An Efficacy Assessment of Phosphate Removal from Drainage Waters by Modified Reactive Material
Abstract
1. Introduction
2. Reactive Materials Used for Phosphate Removal
2.1. Zeolites as Reactive Materials
2.2. Reactive Materials Modification
3. Materials and Methods
3.1. Assumptions for the Experiment Related to the Choice of Ca(OH)2 Concentration Used for Modification
3.2. Determining The Chemical and Mineral Composition, As Well As The Porous Texture and Morphology of The Modified Reactive Material
- Specific surface area (SSABET) according to the methodology by Brunauer–Emmett–Teller;
- Pore total volume V0,99tot for relative pressure p/p0 = 0.99;
- Micropore volume VDRmik (pores of width smaller than 2 nm) according to the methodology by Dubinin–Radushkevich;
- Mesopore volume VBJHmez (pores of width greater than 2 nm and smaller than 50 nm) according to the methodology by Barrett–Joyner–Halenda (BJH).
3.3. The Experiment Methodology of Phosphate Removal Using Calcium Hydroxide Modified Reactive Material
4. Results and Discussion
4.1. Reactive Materials Modification With Calcium Hydroxide
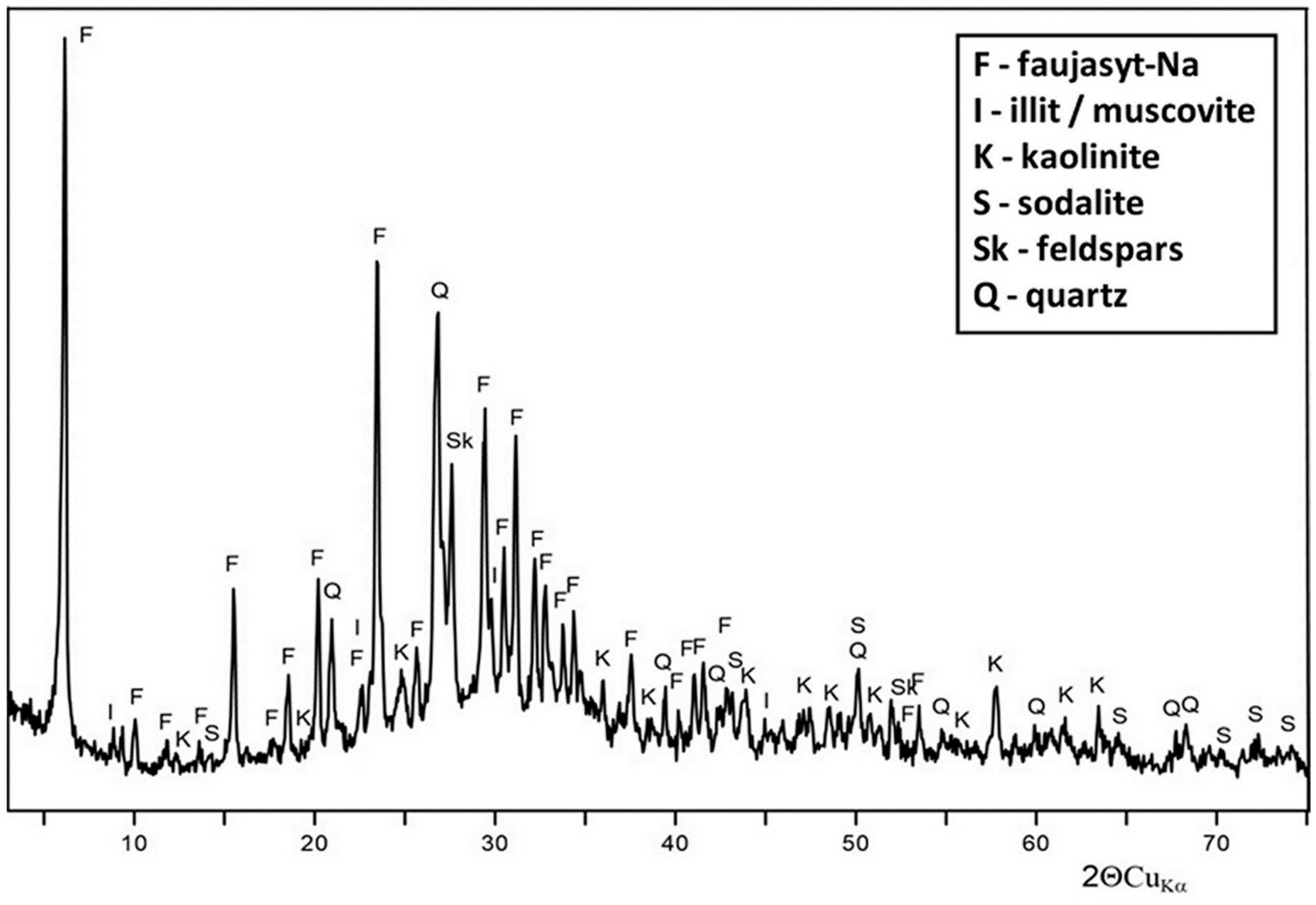
4.2. Modified Reactive Material Characteristics
4.3. The Efficacy of Phosphate Removal Using Modified Reactive Material
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dao, T.H.; Schwartz, R.C. Effects of Manure Management on Phosphorus Biotransformations and Losses During Animal Production. In Soil Biology; Springer Science and Business Media LLC: Berlin, Germany, 2010; Volume 26, pp. 407–429. [Google Scholar]
- Vadas, P.; Kleinman, P.J.A.; Sharpley, A.N.; Turner, B.L. Relating Soil Phosphorus to Dissolved Phosphorus in Runoff: A Single Extraction Coefficient for Water Quality Modeling. J. Environ. Qual. 2005, 34, 572–580. [Google Scholar] [CrossRef] [PubMed]
- McDowell, R.; Snelder, T.; Littlejohn, R.; Hickey, M.; Cox, N.; Booker, D.J. State and potential management to improve water quality in an agricultural catchment relative to a natural baseline. Agric. Ecosyst. Environ. 2011, 144, 188–200. [Google Scholar] [CrossRef]
- Heathwaite, L.; Sharpley, A.; Bechmann, M.; Heathwaite, A.L. The conceptual basis for a decision support framework to assess the risk of phosphorus loss at the field scale across Europe. J. Plant Nutr. Soil Sci. 2003, 166, 447–458. [Google Scholar] [CrossRef]
- Igras, J.; Fotyma MJadczyszyn, T.; Lipiński, W.; Radzimierski, M.W.E. Ocena Stanu Zanieczyszczenia Płytkich wód Gruntowych, Narażonych Bezpośrednio na Zrzuty Składników Biogennych, w tym Szczególnie z Rolnictwa oraz Możliwości Potencjalnego Wpływu Zanieczyszczeń Pochodzących z Produkcji Rolnej na Środowisko; Opracowanie wykonane na zlecenie Ministra Rolnictwa i Rozwoju Wsi; IUNG-PIB: Puławy, Poland, 2008. [Google Scholar]
- Ballantine, D.; Tanner, C.C. Substrate and filter materials to enhance phosphorus removal in constructed wetlands treating diffuse farm runoff: A review. N. Z. J. Agric. Res. 2010, 53, 71–95. [Google Scholar] [CrossRef]
- Buda, A.R.; Koopmans, G.F.; Bryant, R.B.; Chardon, W. Emerging Technologies for Removing Nonpoint Phosphorus from Surface Water and Groundwater: Introduction. J. Environ. Qual. 2012, 41, 621–627. [Google Scholar] [CrossRef]
- Kaasik, A.; Vohla, C.; Mõtlep, R.; Mander, Ü.; Kirsimäe, K. Hydrated calcareous oil-shale ash as potential filter media for phosphorus removal in constructed wetlands. Water Res. 2008, 42, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P.; Amsinck, S.L. Water Framework Directive: ecological classification of Danish lakes. J. Appl. Ecol. 2005, 42, 616–629. [Google Scholar] [CrossRef]
- European Environment Agency. European Environment—State and Outlook 2015: Assessment of Global Megatrends; European Environment Agency: Copenhagen, Denmark, 2015. [Google Scholar]
- Bryant, R.; Buda, A.R.; Kleinman, P.; Church, C.; Saporito, L.S.; Folmar, G.J.; Bose, S.; Allen, A.L. Using Flue Gas Desulfurization Gypsum to Remove Dissolved Phosphorus from Agricultural Drainage Waters. J. Environ. Qual. 2012, 41, 664–671. [Google Scholar] [CrossRef]
- Kronvang, B.; Rubaek, G.H.; Heckrath, G.; Rubæk, G.H. International Phosphorus Workshop: Diffuse Phosphorus Loss to Surface Water Bodies-Risk Assessment, Mitigation Options, and Ecological Effects in River Basins. J. Environ. Qual. 2009, 38, 1924–1929. [Google Scholar] [CrossRef]
- Smith, K.; Jackson, D.; Withers, P. Nutrient losses by surface run-off following the application of organic manures to arable land. 2. Phosphorus. Environ. Pollut. 2001, 112, 53–60. [Google Scholar] [CrossRef]
- Reinhardt, M.; Gächter, R.; Wehrli, B.; Müller, B. Phosphorus Retention in Small Constructed Wetlands Treating Agricultural Drainage Water. J. Environ. Qual. 2005, 34, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Bryant, R.B; Kleinman, P.J.A.; Allen, A.L. Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. J. Soil Water Conserv. 2007, 62, 269–276. [Google Scholar]
- Groenenberg, J.; Chardon, W.; Koopmans, G.F. Reducing Phosphorus Loading of Surface Water Using Iron-Coated Sand. J. Environ. Qual. 2013, 42, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bell, G.E.; Penn, C.J.; Moss, J.Q.; Payton, M.E. Phosphorus Reduction in Turfgrass Runoff Using a Steel Slag Trench Filter System. Crop. Sci. 2014, 54, 1859–1867. [Google Scholar] [CrossRef]
- Uusitalo, R.; Närvänen, A.; Kaseva, A.; Launto-Tiuttu, A.; Heikkinen, J.; Joki-Heiskala, P.; Rasa, K.; Salo, T.J. Conversion of dissolved phosphorus in runoff by ferric sulfate to a form less available to algae: Field performance and cost assessment. Ambio 2015, 44, 286–296. [Google Scholar] [CrossRef]
- Cucarella, V.; Renman, G. Phosphorus Sorption Capacity of Filter Materials Used for On-site Wastewater Treatment Determined in Batch Experiments-A Comparative Study. J. Environ. Qual. 2009, 38, 381–392. [Google Scholar] [CrossRef]
- Johansson Westholm, L. Substrates for phosphorus removal—Potential benefits for on-site wastewater treatment? Water Res. 2006, 40, 23–36. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Shilton, A.N.; Elmetri, I.; Drizo, A.; Pratt, S.; Haverkamp, R.; Bilby, S.C. Phosphorus removal by an ‘active’ slag filter–a decade of full scale experience. Water Res. 2006, 40, 113–118. [Google Scholar] [CrossRef]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef]
- Kholoma, E.; Renman, G.; Renman, A. Phosphorus removal from wastewater by field-scale fortified filter beds during a one-year study. Environ. Technol. 2016, 37, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Sibrell, P.; Montgomery, G.A.; Ritenour, K.L.; Tucker, T.W. Removal of phosphorus from agricultural wastewaters using adsorption media prepared from acid mine drainage sludge. Water Res. 2009, 43, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Christianson, L.E.; Lepine, C.; Sibrell, P.; Penn, C.; Summerfelt, S.T. Denitrifying woodchip bioreactor and phosphorus filter pairing to minimize pollution swapping. Water Res. 2017, 121, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Bowen, J.M. Design and Construction of Phosphorus Removal Structures for Improving Water Quality; Springer: Basel, Switzerland, 2018. [Google Scholar]
- Brooks, A.S.; Rozenwald, M.N.; Geohring, L.D.; Lion, L.W.; Steenhuis, T.S. Phosphorus removal by wollastonite: A constructed wetland substrate. Ecol. Eng. 2000, 15, 121–132. [Google Scholar] [CrossRef]
- Khadhraoui, M.; Watanabe, T.; Kuroda, M. The effect of the physical structure of a porous Ca-based sorbent on its phosphorus removal capacity. Water Res. 2002, 36, 3711–3718. [Google Scholar] [CrossRef]
- Berg, U.; Donnert, D.; Ehbrecht, A.; Bumiller, W.; Kusche, I.; Weidler, P.; Nüesch, R. “Active filtration” for the elimination and recovery of phosphorus from waste water. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 265, 141–148. [Google Scholar] [CrossRef]
- Cucarella, V.; Zaleski, T.; Mazurek, R. Phosphorus sorption capacity of different types of opoka. Ann. Wars. Univ. Life Sci. SGGW Land Reclam. 2007, 38, 11–18. [Google Scholar] [CrossRef]
- Del Bubba, M.; A Arias, C.; Brix, H. Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm. Water Res. 2003, 37, 3390–3400. [Google Scholar] [CrossRef]
- Xu, D.; Xu, J.; Wu, J.; Muhammad, A. Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere 2006, 63, 344–352. [Google Scholar] [CrossRef]
- Molle, P.; Liénard, A.; Grasmick, A.; Iwema, A.; Kabbabi, A. Apatite as an interesting seed to remove phosphorus from wastewater in constructed wetlands. Water Sci. Technol. 2005, 51, 193–203. [Google Scholar] [CrossRef]
- Penn, C.J.; Bryant, R.B. Application of phosphorus sorbing materials to streamside cattle loafing areas. J. Soil Water Conserv. 2006, 61, 303–310. [Google Scholar]
- Stoner, D.; Penn, C.J.; McGrath, J.; Warren, J.G. Phosphorus Removal with By-Products in a Flow-Through Setting. J. Environ. Qual. 2012, 41, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Bryant, R.B.; Callahan, M.P.; McGrath, J. Use of Industrial By-products to Sorb and Retain Phosphorus. Commun. Soil Sci. Plant Anal. 2011, 42, 633–644. [Google Scholar] [CrossRef]
- Penn, C.J.; Bowen, J.; McGrath, J.; Nairn, R.; Fox, G.; Brown, G.; Wilson, S.; Gill, C. Evaluation of a universal flow-through model for predicting and designing phosphorus removal structures. Chemosphere 2016, 151, 345–355. [Google Scholar] [CrossRef]
- Kõiv, M.; Liira, M.; Mander, Ü.; Mõtlep, R.; Vohla, C.; Kirsimäe, K. Phosphorus removal using Ca-rich hydrated oil shale ash as filter material – The effect of different phosphorus loadings and wastewater compositions. Water Res. 2010, 44, 5232–5239. [Google Scholar] [CrossRef]
- Renman, G.; Renman, A. Sustainable Use of Crushed Autoclaved Aerated Concrete (CAAC) as a Filter Medium in Wastewater Purification. In Proceedings of the 8th International Conference on Sustainable Management of Waste and Recycled Materials in Construction, Gothenburg, Sweden, 30 May–1 June 2012. [Google Scholar]
- Dunets, C.S.; Zheng, Y.; Dixon, M. Use of phosphorus-sorbing materials to remove phosphate from greenhouse wastewater. Environ. Technol. 2015, 36, 1759–1770. [Google Scholar] [CrossRef]
- Roseth, R. Shell sand: A new filter medium for constructed wetlands and wastewater treatment. J. Environ. Sci. Health. A 2000, 35, 1335–1355. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Bus, A. Testing of reactive materials for phosphorus removal from water and wastewater – comparative study. Ann. Wars. Univ. Life Sci. SGGW Land Reclam. 2014, 46, 57–67. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.; Delfino, J. Influence of redox potential on phosphorus solubility in chemically amended wetland organic soils. Ecol. Eng. 1999, 14, 169–180. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.; Delfino, J. Influence of chemical amendments on phosphorus immobilization in soils from a constructed wetland. Ecol. Eng. 1999, 14, 157–167. [Google Scholar] [CrossRef]
- Comeau, Y.; Brisson, J.; Réville, J.-P.; Forget, C.; Drizo, A. Phosphorus removal from trout farm effluents by constructed wetlands. Water Sci. Technol. 2001, 44, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shilton, A.; Pratt, S.; Drizo, A.; Mahmood, B.; Banker, S.; Billings, L.; Glenny, S.; Luo, D. ‘Active’ filters for upgrading phosphorus removal from pond systems. Water Sci. Technol. 2005, 51, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Duxbury, J.; Geohring, L.; Peck, T. Designing constructed wetlands to remove phosphorus from barnyard runoff: A comparison of four alternative substrates. J. Environ. Sci. Health A 2000, 35, 1357–1375. [Google Scholar] [CrossRef]
- Boujelben, N.; Bouzid, J.; Elouear, Z.; Feki, M.; Jamoussi, F.; Montiel, A. Phosphorus removal from aqueous solution using iron coated natural and engineered sorbents. J. Hazard. Mater. 2008, 151, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chardon, W.; Groenenberg, J.; Temminghoff, E.J.M.; Koopmans, G.F. Use of Reactive Materials to Bind Phosphorus. J. Environ. Qual. 2012, 41, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, S.H.; Hartikainen, H.H. Phosphorus retention by phlogopite-rich mine tailings. Appl. Geochem. 2008, 23, 2716–2723. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, S.; He, J.; Gan, F.; Ho, Y.-S. Adsorption characteristic studies of phosphorus onto laterite. Desalination Water Treat. 2011, 25, 98–105. [Google Scholar] [CrossRef]
- Erickson, A.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar] [CrossRef]
- Forbes, M.G.; Dickson, K.L.; Saleh, F.; Waller, W.T.; Doyle, R.D.; Hudak, P. Recovery and Fractionation of Phosphorus Retained by Lightweight Expanded Shale and Masonry Sand Used as Media in Subsurface Flow Treatment Wetlands. Environ. Sci. Technol. 2005, 39, 4621–4627. [Google Scholar] [CrossRef]
- Yaghi, N.; Hartikainen, H. Enhancement of phosphorus sorption onto light expanded clay aggregates by means of aluminum and iron oxide coatings. Chemosphere 2013, 93, 1879–1886. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baryła, A.; Bus, A. Effect of P-Reactive Drainage Aggregates on Green Roof Runoff Quality. Water 2014, 6, 2575–2589. [Google Scholar] [CrossRef]
- Ma, J.; Lenhart, J.H.; Tracy, K. Orthophosphate Adsorption Equilibrium and Breakthrough on Filtration Media for Storm-Water Runoff Treatment. J. Irrig. Drain. Eng. 2011, 137, 244–250. [Google Scholar] [CrossRef]
- Srinivasan, R.; Hoffman, D.W.; Wolfe, J.E.; Prcin, L.J. Evaluation of removal of orthophosphate and ammonia from rainfall runoff using aboveground permeable reactive barrier composed of limestone and zeolite. J. Environ. Sci. Health A 2008, 43, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Selim, A.Q.; Seliem, M.K.; Abukhadra, M.R. Modeling and Optimizations of Phosphate Removal from Aqueous Solutions Using Synthetic Zeolite Na-A. J. Mater. Sci. Chem. Eng. 2015, 3, 15–29. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Luan, Z.; Peng, X.; Zhu, C.; Chen, Z.; Zhang, Z.; Fan, J.; Jia, Z. Phosphate removal from aqueous solutions using raw and activated red mud and fly ash. J. Hazard. Mater. 2006, 137, 374–383. [Google Scholar] [CrossRef]
- Makris, K.; Harris, W.G.; O’Connor, G.A.; Obreza, T.A.; Elliott, H.A. Physicochemical Properties Related to Long-Term Phosphorus Retention by Drinking-Water Treatment Residuals. Environ. Sci. Technol. 2005, 39, 4280–4289. [Google Scholar] [CrossRef]
- Makris, K.; Harris, W.G. Time dependency and irreversibility of water desorption by drinking-water treatment residuals: Implications for sorption mechanisms. J. Colloid Interface Sci. 2006, 294, 151–154. [Google Scholar] [CrossRef]
- Liu, J.; Davis, A.P. Phosphorus Speciation and Treatment Using Enhanced Phosphorus Removal Bioretention. Environ. Sci. Technol. 2013, 48, 607–614. [Google Scholar] [CrossRef]
- Oguz, E. Removal of phosphate from aqueous solution with blast furnace slag. J. Hazard. Mater. 2004, 114, 131–137. [Google Scholar] [CrossRef]
- Kostura, B.; Kulveitová, H.; Leško, J. Blast furnace slags as sorbents of phosphate from water solutions. Water Res. 2005, 39, 1795–1802. [Google Scholar] [CrossRef]
- Drizo, A.; Forget, C.; Chapuis, R.P.; Comeau, Y. Phosphorus removal by electric arc furnace steel slag and serpentinite. Water Res. 2006, 40, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.; Shilton, A.; Pratt, S.; Haverkamp, R.; Bolan, N. Phosphorus Removal Mechanisms in Active Slag Filters Treating Waste Stabilization Pond Effluent. Environ. Sci. Technol. 2007, 41, 3296–3301. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.; Shilton, A.; Pratt, S.; Haverkamp, R.; Elmetri, I. Effects of Redox Potential and pH Changes on Phosphorus Retention by Melter Slag Filters Treating Wastewater. Environ. Sci. Technol. 2007, 41, 6585–6590. [Google Scholar] [CrossRef] [PubMed]
- Agyei, N.; Strydom, C.; Potgieter, J. The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends. Cem. Concr. Res. 2002, 32, 1889–1897. [Google Scholar] [CrossRef]
- Zhang, W.; Brown, G.; Storm, D.E.; Zhang, H. Fly-ash-amended sand as filter media in bioretention cells to improve phosphorus removal. Water Environ. Res. 2008, 80, 507–516. [Google Scholar] [CrossRef]
- Drizo, A.; Comeau, Y.; Forget, C.; Chapuis, R.P. Phosphorus Saturation Potential: A Parameter for Estimating the Longevity of Constructed Wetland Systems. Environ. Sci. Technol. 2002, 36, 4642–4648. [Google Scholar] [CrossRef]
- McDowell, R.; Sharpley, A.N.; Bourke, W. Treatment of Drainage Water with Industrial By-Products to Prevent Phosphorus Loss from Tile-Drained Land. J. Environ. Qual. 2008, 37, 1575–1582. [Google Scholar] [CrossRef]
- Bowden, L.I.; Jarvis, A.P.; Younger, P.; Johnson, K.L. Phosphorus Removal from Waste Waters Using Basic Oxygen Steel Slag. Environ. Sci. Technol. 2009, 43, 2476–2481. [Google Scholar] [CrossRef]
- Claveau-Mallet, D.; Wallace, S.; Comeau, Y. Removal of phosphorus, fluoride and metals from a gypsum mining leachate using steel slag filters. Water Res. 2013, 47, 1512–1520. [Google Scholar] [CrossRef]
- Claveau-Mallet, D.; Wallace, S.; Comeau, Y. Model of Phosphorus Precipitation and Crystal Formation in Electric Arc Furnace Steel Slag Filters. Environ. Sci. Technol. 2012, 46, 1465–1470. [Google Scholar] [CrossRef]
- Klimeski, A.; Uusitalo, R.; Turtola, E. Screening of Ca- and Fe-rich materials for their applicability as phosphate-retaining filters. Ecol. Eng. 2014, 68, 143–154. [Google Scholar] [CrossRef]
- Hylander, L.D.; Kietlińska, A.; Renman, G.; Siman, G. Phosphorus retention in filter materials for wastewater treatment and its subsequent suitability for plant production. Bioresour. Technol. 2006, 97, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Klimeski, A.; Chardon, W.; Turtola, E.; Uusitalo, R. Potential and limitations of phosphate retention media in water protection: A process-based review of laboratory and field-scale tests. Agric. Food Sci. 2012, 21, 206–223. [Google Scholar] [CrossRef]
- Masters, A.F.; Maschmeyer, T. Zeolites – From curiosity to cornerstone. Microporous Mesoporous Mater. 2011, 142, 423–438. [Google Scholar] [CrossRef]
- Valdés, M.G.; Pérez-Cordoves, A.; Díaz-García, M. Zeolites and zeolite-based materials in analytical chemistry. TrAC Trends Anal. Chem. 2006, 25, 24–30. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Auerbach, S.; Carrado, K.; Dutta, P. Handbook of Zeolite Science and Technology; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef]
- Franus, W.; Jozefaciuk, G.; Bandura, L.; Franus, M. Use of Spent Zeolite Sorbents for the Preparation of Lightweight Aggregates Differing in Microstructure. Minerals 2017, 7, 25. [Google Scholar] [CrossRef]
- Jiao, G.-F.; Pu, M.; Chen, B.-H. Theoretical study of formation mechanism of aluminosilicate in the synthesis of zeolites. Struct. Chem. 2008, 19, 481–487. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Hydrothermal Synthesis and Growth of Zeolites. In Handbook of Hydrothermal Technology; Elsevier BV: Amsterdam, The Netherlands, 2013; pp. 269–347. [Google Scholar]
- Franus, W. Characterization of X-type zeolite prepared from coal fly ash. Pol. J. Environ. Stud. 2012, 21, 337–343. [Google Scholar]
- Yin, H.; Han, M.; Tang, W. Phosphorus sorption and supply from eutrophic lake sediment amended with thermally-treated calcium-rich attapulgite and a safety evaluation. Chem. Eng. J. 2016, 285, 671–678. [Google Scholar] [CrossRef]
- Haghseresht, F.; Wang, S.; Do, D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Yang, M.; Lin, J.; Zhan, Y.; Zhu, Z.; Zhang, H. Immobilization of phosphorus from water and sediment using zirconium-modified zeolites. Environ. Sci. Pollut. Res. 2014, 22, 3606–3619. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Markou, G.; Inglezakis, V.J.; Mitrogiannis, D.; Efthimiopoulos, I.; Psychoyou, M.; Koutsovitis, P.; Muylaert, K.; Baziotis, I. Sorption mechanism(s) of orthophosphate onto Ca(OH)2 pretreated bentonite. RSC Adv. 2016, 6, 22295–22305. [Google Scholar] [CrossRef]
- Ma, J.; Qi, J.; Yao, C.; Cui, B.; Zhang, T.; Li, D. A novel bentonite-based adsorbent for anionic pollutant removal from water. Chem. Eng. J. 2012, 200, 97–103. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.I.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH) 2 treated natural clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Perassi, I.; Borgnino, L. Adsorption and surface precipitation of phosphate onto CaCO3–montmorillonite: effect of pH, ionic strength and competition with humic acid. Geoderma 2014, 232, 600–608. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.; Cortina, J.L. Powdered Ca-activated zeolite for phosphate removal from treated waste-water. J. Chem. Technol. Biotechnol. 2016, 91, 1962–1971. [Google Scholar] [CrossRef]
- Randall, D.; Krähenbühl, M.; Köpping, I.; Larsen, T.A.; Udert, K. A novel approach for stabilizing fresh urine by calcium hydroxide addition. Water Res. 2016, 95, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wu, F.-C.; Liu, G.; Mu, Y.; Feng, C.; Wang, H.; Giesy, J.P. Removal of Phosphate from Eutrophic Lakes through Adsorption by in Situ Formation of Magnesium Hydroxide from Diatomite. Environ. Sci. Technol. 2013, 48, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N. Removal and Recovery of Phosphate From Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Grela, A.; Łach, M.; Bajda, T.; Mikuła, J.; Hebda, M. Characterization of the products obtained from alkaline conversion of tuff and metakaolin. J. Therm. Anal. Calorim. 2018, 133, 217–226. [Google Scholar] [CrossRef]
- Marczenko, Z.B.M. Spektrofotometryczne Metody w Analizie Nieorganicznej; PWN: Warszawa, Poland, 1998; pp. 250–265. [Google Scholar]
- Kubiak, J.; Tórz, A.N.; Nędzarek, A. Analityczne Podstawy Hydrochemii; Wydawnictwo Akademii Rolniczej w Szczecinie: Szczecin, Poland, 1999; pp. 151–158. [Google Scholar]
- Zhu, T.; Jenssen, P.D.; Mæhlum, T.; Krogstad, T. Phosphorus sorption and chemical characteristics of lightweight aggregates (LWA)—Potential filter media in treatment wetlands. Water Sci. Technol. 1997, 35, 103–108. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A. Supporting constructed wetlands in P removal efficiency from surface water. Water Sci. Technol. 2017, 75, 2554–2561. [Google Scholar] [CrossRef]
- Vohla, C.; Põldvere, E.; Noorvee, A.; Kuusemets, V.; Mander, Ü. Alternative filter media for phosphorous removal in a horizontal subsurface flow constructed wetland. J. Environ. Sci. Health A 2005, 40, 1251–1264. [Google Scholar] [CrossRef]
- Allred, B.; Racharaks, R. Laboratory comparison of four iron-based filter materials for drainage water phosphate treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef]
- Pollytag, P.; Bus, A.; Karczmarczyk, A.; Baryła, A. Wybór Materiału Reaktywnego Do Usuwania Fosforu Z Wód I Ścieków Na Przykładzie Kruszywa Choosing of Reactive Material for Phosphorous Removal from Water and Wastewater on the Example of Lightweight. Inżynieria Ekologiczna 2014, 39, 33–41. [Google Scholar]
- Karczmarczyk, A.; Bus, A.; Baryła, A. Wykorzystanie materiałów reaktywnych w systemach zagospodarowania wody opadowej na osiedlach mieszkaniowych. Infrastruktura i Ekologia Terenów Wiejskich 2015, 4, 1089–1096. [Google Scholar]


| Component | Facility | Quality Classification | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| PO4 [mg/dm3] | Drains | <0.1 | 0.1–0.2 | 0.3–0.4 | 0.5–3.3 | >3.3 |
| Drainage ditches | <0.1 | 0.1–0.5 | 0.6–2.0 | 2.1–6.4 | >6.4 | |
| Reactive Material | Main Component (Al, Ca, Fe, Mg) | Literature |
|---|---|---|
| Marls and sands | Al | [32,33] |
| Apatites | Ca | [34] |
| FGD gypsum | [35,36,37,38] | |
| An oil shale | [39] | |
| Crushed concrete | [40,41] | |
| Crushed seashells/marls | [42,43] | |
| Limestones | [43,44,45,46,47] | |
| Wollastonite | [28,48] | |
| Fe modified sand | Fe | [16,49,50] |
| Biotites | Al, Fe | [51] |
| Laterites | [52] | |
| Metal chips and iron dust | [53] | |
| Lightweight aggregate (LECA) | Al, Ca, Fe | [43,54,55,56] |
| Zeolites | [57,58,59] | |
| Bauxite production waste | Al, Ca, Fe, Mg | [37,60] |
| Drinking water treatment waste | [35,36,37,61,62,63] | |
| Fly ash | [22,35,36,37,38,41,60,64,65,66,67,68,69,70,71] | |
| Slag | [64,65,66,67,68,72,73,74,75,76] |
| Material | Calcium Compound | Literature |
|---|---|---|
| bentonite | Ca(OH)2 | [93] |
| CaO | [94] | |
| clinoptylolite | Ca(OH)2 | [95] |
| montmorillonite | CaCl2·2H2O | [96] |
| NaP1–FA zeolite | CaCl2 | [97] |
| Dose [g/dm3] | Literature | Doses Tested |
|---|---|---|
| 1.0 | [105] | + |
| 5.0 | + | |
| 10.0 | + | |
| 3.0 | [106] | — |
| 5.0 | [107] | + |
| 8.0 | [104] | — |
| 10.0 | [95] | + |
| Material | Oxide Composition/Mass% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaTF | SiO2 | TiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | Na2O | Ig |
| 37.07 | 0.57 | 3.32 | 16.03 | 15.72 | 1.15 | 2.64 | 1.54 | 21.52 | |
| Sample Name | SSABET 1 [m2/g] | V0.99tot 2 [cm3/g] | VDRmik 3 [cm3/g] | VBJHmez 4 [cm3/g] |
|---|---|---|---|---|
| CaTF | 276.0 | 0.292 | 0.105 | 0.162 |
| No. | Reactive Material | Initial Phosphate Concentration [mg/dm3] | Reactive Material Dose [g/dm3] | Contact Time [h] | Phosphate Removal Efficacy [%] | Literature |
|---|---|---|---|---|---|---|
| 1 | zeolite from Thessaloniki modified with 0.25 M solution of Ca(OH)2 | 10.00 | 10.0 | 120 | 93.5 | [95] |
| 2 | autoclaved aerated concrete (AAC) | 2.97 | 1.0 | 24 | 33.0 | [105] |
| 5.0 | 56.0 | |||||
| 10.0 | 80.0 | |||||
| 1.0 | 48 | 50.0 | ||||
| 5.0 | 80.0 | |||||
| 10.0 | 90.0 | |||||
| 1.0 | 144 | 56.0 | ||||
| 5.0 | 83.0 | |||||
| 10.0 | 93.0 | |||||
| 1.0 | 720 | 88.5 | ||||
| 5.0 | 99.8 | |||||
| 10.0 | 99.9 | |||||
| 3 | autoclaved aerated concrete (AAC) | 0.56 | - | 10 | 70.0 | [56] |
| 1.00 | 48 | 90.0 | ||||
| 4 | lightweight fly ash aggregate Pollytag® | 1.00–3.00 | - | - | 1.0–2.5 | [108] |
| 10.00 | 34.0 | |||||
| 5 | white brick | 1.20 | 10.0 | 0.25 | 6.8 | [109] |
| 6 | crushed seashells | 10.00 | 10.0 | 0.25 | 20.0 | |
| 7 | Philippines limestone | 1.40 | 10.0 | 0.25 | 14.6 | |
| 5.60 | 10.0 | 0.25 | 7.6 | |||
| 8 | limestone | 5.00 | 10.0 | 0.25 | 36.0 | |
| 9 | Polonite® | 1.00 | 50.0 | 0.25 | 98.8 | |
| 10 | “opoka” rock | 2.00 | 1.0 | 5 min | 95.0 | |
| 11 | FerroSorp® | 2.00 | 1.0 | 5 min | 13.0 | |
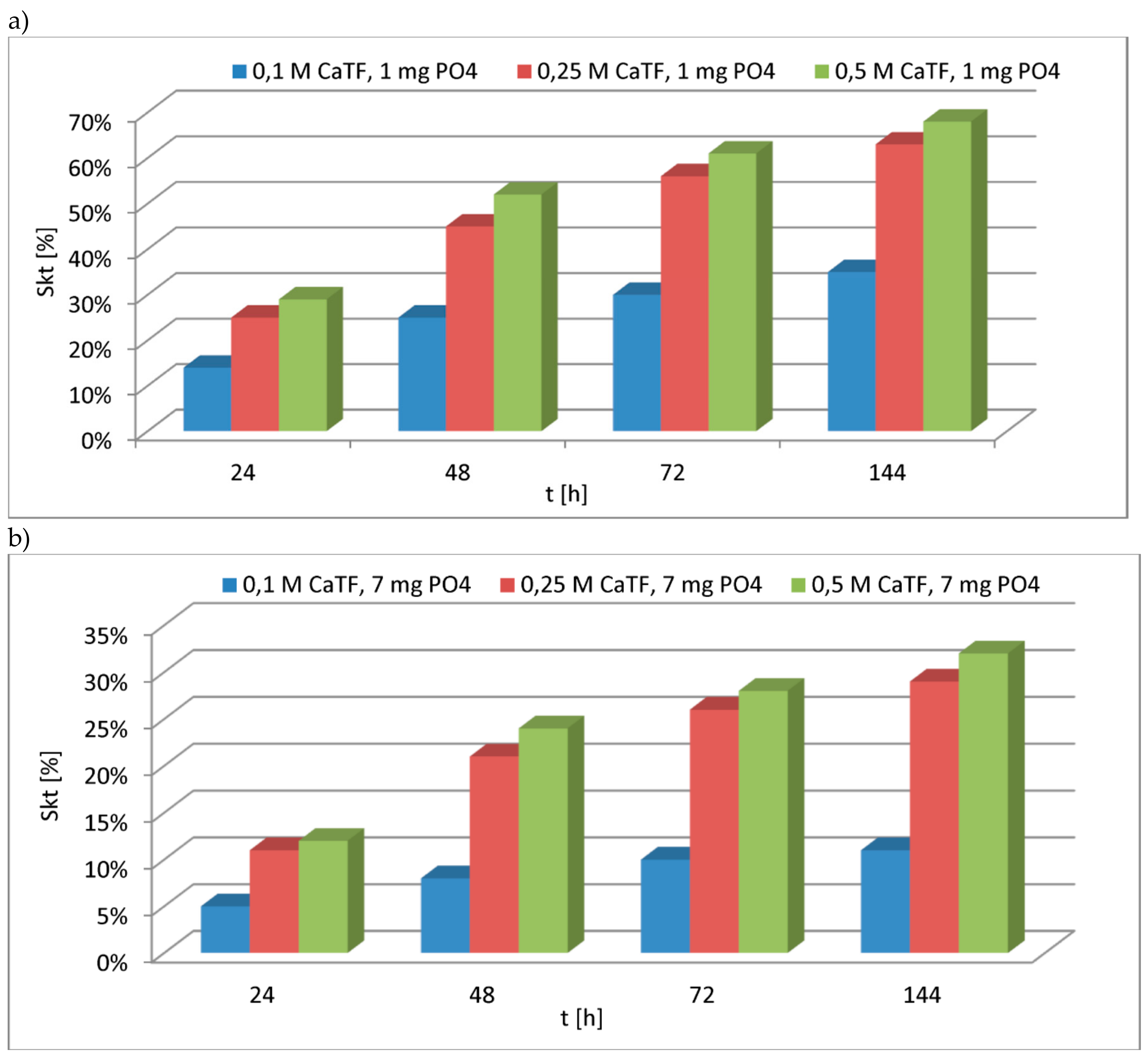
| 12 | CaTF | 1.031 | 2.5 | 1 | 3.0 | in article |
| 5.0 | 4.0 | |||||
| 10.0 | 16.0 | |||||
| 2.5 | 4 | 10.0 | ||||
| 5.0 | 11.0 | |||||
| 10.0 | 31.0 | |||||
| 2.5 | 24 | 25.0 | ||||
| 5.0 | 29.0 | |||||
| 10.0 | 61.0 | |||||
| 2.5 | 48 | 32.0 | ||||
| 5.0 | 46.0 | |||||
| 10.0 | 73.0 | |||||
| 2.5 | 72 | 38.0 | ||||
| 5.0 | 56.0 | |||||
| 10.0 | 77.0 | |||||
| 2.5 | 144 | 43.0 | ||||
| 5.0 | 66.0 | |||||
| 10.0 | 82.0 | |||||
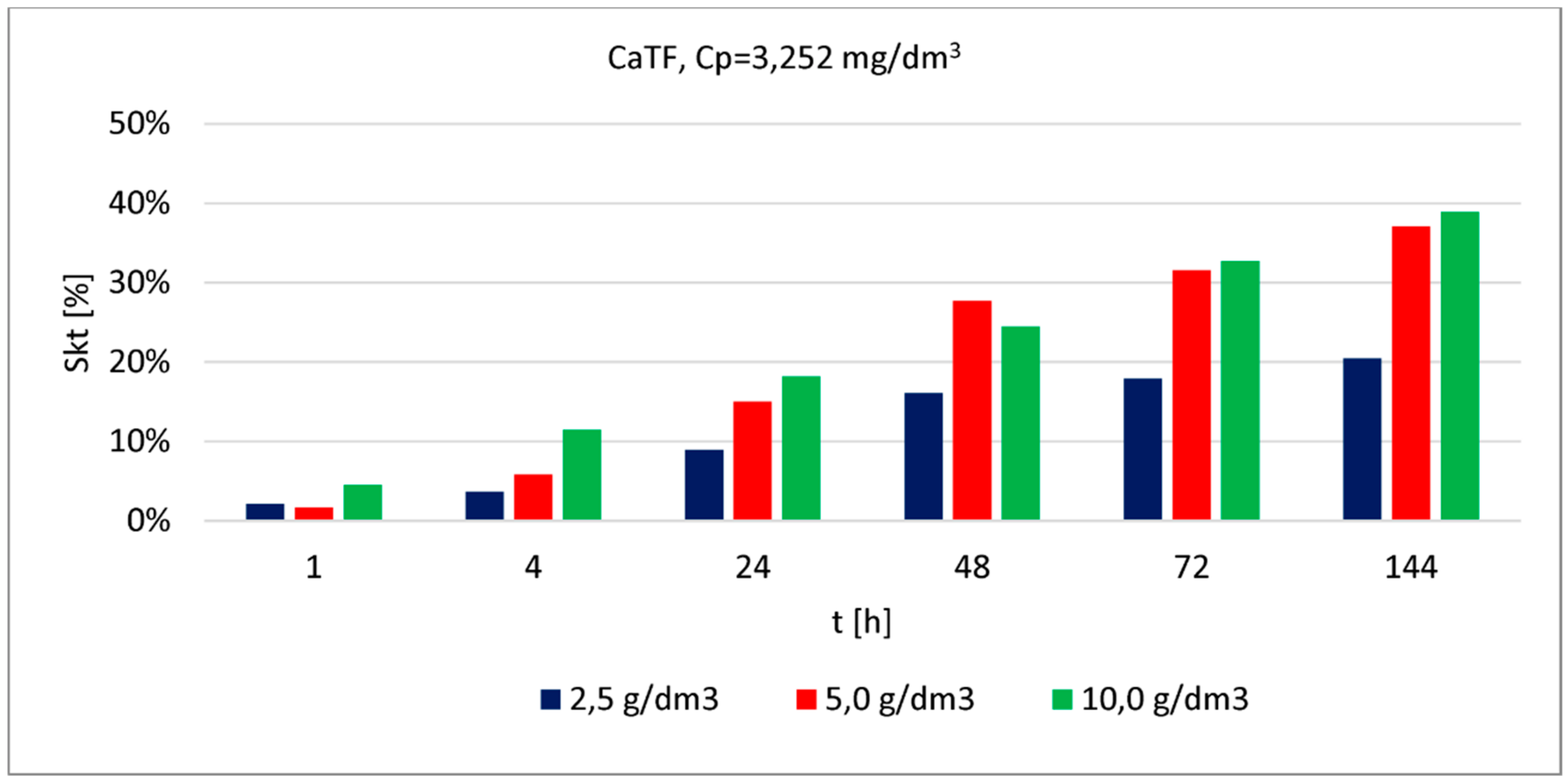
| CaTF | 3.252 | 2.5 | 1 | 2.0 | in article | |
| 5.0 | 2.0 | |||||
| 10.0 | 5.0 | |||||
| 2.5 | 4 | 4.0 | ||||
| 5.0 | 6.0 | |||||
| 10.0 | 11.0 | |||||
| 2.5 | 24 | 9.0 | ||||
| 5.0 | 15.0 | |||||
| 10.0 | 18.0 | |||||
| 2.5 | 48 | 16.0 | ||||
| 5.0 | 28.0 | |||||
| 10.0 | 24.0 | |||||
| 2.5 | 72 | 18.0 | ||||
| 5.0 | 32.0 | |||||
| 10.0 | 33.0 | |||||
| 2.5 | 144 | 20.0 | ||||
| 5.0 | 37.0 | |||||
| 10.0 | 39.0 | |||||
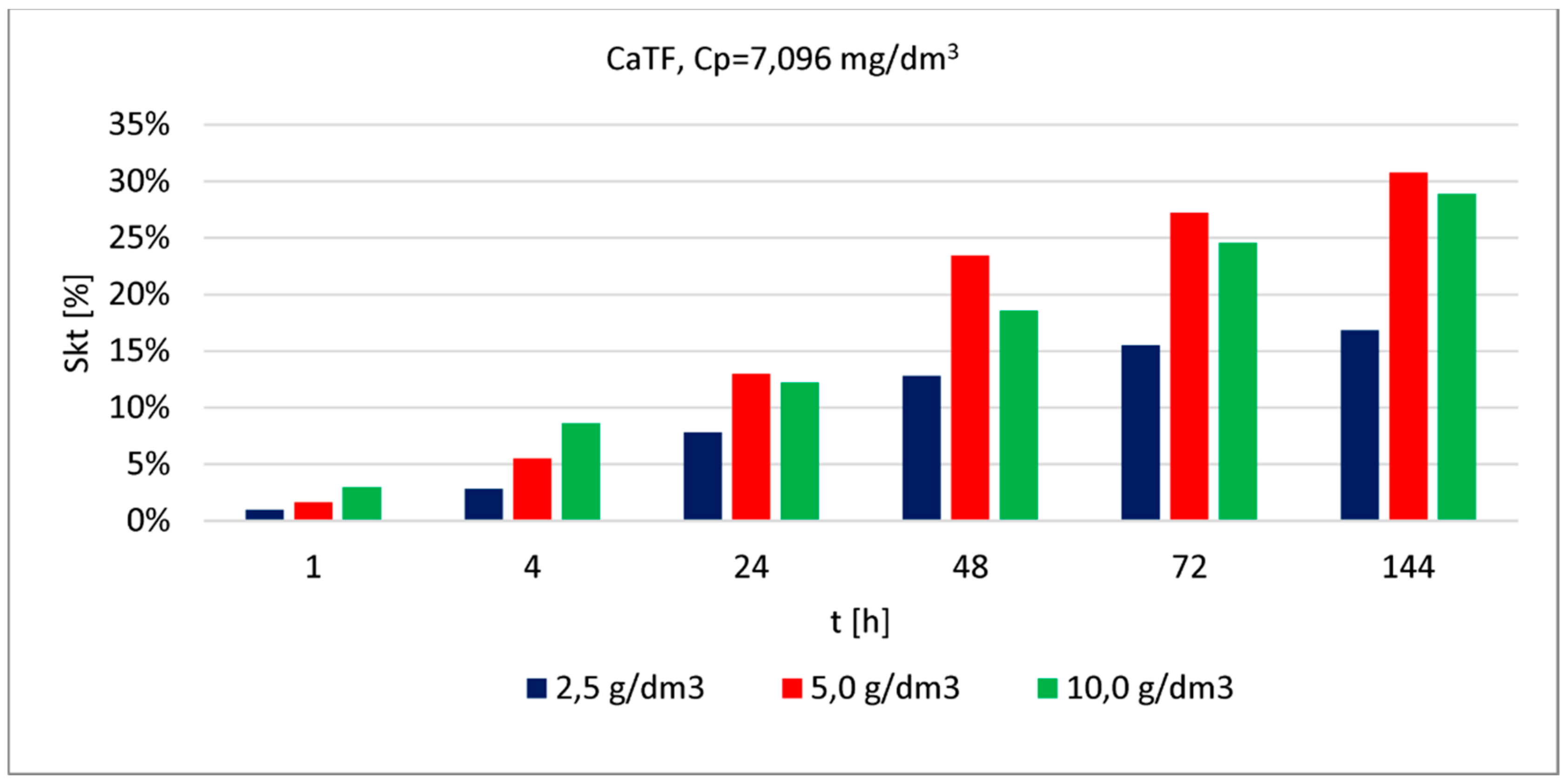
| CaTF | 7.096 | 2.5 | 1 | 1.0 | in article | |
| 5.0 | 2.0 | |||||
| 10.0 | 3.0 | |||||
| 2.5 | 4 | 3.0 | ||||
| 5.0 | 6.0 | |||||
| 10.0 | 9.0 | |||||
| 2.5 | 24 | 8.0 | ||||
| 5.0 | 13.0 | |||||
| 10.0 | 12.0 | |||||
| 2.5 | 48 | 13.0 | ||||
| 5.0 | 23.0 | |||||
| 10.0 | 19.0 | |||||
| 2.5 | 72 | 16.0 | ||||
| 5.0 | 27.0 | |||||
| 10.0 | 25.0 | |||||
| 2.5 | 144 | 17.0 | ||||
| 5.0 | 31.0 | |||||
| 10.0 | 29.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grela, A.; Łach, M.; Mikuła, J. An Efficacy Assessment of Phosphate Removal from Drainage Waters by Modified Reactive Material. Materials 2020, 13, 1190. https://doi.org/10.3390/ma13051190
Grela A, Łach M, Mikuła J. An Efficacy Assessment of Phosphate Removal from Drainage Waters by Modified Reactive Material. Materials. 2020; 13(5):1190. https://doi.org/10.3390/ma13051190
Chicago/Turabian StyleGrela, Agnieszka, Michał Łach, and Janusz Mikuła. 2020. "An Efficacy Assessment of Phosphate Removal from Drainage Waters by Modified Reactive Material" Materials 13, no. 5: 1190. https://doi.org/10.3390/ma13051190
APA StyleGrela, A., Łach, M., & Mikuła, J. (2020). An Efficacy Assessment of Phosphate Removal from Drainage Waters by Modified Reactive Material. Materials, 13(5), 1190. https://doi.org/10.3390/ma13051190







