Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermal Analysis
Friedman Analysis
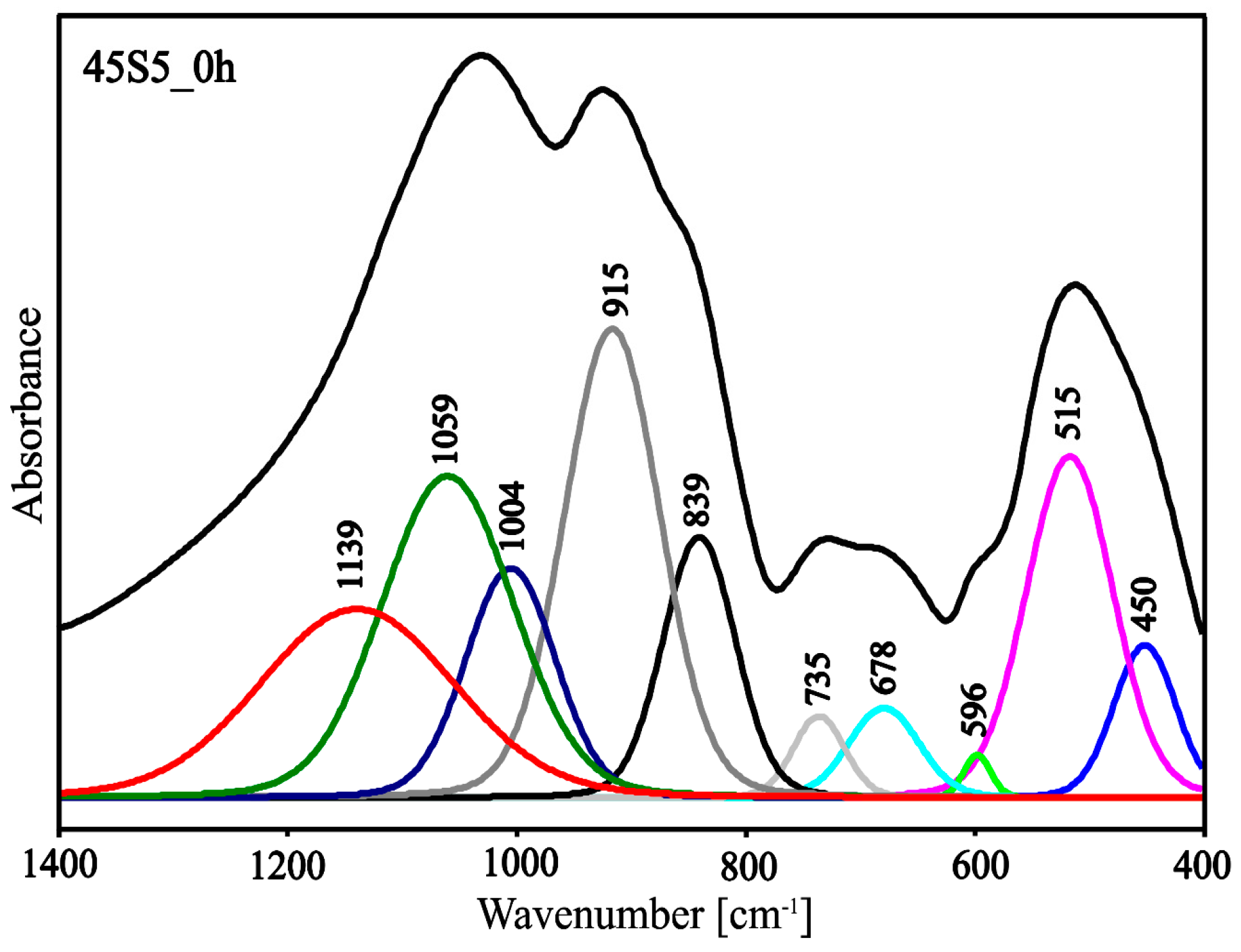
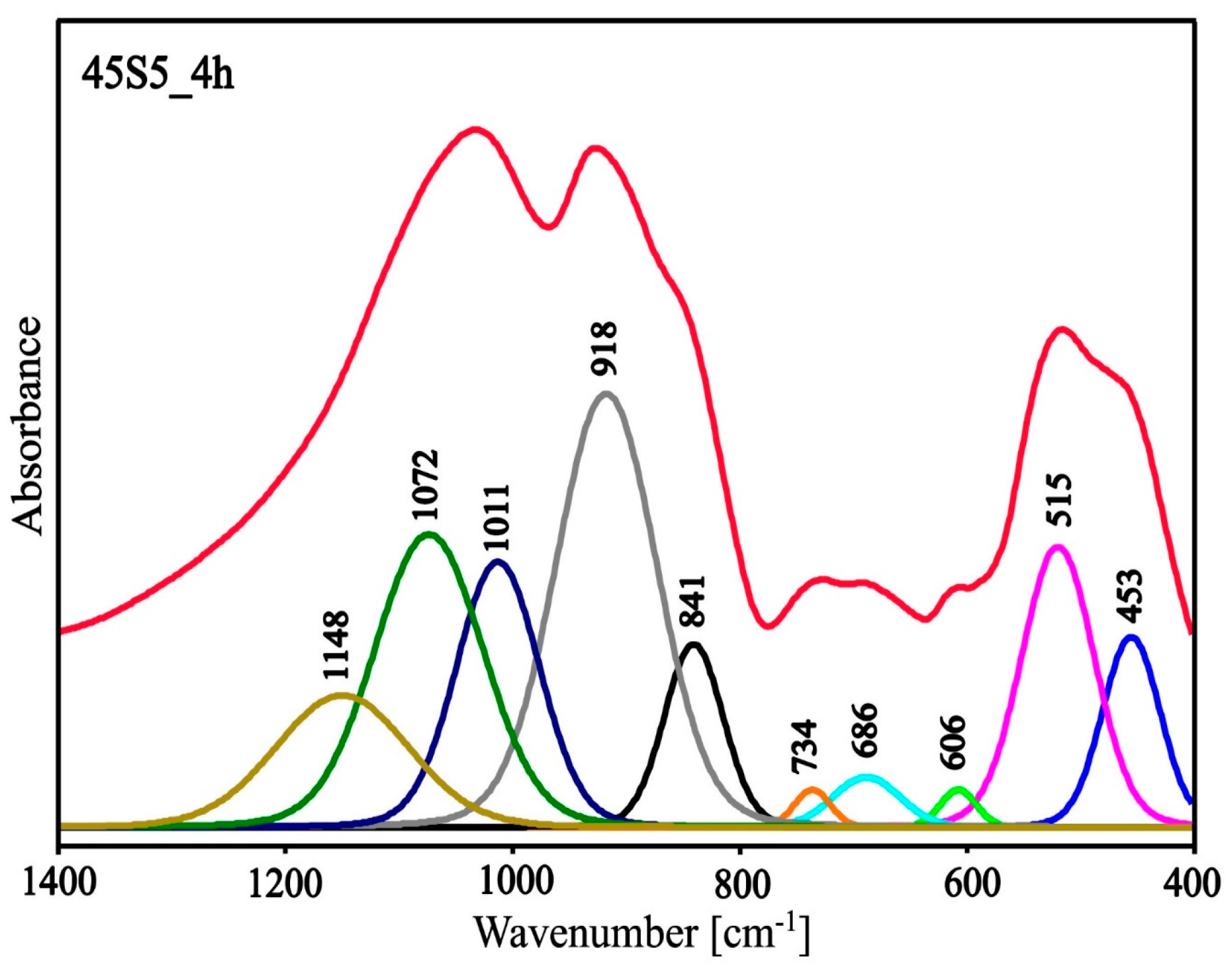
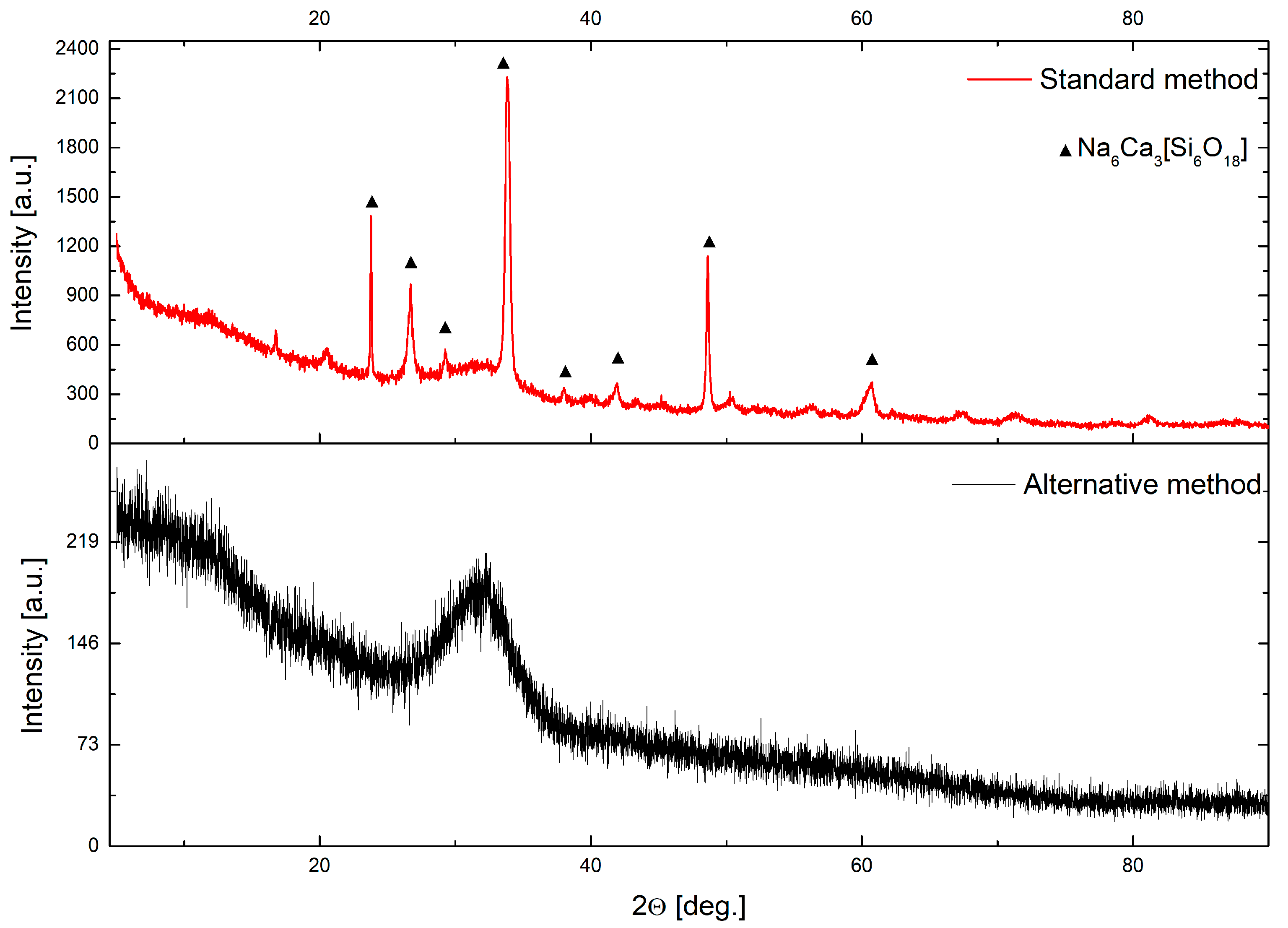
3.2. Structure Study
3.3. Bioactivity Test
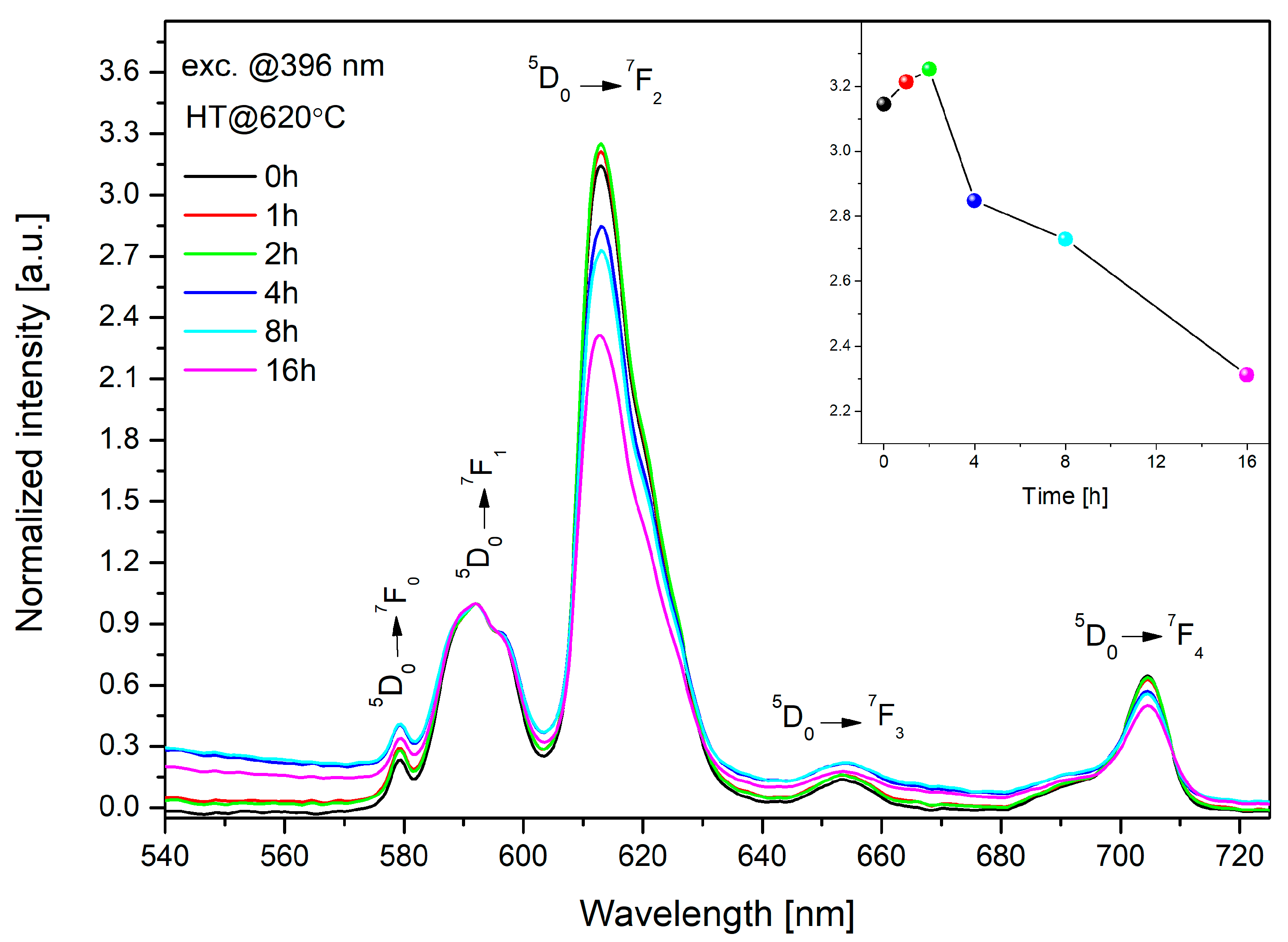
3.4. Luminescent Properties of Glass and Glass-Ceramics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hench, L.L.; Splinter, R.J.; Allen, W.; Greenlee, T. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. A 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Suominen, E.; Kinnunen, J. Bioactive glass granules and plates in the reconstruction of defects of the facial bones. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1996, 30, 281–289. [Google Scholar] [CrossRef]
- Durand, L.A.H.; Vargas, G.E.; Gomez-Gramajo, F.; Vera-Mesones, R.; Miguez-Pacheco, V.; Boccaccini, A.R.; Gorustovich, A. Lithium-Containing Bioactive Glasses for Bone Regeneration. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–217. [Google Scholar]
- Hench, L.L.; West, J.K. Biological Applications of Bioactive Glasses; Harwood Academic Publishers: Reading, UK, 1996. [Google Scholar]
- Goh, Y.F.; Alshemary, A.Z.; Akram, M.; Abdul Kadir, M.R.; Hussain, R. Bioactive glass: An in vitro comparative study of doping with nanoscale copper and silver particles. Int. J. Appl. Glass Sci. 2014, 5, 255–266. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Lin, C.-C.; Huang, L.-C.; Shen, P. Na2CaSi2O6–P2O5 based bioactive glasses. Part 1: Elasticity and structure. J. Non-Cryst. Solids 2005, 351, 3195–3203. [Google Scholar] [CrossRef]
- Lefebvre, L.; Chevalier, J.; Gremillard, L.; Zenati, R.; Thollet, G.; Bernache-Assolant, D.; Govin, A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007, 55, 3305–3313. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Roman, J.; Padilla, S.; Doadrio, J.; Gil, F. Bioactivity and mechanical properties of SiO 2–CaO–P 2 O 5 glass-ceramics. J. Mater. Chem. 2005, 15, 1353–1359. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Filho, O.P.; La Torre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Ylänen, H. Bioactive glasses and glass ceramics. In Wiley Encyclopedia of Biomedical Engineering; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. Ceramics as bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–178. [Google Scholar]
- Żur, L.; Janek, J.; Sołtys, M.; Goryczka, T.; Pisarska, J.; Pisarski, W.A. Structural and optical investigations of rare earth doped lead-free germanate glasses modified by MO and MF2 (M = Ca, Sr, Ba). J. Non-Cryst. Solids 2016, 431, 145–149. [Google Scholar] [CrossRef]
- Baranowska, A.; Dąbrowski, J.R.; Kochanowicz, M.; Dorosz, J. Effect of biodegradation on spectroscopic properties of Sm3+ doped 45S5 bioglass. Proc. SPIE 2018, 10808, 1080833. [Google Scholar] [CrossRef]
- Massera, J.; Ahmed, I.; Petit, L.; Aallos, V.; Hupa, L. Phosphate-based glass fiber vs. bulk glass: Change in fiber optical response to probe in vitro glass reactivity. Mater. Sci. Eng. C 2014, 37, 251–257. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Ojha, N.; Pugliese, D.; Mishra, A.; Gumenyuk, R.; Boetti, N.G.; Janner, D.; Troles, J.; Bureau, B.; Boussard-Plédel, C. Design, processing and characterization of an optical core bioactive clad phosphate fiber for biomedical applications. J. Am. Ceram. Soc. 2019, 102, 6882–6892. [Google Scholar] [CrossRef]
- Clupper, D.C.; Gough, J.E.; Embanga, P.M.; Notingher, I.; Hench, L.L.; Hall, M.M. Bioactive evaluation of 45S5 bioactive glass fibres and preliminary study of human osteoblast attachment. J. Mater. Sci. Mater. Med. 2004, 15, 803–808. [Google Scholar] [CrossRef]
- De Diego, M.A.; Coleman, N.J.; Hench, L.L. Tensile properties of bioactive fibers for tissue engineering applications. J. Biomed. Mater. Res. 2000, 53, 199–203. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Preparation and in vitro characterization of electrospun 45S5 bioactive glass nanofibers. Ceram. Int. 2015, 41, 417–425. [Google Scholar] [CrossRef]
- Asgharnia, S.; Alizadeh, P. Synthesis and characterization of SiO2–CaO–P2O5–MgO based bioactive glass and glass-ceramic nanofibres by electrospinning. Mater. Lett. 2013, 101, 107–110. [Google Scholar] [CrossRef]
- Charvet, J.L.; Cordes, J.A.; Alexander, H. Mechanical and fracture behavior of a fiber-reinforced bioabsorbable material for orthopaedic applications. J. Mater. Sci. Mater. Med. 2000, 11, 101–109. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer formula for X-ray particle size determination. PhRv 1939, 56, 978. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Guérineau, T.; Strutynski, C.; Skopak, T.; Morency, S.; Hanafi, A.; Calzavara, F.; Ledemi, Y.; Danto, S.; Cardinal, T.; Messaddeq, Y.; et al. Extended germano-gallate fiber drawing domain: From germanates to gallates optical fibers. Opt. Mater. Express 2019, 9, 2437–2445. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Chen, Q.; Lefebvre, L.; Gremillard, L.; Chevalier, J. Sintering, crystallisation and biodegradation behaviour of Bioglass®-derived glass–ceramics. Faraday Discuss. 2007, 136, 27–44. [Google Scholar] [CrossRef]
- Clupper, D.; Hench, L. Crystallization kinetics of tape cast bioactive glass 45S5. J. Non-Cryst. Solids 2003, 318, 43–48. [Google Scholar] [CrossRef]
- Massera, J.; Fagerlund, S.; Hupa, L.; Hupa, M. Crystallization mechanism of the bioactive glasses, 45S5 and S53P4. J. Am. Ceram. Soc. 2012, 95, 607–613. [Google Scholar] [CrossRef]
- Friedman, H. Simulation models of analyze by using isoconversional methods. J. Polym. Sci. (Part C) Polym. Symp. (6PC) 1964, 183, 25–32. [Google Scholar]
- Augis, J.; Bennett, J. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J. Therm. Anal. Calorim. 1978, 13, 283–292. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Sola, A. An overview of the effects of thermal processing on bioactive glasses. Sci. Sinter. 2010, 42, 307–320. [Google Scholar] [CrossRef]
- Cannillo, V.; Chiellini, F.; Fabbri, P.; Sola, A. Production of Bioglass® 45S5 – Polycaprolactone composite scaffolds via salt-leaching. Compos. Struct. 2010, 92, 1823–1832. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W.; Galuskin, E.; Galuskina, I. The non-ring cations influence on silicooxygen ring vibrations. J. Mol. Struct. 2000, 555, 357–362. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Mozgawa, W. Calculations of silicooxygen ring vibration frequencies. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 1999, 55, 2831–2837. [Google Scholar] [CrossRef]
- Sitarz, M.; Mozgawa, W.; Handke, M. Vibrational spectra of complex ring silicate anions—Method of recognition. J. Mol. Struct. 1997, 404, 193–197. [Google Scholar] [CrossRef]
- Kuczek, J.; Jeleń, P.; Stoch, P.; Błachowski, A.; Wacławska, I.; Szumera, M. Raman and Mössbauer studies of iron phosphate-silicate glasses. J. Mol. Struct. 2018, 1170, 82–89. [Google Scholar] [CrossRef]
- Szumera, M. Structural investigations of silicate–phosphate glasses containing MoO3 by FTIR, Raman and 31P MAS NMR spectroscopies. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 2014, 130, 1–6. [Google Scholar] [CrossRef]
- Sitarz, M.; Handke, M.; Fojud, Z.; Jurga, S. Spectroscopic studies of glassy phospho-silicate materials. J. Mol. Struct. 2005, 744, 621–626. [Google Scholar] [CrossRef]
- Sitarz, M.; Rokita, M.; Handke, M.; Galuskin, E. Structural studies of the NaCaPO4–SiO2 sol–gel derived materials. J. Mol. Struct. 2003, 651, 489–498. [Google Scholar] [CrossRef]
- Szumera, M.; Wacławska, I.; Mozgawa, W.; Sitarz, M. Spectroscopic study of biologically active glasses. J. Mol. Struct. 2005, 744, 609–614. [Google Scholar] [CrossRef]
- Sitarz, M. Influence of modifying cations on the structure and texture of silicate–phosphate glasses. J. Mol. Struct. 2008, 887, 237–248. [Google Scholar] [CrossRef]
- Hench, L.L.; Clark, D.E. Physical chemistry of glass surfaces. J. Non-Cryst. Solids 1978, 28, 83–105. [Google Scholar] [CrossRef]
- Montinaro, S.; Desogus, L.; Orrù, R.; Garroni, S.; Delogu, F.; Ricci, P.C.; Cao, G. A comprehensive study on compositional and structural changes in 45S5 bioglass products exposed to simulated body fluid. J. Am. Ceram. Soc. 2018, 101, 116–130. [Google Scholar] [CrossRef]
- Zhao, D.; Qiao, X.; Fan, X.; Wang, M. Local vibration around rare earth ions in SiO2–PbF2 glass and glass ceramics using Eu3+ probe. Phys. B Condens. Matter 2007, 395, 10–15. [Google Scholar] [CrossRef]
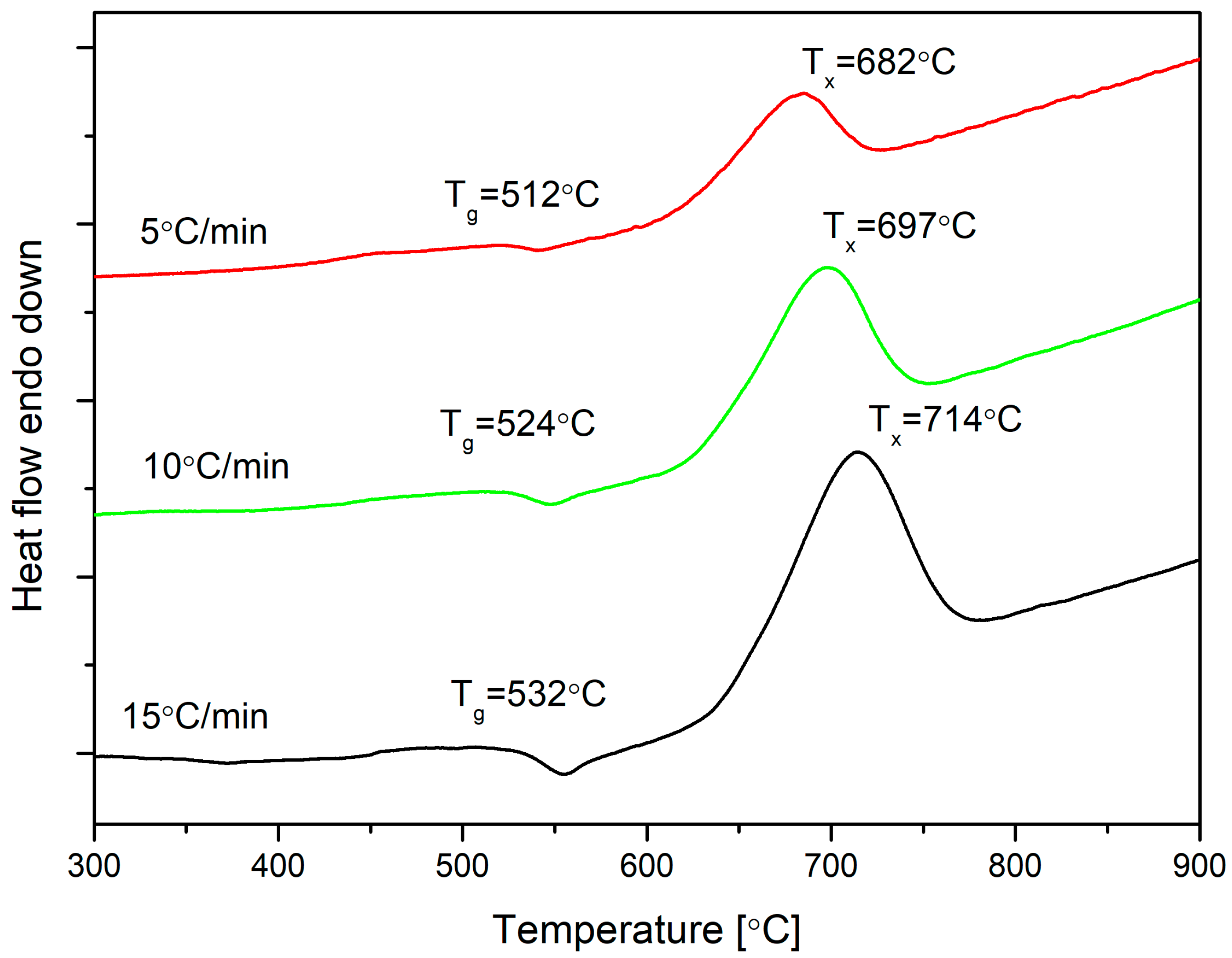
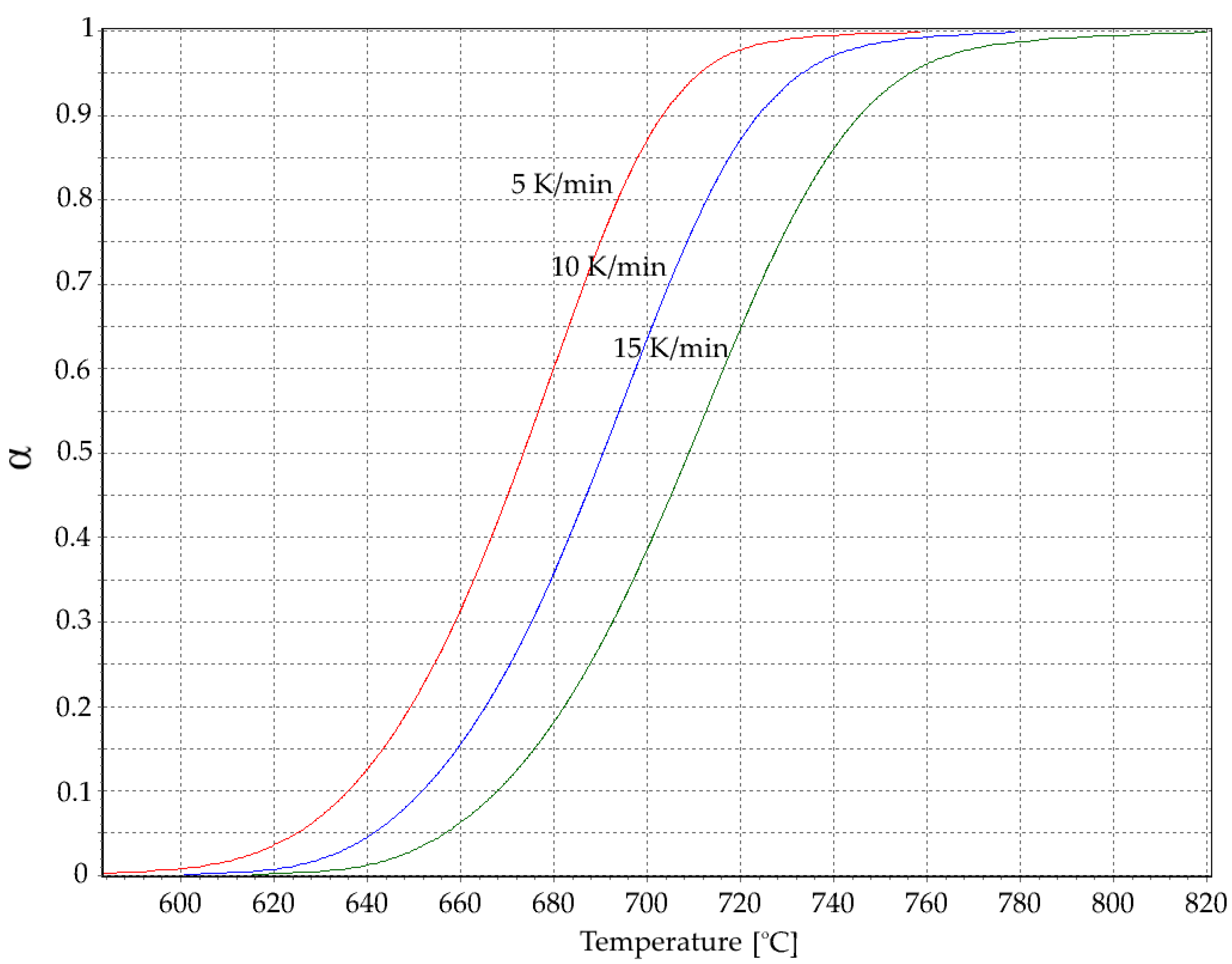
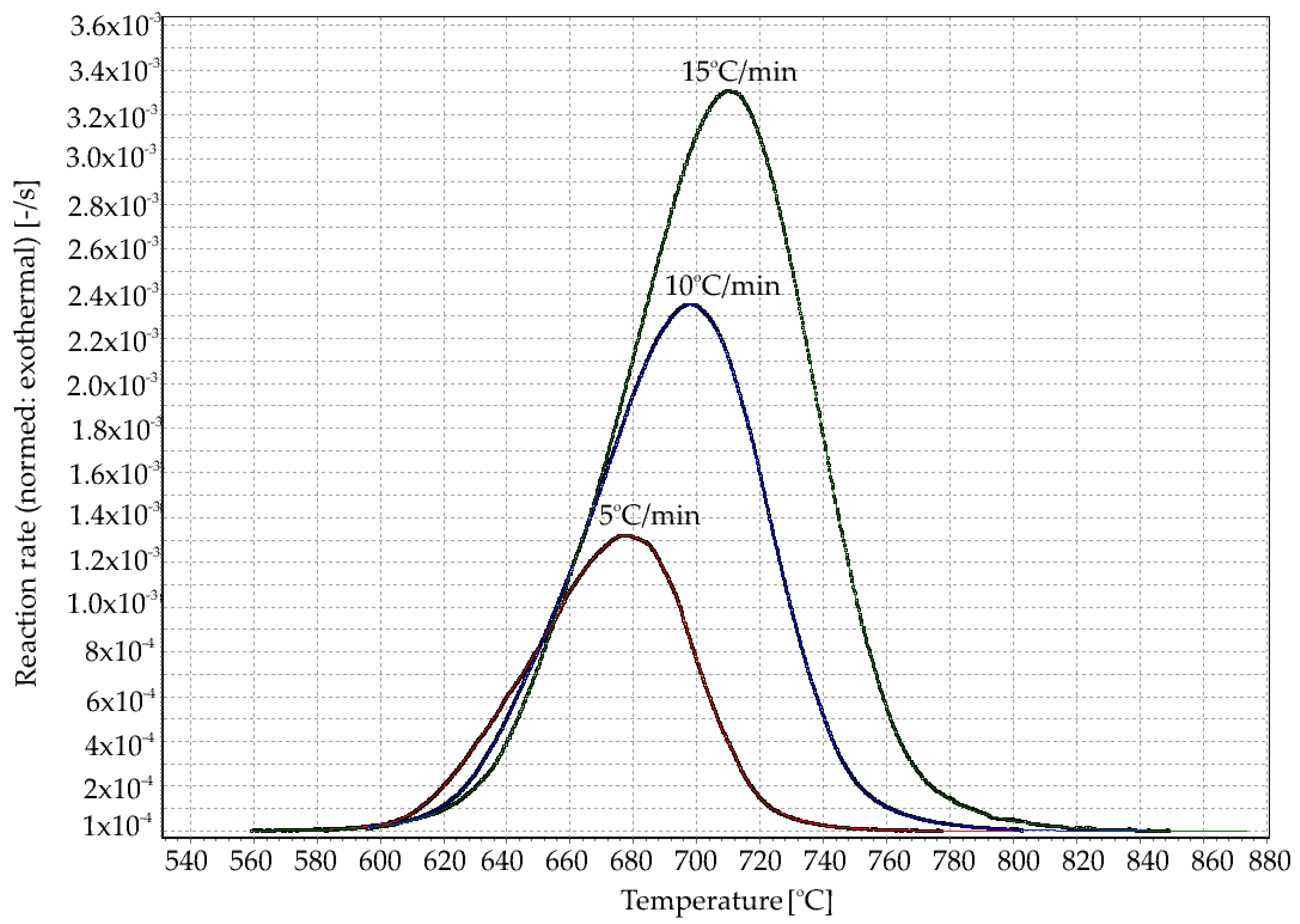
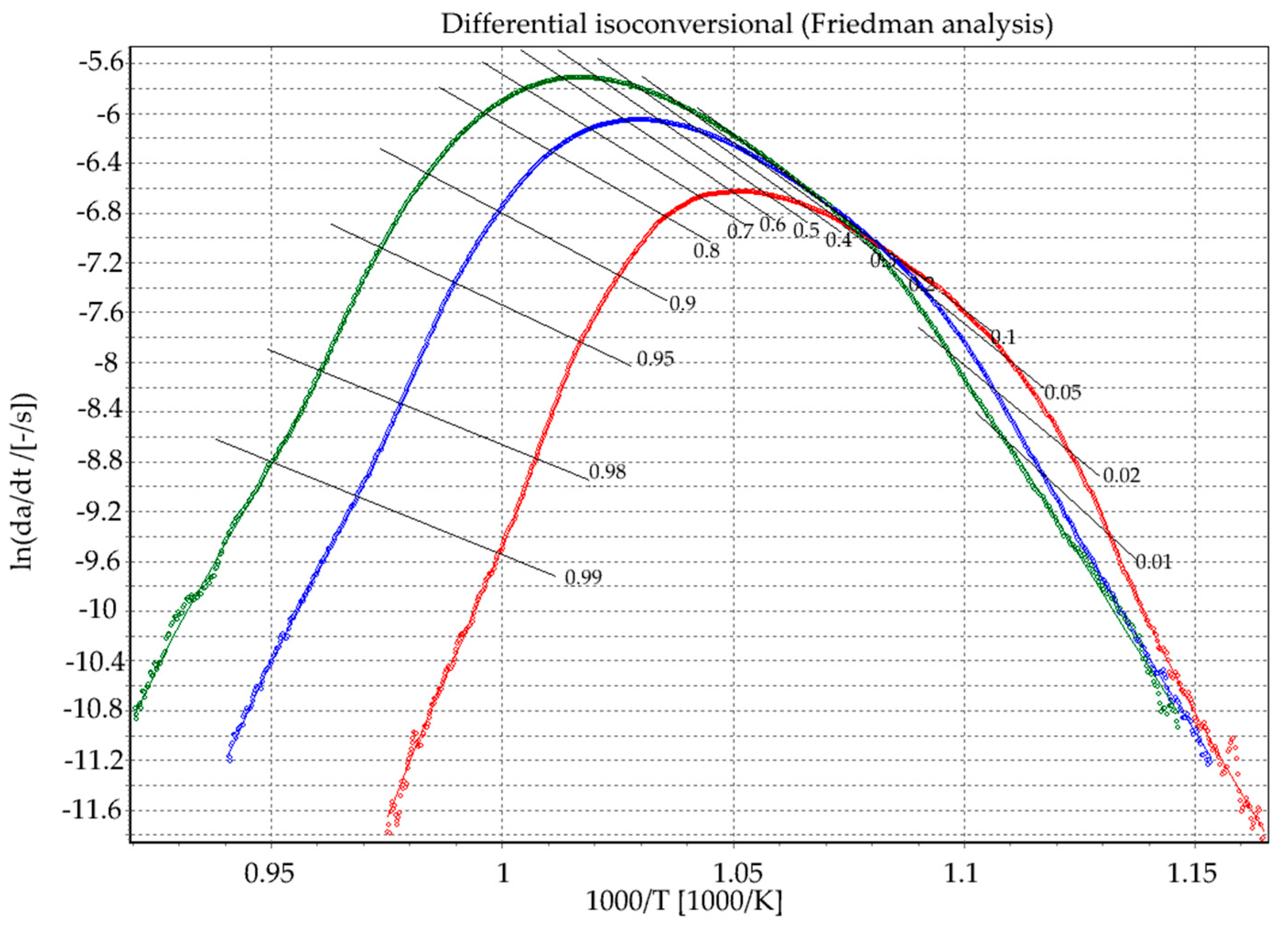
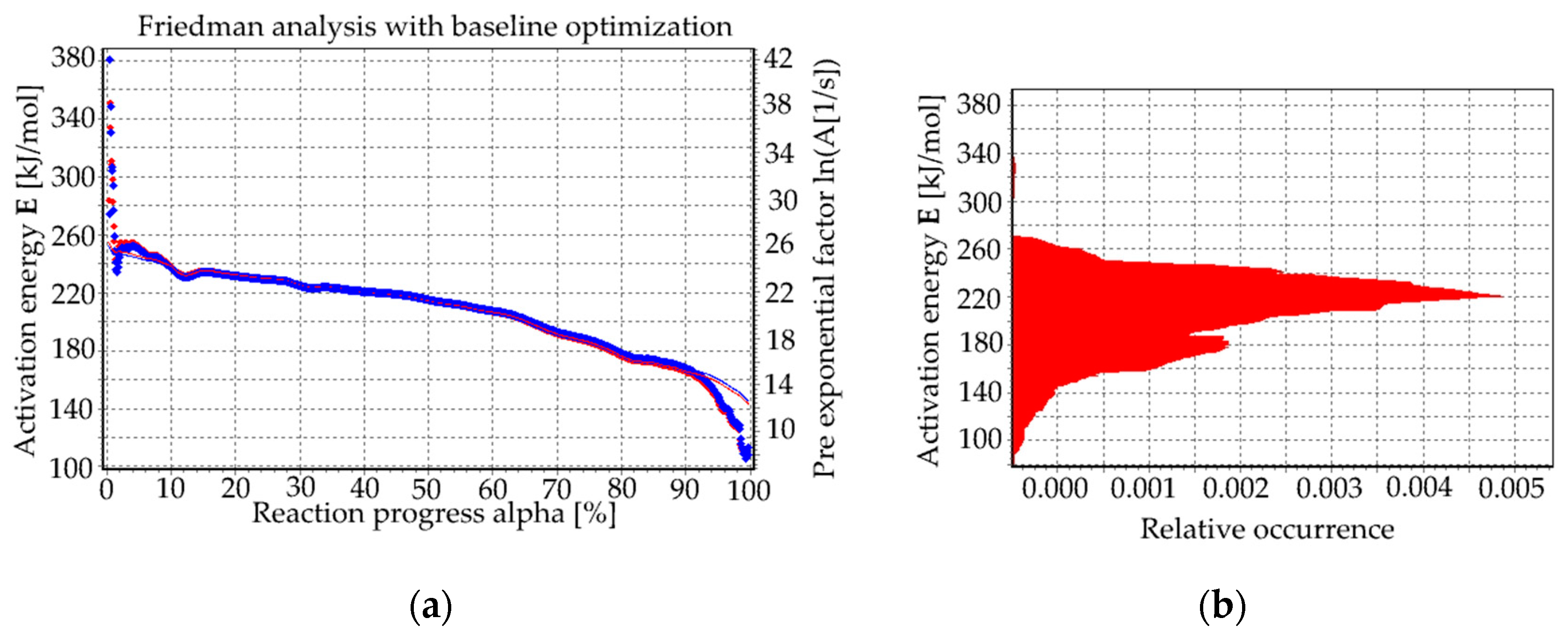
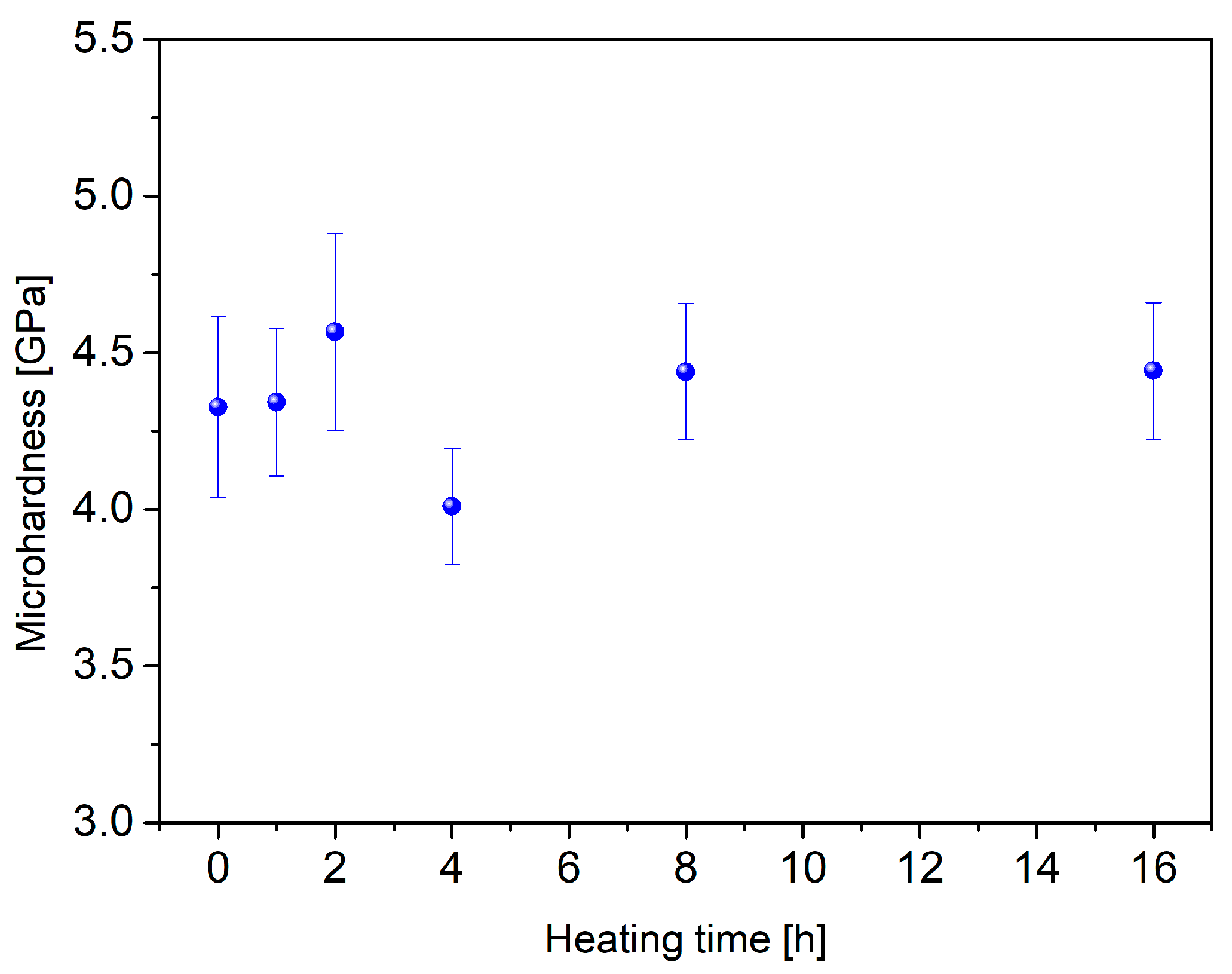
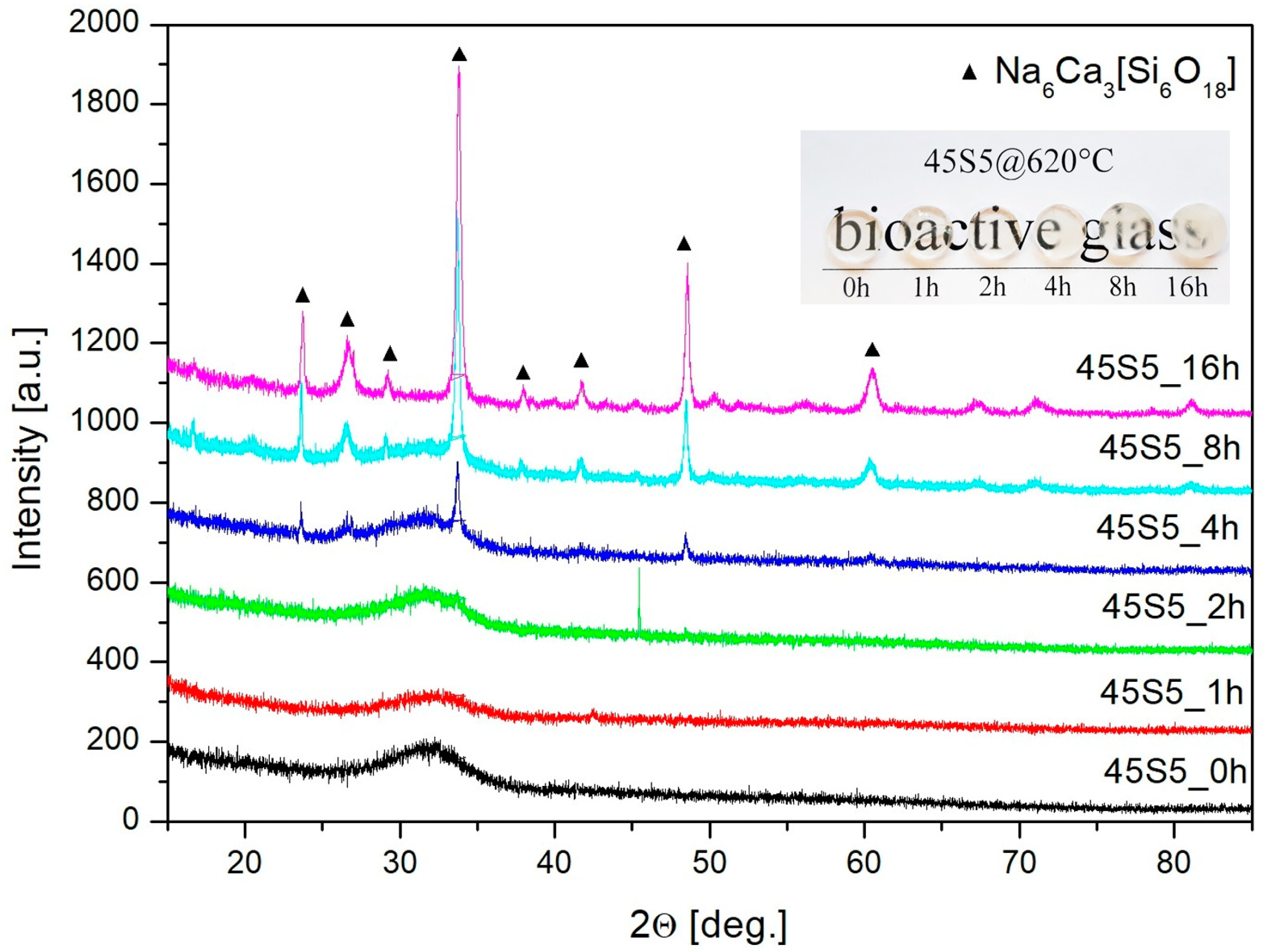
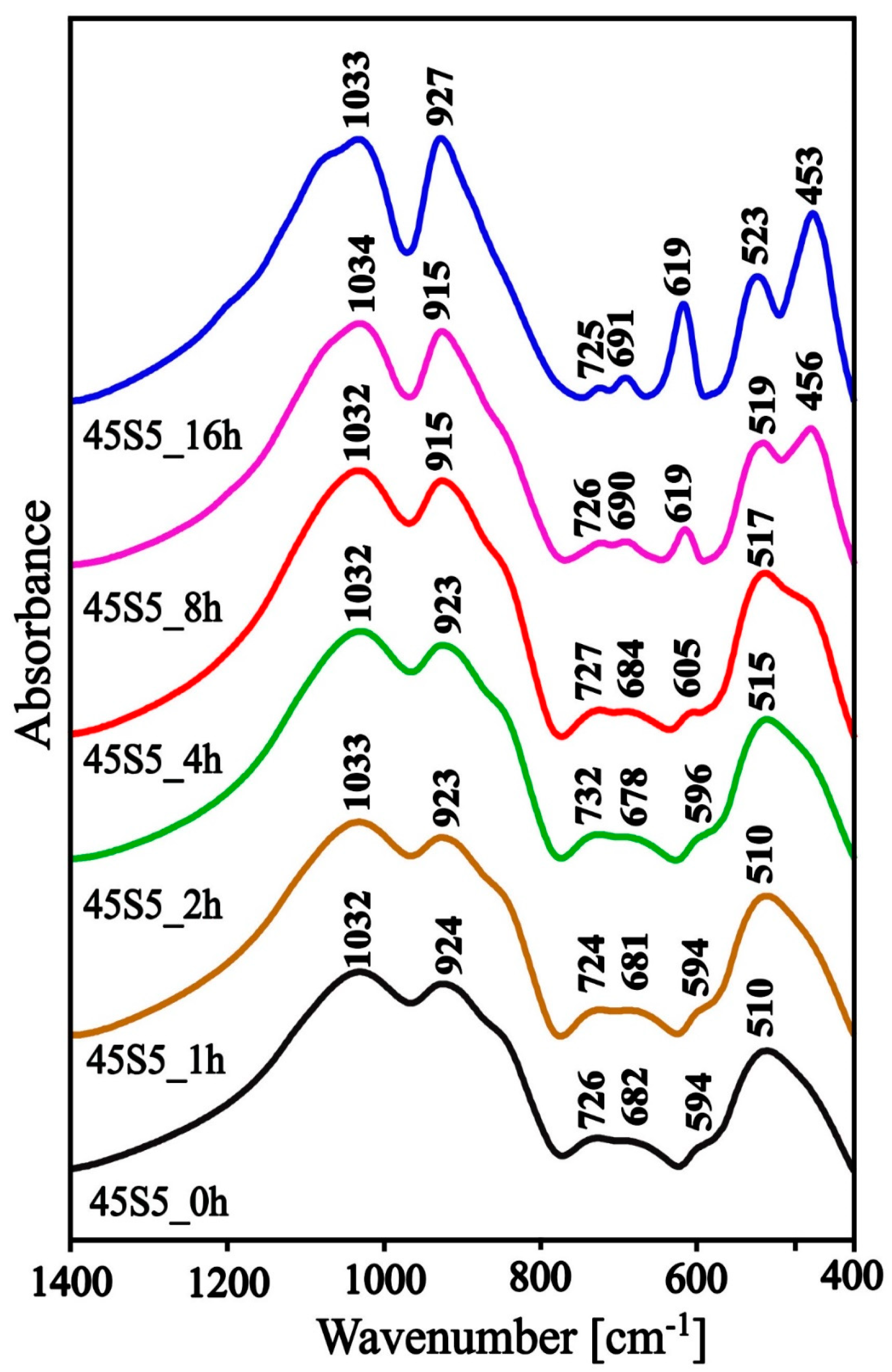


| Rate β (°C/min) | Tg (°C) | Tx (°C) | ∆T (°C) |
|---|---|---|---|
| 5 | 521 | 682 | 161 |
| 10 | 524 | 697 | 173 |
| 15 | 532 | 714 | 182 |
| β °C/min | 5 | 10 | 15 | Avg. | SD |
|---|---|---|---|---|---|
| n | 1.53 | 1.42 | 1.34 | 1.43 | 0.09 |
| Center (cm−1) | Assignment Vibration |
|---|---|
| 1150 | asymmetric stretching vibrations of the Si=O and P=O |
| 1100–1000 | asymmetric stretching vibrations of the asymmetric Si-O-Si |
| 1000–1050 | asymmetric stretching vibrations of the asymmetric Si-O(P) |
| 1040–840 | asymmetric stretching vibrations of the asymmetric Si-O- and P-O- |
| 800–730 | symmetric stretching vibrations of the Si-O(Si) and Si-O(P) bridging bonds |
| 720–590 | silico‒oxygen rings of different numbers of ring members |
| 550–400 | bending vibrations of the O-P-O and O-Si-O bridges |
| Center (cm−1) | Area | Height | FWHM | Assignment Vibration |
|---|---|---|---|---|
| 450 | 13.88 | 0.19 | 64.31 | bending vibrations of O-Si-O and O-P-O |
| 515 | 43.36 | 0.43 | 89.47 | |
| 596 | 1.68 | 0.05 | 27.72 | 6-membered silico‒oxygen ring vibrations |
| 678 | 9.26 | 0.11 | 72.61 | 3- and 4-membered silico‒oxygen rings vibrations |
| 735 | 6.16 | 0.10 | 53.29 | symmetric stretching vibrations of the Si-O(Si) and Si-O(P) |
| 839 | 27.78 | 0.33 | 75.05 | asymmetric stretching vibrations of the Si-O- and P-O- |
| 915 | 65.73 | 0.59 | 98.84 | |
| 1004 | 30.12 | 0.29 | 92.50 | asymmetric stretching Si-O(P) vibrations |
| 1059 | 61.78 | 0.40 | 135.41 | asymmetric stretching Si-O(Si) vibrations |
| 1139 | 53.45 | 0.23 | 200.13 | asymmetric stretching Si=O and P=O vibrations |
| Center (cm−1) | Area | Height | FWHM | Assignment Vibration |
|---|---|---|---|---|
| 453 | 52.29 | 0.77 | 60.34 | bending vibrations of O-Si-O and O-P-O |
| 515 | 96.79 | 1.14 | 76.07 | |
| 606 | 6.45 | 0.15 | 37.60 | 6-membered silico‒oxygen ring vibrations |
| 686 | 15.97 | 0.20 | 69.58 | 3- and 4-membered silico‒oxygen rings vibrations |
| 734 | 6.43 | 0.15 | 37.48 | symmetric stretching vibrations of the Si-O(Si) and Si-O(P) |
| 839 | 51.16 | 0.74 | 61.42 | asymmetric stretching vibrations of the Si-O- and P-O- |
| 915 | 207.15 | 1.76 | 105.34 | |
| 1011 | 104.16 | 1.08 | 86.50 | asymmetric stretching Si-O(P) vibrations |
| 1072 | 150.53 | 1.19 | 113.34 | asymmetric stretching Si-O(Si) vibrations |
| 1148 | 83.37 | 0.53 | 138.89 | asymmetric stretching Si=O and P=O vibrations |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska, A.; Leśniak, M.; Kochanowicz, M.; Żmojda, J.; Miluski, P.; Dorosz, D. Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials 2020, 13, 1281. https://doi.org/10.3390/ma13061281
Baranowska A, Leśniak M, Kochanowicz M, Żmojda J, Miluski P, Dorosz D. Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials. 2020; 13(6):1281. https://doi.org/10.3390/ma13061281
Chicago/Turabian StyleBaranowska, Agata, Magdalena Leśniak, Marcin Kochanowicz, Jacek Żmojda, Piotr Miluski, and Dominik Dorosz. 2020. "Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+" Materials 13, no. 6: 1281. https://doi.org/10.3390/ma13061281
APA StyleBaranowska, A., Leśniak, M., Kochanowicz, M., Żmojda, J., Miluski, P., & Dorosz, D. (2020). Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials, 13(6), 1281. https://doi.org/10.3390/ma13061281





