Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications
Abstract
1. Introduction
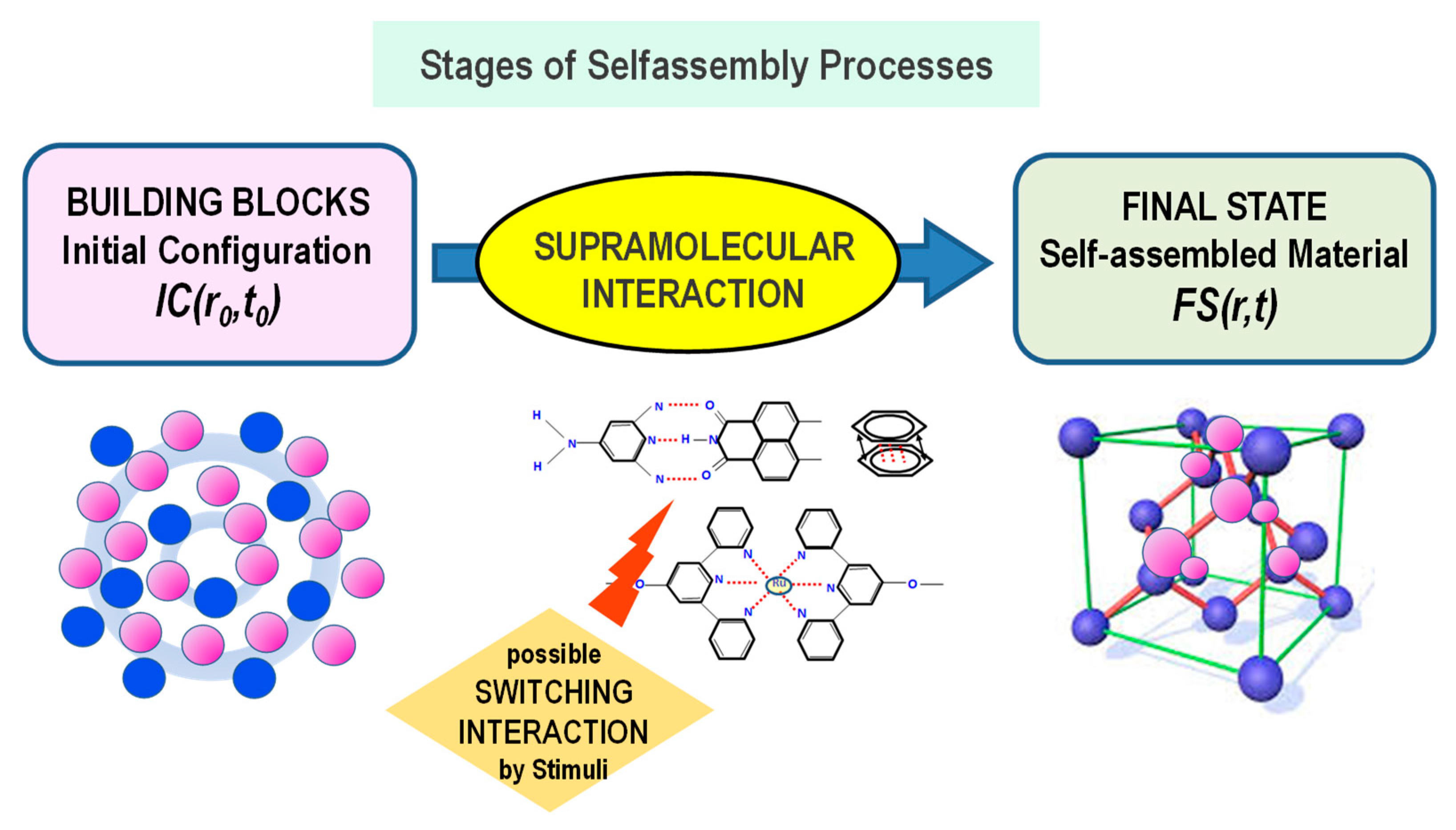
2. Common Features of the Self-Assembly Processes in Nanoscience
2.1. Initial State: Spatial Configuration of Basic Components (Building Blocks)
2.2. (Supra) Molecular Interaction
2.3. Final State: Formation of Functional Supramolecular Structures
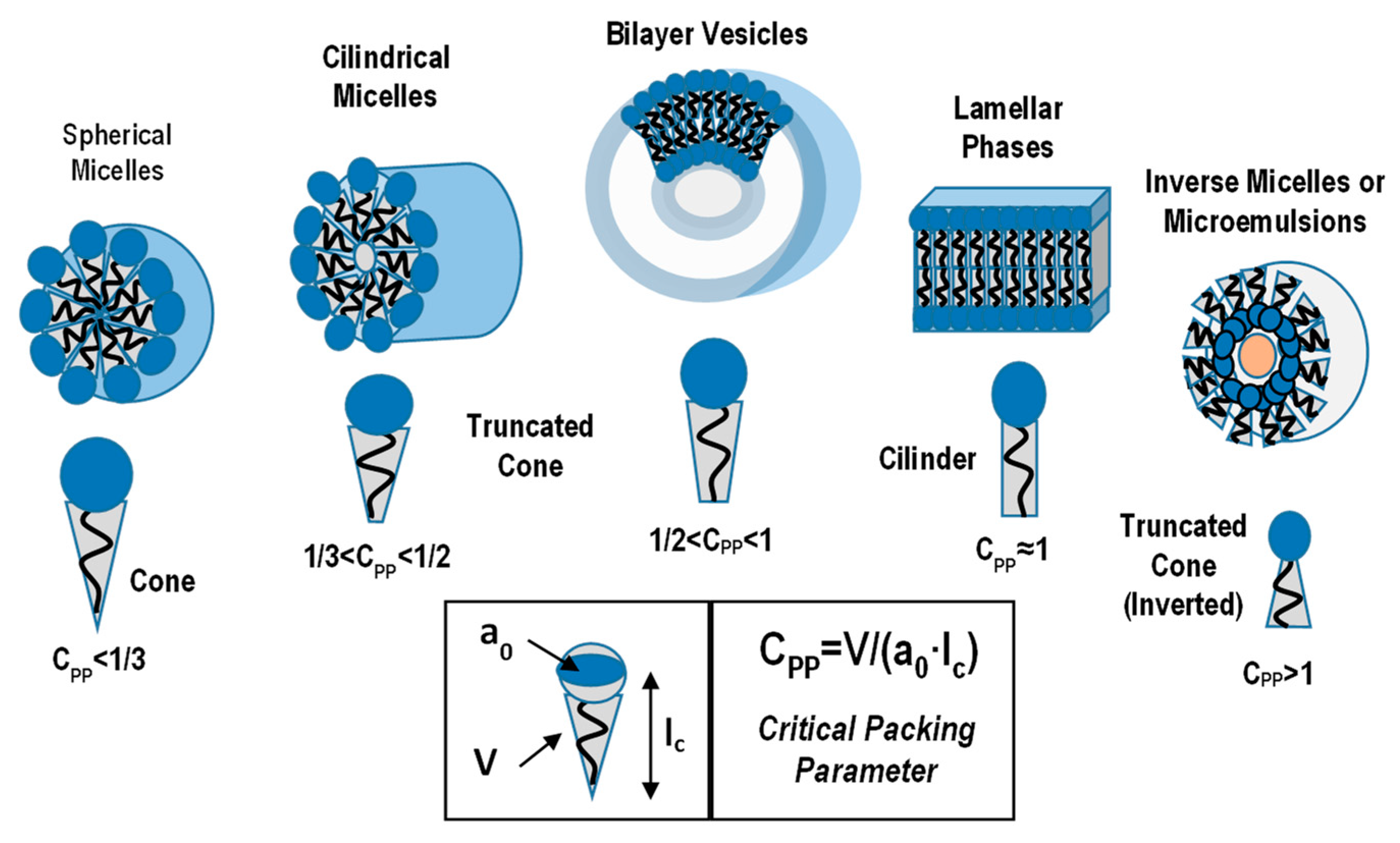
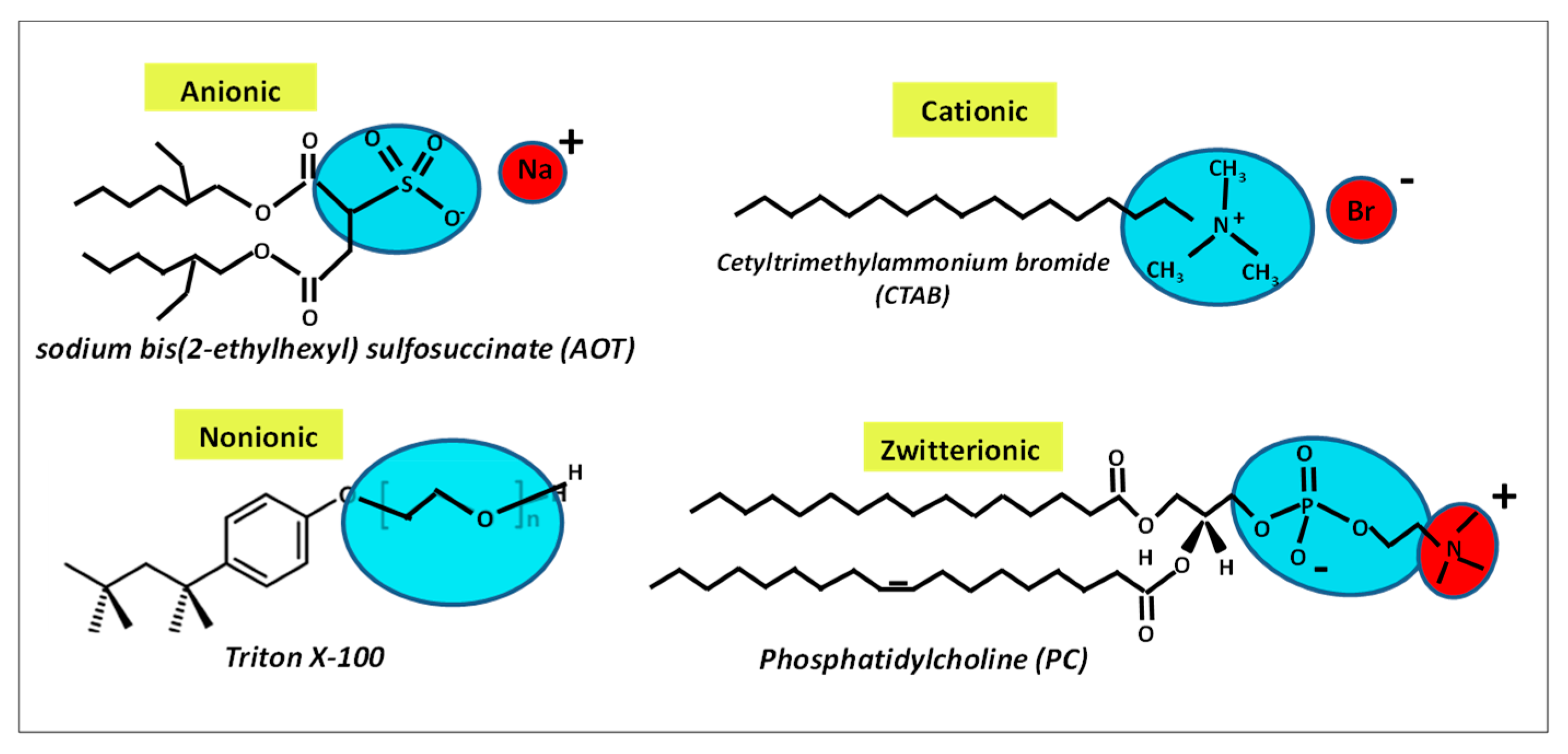
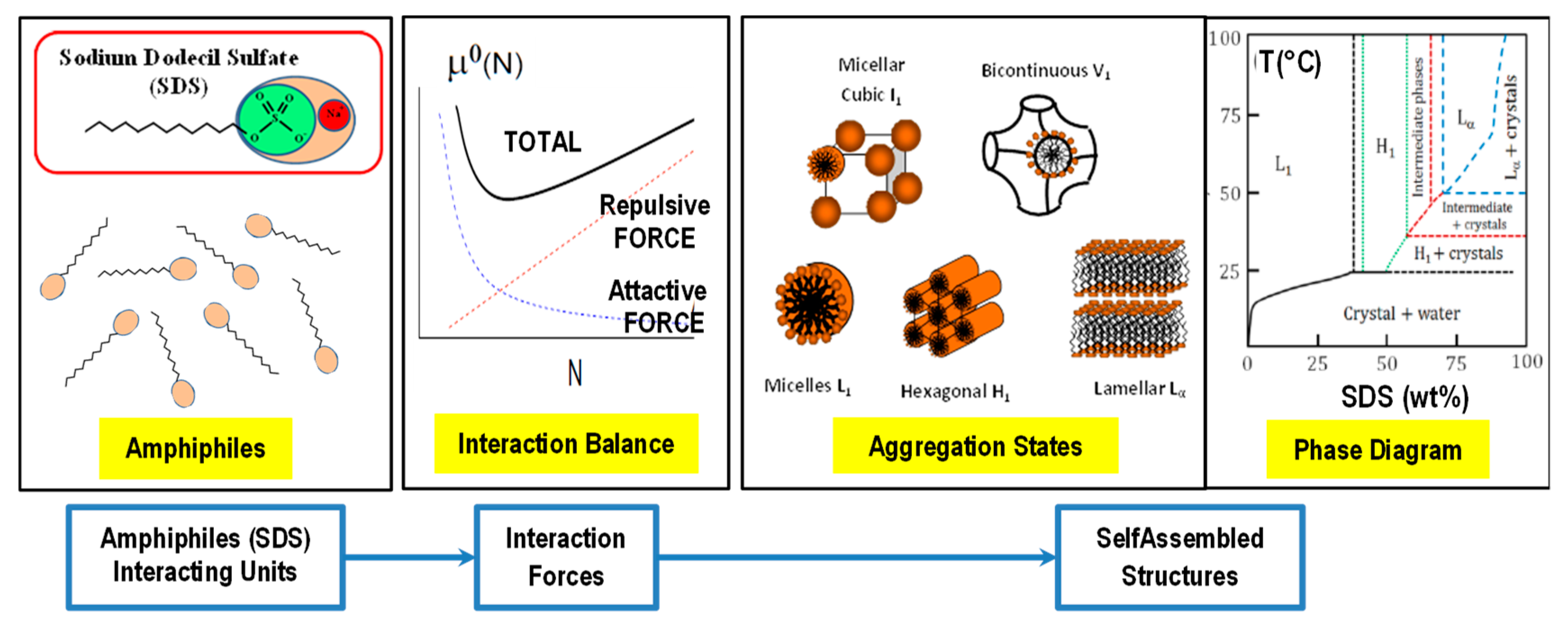
3. Traditional Amphiphile Building Blocks: Micelles, Vesicles, Liquid Crystalline Nanostructures and Microemulsions
3.1. Micelles and Vesicles Nanostructures
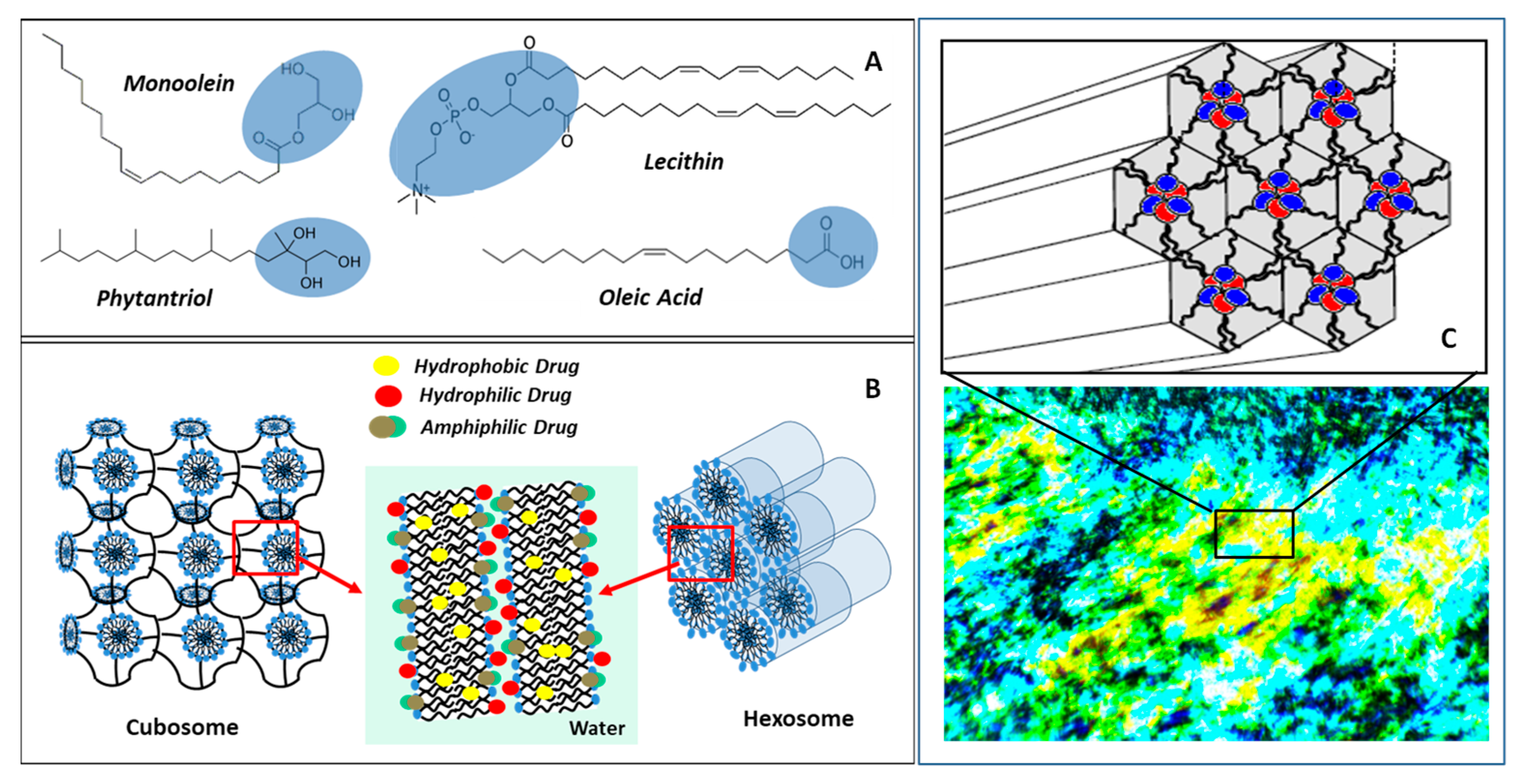
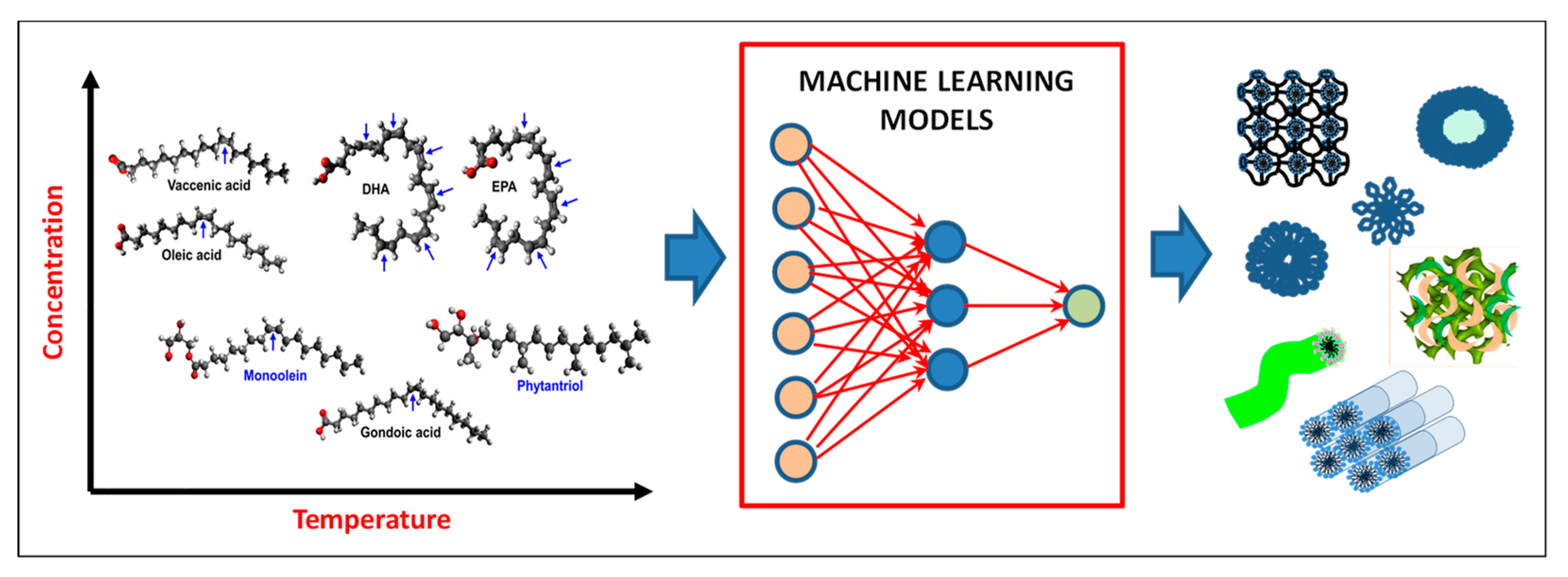
3.2. Liquid Crystalline Nanostructures
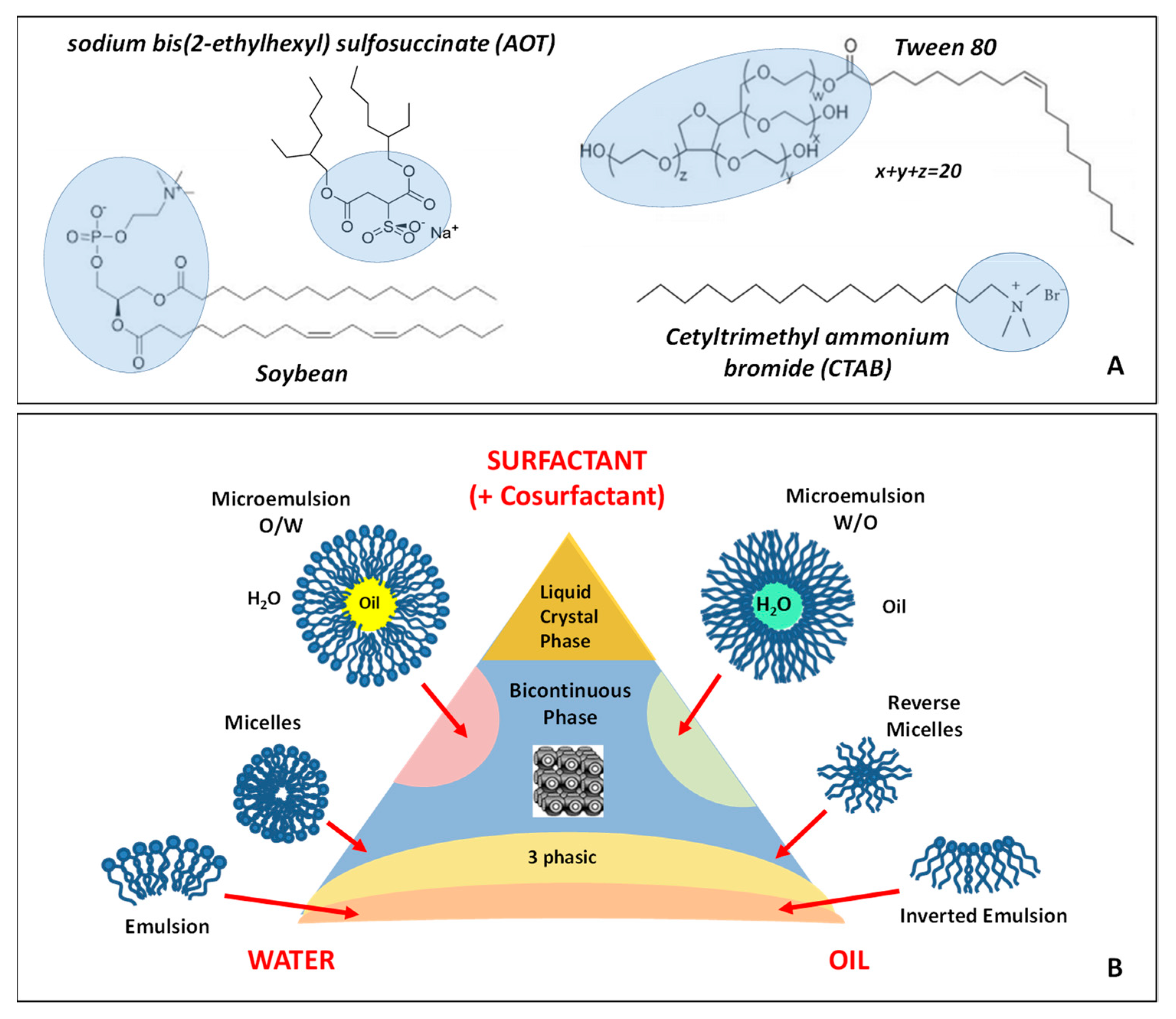
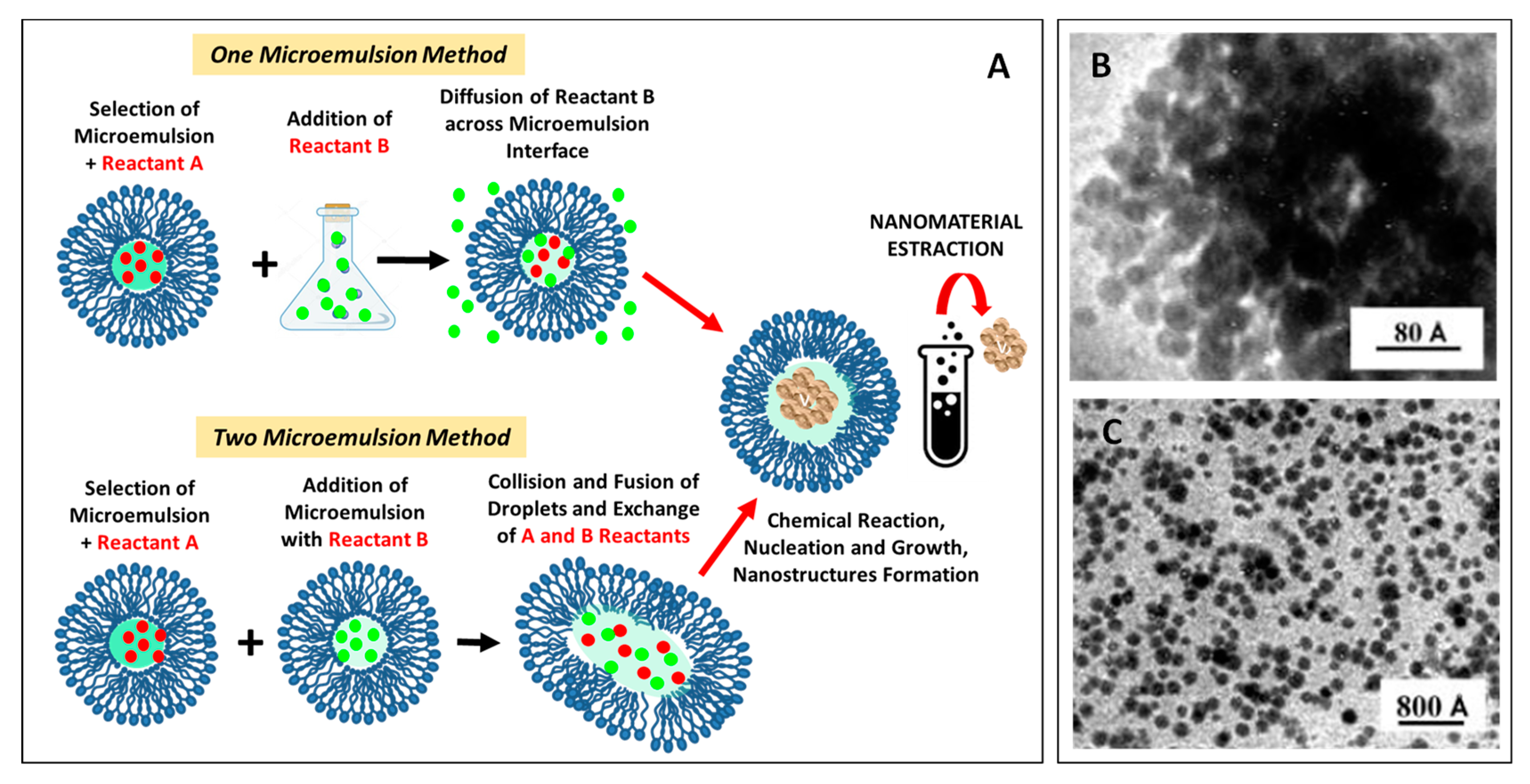
3.3. Self-assembly in Ternary Systems: Microemulsions
4. Polymer-based Building Blocks: Linear, Cross-linked and Hyperbranched/Dendritic Morphologies
- Linear polymers
- Cross-linked polymers (nano-gels)
- Hyperbranched/dendritic polymers
4.1. Linear Polymers
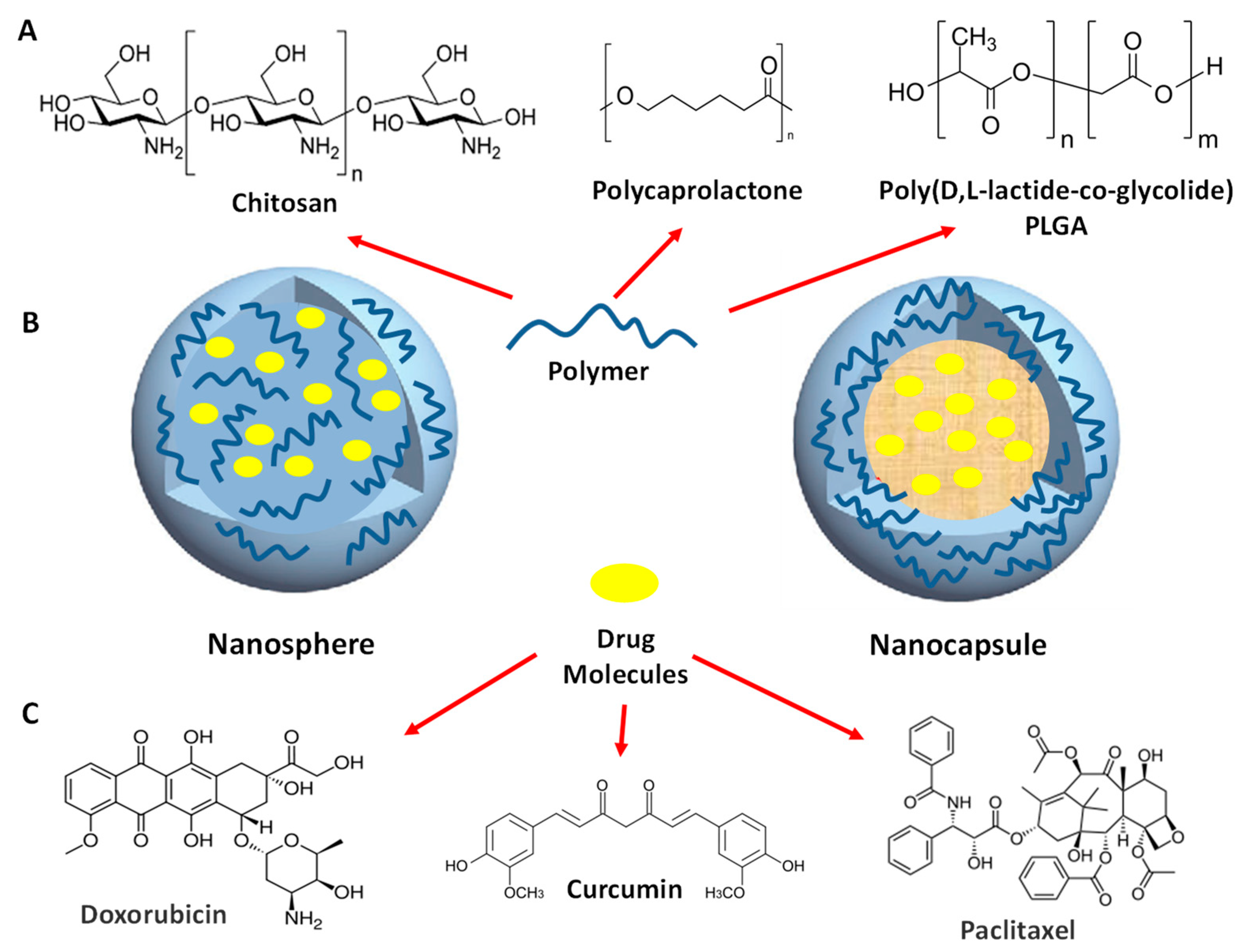
4.1.1. Homopolymers Building Blocks: Polymer-based Nanoparticles
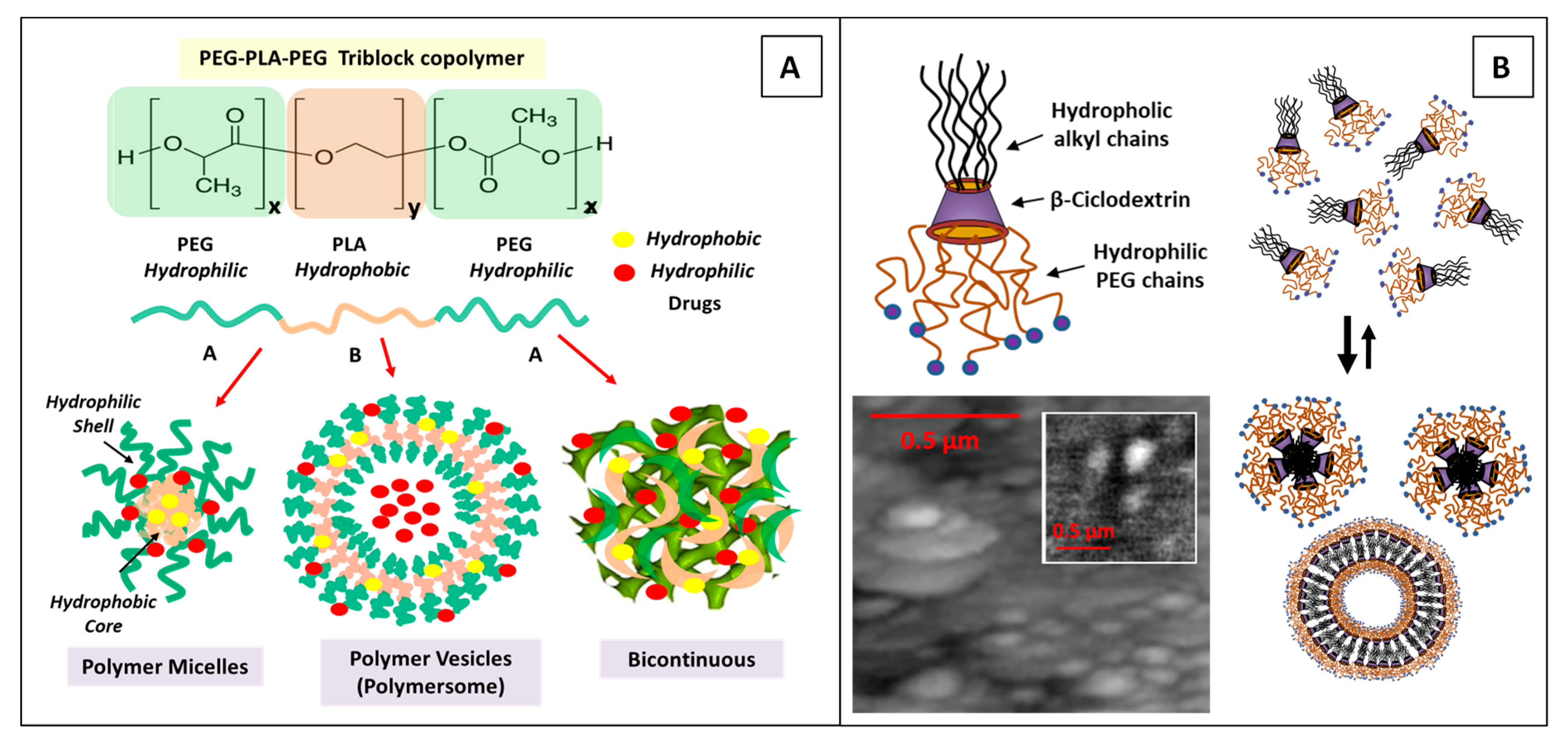
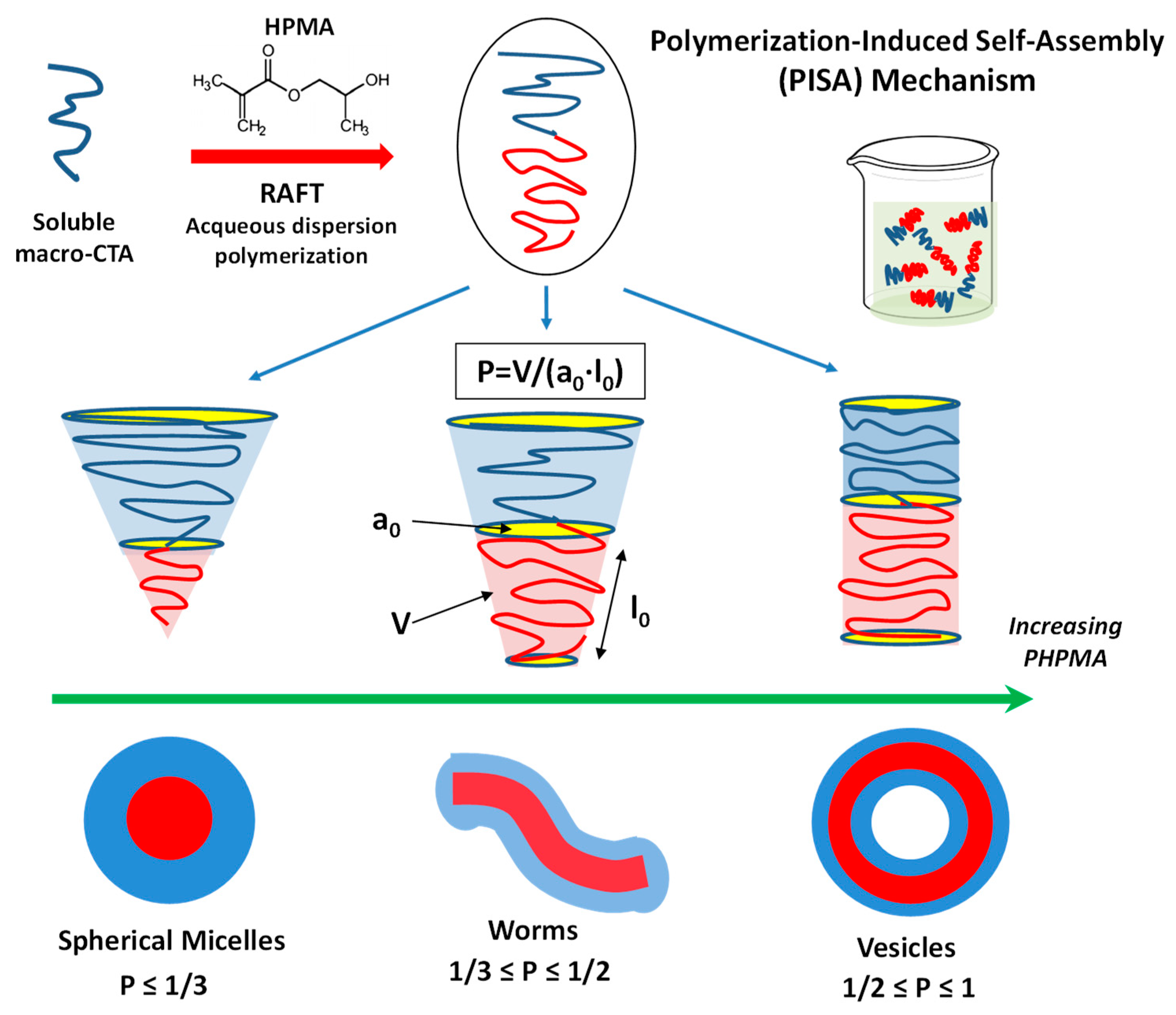
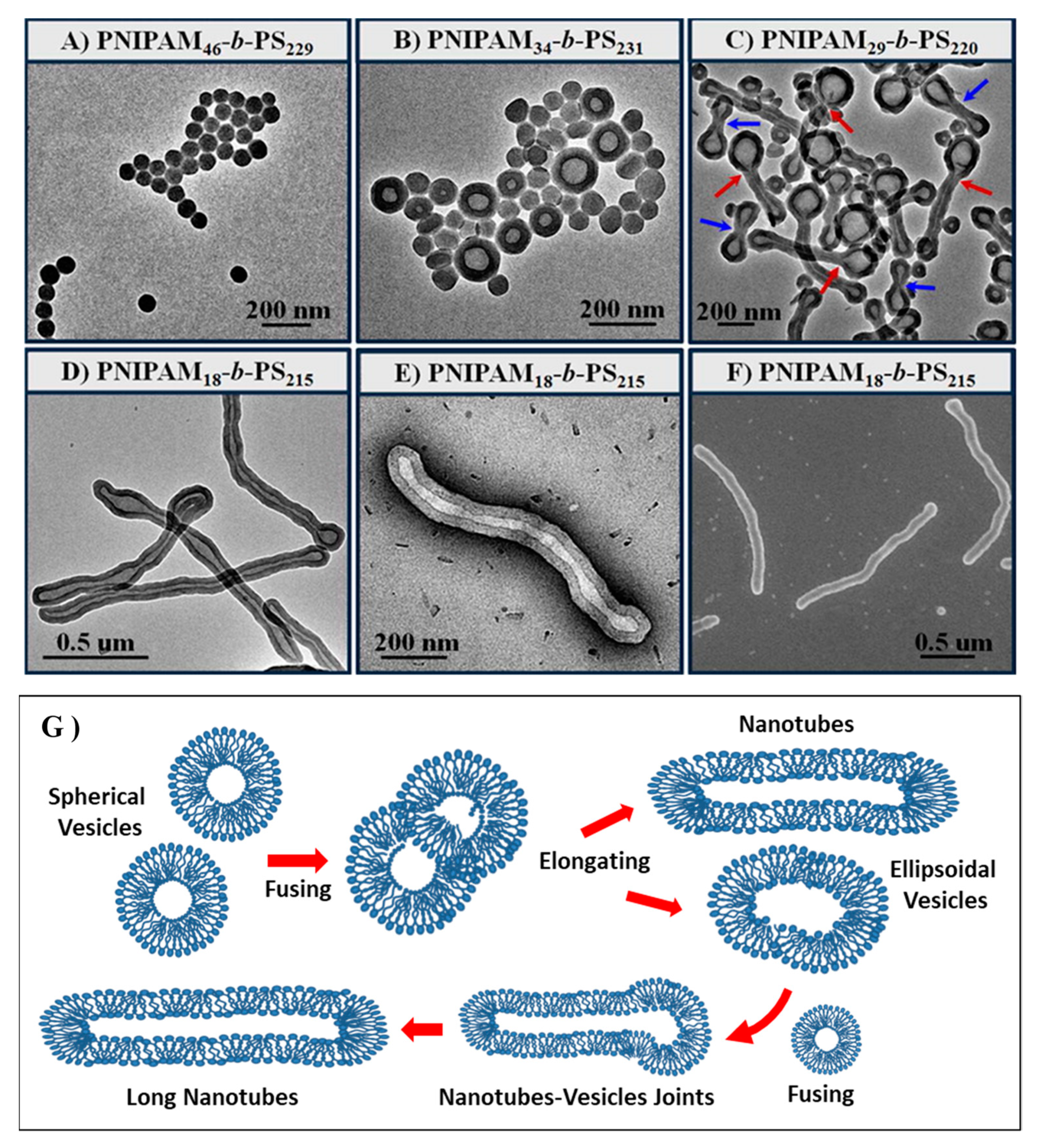
4.1.2. Amphiphilic Block Copolymers
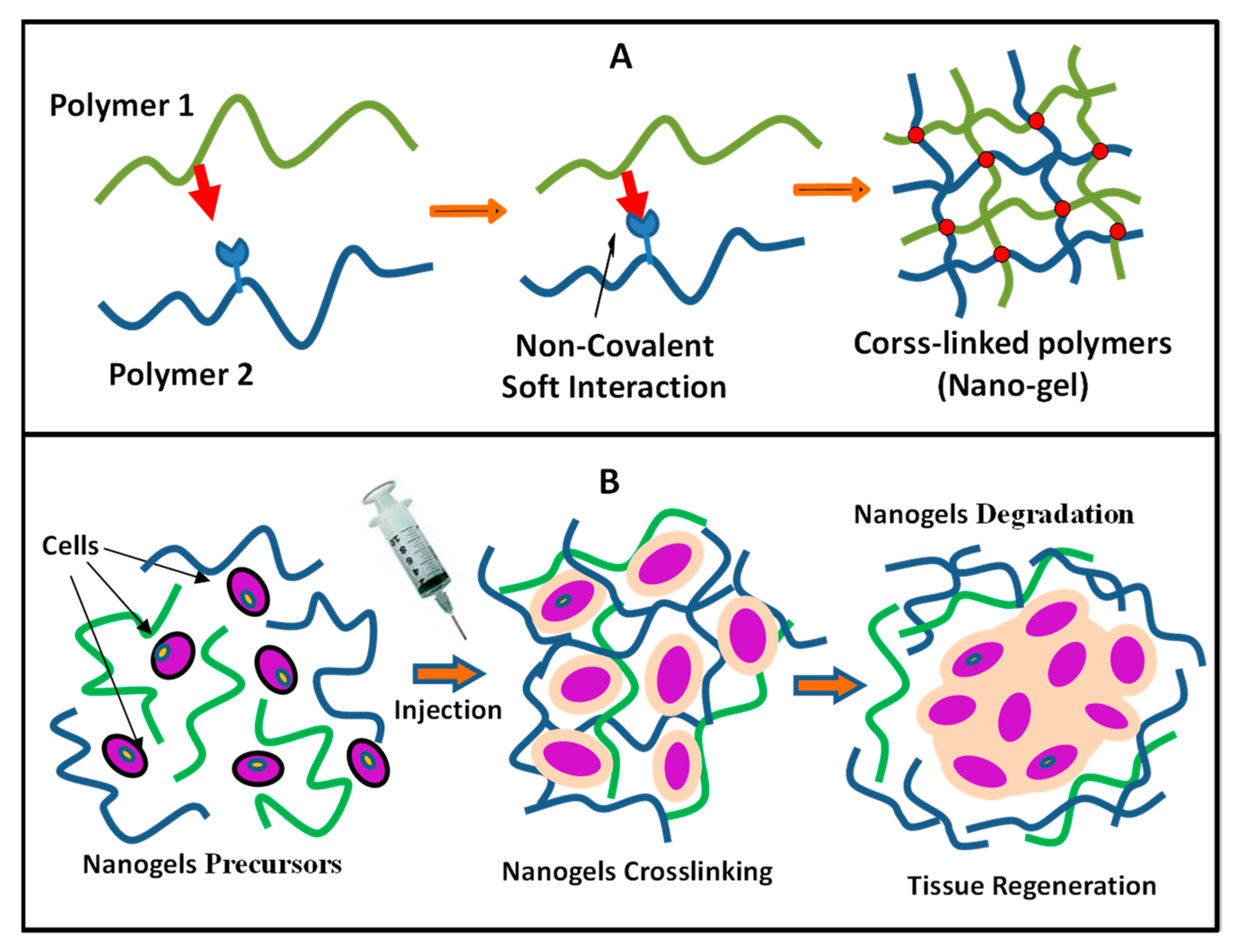
4.2. Cross-linked Polymers (Nanogels)
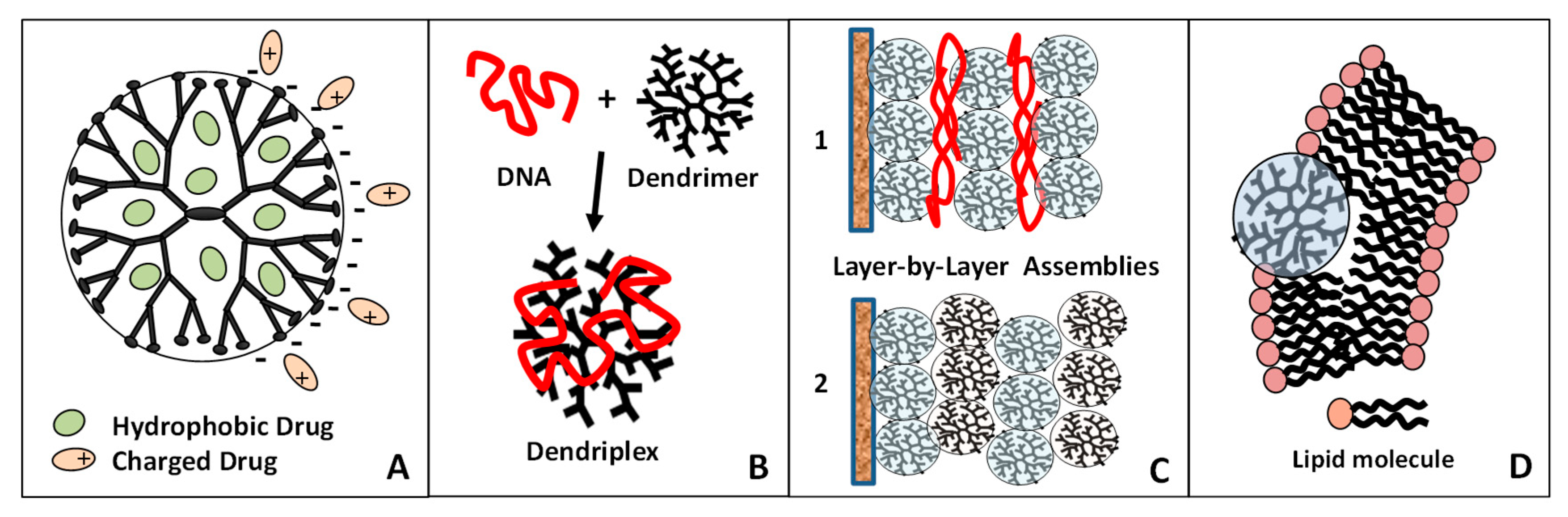
4.3. Hyperbranched/Dendritic Polymers: Self-assembly and Co-assembly of Dendrimers
5. Self-assembly by Biomolecules Building Blocks
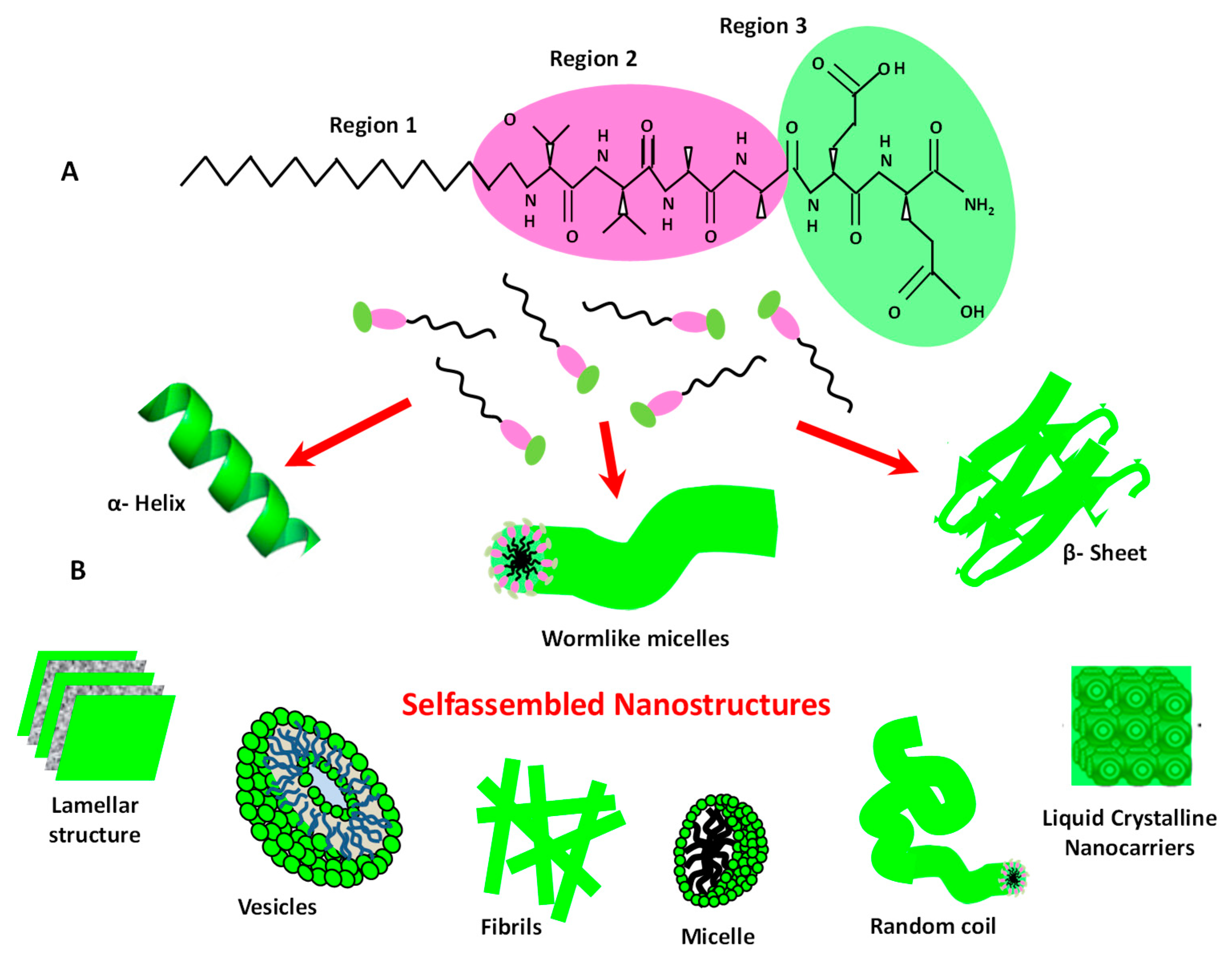
5.1. Peptide and Protein Based Bio-Nanomaterials
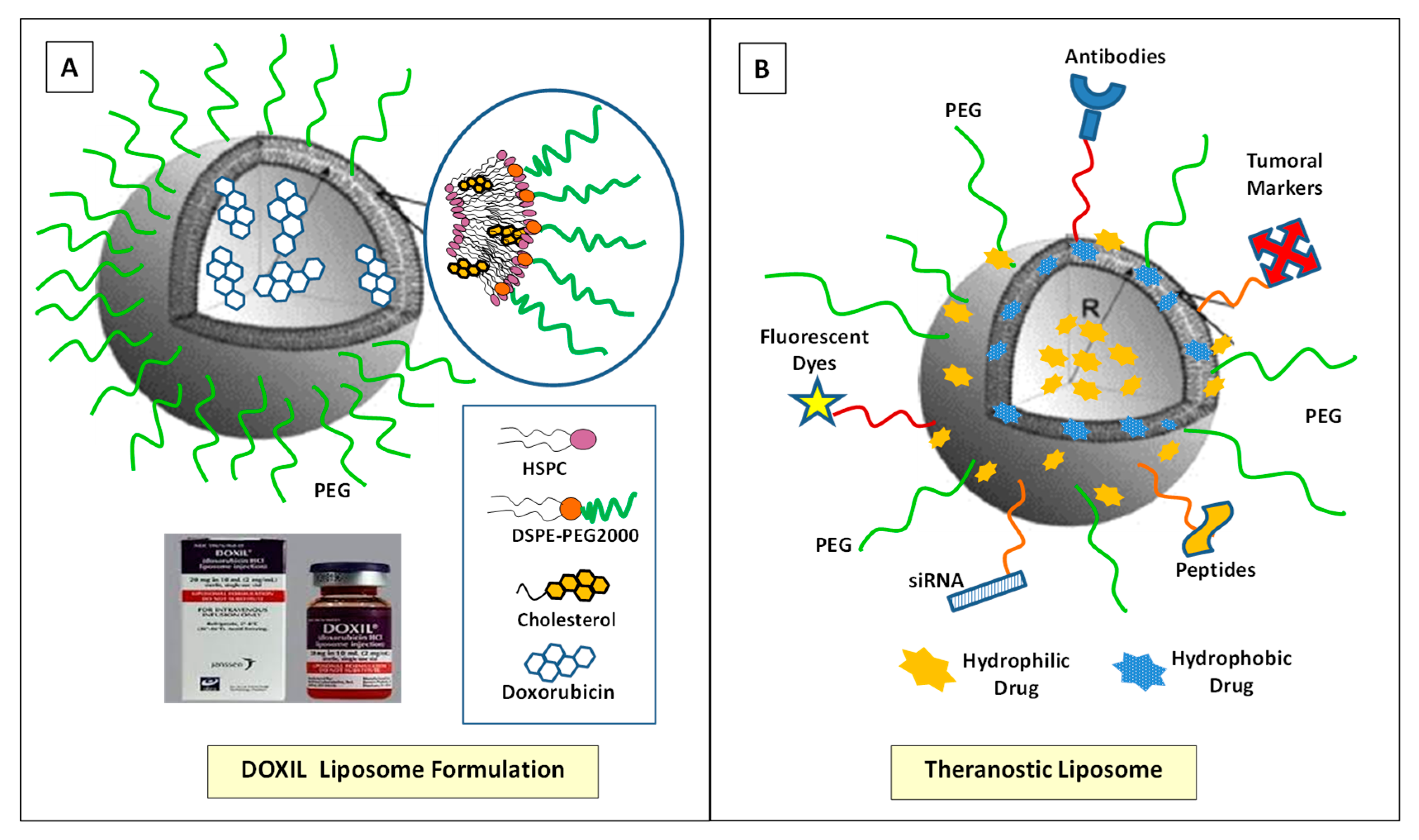
5.2. Lipids
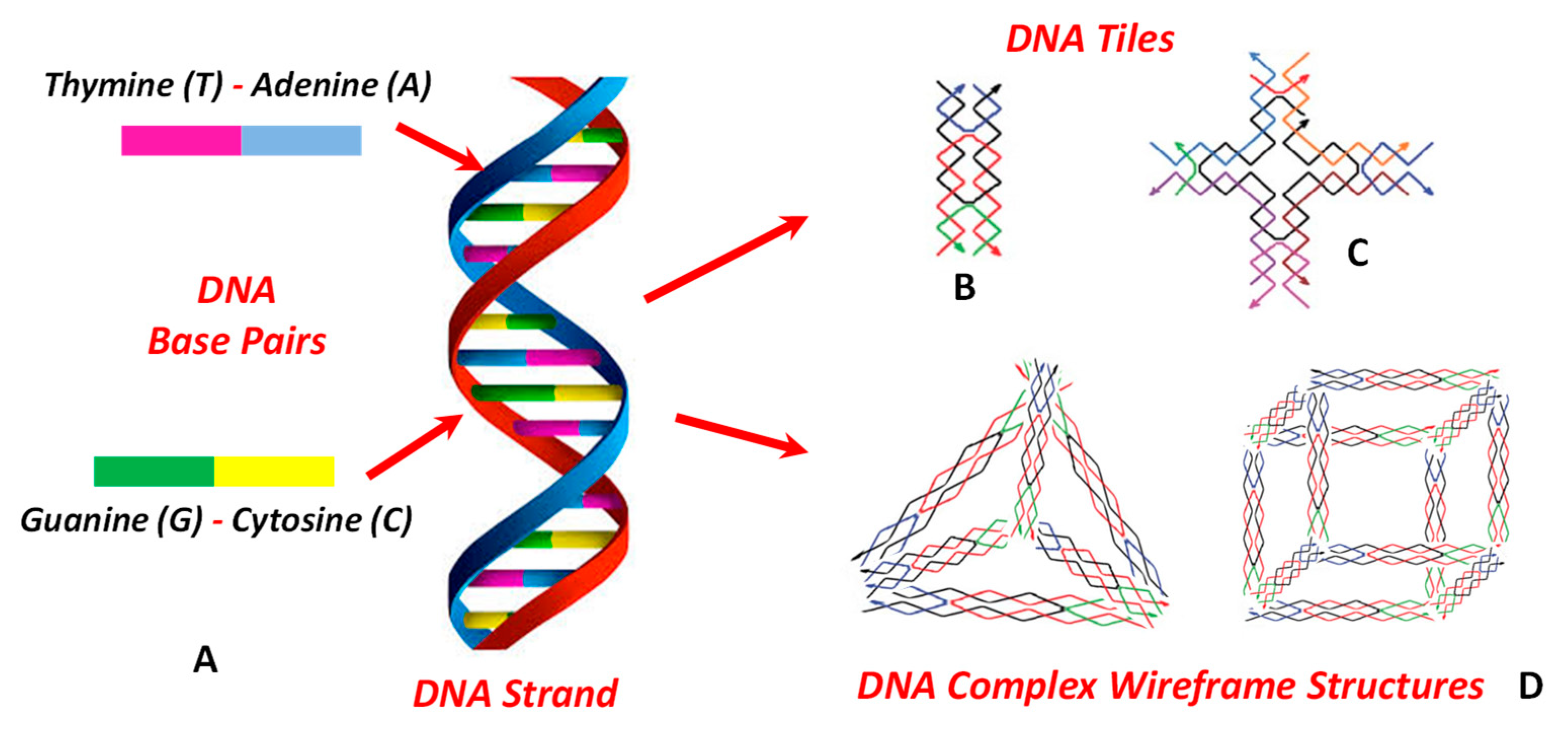
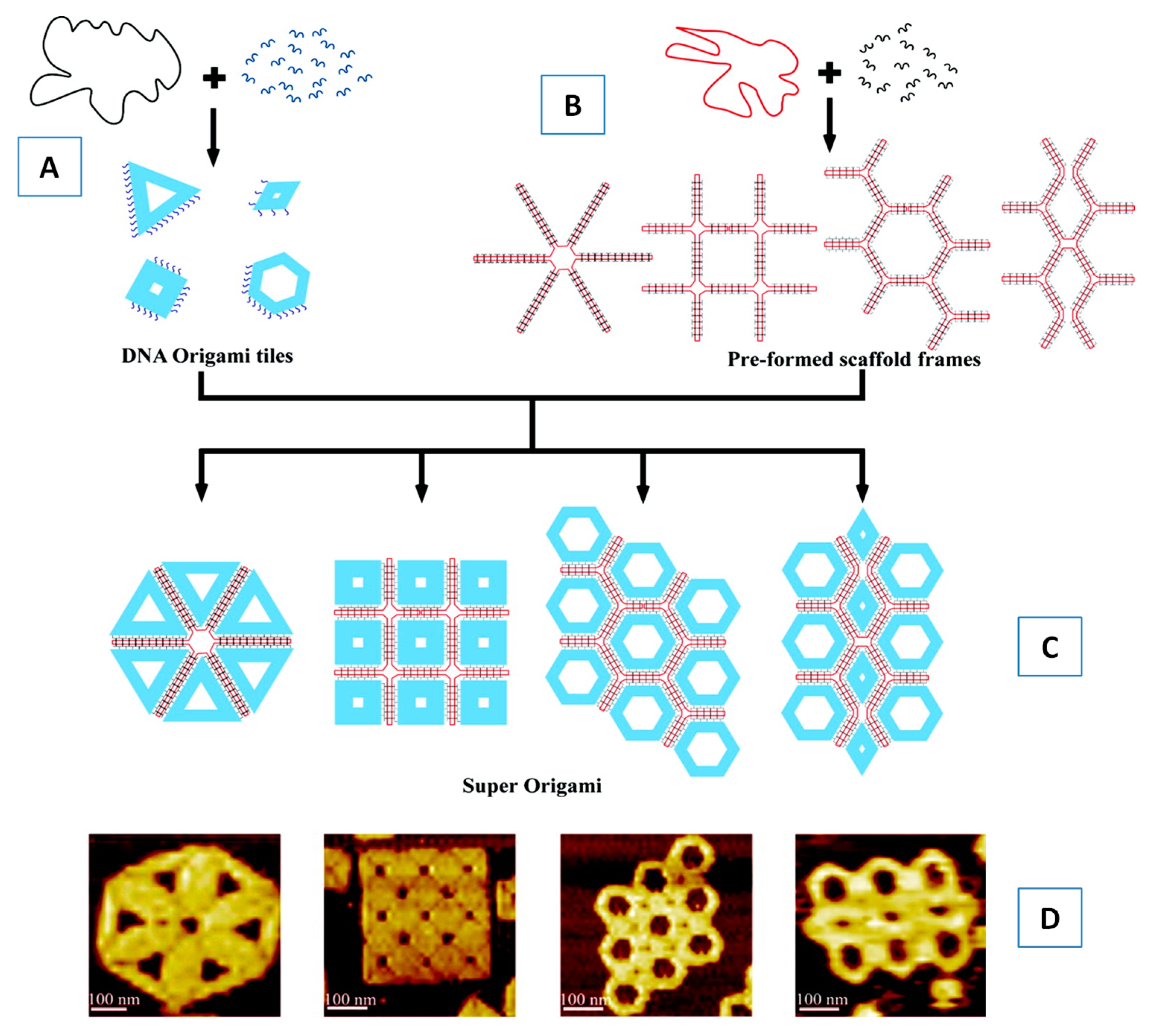
5.3. Self-assembly of Oligonucleotides (DNA and RNA)
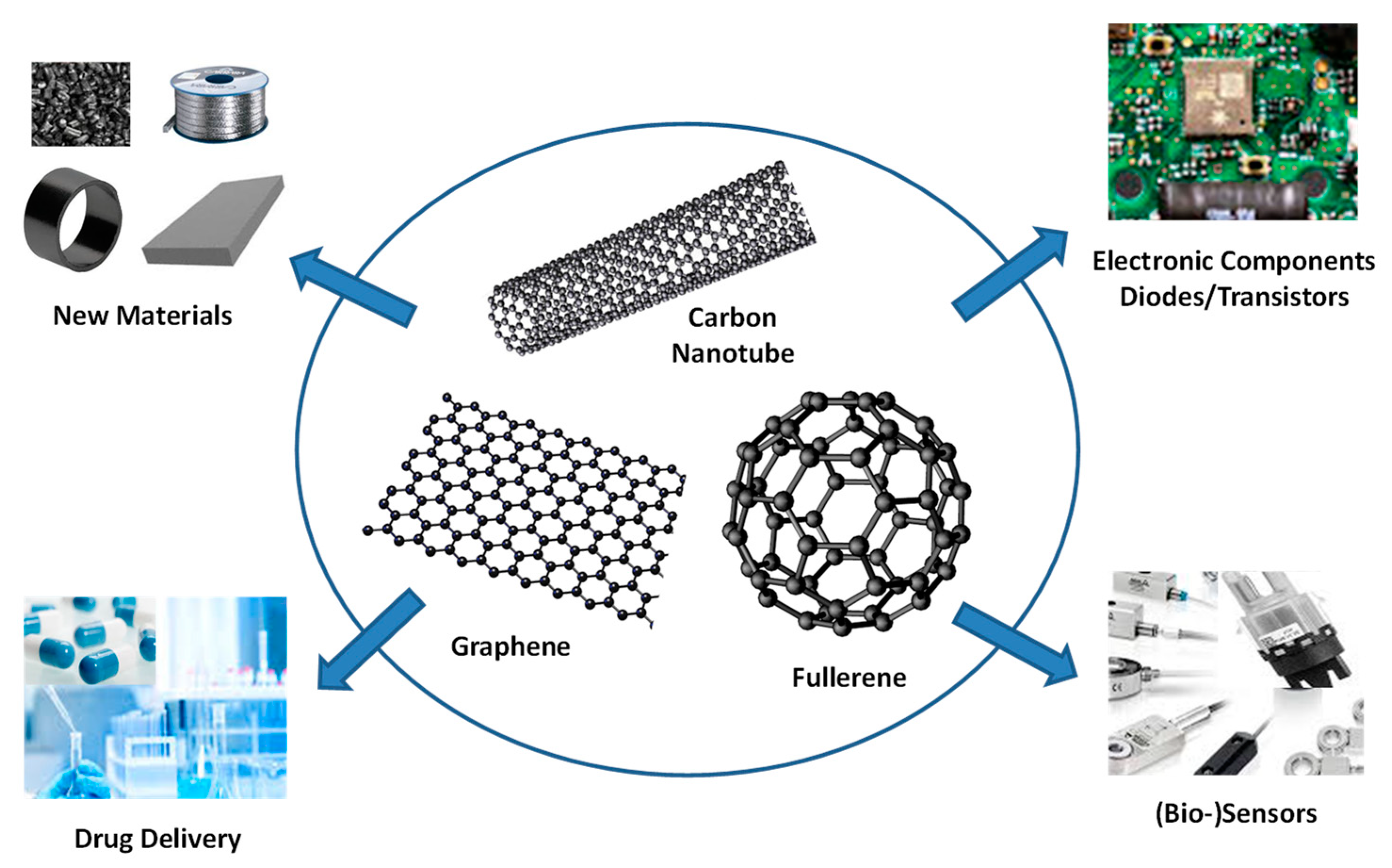
6. Emerging Technologies: Self-assembly of Carbon-based Nanostructured Materials
6.1. Carbon Nanotubes
6.2. Graphene
6.3. Fullerene
7. Nature-Inspired Nanomaterials: Self-Assembly of Nanostructured Dyes for Solar Cells Applications
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular Chemistry; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1995. [Google Scholar]
- Gale, P.; Steed, J. Supramolecular Chemistry: From Molecules to Nanomaterials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Tiwari, A.; Tiwari, A. Nanomaterials in Drug Delivery, Imaging, and Tissue Engineerin; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Aguilar, Z.P. Nanomaterials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomat. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Malmsten, M. Surfactants and Polymers in Drug Delivery; Marcel Dekker Inc.: New York, NY, USA, 2006; pp. 116–212. [Google Scholar]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Caschera, D.; Liveri, V.T.; Lombardo, D. How self-assembly of amphiphilic molecules can generate complexity in the nanoscale. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 164–183. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/molecular nanoarchitectonics for devices and related applications. Nano Today 2019, 28, 100762. [Google Scholar] [CrossRef]
- Grzelczak, M.; Liz-Marzán, L.M.; Klajn, R. Stimuli-responsive self-assembly of nanoparticles. Chem. Soc. Rev. 2019, 48, 1342–1361. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chi-Leung Hui, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef] [PubMed]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Tang, Z. Chiral Nanomaterials: Preparation, Properties and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar]
- Lee, Y.S. Self-Assembly and Nanotechnology, a Force Balance Approach; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zana, R. Dynamics of Surfactant Self-Assemblies: Micelles, Microemulsions, Vesicles and Lyotropic Phases; Taylor & Francis: London, UK, 2005. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: New York, NY, USA, 1992. [Google Scholar]
- Lombardo, D.; Kiselev, M.A.; Magazu, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Cond. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]
- Holmberg, K.; Jonsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; Wiley and Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Domb, C.; Green, M.S.; Lebowitz, J.L.; Gompper, G.; Schick, M. Self-Assembling Amphiphilic Systems (Phase Transitions and Critical Phenomena); Academic Press: London, UK, 1994. [Google Scholar]
- Laughlin, R.G. The Aqueous Phase Behavior of Surfactants; Academic Press: London, UK, 1994. [Google Scholar]
- LeBard, D.N.; Levine, B.G.; DeVane, R.; Shinoda, W.; Klein, M.L. Premicelles and monomer exchange in aqueous surfactant solutions above and below the critical micelle concentration. Chem. Phys. Lett. 2012, 522, 38–42. [Google Scholar] [CrossRef]
- Lombardo, D.; Munaò, M.; Calandra, P.; Pasqua, L.; Caccamo, M.T. Evidence of pre-micellar aggregates in water solution of amphiphilic PDMS-PEO block copolymer. Phys. Chem. Chem. Phys. 2019, 21, 11983–11991. [Google Scholar] [CrossRef]
- Cui, X.; Mao, S.; Liu, M.; Yuan, H.; Du, Y. Mechanism of Surfactant Micelle Formation. Langmuir 2008, 24, 10771–10775. [Google Scholar] [CrossRef] [PubMed]
- Turco Liveri, V.; Lombardo, D.; Pochylski, M.; Calandra, P. Molecular association of small amphiphiles: Origin of ionic liquid properties in dibutyl phosphate/propylamine binary mixtures. J. Mol. Liq. 2018, 263, 274–281. [Google Scholar] [CrossRef]
- Lyu, X.; Xiao, A.; Zhang, W.; Hou, P.; Gu, K.; Tang, Z.; Pan, H.; Wu, F.; Shen, Z.; Fan, X.H. Head-Tail Asymmetry as the Determining Factor in the Formation of Polymer Cubosomes or Hexasomes in a Rod-Coil Amphiphilic Block Copolymer. Angew. Chem. Int. Ed. Engl. 2018, 57, 10132–10136. [Google Scholar] [CrossRef] [PubMed]
- Israelachvilli, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. II 1976, 72, 1525–1567. [Google Scholar] [CrossRef]
- Wu, A.; Gao, Y.; Zheng, L. Zwitterionic amphiphiles: Their aggregation behavior and applications. Green Chem. 2019, 21, 4290–4312. [Google Scholar] [CrossRef]
- Abe, M. Mixed Surfactant Systems, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: New York, NY, USA, 2004. [Google Scholar]
- Calandra, P.; Nicotera, I.; Rossi, C.O.; Turco Liveri, V. Dynamical Properties of Self-Assembled Surfactant-Based Mixtures: Triggering of One-Dimensional Anomalous Diffusion in Bis (2-ethylhexyl) phosphoric Acid/n-Octylamine Systems. Lamgmuir 2013, 29, 14848–14854. [Google Scholar] [CrossRef]
- Calandra, P.; Ruggirello, A.; Mele, A.; Turco Liveri, V. Self-assembly in surfactant-based liquid mixtures: Bis (2-ethylhexyl) phosphoric acid/bis (2-ethylhexyl)amine systems. J. Colloid Interf. Sci. 2010, 348, 183–188. [Google Scholar] [CrossRef]
- Sachin, K.M.; Karpe, S.A.; Singh, M.; Bhattarai, A. Self-assembly of sodium dodecylsulfate and dodecyltrimethylammonium bromide mixed surfactants with dyes in aqueous mixtures. R. Soc. Open Sci. 2019, 6, 181979. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering-ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. Liquid Crystals with Nano and Microparticles; World Scientific: Singapore, 2017. [Google Scholar]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Calandra, P.; Turco Liveri, V.; Ruggirello, A.M.; Licciardi, M.; Lombardo, D.; Mandanici, A. Anti-Arrhenian behaviour of conductivity in octanoic acid–bis (2-ethylhexyl) amine systems: A physico-chemical study. J. Mater. Chem. C 2015, 3, 3198–3210. [Google Scholar] [CrossRef]
- Calandra, P.; Caponetti, E.; Chillura Martino, D.; D’Angelo, P.; Minore, A.; Turco Liveri, V. FT-IR and Dielectric study of water/AOT liquid crystals. J. Mol. Struct. 2000, 522, 165–178. [Google Scholar] [CrossRef]
- Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef]
- Popov, N.; Honaker, L.W.; Popova, M.; Usol’tseva, N.; Mann, E.K.; Jákli, A.; Popov, P. Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials 2017, 11, 20. [Google Scholar] [CrossRef]
- Calandra, P.; Turco Liveri, V.; Riello, P.; Freris, I.; Mandanici, A. Self-assembly in surfactant-based liquid mixtures: Octanoic acid/Bis (2-ethylhexyl) amine systems. J. Colloid Interf. Sci. 2012, 367, 280–285. [Google Scholar] [CrossRef]
- Lugger, J.; Mulder, D.J.; Sijbesma, R.; Schenning, A. Nanoporous Polymers Based on Liquid Crystals. Materials 2018, 11, 104. [Google Scholar] [CrossRef]
- Iino, H.; Hanna, J. Liquid crystalline organic semiconductors for organic transistor applications. Polym. J. 2017, 49, 23–30. [Google Scholar] [CrossRef]
- Garti, N.; Libster, D.; Aserin, A. Solubilization and Delivery of Drugs from GMO-Based Lyotropic Liquid Crystals. In Nanoscience with Liquid Crystals; Li, Q., Ed.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Mo, J.; Milleret, G.; Nagaraj, M. Liquid crystal nanoparticles for commercial drug delivery. Liq. Cryst. Rev. 2017, 5, 69–85. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Ustunel, S.; Hegmann, E. Liquid Crystal Elastomers-A Path to Biocompatible and Biodegradable 3D-LCE Scaffolds for Tissue Regeneration. Materials 2018, 11, 377. [Google Scholar] [CrossRef]
- Le, T.C.; Tran, N. Using Machine Learning to Predict the Self-Assembled Nanostructures of Monoolein and Phytantriol as a Function of Temperature and Fatty Acid Additives for Effective Lipid-Based Delivery Systems. ACS Appl. Nano Mater. 2019, 2, 1637–1647. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.C.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Wei, L.; Li, X.; Guo, F.; Liu, X.; Wang, Z. Structural properties, in vitro release and radical scavenging activity of lecithin based curcumin-encapsulated inverse hexagonal (HII) liquid crystals. Colloids Surf. A 2018, 539, 124–131. [Google Scholar] [CrossRef]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid crystalline nanostructures as pegylated reservoirs of omega-3 polyunsaturated fatty acids: Structural insights toward delivery formulations against neurodegenerative disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef]
- Baskaran, R.; Madheswaran, T.; Sundaramoorthy, P.; Kim, H.M.; Yoo, B.K. Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. Int. J. Nanomed. 2014, 9, 3119–3130. [Google Scholar] [CrossRef]
- Iqbal, D.; Samiullah, M.H. Photo-Responsive Shape-Memory and Shape-Changing Liquid-Crystal Polymer Networks. Materials 2013, 6, 116–142. [Google Scholar] [CrossRef]
- Attwood, D. Microemulsions. In Colloidal Drug Delivery Systems; Kreuter, J., Ed.; Dekker: New York, NY, USA, 1994; pp. 31–71. [Google Scholar]
- Eccleston, G.M.; Swarbick, J.; Boylan, J.C. Emulsion and Microemulsions. Encycl. Pharm. Technol. 2002, 2, 1080–1085. [Google Scholar]
- Acharya, D.P.; Hartley, P.G. Progress in microemulsion characterization. Curr. Opin. Colloid Interface 2012, 17, 274–280. [Google Scholar] [CrossRef]
- Richard, B.; Lemyre, J.-L.; Ritcey, A.M. Nanoparticle Size Control in Microemulsion Synthesis. Langmuir 2017, 33, 4748–4757. [Google Scholar] [CrossRef]
- Calandra, P.; Di Marco, G.; Ruggirello, A.; Turco Liveri, V. Physico-chemical investigation of nanostructures in liquid phases: Nickel chloride ionic clusters confined in sodium bis (2-ethylhexyl) sulfosuccinate reverse micelles. J. Colloid Interface Sci. 2009, 336, 176–182. [Google Scholar] [CrossRef]
- Perazzo, A.; Tomaiuolo, G.; Preziosi, V.; Guido, S. Emulsions in porous media: From single droplet behavior to applications for oil recovery. Adv. Colloid Interface Sci. 2018, 256, 305–325. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Boonme, P.; Junyaprasert, V.B.; Suksawad, N.; Songkro, S. Microemulsions and nano-emulsions: Novel vehicles for whitening cosmeceuticals. J. Biomed. Nanotechnol. 2009, 5, 373–383. [Google Scholar] [CrossRef]
- Hussain, T.; Batool, R. Microemulsion route for the synthesis of nano-structured catalytic materials. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Theochari, I.; Goulielmaki, M.; Danino, D.; Papadimitriou, V.; Pintzas, A.; Xenakis, A. Drug nanocarriers for cancer chemotherapy based on microemulsions: The case of Vemurafenib analog PLX4720. Colloids Surf. B Biointerfaces 2017, 154, 350–356. [Google Scholar] [CrossRef]
- Calandra, P.; Longo, A.; Turco Liveri, V. Preparation and Characterisation of Na2S and ZnSO4 Nanoparticles in Water/AOT/n-Heptane Microemulsions. Coll. Pol. Sci. 2001, 279, 1112–1117. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A review on preparation, properties and applications of polymeric nanoparticlebased materials. Polym. Plast. Technol. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated Polymer Nanoparticles for Bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef]
- Canfarotta, F.; Whitcombe, M.J.; Piletsky, S.A. Polymeric nanoparticles for optical sensing. Biotechnol. Adv. 2013, 31, 1585–1599. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J.S. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, Y.; Wang, Q.; Zhao, L. Curcumin-loaded chitosan–bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Mora-Huertasa, C.E.; Fessia, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Feng, H.; Lu, L.; Wang, W.; Kang, N.-G.; Mays, J.W. 2017: Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications (Studies in Surface Science and Catalysis); Elsevier Science B.V.: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Mallamace, F.; Beneduci, R.; Gambadauro, P.; Lombardo, D.; Chen, S.H. Glass and percolation transitions in dense attractive micellar system. Phys. A Stat. Mech. Appl. 2001, 302, 202–219. [Google Scholar] [CrossRef]
- Chen, S.H.; Mallamace, F.; Faraone, A.; Gambadauro, P.; Lombardo, D.; Chen, W.R. Observation of a re-entrant kinetic glass transition in a micellar system with temperature-dependent attractive interaction. Eur. Phys. J. E Soft Matter. 2002, 9, 283–286. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Gurevich, L. Drug Delivery with Polymeric Nanocarriers-Cellular Uptake Mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef]
- Lombardo, D.; Micali, N.; Villari, V.; Kiselev, M.A. Large structures in diblock copolymer micellar solution. Phys. Rev. E 2004, 70, 021402. [Google Scholar] [CrossRef]
- Stein, A.; Rudisill, S.G.; Petkovich, N.D. Perspective on the influence of interactions between hard and soft templates and precursors on morphology of hierarchically structured porous materials. Chem. Mater. 2013, 26, 259–276. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calandra, P.; Kiselev, M.A.; Amenitsch, H.; Proverbio, E.; Lombardo, D. Self-assembly in poly(dimethylsiloxane)-poly(ethylene oxide) block copolymer template directed synthesis of linde type A zeolite. Langmuir 2013, 29, 7079–7086. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, L.; Moingeon, F.; Gauthier, M.; Yang, G. PH-Responsive Poly(Ethylene Glycol)-block-Polylactide Micelles for Tumor-Targeted Drug Delivery. Biomacromolecules 2017, 18, 2711–2722. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly (Ethylene Glycol)-Polylactide Micelles for Cancer Therapy. Front. Pharmacol. 2018, 9, 202. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Cucchiarini, M. PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine-An Overview. Int. J. Mol. Sci. 2018, 19, 775. [Google Scholar] [CrossRef]
- Cheng, H.; Yuan, X.; Sun, X.; Li, K.; Zhou, Y.; Yan, D. Effect of Degree of Branching on the Self-Assembly of Amphiphilic Hyperbranched Multiarm Copolymers. Macromolecules 2010, 43, 1143–1147. [Google Scholar] [CrossRef]
- Lombardo, D.; Longo, A.; Darcy, R.; Mazzaglia, A. Structural Properties of Nonionic Cyclodextrin Colloids in Water. Langmuir 2004, 20, 1057–1064. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Angelini, N.; Lombardo, D.; Micali, N.; Patané, S.; Villari, V.; Scolaro, L.M. Amphiphilic cyclodextrin carriers embedding porphyrins: Charge and size modulation of colloidal stability in heterotopic aggregates. J. Phys. Chem. B 2005, 109, 7258–7565. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures--synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.O.; Zhulina, E.B.; Borisov, O.V. Self-Assembly of Linear-Dendritic and Double Dendritic Block Copolymers: From Dendromicelles to Dendrimersomes. Macromolecules 2019, 52, 3655–3667. [Google Scholar] [CrossRef]
- Khor, S.Y.; Quinn, J.F.; Whittaker, M.R.; Truong, N.P.; Davis, T.P. Controlling Nanomaterial Size and Shape for Biomedical Applications via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800438. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.Y.; Vu, M.N.; Pilkington, E.H.; Johnston, A.P.R.; Whittaker, M.R.; Quinn, J.F.; Truong, N.P.; Davis, T.P. Elucidating the Influences of Size, Surface Chemistry, and Dynamic Flow on Cellular Association of Nanoparticles Made by Polymerization-Induced Self-Assembly. Small 2018, 14, 1801702. [Google Scholar] [CrossRef]
- Zhao, W.; Ta, H.T.; Zhang, C.; Whittaker, A.K. Polymerization-Induced Self-Assembly (PISA)-Control over the Morphology of 19F-Containing Polymeric Nano-objects for Cell Uptake and Tracking. Biomacromolecules 2017, 18, 1145–1156. [Google Scholar] [CrossRef]
- Kaga, S.; Truong, N.P.; Esser, L.; Senyschyn, D.; Sanyal, A.; Sanyal, R.; Quinn, J.F.; Davis, T.P.; Kaminskas, L.M.; Whittaker, M.R. Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly. Biomacromolecules 2017, 18, 3963–3970. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, H.; Qu, Y.; Wang, W.; Khan, H.; Zhang, W. In Situ Synthesis of Block Copolymer Nanoassemblies via Polymerization-Induced Self-Assembly in Poly (ethylene glycol). Macromolecules 2016, 49, 3789–3798. [Google Scholar] [CrossRef]
- Truong, N.P.; Zhang, C.; Nguyen, T.A.H.; Anastasaki, A.; Schulze, M.W.; Quinn, J.F.; Whittaker, A.K.; Hawker, C.J.; Whittaker, M.R.; Davis, T.P. Overcoming Surfactant-Induced Morphology Instability of Noncrosslinked Diblock Copolymer Nano-Objects Obtained by RAFT Emulsion Polymerization. ACS Macro Lett. 2018, 7, 159–165. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 105, 21. [Google Scholar] [CrossRef] [PubMed]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting Polymers, Hydrogels and Their Composites: Preparation, Properties and Bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, Y.; Shen, L.; Sheng, Q.; Fu, S.; Chen, S.; Du, Y.; Chen, Y.; Liu, Y. High Mechanical Performance Based on Physically Linked Double Network (DN) Hydrogels. Materials 2019, 12, 3333. [Google Scholar] [CrossRef] [PubMed]
- Charitidis, C.A.; Dragatogiannis, D.A.; Milioni, E.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Synthesis, Nanomechanical Characterization and Biocompatibility of a Chitosan-Graft-Poly (ε-caprolactone) Copolymer for Soft Tissue Regeneration. Materials 2019, 12, 150. [Google Scholar] [CrossRef]
- Konkolewicz, D.M.; Monteiro, J.; Perrier, S. Dendritic and Hyperbranched Polymers from Macromolecular Units: Elegant Approaches to the Synthesis of Functional Polymers. Macromolecules 2011, 44, 7067–7708. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Noh, H.J.; Baek, J.B. Hyperbranched Macromolecules: From Synthesis to Applications. Molecules 2018, 23, 657. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated nanoparticles: Synthesis, characterization, and application to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef]
- Lombardo, D. Modeling Dendrimers Charge Interaction in Solution: Relevance in Biosystems. Biochem. Res. Int. 2014, 837651, 22014. [Google Scholar] [CrossRef]
- Micali, N.; Scolaro, L.M.; Romeo, A.; Lombardo, D.; Lesieur, P.; Mallamace, F. Structural properties of methanol-polyamidoamine dendrimer solutions. Phys. Rev. E 1998, 58, 6229–6235. [Google Scholar] [CrossRef]
- Nisato, G.; Ivkov, R.; Amis, E.J. Structure of Charged Dendrimer Solutions as Seen by Small-Angle Neutron Scattering. Macromolecules 1999, 32, 5895–5900. [Google Scholar] [CrossRef]
- Lombardo, D. Liquid-like ordering of negatively charged poly (amidoamine) (PAMAM) dendrimers in solution. Langmuir 2009, 25, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Fréchet, J.M.J. Dendrimers and other dendritic macromolecules: From building blocks to functional assemblies in nanoscience and nanotechnology. J. Polym. Sci. 2003, 41, 3713–3725. [Google Scholar] [CrossRef]
- Nemanashi, M.; Noh, J.; Meijboom, R. Dendrimers as alternative templates and pore-directing agents for the synthesis of micro- and mesoporous materials. J. Mater. Sci. 2018, 53, 12663–12678. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Lombardo, D.; Longo, A.; Proverbio, E.; Triolo, A. Dendrimer template directed self-assembly during zeolite formation. Macromolecules 2009, 42, 1239–1243. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, S.; Kim, I. Hyperbranched Polymers and Dendrimers as Templates for Organic/Inorganic Hybrid Nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 1631–1646. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calandra, P.; Amenitsch, H.; Proverbio, E.; Lombardo, D. Growth of fractal aggregates during template directed SAPO-34 zeolite formation. Micropor. Mesopor. Mat. 2013, 167, 3–9. [Google Scholar] [CrossRef]
- Vieira, N.C.S.; Figueiredo, A.; de Queiroz, A.A.A.; Zucolotto, V.; Guimarães, F.E.G. Self-assembled films of dendrimers and metallophthalocyanines as FET-based glucose biosensors. Sensors 2011, 11, 9442–9449. [Google Scholar] [CrossRef]
- Sato, K.; Anzai, J. Dendrimers in layer-by-layer assemblies: Synthesis and applications. Molecules 2013, 18, 8440–8460. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.V.; Liroff, M.G.; Triplett, L.D. Stoichiometry and structure of poly (amidoamine) dendrimer- Lipid complexes. ACS Nano 2009, 3, 1886–1896. [Google Scholar] [CrossRef]
- Åkesson, A.; Bendtsen, K.M.; Beherens, M.A.; Pedersen, J.S.; Alfredsson, V.; Gómez, M.C. The effect of PAMAM G6 dendrimers on the structure of lipid vesicles. Phys. Chem. Chem. Phys. 2010, 12, 12267–12272. [Google Scholar] [CrossRef] [PubMed]
- Tiriveedhi, V.; Kitchens, K.M.; Nevels, K.J.; Ghandehari, H.; Butko, P. Kinetic analysis of the interaction between poly (amidoamine) dendrimers and model lipid membranes. Biochim. Biophys. Acta 2011, 1808, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Bellocco, E.; Laganà, G.; Barreca, D.; Magazù, S.; Wanderlingh, U.; Kiselev, M.A. Effect of anionic and cationic polyamidoamine (PAMAM) dendrimers on a model lipid membrane. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Magazù, S.; Wanderlingh, U.; Barreca, D.; Pasqua, L.; Kiselev, M.A. Soft nanoparticles charge expression within lipid membranes: The case of amino terminated dendrimers in bilayers vesicles. Colloids Surf. B Biointerfaces 2018, 170, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R., Jr. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.S.; Vetro, J.A.; Tomalia, D.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Structure/function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J. Pharm. Sci. 2005, 94, 423–436. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Yang, H.; Kao, W.J. Dendrimers for pharmaceutical and biomedical applications. J. Biomater. Sci. Polym. Ed. 2006, 17, 3–19. [Google Scholar] [CrossRef]
- Araújo, R.V.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhang, S.; Percec, V. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Ding, L.; Huang, A.Y.-T.; Kaob, C.-L.; Peng, L. Poly(amidoamine) dendrimers: Covalent and supramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y.; Li, Z.; Wu, A.; Wei, G. Bottom-Up Synthesis and Sensor Applications of Biomimetic Nanostructures. Materials 2016, 9, 53. [Google Scholar] [CrossRef]
- Liu, R.; Hudalla, G.A. Using Self-Assembling Peptides to Integrate Biomolecules into Functional Supramolecular Biomaterials. Molecules 2019, 24, 1450. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef]
- Ke, P.C.; Sani, M.A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of peptide assemblies in amyloid diseases. Chem. Soc. Rev. 2017, 46, 6492–6531. [Google Scholar] [CrossRef]
- Dasgupta, A.; Das, D. Designer Peptide Amphiphiles: Self-Assembly to Applications. Langmuir 2019, 35, 10704–10724. [Google Scholar] [CrossRef]
- Chu-Kung, A.F.; Bozzelli, K.N.; Lockwood, N.A.; Haseman, J.R.; Mayo, K.H.; Tirrell, M.V. Inhibition of Fungal and Bacterial Plant Pathogens In Vitro and In Planta with Ultrashort Cationic Lipopeptides. Bioconjugate Chem. 2004, 15, 530–535. [Google Scholar] [CrossRef]
- Goktas, M.; Cinar, G.; Orujalipoor, I.; Ide, S.; Tekinay, A.B.; Guler, M.O. Self-assembled peptide amphiphile nanofibers and peg composite hydrogels as tunable ECM mimetic microenvironment. Biomacromolecules 2015, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef] [PubMed]
- LeCher, J.C.; Nowak, S.J.; McMurry, J.L. Breaking in and busting out: Cell-penetrating peptides and the endosomal escape problem. Biomol. Concepts 2017, 8, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Váňová, J.; Hejtmánková, A.; Kalbáčová, M.H.; Španielová, H. The Utilization of Cell-Penetrating Peptides in the Intracellular Delivery of Viral Nanoparticles. Materials 2019, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-Engineered Functional Materials. Adv. Healthc. Mater. 2019, 8, 1801374. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Luo, Q.; Liu, J. Protein self-assembly via supramolecular strategies. Chem. Soc. Rev. 2016, 45, 2756–2767. [Google Scholar] [CrossRef]
- Mason, T.O.; Shimanovich, U. Fibrous Protein Self-Assembly in Biomimetic Materials. Adv. Mater. 2018, 30, 1706462. [Google Scholar] [CrossRef]
- Iglic, A.; Garcia-Saez, A.; Rappolt, M. Advances in Biomembranes and Lipid Self-Assembly; Academic Press: Londin, UK, 2019. [Google Scholar]
- Sackmann, E. Physical basis of self-organization and function of membranes: Physics of vesicles. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Katsaras, J.; Gutberlet, T. Lipid Bilayers: Structure and Interactions; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Lesieur, P.; Kiselev, M.A.; Barsukov, L.I.; Lombardo, D. Temperature-induced micelle to vesicle transition: Kinetic effects in the DMPC/NaC system. J. Appl. Cryst. 2000, 33, 623–627. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazù, S.; Kiselev, M.A. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Lesieur, P.; Kisselev, A.M.; Lombardo, D.; Killany, M.; Lesieur, S.; Ollivon, M. A sucrose solutions application to the study of model biological membranes. Nucl. Instrum. Methods Phys. Res. A 2001, 470, 409–416. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Nkepang, G.; Awasthi, V. Surface Modification of Liposomes by a Lipopolymer Targeting Prostate Specific Membrane Antigen for Theranostic Delivery in Prostate Cancer, settings. Materials 2019, 12, 756. [Google Scholar] [CrossRef]
- Olguín, Y.; Campos, C.; Catalán, J.; Velásque, Z.L.; Osorio, F.; Montenegro, I.; Madrid, A.; Acevedo, C. Effects of Liposomes Contained in Thermosensitive Hydrogels as Biomaterials Useful in Neural Tissue Engineering. Materials 2017, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Sapra, K.; Bayley, H. Lipid-coated hydrogel shapes as components of electrical circuits and mechanical devices. Sci. Rep. 2012, 2, 848. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Kulkarni, C.V. Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations. Cosmetics 2016, 3, 37. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Bourgaux, C.; Couvreur, P. Interactions of anticancer drugs with biomembranes: What can we learn from model membranes? J. Control. Release 2014, 190, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, M.A.; Lombardo, D.; Lesieur, P.; Kisselev, A.M.; Borbely, S.; Simonova, T.N.; Barsukov, L.I. Membrane self assembly in mixed DMPC/NaC systems by SANS. Chem. Phys. 2008, 345, 173–180. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Janich, M.; Hildebrand, A.; Strunz, P.; Neubert, R.H.H.; Lombardo, D. Structural transition in aqueous lipid/bile salt [DPPC/NaDC] supramolecular aggregates: SANS and DLS study. Chem. Phys. 2013, 424, 93–99. [Google Scholar] [CrossRef]
- Daniel, M.; Řezníčková, J.; Handl, M.; Iglič, A.; Kralj-Iglič, V. Clustering and separation of hydrophobic nanoparticles in lipid bilayer explained by membrane mechanics. Sci. Rep. 2018, 8, 10810. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Lombardo, D. Structural characterization in mixed lipid membrane systems by neutron and X-ray scattering. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3700–3717. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Q. Development of liposomal formulations: From concept to clinical investigations. Asian J. Pharm. Sci. 2013, 8, 81–87. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil ®-the first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Mohamed, M.; Lila, A.S.A.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly (ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., II; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, B.; Sun, Z.; Cheng, J.; Zhao, H.; Wu, K.; Sun, P.; Shen, Q.; Li, M.; Fan, Q. Multifunctional Theranostic Liposomes Loaded with a Hypoxia-Activated Prodrug for Cascade-Activated Tumor Selective Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 39410–39423. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Liu, B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics 2019, 11, 381. [Google Scholar] [CrossRef]
- Kumar Nayak, A.; Rath, S.K.; Subudhi, U. Preparation of Stable Branched DNA Nanostructures: Process of Cooperative Self-Assembly. J. Phys. Chem. B 2019, 123, 3591–3597. [Google Scholar] [CrossRef]
- Salam, A.; Makhlouf, H.; Barhoum, A. DNA Nanostructures: Chemistry, Self-Assembly, and Applications. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Li, Y.; Liu, X.; Yuan, Q. Self-assembled DNA nanomaterials with highly programmed structures and functions. Mater. Chem. Front. 2018, 2, 423–436. [Google Scholar] [CrossRef]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.E.; Kilchherr, F.; Kim, D.; Shiao, E.L.; Wauer, T.; Wortmann, P.; Bathe, M.; Dietz, H. A primer to scaffolded DNA origami. Nat. Methods 2011, 8, 221–229. [Google Scholar] [CrossRef]
- Halley, P.D.; Patton, R.A.; Chowdhury, A.; Byrd, J.C.; Castro, C.E. Low-cost, simple, and scalable self-assembly of DNA origami nanostructures. Nano Res. 2019, 12, 1207–1215. [Google Scholar] [CrossRef]
- Rothemund, P. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Nummelin, S.; Kommeri, J.; Kostiainen, M.A.; Linko, V. Evolution of Structural DNA Nanotechnology. Adv. Mater. 2018, 30, 1703721. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Liu, Y.; Yan, H. Organizing DNA origami tiles into larger structures using preformed scaffold frames. Nano Lett. 2011, 11, 2997–3002. [Google Scholar] [CrossRef] [PubMed]
- Hudoba, M.W.; Luo, Y.; Zacharias, A.; Poirier, M.G.; Castro, C.E. Dynamic DNA Origami Device for Measuring Compressive Depletion Forces. ACS Nano 2017, 11, 6566–6573. [Google Scholar] [CrossRef]
- Wickham, S.F.; Endo, M.; Katsuda, Y.; Hidaka, K.; Bath, J.; Sugiyama, H.; Turberfield, A.J. Direct observation of stepwise movement of a synthetic molecular transporter. Nat. Nanotechnol. 2011, 6, 166–169. [Google Scholar] [CrossRef]
- Wickham, S.F.; Bath, J.; Katsuda, Y.; Endo, M.; Hidaka, K.; Sugiyama, H.; Turberfield, A.J. A DNA-based molecular motor that can navigate a network of tracks. Nat. Nanotechnol. 2012, 7, 169–173. [Google Scholar] [CrossRef]
- Surana, S.; Shenoy, A.R.; Krishnan, Y. Designing DNA nanodevices for compatibility with the immune system of higher organisms. Nat. Nanotechnol. 2015, 10, 741–747. [Google Scholar] [CrossRef]
- Linko, V.; Ora, A.; Kostiainen, M.A. DNA Nanostructures as Smart Drug-Delivery Vehicles and Molecular Devices. Trends Biotechnol. 2015, 33, 586–594. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, J.; Wang, D.; Zhu, H.; Maity, S.K.; Qu, X.; Bogaert, B.; Pei, H.; Zhang, H. Programmable and Multifunctional DNA-Based Materials for Biomedical Applications. Adv. Mater. 2018, 30, 1703658. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Ijäs, H.; Linko, V.; Keller, A. Structural stability of DNA origami nanostructures under application-specific conditions. Comput. Struct. Biotechnol. J. 2018, 16, 342–349. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, H.; Shi, X.; Zheng, P.A. Programming 20–30nm Rectangular DNA Origami for Loading Doxorubicin to Penetrate Ovarian Cancer Cells. IEEE Trans. Nanobiosci. 2020, 19, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, F.; Kick, B.; Behler, K.; Honemann, M.N.; Weuster-Botz, D.; Dietz, H. Biotechnological mass production of DNA origami. Nature 2017, 552, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.E. The Business of DNA Nanotechnology: Commercialization of Origami and Other Technologies. Molecules 2020, 25, 377. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, H.; Zhang, H.; Nonappa; Kopilow, A.; Niemelä, E.H.; Nummelin, S.; Correia, A.; Santos, H.A.; Linko, V.; Kostiainen, M.A. Protein Coating of DNA Nanostructures for Enhanced Stability and Immunocompatibility. Adv. Healthc. Mater. 2017, 6, 28738444. [Google Scholar] [CrossRef] [PubMed]
- Ponnuswamy, N.; Bastings, M.M.C.; Nathwani, B.; Ryu, J.H.; Chou, L.Y.T.; Vinther, M.; Li, W.A.; Anastassacos, F.M.; Mooney, D.J.; Shih, W.M. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 2017, 8, 15654. [Google Scholar] [CrossRef]
- Bila, H.; Kurisinkal, E.E.; Bastings, M.M.C. Engineering a stable future for DNA-origami as a biomaterial. Biomater. Sci. 2019, 7, 532–541. [Google Scholar] [CrossRef]
- Agarwal, N.P.; Matthies, M.; Gür, F.N.; Osada, K.; Schmidt, T.L. Block Copolymer Micellization as a Protection Strategy for DNA Origami. Angew. Chem. Int. Ed. Engl. 2017, 56, 5460–5464. [Google Scholar] [CrossRef]
- Gerling, T.; Kube, M.; Kick, B.; Dietz, H. Sequence-programmable covalent bonding of designed DNA assemblies. Sci. Adv. 2018, 4, eaau1157. [Google Scholar] [CrossRef]
- Gerling, T.; Dietz, H. Reversible Covalent Stabilization of Stacking Contacts in DNA Assemblies. Angew. Chem. Int. Ed. Engl. 2019, 58, 2680–2684. [Google Scholar] [CrossRef] [PubMed]
- MacCulloch, T.; Buchberger, A.; Stephanopoulos, N. Emerging applications of peptide-oligonucleotide conjugates: Bioactive scaffolds, self-assembling systems, and hybrid nanomaterials. Org. Biomol. Chem. 2019, 17, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Jaekel, A.; Stegemann, P.; Saccà, B. Manipulating Enzymes Properties with DNA Nanostructures. Molecules 2019, 24, 3694. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.P.; Knez, K.; Spasic, D.; Lammertyn, J. Nucleic acids for ultra-sensitive protein detection. Sensors 2013, 13, 1353–1384. [Google Scholar] [CrossRef]
- Miao, P.; Wang, B.; Meng, F.; Yin, J.; Tang, Y. Ultrasensitive detection of microRNA through rolling circle amplification on a DNA tetrahedron decorated electrode. Bioconjug. Chem. 2015, 26, 602–607. [Google Scholar] [CrossRef]
- Grabow, W.W.; Jaeger, L. RNA Self-Assembly and RNA Nanotechnology. Acc. Chem. Res. 2014, 47, 1871–1880. [Google Scholar] [CrossRef]
- Li, M.; Zheng, M.; Wu, S.; Tian, C.; Liu, D.; Weizmann, Y.; Jiang, W.; Wang, G.; Mao, C. In vivo production of RNA nanostructures via programmed folding of single-stranded RNAs. Nat. Commun. 2018, 9, 2196. [Google Scholar] [CrossRef]
- Jasinski, D.; Haque, F.; Binzel, D.W.; Guo, P. Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano 2017, 11, 1142–1164. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Wang, Y.; Maspoch, D.; Zou, S.; Schatz, G.C.; Smalley, R.E.; Mirkin, C.A. Controlling the shape, orientation, and linkage of carbon nanotube features with nano affinity templates. Proc. Natl. Acad. Sci. USA 2006, 103, 2026–2031. [Google Scholar] [CrossRef]
- Zuber, M.; Sherman, D.M.; Cho, Y. Carbon Nanotube Microspheres Produced by Surfactant-Mediated Aggregation. J. Phys. Chem. C 2011, 115, 3881–3887. [Google Scholar] [CrossRef]
- Yi, H.; Song, H.; Chen, X. Carbon nanotube capsules self-assembled by w/o emulsion technique. Langmuir 2007, 23, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, J.J.; Villa, C.H.; McDevitt, M.R.; Escorcia, F.E.; Casey, E.; Scheinberg, D.A. Self-assembly of carbon nanotubes and antibodies on tumours for targeted amplified delivery. Nat. Nanotechnol. 2013, 8, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ghasemi, A.; Mirkiani, S.; Moosavi Basri, S.M.; Hamblin, M.R. Carbon Nanotubes in Drug and Gene Delivery; IOP Publishing, Temple Circus: Bristol, UK, 2017. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, R.; Hu, Y.; Sun, R.; Song, T.; Shi, X.; Yin, S. Stacking of doxorubicin on folic acid-targeted multiwalled carbon nanotubes for in vivo chemotherapy of tumors. Drug Deliv. 2018, 25, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Small, J.P.; Steiner, M.; Freitag, M.; Green, A.A.; Hersam, M.C.; Avouris, P. Thin film nanotube transistors based on self-assembled, aligned, semiconducting carbon nanotube arrays. ACS Nano 2008, 2, 2445–2452. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Yavari, F.; Koratkar, N. Graphene-Based Chemical Sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef]
- El-Kady, M.; Shao, Y.; Kaner, R. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, S.; Zhang, S.; Wang, Y.; Yang, X.; Duan, H.; Huang, Y.; Chen, Y. Functionalized Graphene Oxide with Hepatocyte Targeting as Anti-Tumor Drug and Gene Intracellular Transporters. J. Nanosci. Nanotechnol. 2015, 15, 2052–2059. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.; Kwon, S.W.; Kim, Y.; Park, W.K.; Yoon, D.H.; Jang, A.-R.; Shin, H.S.; Suh, K.S.; Yang, W.S. Large-scale graphene micropatterns via self-assembly-mediated process for flexible device application. Nano Lett. 2012, 12, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, F.; Martín, N. Fullerene-Polymers: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Shrestha, L.K.; Shrestha, R.G.; Hill, J.P.; Ariga, K. Fullerene self-assembly and supramolecular nanostructures. J. Oleo Sci. 2013, 62, 541–553. [Google Scholar] [CrossRef][Green Version]
- Chen, C.; Wang, H. Biomedical Applications and Toxicology of Carbon Nanomaterials; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016. [Google Scholar]
- Coro, J.; Suárez, M.; Silva, L.S.R.; Eguiluz, K.I.B.; Salazar-Bandab, G.R. Fullerene applications in fuel cells: A review. Int. J. Hydrog. Energy 2016, 41, 17944–17959. [Google Scholar] [CrossRef]
- Ji, H.X.; Hu, J.S.; Tang, Q.X.; Song, W.G.; Wang, C.R.; Hu, W.P.; Wan, L.J.; Lee, S.T. Controllable Preparation of Submicrometer Single-Crystal C60 Rods and Tubes Trough Concentration Depletion at the Surfaces of Seeds. J. Phys. Chem. C 2007, 111, 10498–10502. [Google Scholar] [CrossRef]
- Maeda-Mamiya, R.; Noiri, E.; Isobe, H.; Nakanishi, W.; Okamoto, K.; Doi, K.; Sugaya, T.; Izumi, T.; Homma, T.; Nakamura, E. In vivo gene delivery by cationic tetraamino fullerene. Proc. Natl. Acad. Sci. USA 2010, 107, 5339–5344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, H.; Tan, L. A novel fullerene-based drug delivery system delivering doxorubicin for potential lung cancer therapy. J. Nanosci. Nanotechnol. 2017, 17, 5147–5154. [Google Scholar] [CrossRef]
- Hung, C.-H.; Chang, W.-W.; Liu, S.-C.; Wu, S.-J.; Chu, C.-C.; Tsai, Y.-J.; Imae, T. Self-aggregation of amphiphilic [60]fullerenyl focal poInt. functionalized PAMAM dendrons into pseudodendrimers: DNA binding involving dendriplex formation. J. Biomed. Mater. Res. A 2015, 103, 1595–1604. [Google Scholar] [CrossRef]
- Wei, L.; Yao, J.; Fu, H. Solvent-Assisted Self-Assembly of Fullerene into Single-Crystal Ultrathin Microribbons as Highly Sensitive UV–Visible Photodetectors. ACS Nano 2013, 7, 7573–7582. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Calogero, G.; Citro, I.; Calandra Sebastianella, G.; Di Marco, G.; Diniz, A.M.; Parola, A.J.; Pina, F. A Photoelectrochemical Study of Bioinspired 2-Styryl-1-Benzopyrylium Cations on TiO2 Nanoparticle Layer for Application in Dye-Sensitized Solar Cells. Materials 2020, 12, 4060. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Hou, Z.; Xing, Y.; Liu, X.; Yin, X.; Que, M.; Shao, J.; Que, W.; Stang, P.J. Enhanced Conversion Efficiencies in Dye-Sensitized Solar Cells Achieved through Self-Assembled Platinum (II) Metallacages. Sci. Rep. 2016, 6, 29476. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Citro, I.; Calogero, G.; Bartolotta, A. Combined experimental and DFT-TDDFT investigation on anthocyanidins for application in dye-sensitised solar cells. Dye. Pigment. 2017, 143, 291–300. [Google Scholar] [CrossRef]
- Bartolotta, A.; Calogero, G. Solar Cells and Light Management, Dye-sensitized solar cells: From synthetic dyes to natural pigments. In Solar Cells and Light Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–161. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G.; Caramori, S.; Cazzanti, S.; Argazzi, R.; Bignozzi, C.A. Natural dye senstizers for photoelectrochemical cells. Energy Environ. Sci. 2009, 2, 1162–1172. [Google Scholar] [CrossRef]
- Chiappara, C.; Figà, V.; Di Marco, G.; Calogero, G.; Citro, I.; Scuto, A.; Lombardo, S.; Pignataro, B.; Principato, F. Investigation of recovery mechanisms in dye sensitized solar cells. Sol. Energy 2016, 127, 56–66. [Google Scholar] [CrossRef]
- Högberg, D.; Soberats, B.; Yoshio, M.; Mizumura, Y.; Uchida, S.; Kloo, L.; Segawa, H.; Kato, T. Self-Assembled Liquid-Crystalline Ion Conductors in Dye-Sensitized Solar Cells: Effects of Molecular Sensitizers on Their Performance. ChePlusChem 2017, 82, 834–840. [Google Scholar] [CrossRef]
- Cassone, G.; Calogero, G.; Sponer, J.; Saija, F. Mobilities of iodide anions in aqueous solutions for applications in natural dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2018, 20, 13038–13046. [Google Scholar] [CrossRef]
- Calogero, G.; Citro, I.; Crupi, C.; Di Marco, G. Absorption spectra and photovoltaic characterization of chlorophyllins as sensitizers for dye-sensitized solar cells. Spectrochim. Acta A 2014, 132, 477–484. [Google Scholar] [CrossRef]
- Calogero, G.; Citro, I.; Di Marco, G.; Minicante, S.A.; Morabito, M.; Genovese, G. Brown seaweed pigment as a dye source for photoelectrochemical solar cells. Spectrochim. Acta A 2014, 117, 702–706. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3294. [Google Scholar] [CrossRef]
- Jia, H.-L.; Peng, Z.-J.; Li, S.-S.; Huang, C.-Y.; Guan, M.-Y. Self-Assembly by Coordination with Organic Antenna Chromophores for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 15845–15852. [Google Scholar] [CrossRef] [PubMed]
- Delekar, S.D.; More, K.V.; Dhodamani, A.G.; Maity, K.; Acquah, S.F.A.; Dalal, N.; Panda, D.K. Noncovalent interactions based self-assembled bichromophoric sensitizer for dye-sensitized solar cells. Solid State Electrochem. 2019, 23, 1099. [Google Scholar] [CrossRef]
| Bonding and Interaction Type | kJ/mol |
|---|---|
| Covalent bond | 100–400 |
| Van-der-Waals interaction | <5 |
| Hydrogen bond | 4–120 |
| Hydrophobic effects | Entropy |
| π–π interaction | 0–50 |
| Metal–Ligand | 0–400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials 2020, 13, 1048. https://doi.org/10.3390/ma13051048
Lombardo D, Calandra P, Pasqua L, Magazù S. Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials. 2020; 13(5):1048. https://doi.org/10.3390/ma13051048
Chicago/Turabian StyleLombardo, Domenico, Pietro Calandra, Luigi Pasqua, and Salvatore Magazù. 2020. "Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications" Materials 13, no. 5: 1048. https://doi.org/10.3390/ma13051048
APA StyleLombardo, D., Calandra, P., Pasqua, L., & Magazù, S. (2020). Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials, 13(5), 1048. https://doi.org/10.3390/ma13051048








