Optimal Hot-Dipped Tinning Process Routine for the Fabrication of Solderable Sn Coatings on Circuit Lead Frames
Abstract
1. Introduction
2. Hot-Dipped Tinning Experiments for Copper Foils
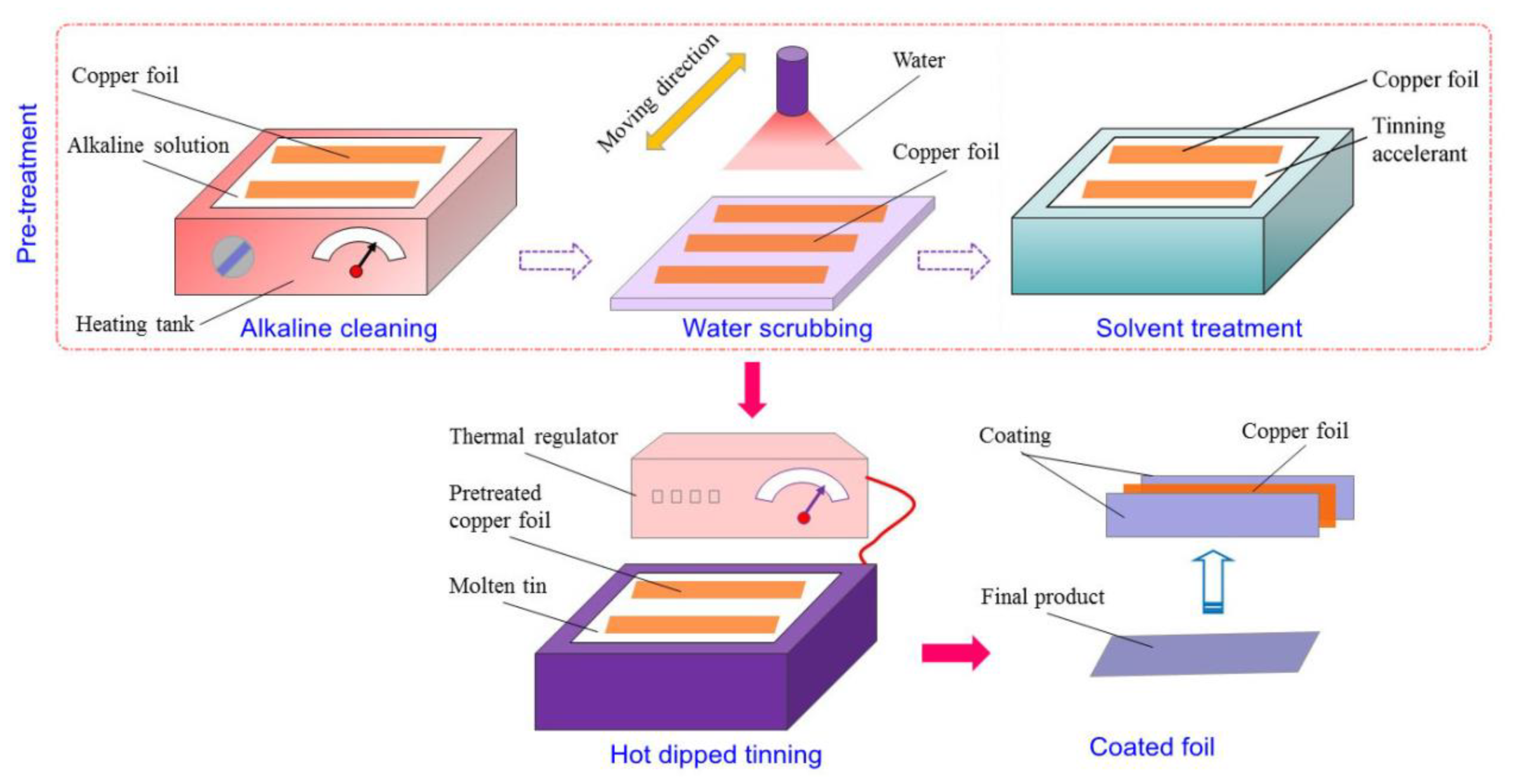
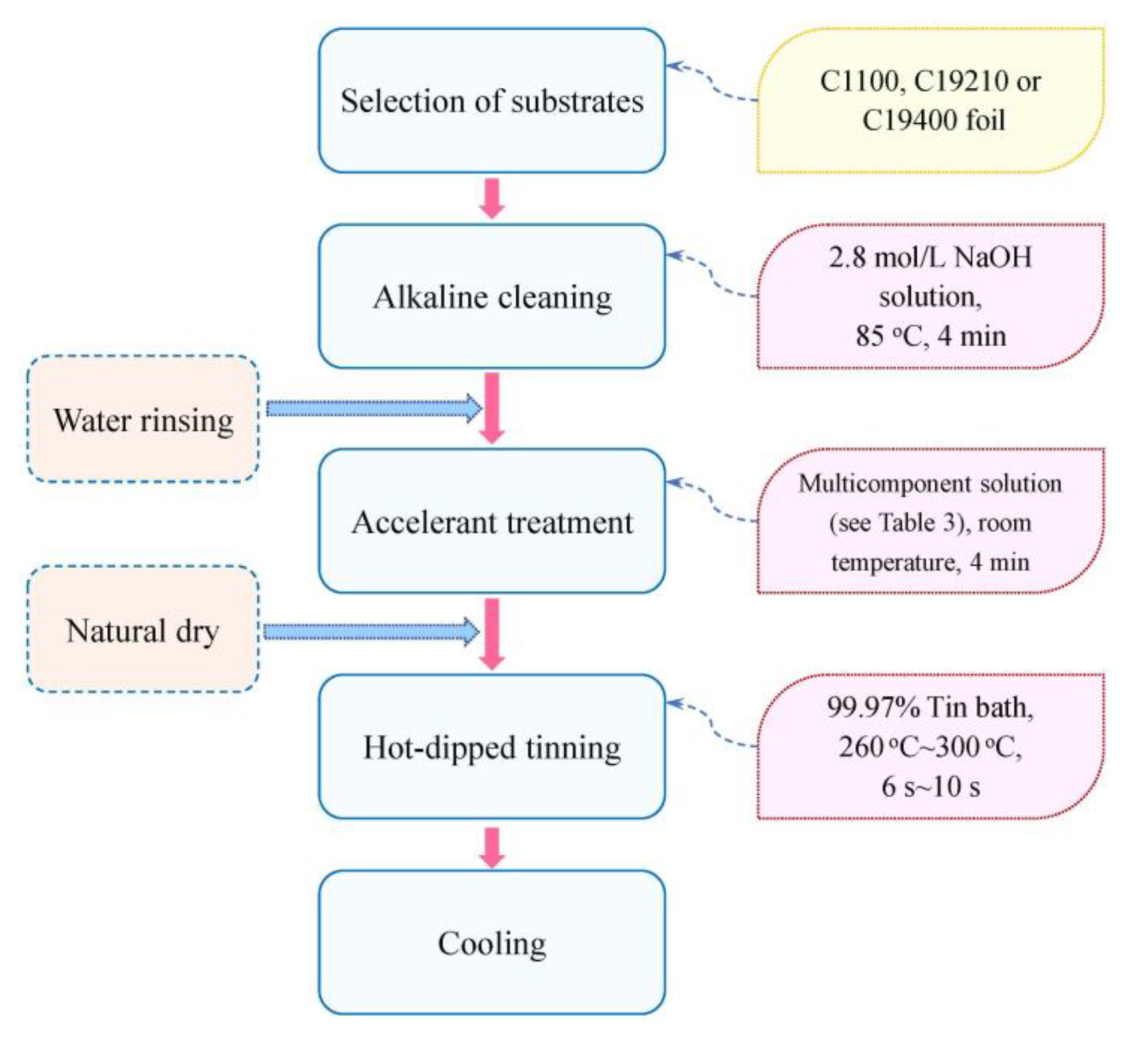
2.1. Introduction to Hot-Dipped Tinning Process
2.2. Materials and Characterization
2.3. Schemes of Pretreatment Experiments
2.4. Schemes of Hot-Dipped Tinning Experiments
3. Results and Discussion
3.1. Optimization of Pretreatment Parameters
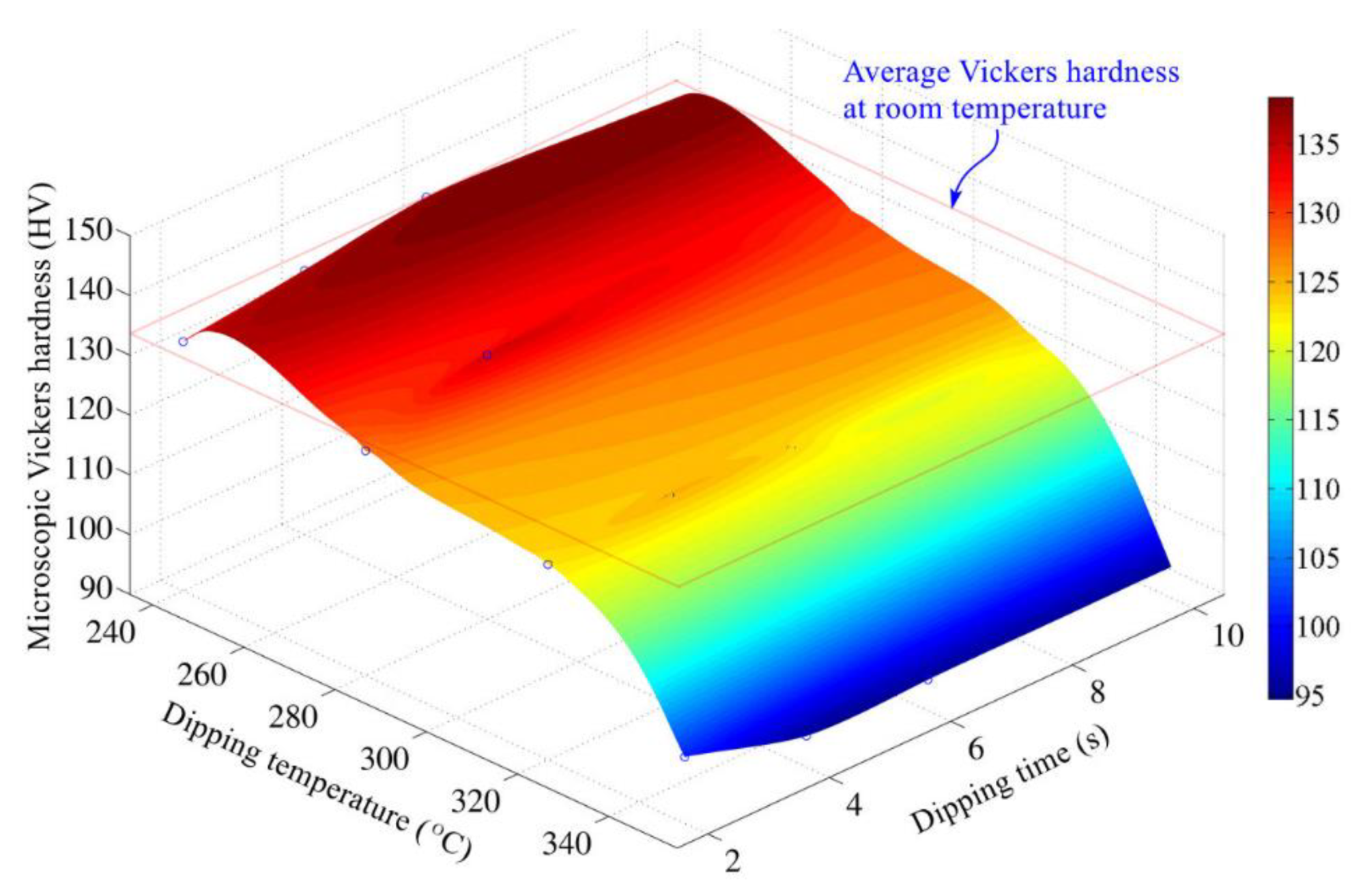
3.2. Effects of Hot Dipping Parameters on Microhardness of Cu Substrate
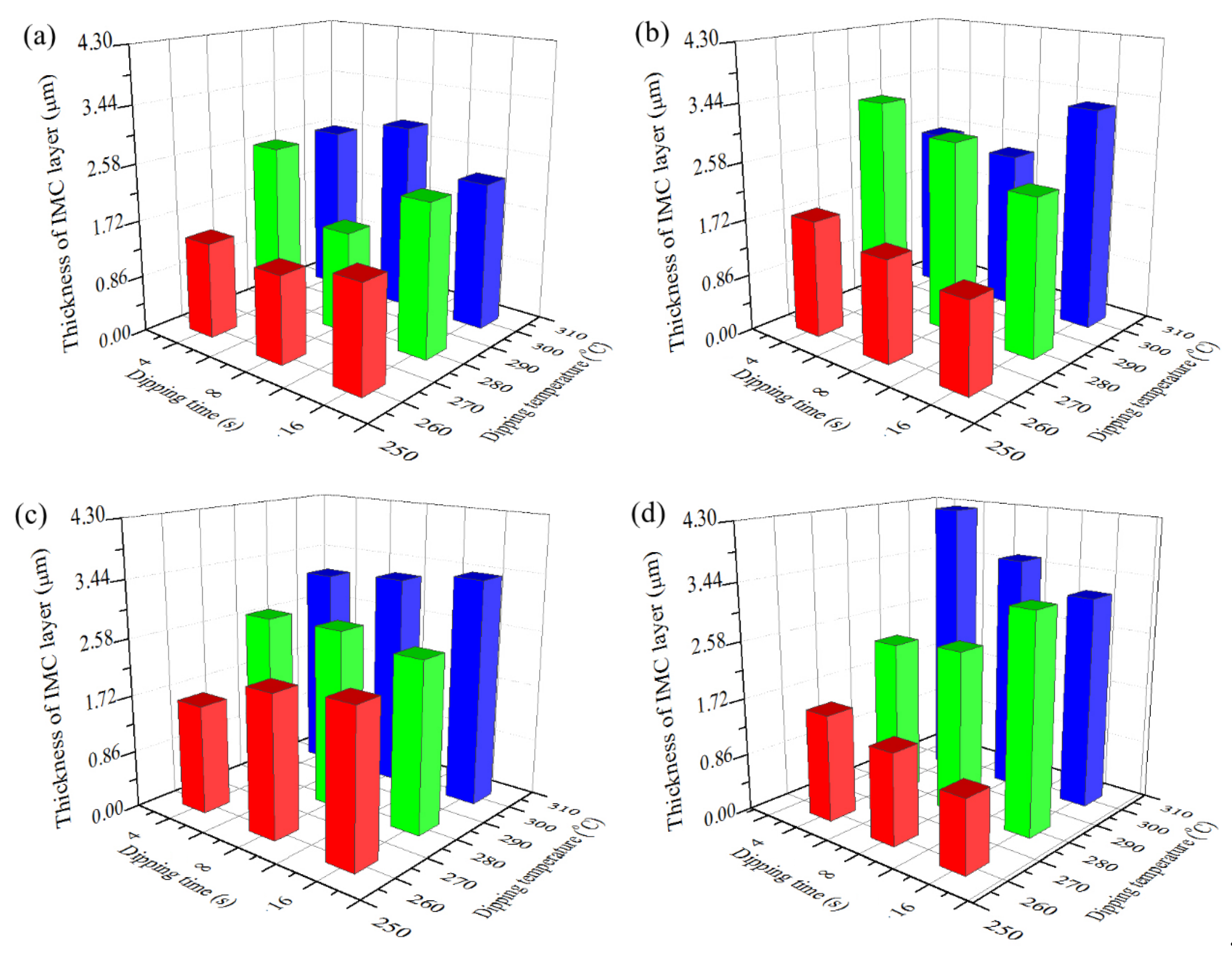
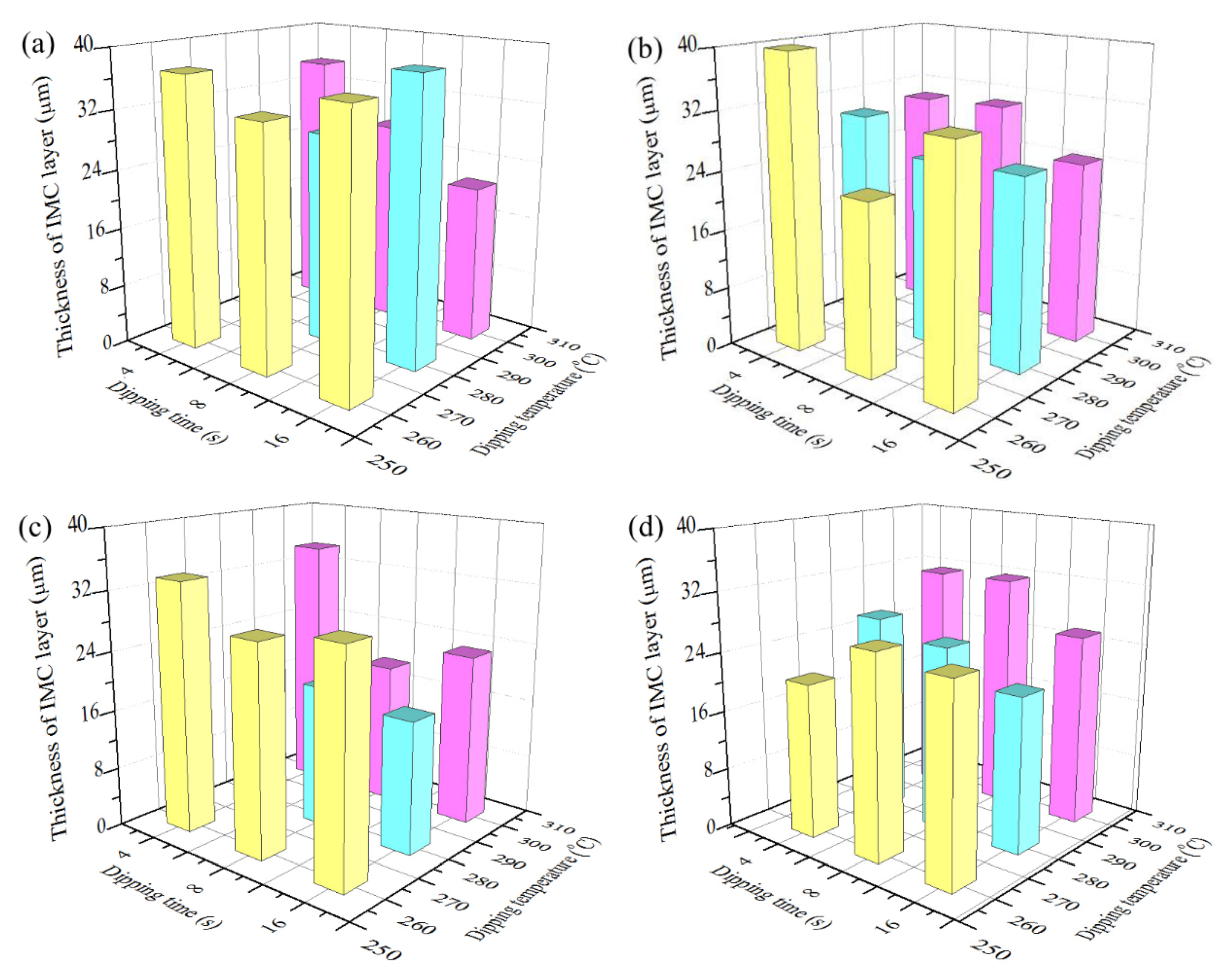
3.3. Variation of Coating Thickness and IMC Layer Thickness with Hot Dipping Parameters
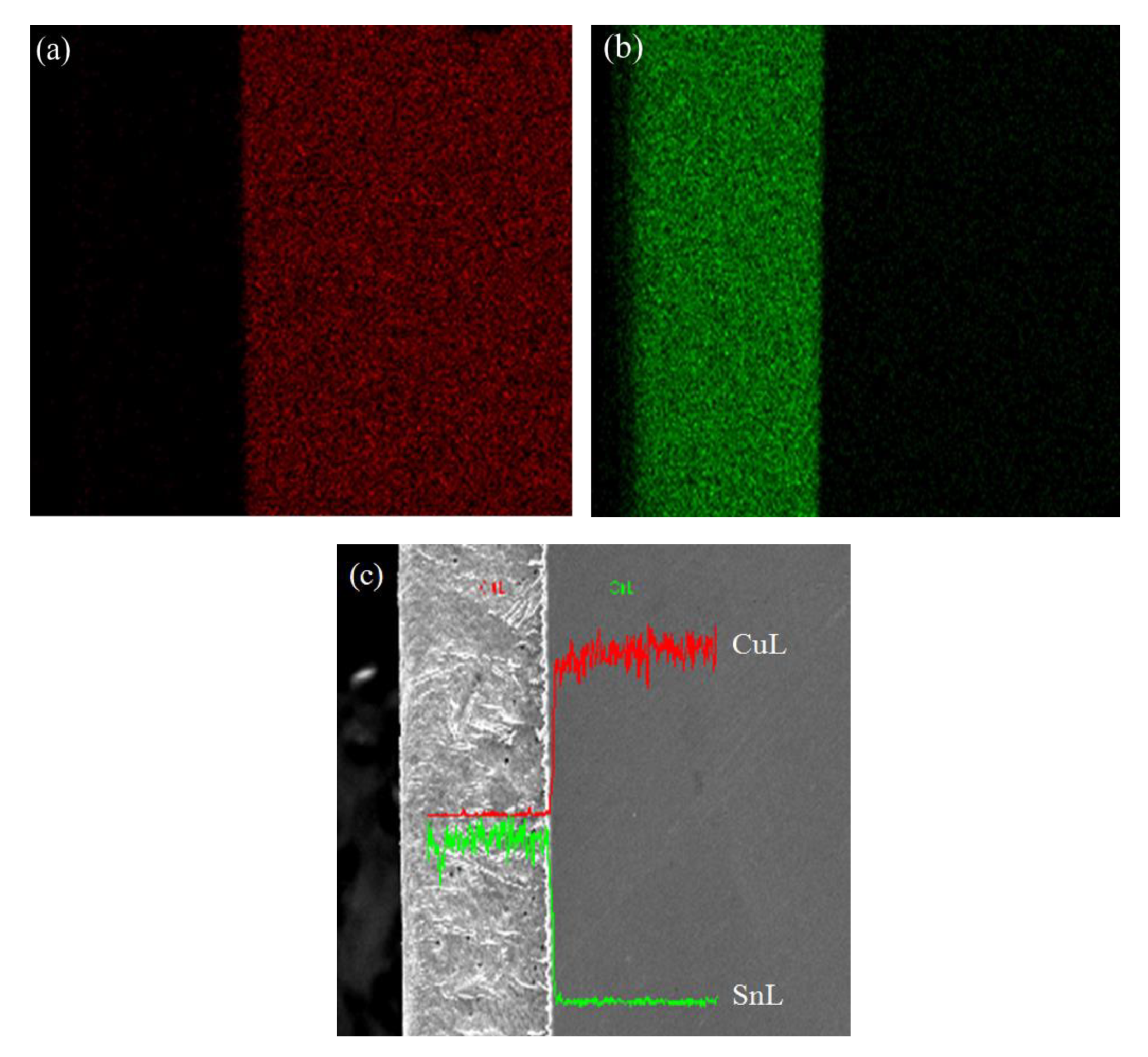
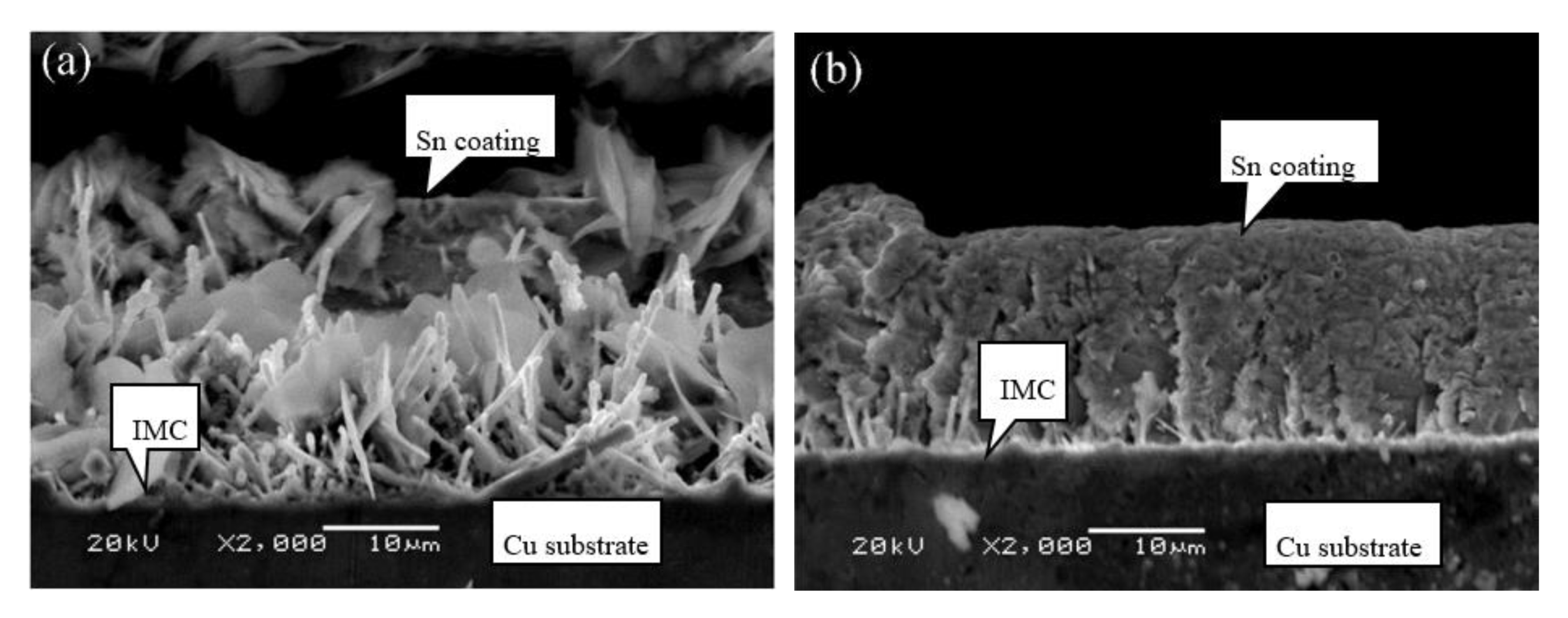
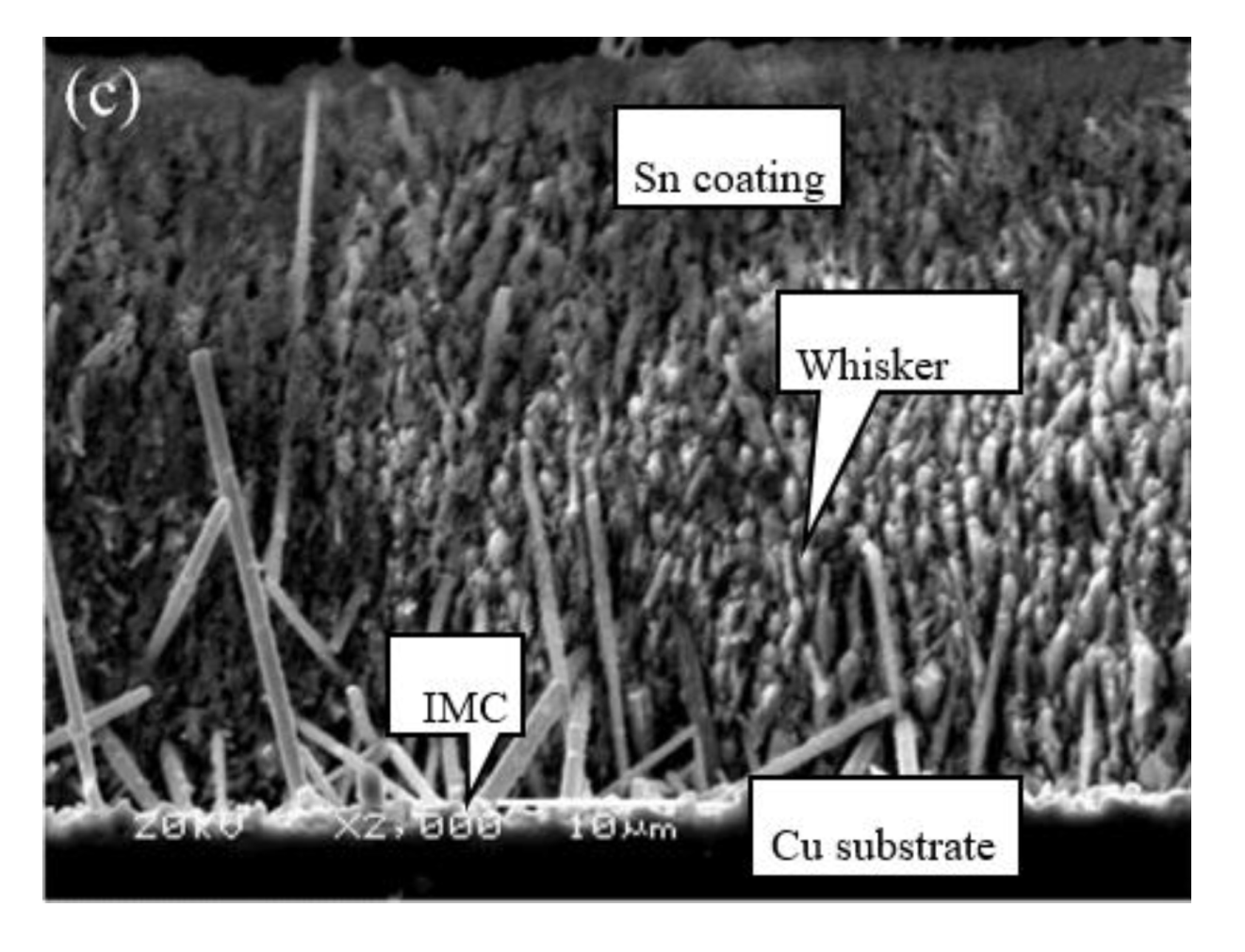
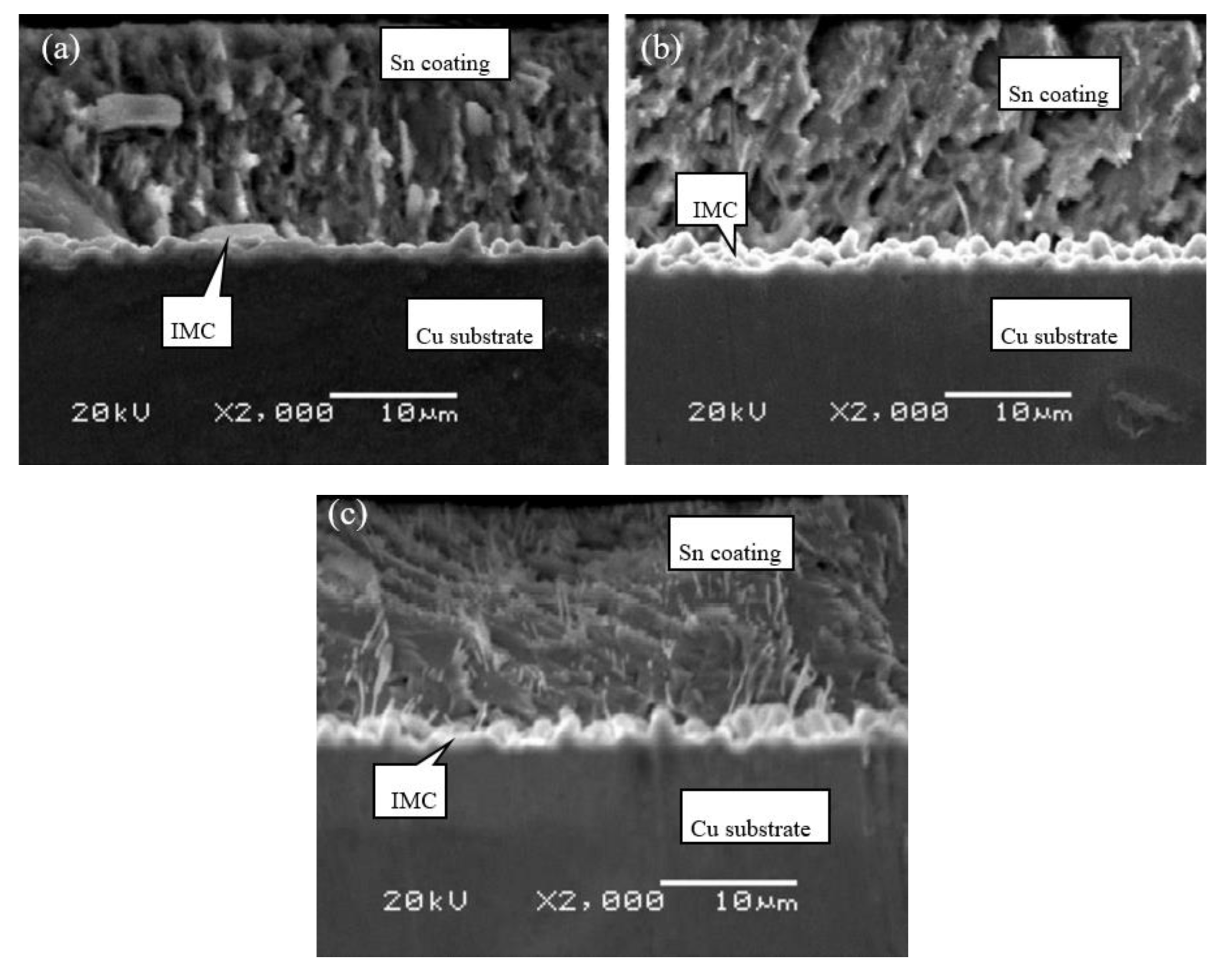
3.4. Coating Layer Structure of Different Cu Foils
3.5. Optimal Hot-Dipped Tinning Process Routine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manti, P.; Watkinson, D. Hot tinning of low-tin bronzes. In Metal 2010, Proceedings of the Interim Meeting of the ICOM-CC Metal Working Group, Charleston, SC, USA, 11–15 October 2010; Mardikian, P., Chemello, C., Watters, C., Hull, P., Eds.; Clemson University: Clemson, SC, USA; pp. 92–98.
- Zhang, Z.G.; Wang, Y.J.; Xiao, L.J.; Zhang, L.Q.; Su, Y.; Lin, J.S. High-temperature oxidation of hot-dipped aluminizing coatings on a Ti-3Al-Nb alloy and the effects of element additions. Corros. Sci. 2012, 64, 137–144. [Google Scholar] [CrossRef]
- Cao, R.; Chang, J.H.; Huang, Q.; Zhang, X.B.; Yan, Y.J.; Chen, J.H. Behaviors and effects of Zn coating on welding-brazing process of Al-steel and Mg-steel dissimilar metals. J. Manuf. Process. 2018, 31, 674–688. [Google Scholar] [CrossRef]
- Spitz, M.; Fleischanderl, M.; Sierlinger, R.; Reischauer, M.; Perndorfer, F.; Fafilek, G. Surface lubrication influence on electrode degradation during resistance spot welding of hot dip galvanized steel sheets. J. Mater. Process. Technol. 2015, 216, 339–347. [Google Scholar] [CrossRef]
- Diao, H.; Wang, C.Q.; Wang, L. Bonding of aluminum alloy by hot-dipped tin coating. Adv. Mater. Res. 2008, 32, 93–97. [Google Scholar] [CrossRef]
- Lee, Y.G.; Duh, J.G. Phase analysis in the solder joint of Sn-Cu solder/IMCs/Cu substrate. Mater. Charact. 1999, 42, 143–160. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, M.Y.; Liu, Z.Q.; Yang, S.H. Growth characteristics and formation mechanisms of Cu6Sn5, phase at the liquid-Sn 0.7 Cu/(111) Cu, and liquid-Sn 0.7 Cu/(001) Cu, joint interfaces. Acta Mater. 2016, 104, 1–8. [Google Scholar] [CrossRef]
- Lee, T.Y.; Choi, W.J.; Tu, K.N.; Jang, J.W.; Kuo, S.M.; Lin, J.K. Morphology, kinetics, and thermodynamics of solid-state aging of eutectic SnPb and Pb-free solders (Sn-3.5Ag, Sn-3.8Ag-0.7Cu and Sn-0.7Cu) on Cu. J. Mater. Res. 2002, 17, 291–301. [Google Scholar] [CrossRef]
- Zou, H.F.; Yang, H.J.; Zhang, Z.F. Morphologies, orientation relationships and evolution of CuSn grains formed between molten Sn and Cu single crystals. Acta Mater. 2008, 56, 2649–2662. [Google Scholar] [CrossRef]
- Buresch, I. Yellowishing of tin coatings at elevated temperatures. In Proceedings of the 27th International Conference on Electrical Contacts, Dresden, Germany, 22–26 June 2014; pp. 175–180. [Google Scholar]
- Gagliano, R.A.; Ghosh, G.; Fine, M.E. Nucleation kinetics of Cu6Sn5 by reaction of molten tin with a copper substrate. J. Electron. Mater. 2002, 31, 1195–1202. [Google Scholar] [CrossRef]
- Ohki, M.; Ishibashi, T.; Suzuki, W. Intermetallic layer growth and adhesion strength on hot-dipped tin-plated materials. J. Mater. Test. Res. Assoc. Jpn. 2009, 54, 92–99. [Google Scholar]
- Takaku, Y.; Liu, X.J.; Ohnuma, I.; Kainuma, R.; Ishida, K. Interfacial reaction and morphology between molten Sn base solders and Cu substrate. Mater. Trans. 2004, 45, 646–651. [Google Scholar] [CrossRef]
- Li, J.F.; Mannan, S.H.; Clode, M.P.; Whalley, D.C.; Hutt, D.A. Interfacial reactions between molten Sn-Bi-X solders and Cu substrates for liquid solder interconnects. Acta Mater. 2006, 54, 2907–2922. [Google Scholar] [CrossRef]
- Mendala, J. Mechanical properties of parts with tin coating obtained by hot-dipped method. Solid State Phenom. 2016, 246, 113–116. [Google Scholar] [CrossRef]
- Xiang, N.; Yin, T.; Tian, B.H.; Tang, S.W.; Chen, E. Evaluation on the manufacturability of solderable Sn coatings obtained by employing hot-dipped tinning process. JOM 2019, 71, 4284–4295. [Google Scholar] [CrossRef]
- Braunovic, M. Effect of intermetallic phases on the performance of tin-plated copper connections and conductors. In Proceedings of the 49th Holm Conference on IEEE, Washington, DC, USA, 8–10 September 2003; pp. 124–131. [Google Scholar]
- Chang, T.C.; Hon, M.H.; Wang, M.C. Intermetallic compounds formation and interfacial adhesion strength of Sn-9Zn-0.5Ag solder alloy hot-dipped on Cu substrate. J. Alloy. Compd. 2003, 352, 168–174. [Google Scholar] [CrossRef]
- Lee, H.T.; Chen, M.H. Influence of intermetallic compounds on the adhesive strength of solder joints. Mater. Sci. Eng. A 2002, 333, 24–34. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Zhang, Z.F. In-situ observations on fracture behaviors of Cu–Sn IMC layers induced by deformation of Cu substrates. Mater. Sci. Eng. A 2011, 530, 452–461. [Google Scholar] [CrossRef]
- Lee, H.T.; Chen, M.H.; Jao, H.M.; Liao, T.L. Influence of interfacial intermetallic compound on fracture behavior of solder joints. Mater. Sci. Eng. A 2003, 358, 134–141. [Google Scholar] [CrossRef]
- Park, Y.W.; Bapu, G.N.K.R.; Kang, Y.L. Evaluation of surface characteristics under fretting of electrical contacts: Removal behaviour of hot dipped tin coating. Appl. Surf. Sci. 2009, 255, 4434–4442. [Google Scholar] [CrossRef]
- Zeng, K.; Tu, K.N. Six cases of reliability study of Pb-free solder joints in electronic packaging technology. Mater. Sci. Eng. R Rep. 2002, 38, 55–105. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Qian, Y. Improvement of microstructure and interface structure of eutectic Sn–0.7Cu solder with small amount of Zn addition. Scr. Mater. 2005, 53, 699–702. [Google Scholar] [CrossRef]
- Ren, F.; Nah, J.W.; Tu, K.N.; Xiong, B.; Xu, L.; Pang, J.H.L. Electromigration induced ductile-to-brittle transition in lead-free solder joints. Appl. Phys. Lett. 2006, 89, 141914. [Google Scholar] [CrossRef]
- Saunders, N.; Miodownik, A.P. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990; pp. 1481–1483. [Google Scholar]
- Liu, X.J.; Kainuma, R.; Wang, C.P.; Ohnuma, I.; Ishida, K. Experimental investigation and thermodynamic calculation of the phase equilibria in the Cu-Sn and Cu-Sn-Mn systems. Metall. Mater. Trans. A 2004, 35, 1641–1654. [Google Scholar] [CrossRef]
- Wang, W.; Sun, K.; Liu, H.T. Effects of different aluminum sources on morphologies and properties of ceramic floor tiles from red mud. Constr. Build. Mater. 2020, 241, 118119. [Google Scholar] [CrossRef]

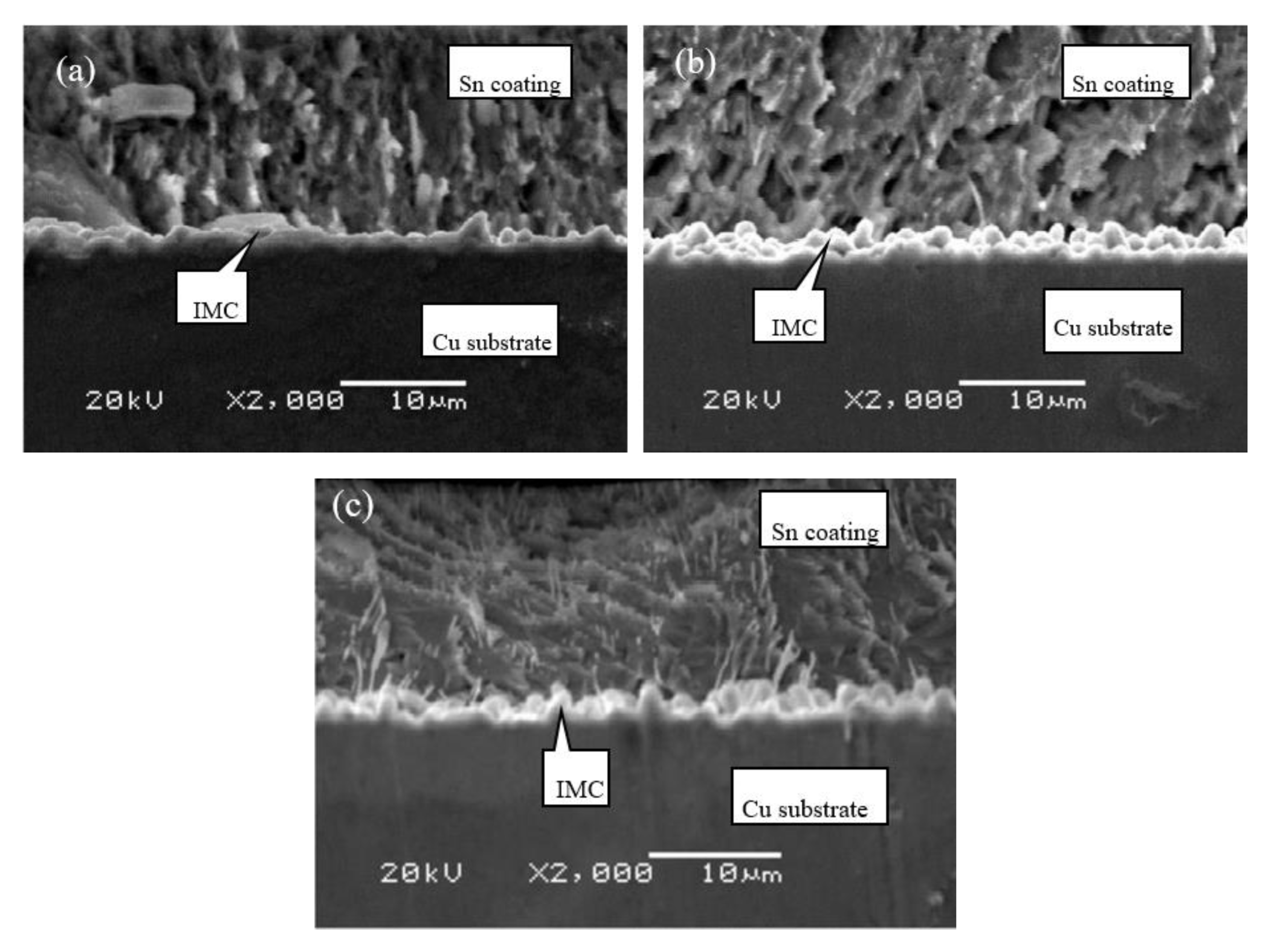
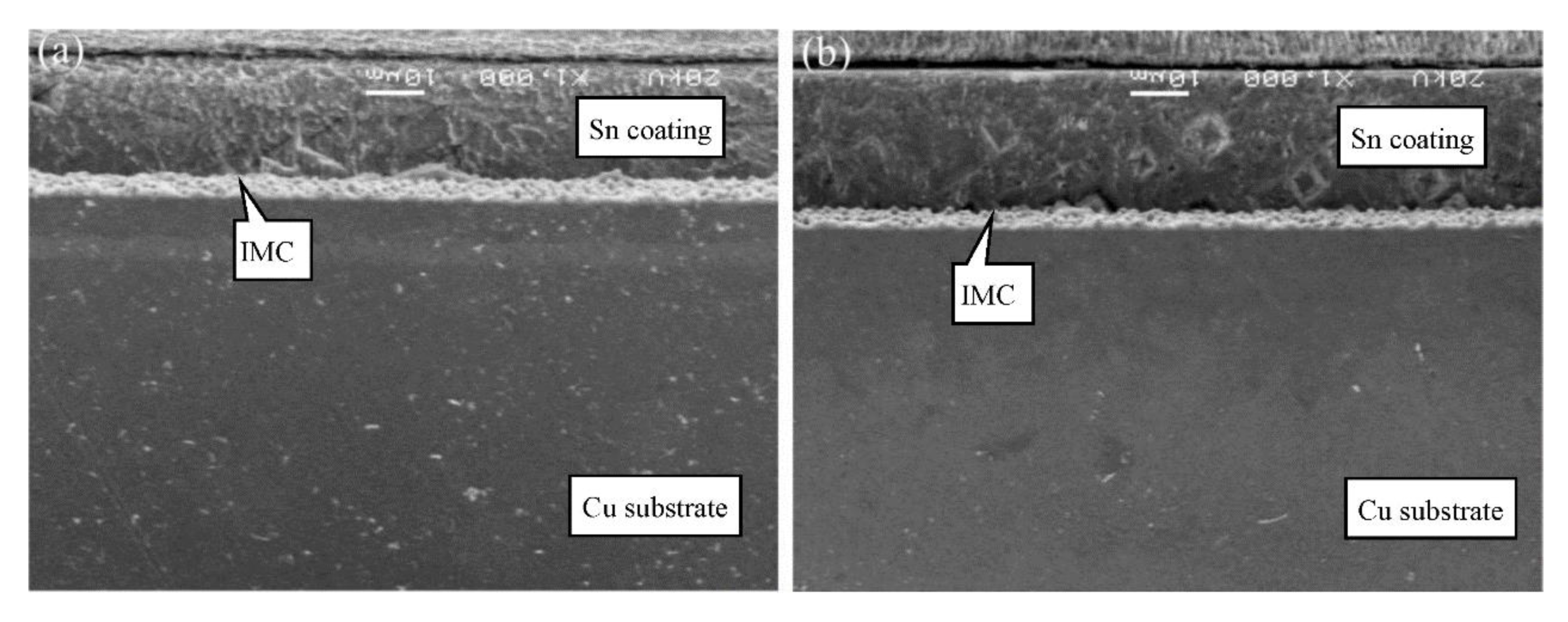
| Category | Grade of Copper Foil | |||
|---|---|---|---|---|
| C70250 | C19400 | C19210 | C11000 | |
| Heat treatment state | TM02 | H00 | H02 | TM02 |
| Thickness, δ0 (mm) | 0.640 | 0.254 | 0.200 | 0.200 |
| Size (mm × mm) | 10 × 44 | 10 × 300 | 10 × 82 | 10 × 200 |
| Tensile strength, σb (MPa) | 658 | 431 | 379 | 350 |
| Yield strength, σs (MPa) | / | 400–402 | / | 245 |
| Elongation, ε (%) | 15.0–16.0 | 7.5 | 19.0 | 8.0 |
| Hardness, H (HV) | 204 | 138 | 121 | 133 |
| Surface roughness, Ra (μm) | 0.082 | 0.069 | 0.075 | ≤ 0.300 |
| Materials | Cu (%) | Fe (%) | P (%) | Zn (%) | Sn (%) | Ni (%) | Si (%) | Mn (%) | Mg (%) |
|---|---|---|---|---|---|---|---|---|---|
| C70250 | 96.67 | / | / | / | / | 2.0 | 0.6 | <0.1 | 0.17 |
| C19400 | 97.54 | 2.3 | 0.028 | 0.1 | / | / | / | / | / |
| C19210 | 99.8 | 0.11 | 0.032 | / | / | / | / | / | / |
| C11000 | 99.97 | 0.005 | / | 0.005 | 0.005 | / | / | / | / |
| ZnCl2 (mol/L) | NH4Cl (mol/L) | NaCl (mol/L) | HCl (mol/L) |
|---|---|---|---|
| 1.80 | 0.58 | 1.14 | 0.08–0.17 |
| No. | Duration of each Procedure (min) | Dry Method | ||
|---|---|---|---|---|
| Acid Pickling | Alkaline Cleaning | Dipping in Accelerant | ||
| 1 | / | / | / | / |
| 2 | 5 | 4 | 4 | Natural dry |
| 3 | 5 | 4 | 4 | / |
| 4 | / | 4 | 4 | Quick dry |
| 5 | / | 4 | 4 | Natural dry |
| 6 | / | 4 | 4 | / |
| Materials | Dipping Temperature, T (°C) | Dipping Time, t (s) |
|---|---|---|
| C70250 | 260, 280, 300 | 4, 8, 16 |
| C19400 | 260, 280, 300 | 4, 8, 16 |
| C19210 | 260, 280, 300 | 4, 8, 16 |
| C11000 | 240, 260, 280, 300, 350 | 2, 4, 8, 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, T.; Xiang, N.; Wang, G.; Tian, B.; Sun, W.; Zhang, X. Optimal Hot-Dipped Tinning Process Routine for the Fabrication of Solderable Sn Coatings on Circuit Lead Frames. Materials 2020, 13, 1191. https://doi.org/10.3390/ma13051191
Yin T, Xiang N, Wang G, Tian B, Sun W, Zhang X. Optimal Hot-Dipped Tinning Process Routine for the Fabrication of Solderable Sn Coatings on Circuit Lead Frames. Materials. 2020; 13(5):1191. https://doi.org/10.3390/ma13051191
Chicago/Turabian StyleYin, Ting, Nan Xiang, Guangxin Wang, Baohong Tian, Wanting Sun, and Xiaoyu Zhang. 2020. "Optimal Hot-Dipped Tinning Process Routine for the Fabrication of Solderable Sn Coatings on Circuit Lead Frames" Materials 13, no. 5: 1191. https://doi.org/10.3390/ma13051191
APA StyleYin, T., Xiang, N., Wang, G., Tian, B., Sun, W., & Zhang, X. (2020). Optimal Hot-Dipped Tinning Process Routine for the Fabrication of Solderable Sn Coatings on Circuit Lead Frames. Materials, 13(5), 1191. https://doi.org/10.3390/ma13051191






