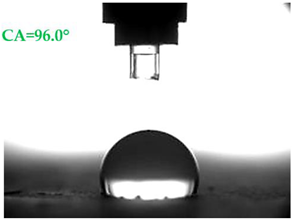 | 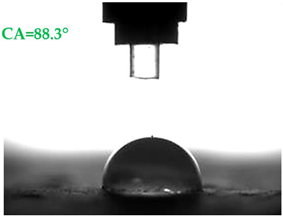 |
| A2.4 |  |  |
| A2.8 |  |  |
| Micro-Roughness Characteristics (µm) | ||
|---|---|---|
| S |  | Ra = 4.29 µm Rp = 10.3 µm Rv = 10.6 µm Rmax = 20.9 µm |
| A1.4 |  | Ra = 0.883 µm Rp = 4.21 µm Rv = 3.01 µm Rmax = 20.9 µm |
| A1.8 | 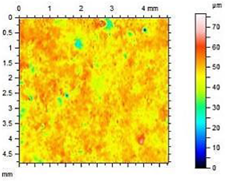 | Ra = 1.04 µm Rp = 3.09 µm Rv = 4.24 µm Rmax = 7.33 µm |
| A2.4 | 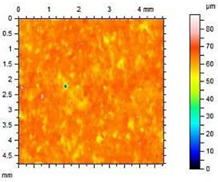 | Ra = 1.40 µm Rp = 4.57 µm Rv = 4.75 µm Rmax = 9.32 µm |
| A2.8 | 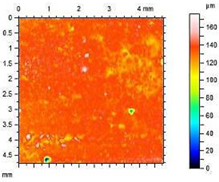 | Ra = 2.13 µm Rp = 6.84 µm Rv = 6.33 µm Rmax = 13.17 µm |
| Mortar | Compound | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al2O5 | SiO2 | Na2O | Fe2O3 | MgO | K2O | CaO | SO3 | P2O5 | ||
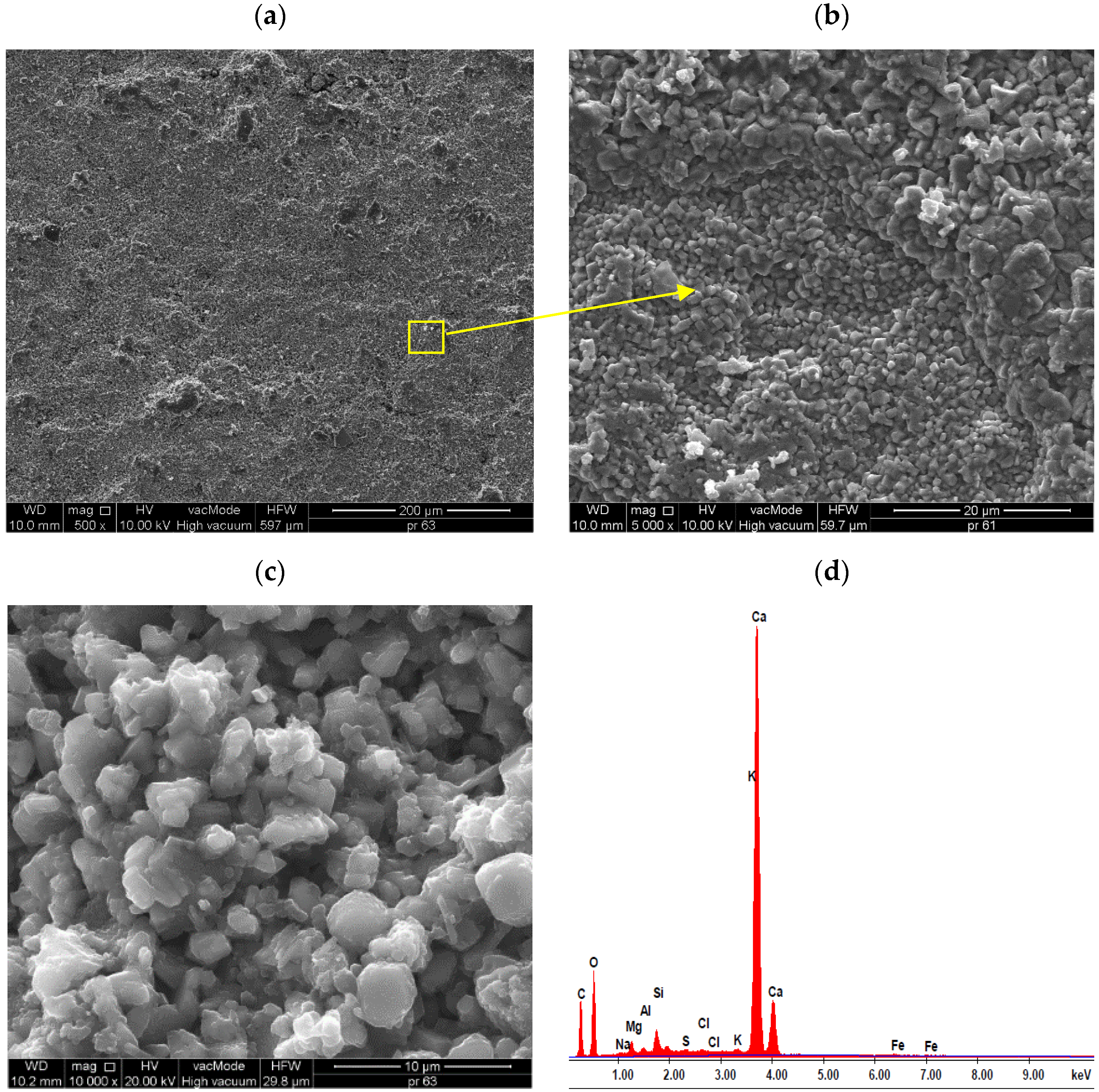
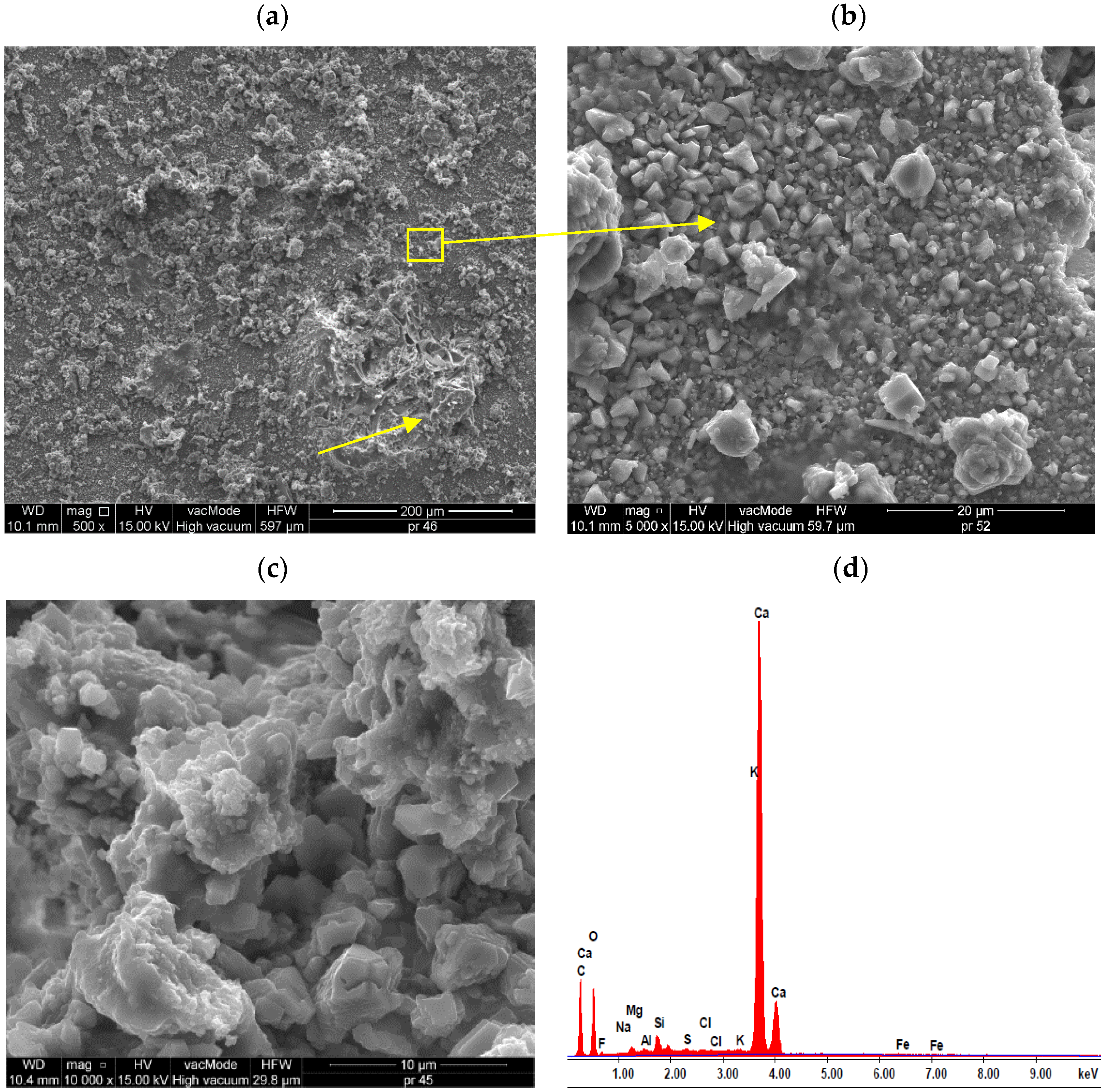
| S | Content (%mass) | 2.30 | 9.77 | 1.17 | 0.50 | 10.0 | 0.56 | 74.93 | 0.38 | - |
| A1.4 | 3.06 | 6.91 | 1.51 | 0.65 | 4.22 | 0.86 | 80.15 | 1.74 | - | |
| A2.4 | 2.57 | 5.60 | 1.25 | 0.46 | 2.50 | 0.71 | 80.96 | 1.71 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafraniec, M.; Barnat-Hunek, D.; Grzegorczyk-Frańczak, M.; Trochonowicz, M. Surface Modification of Lightweight Mortars by Nanopolymers to Improve Their Water-Repellency and Durability. Materials 2020, 13, 1350. https://doi.org/10.3390/ma13061350
Szafraniec M, Barnat-Hunek D, Grzegorczyk-Frańczak M, Trochonowicz M. Surface Modification of Lightweight Mortars by Nanopolymers to Improve Their Water-Repellency and Durability. Materials. 2020; 13(6):1350. https://doi.org/10.3390/ma13061350
Chicago/Turabian StyleSzafraniec, Małgorzata, Danuta Barnat-Hunek, Małgorzata Grzegorczyk-Frańczak, and Maciej Trochonowicz. 2020. "Surface Modification of Lightweight Mortars by Nanopolymers to Improve Their Water-Repellency and Durability" Materials 13, no. 6: 1350. https://doi.org/10.3390/ma13061350
APA StyleSzafraniec, M., Barnat-Hunek, D., Grzegorczyk-Frańczak, M., & Trochonowicz, M. (2020). Surface Modification of Lightweight Mortars by Nanopolymers to Improve Their Water-Repellency and Durability. Materials, 13(6), 1350. https://doi.org/10.3390/ma13061350







