Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants for Oilfield Applications: Effect of Hydrophilicity of Spacer Group
Abstract
1. Introduction
2. Materials and Synthesis
2.1. Materials
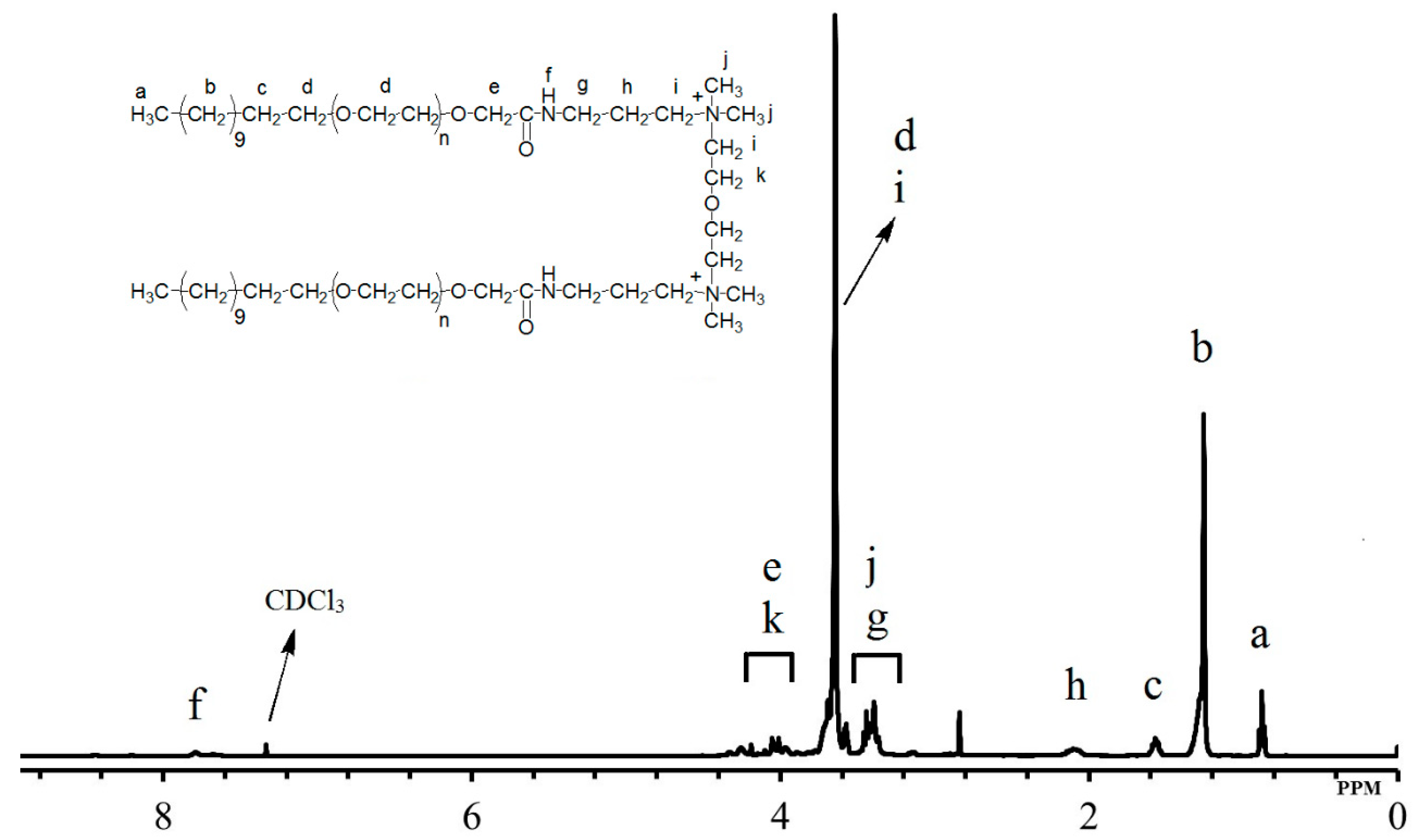
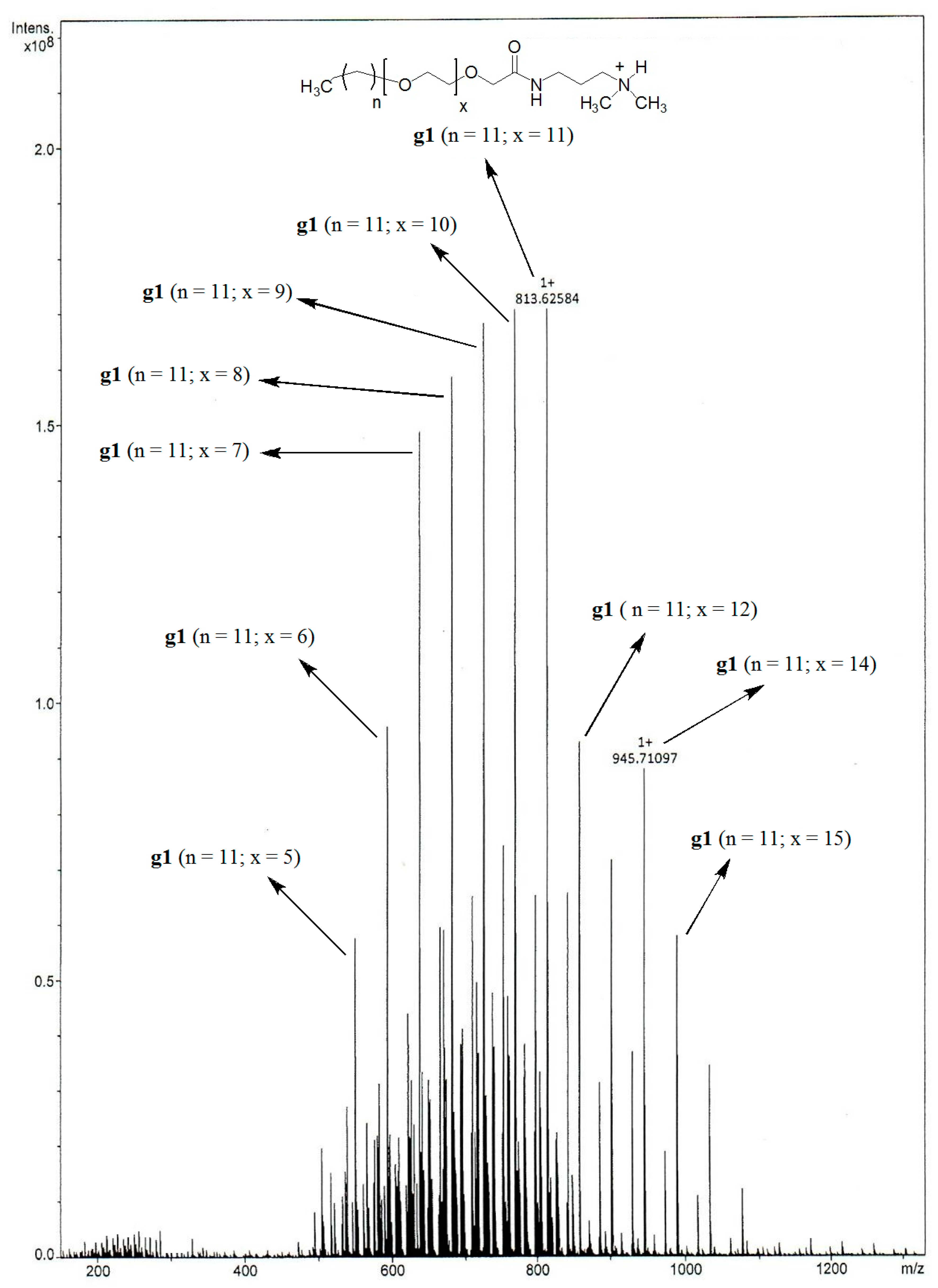
2.2. Structure Determination
2.3. Solubility Tests
2.4. Thermal Gravimetric Analysis (TGA)
2.5. Surface Tension Experiments
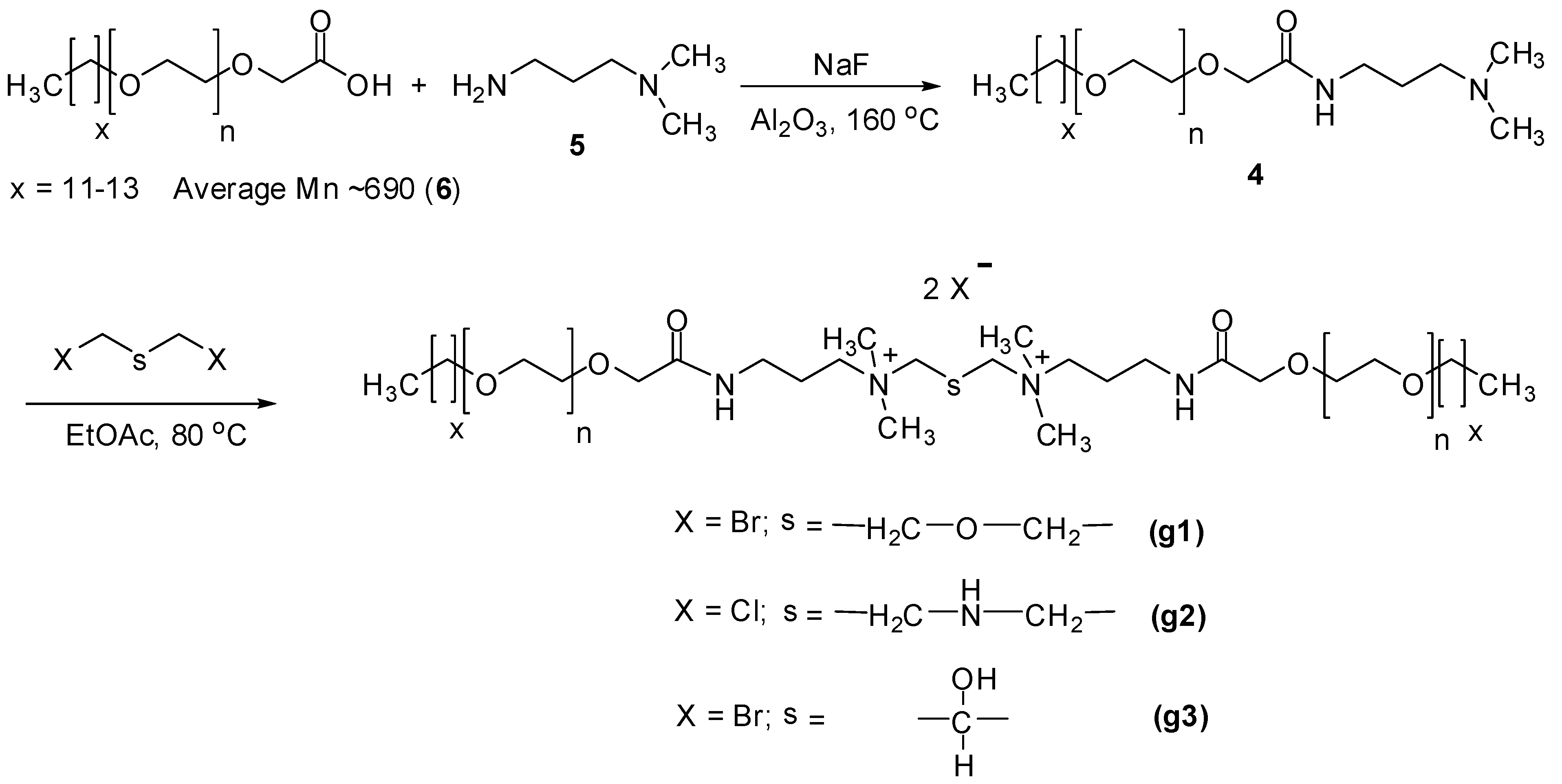
2.6. Synthesis
3. Results and Discussion
3.1. Interpretation of the GS Structure
3.2. Aqueous Stability and Solubility
3.3. Thermal Gravimetric Analysis (TGA)
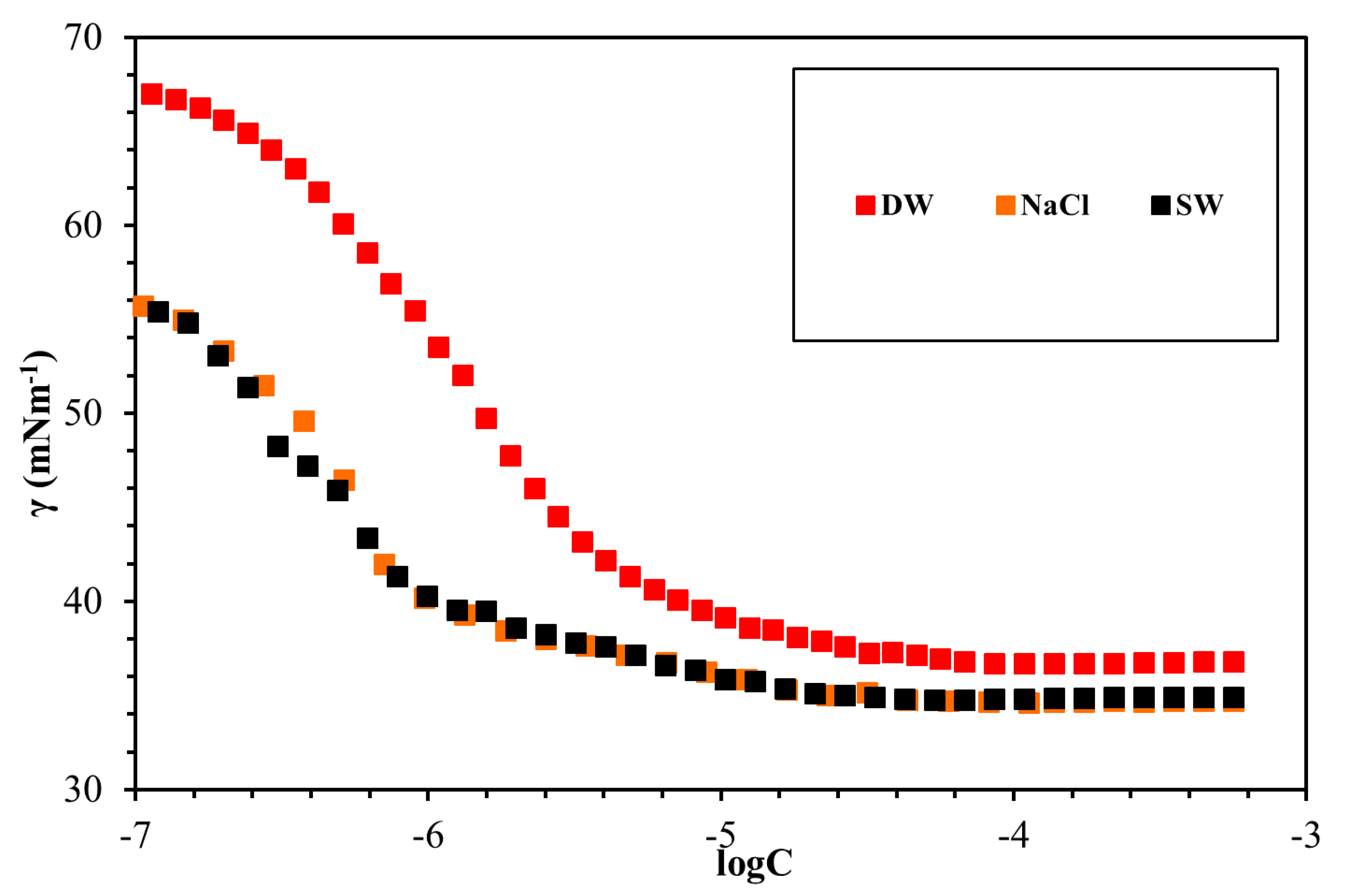
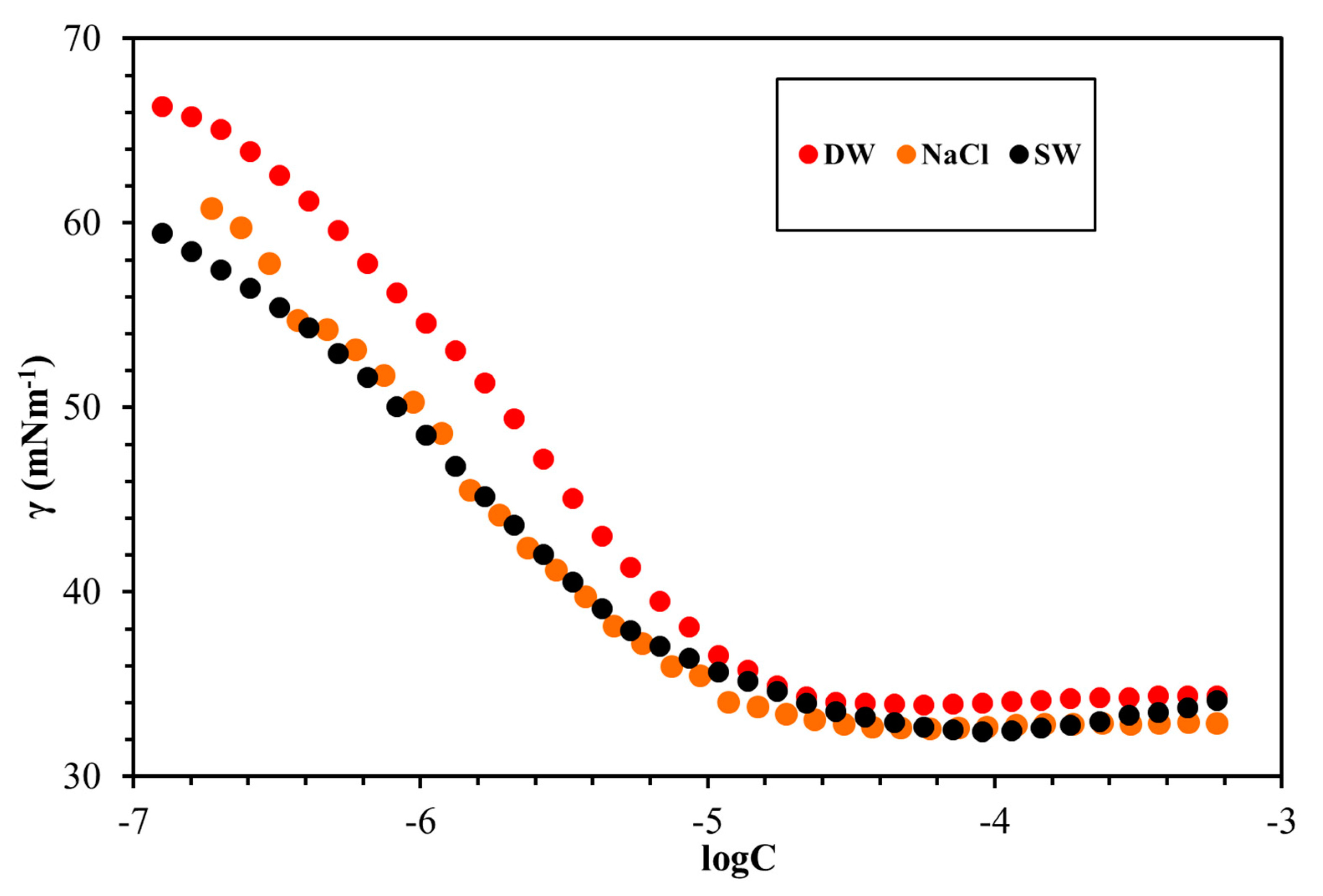
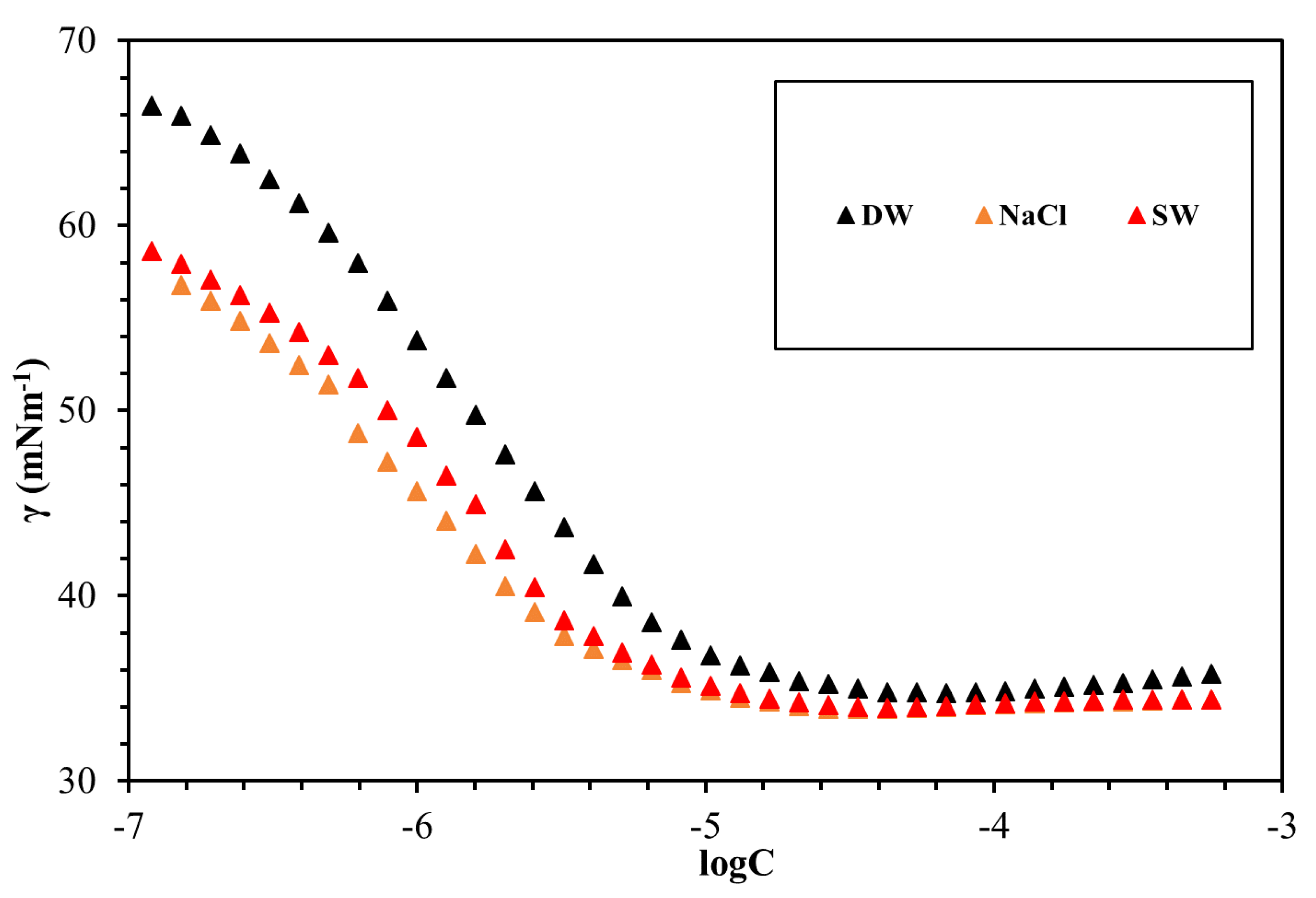
3.4. Surface Tension Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Feng, H.; Wu, H.; Li, Z.; Jiang, J.; Xu, D.; Meng, Z.; Kang, W. Property Evaluation of Synthesized Anionic-Nonionic Gemini Surfactants for Chemical Enhanced Oil Recovery. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123800. [Google Scholar] [CrossRef]
- Kalam, S.; Kamal, M.S.; Patil, S.; Hussain, S.M. Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants. Processes 2019, 7, 502. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussain, S.M.S.; Fogang, L.T. Role of Ionic Headgroups on the Thermal, Rheological, and Foaming Properties of Novel Betaine-Based Polyoxyethylene Zwitterionic Surfactants for Enhanced Oil Recovery. Processes 2019, 7, 908. [Google Scholar] [CrossRef]
- Sagir, M.; Talebian, S.H. Screening of CO2-Philic Surfactants Morphology for High Temperature-Pressure Sandstone Reservoir Conditions. J. Pet. Sci. Eng. 2020, 186. [Google Scholar] [CrossRef]
- Sagir, M.; Tan, I.M.; Mushtaq, M.; Ismail, L.; Nadeem, M.; Azam, M.R.; Hashmet, M.R. Novel Surfactant for the Reduction of CO2/Brine Interfacial Tension. J. Dispers. Sci. Technol. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- SalahEldin Hussien, O.; Elraies, K.A.; Almansour, A.; Husin, H.; Belhaj, A.; Ern, L. Experimental Study on the Use of Surfactant as a Fracking Fluid Additive for Improving Shale Gas Productivity. J. Pet. Sci. Eng. 2019, 183. [Google Scholar] [CrossRef]
- Ghasemi, M.; Moslemizadeh, A.; Shahbazi, K.; Mohammadzadeh, O.; Zendehboudi, S.; Jafari, S. Primary Evaluation of a Natural Surfactant for Inhibiting Clay Swelling. J. Pet. Sci. Eng. 2019, 878–891. [Google Scholar] [CrossRef]
- Mao, J.; Wang, D.; Yang, X.; Zhang, Z.; Yang, B.; Zhang, C. Adsorption of Surfactant on Stratum Rocks: Exploration of Low Adsorption Surfactants for Reservoir Stimulation. J. Taiwan Inst. Chem. Eng. 2019, 95, 424–431. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Cationic Gemini-Surfactants Based on Waste Cooking Oil as New ‘Green’ Inhibitors for N80-Steel Corrosion in Sulphuric Acid: A Combined Empirical and Theoretical Approaches. J. Mol. Struct. 2019. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Mou, J.; Wu, D.; Zheng, S. Research Progress on the Collaborative Drag Reduction Effect of Polymers and Surfactants. Materials 2020, 13, 444. [Google Scholar] [CrossRef]
- Liu, L.; Pu, X.; Zhou, Y.; Zhou, J.; Luo, D.; Ren, Z. Smart Pickering Water-in-Oil Emulsion by Manipulating Interactions between Nanoparticles and Surfactant as Potential Oil-Based Drilling Fluid. Colloids Surfaces A Physicochem. Eng. Asp. 2020. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhizadeh, V.; Kord, S.; Dejam, M. EOR Potential within Iran. Spec. Top. Rev. Porous Media Int. J. 2014, 5, 325–354. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Galedarzadeh, M.; Shadizadeh, S.R. Wettability Alteration in Carbonate Rocks by Implementing New Derived Natural Surfactant: Enhanced Oil Recovery Applications. Transp. Porous Media 2015, 106, 645–667. [Google Scholar] [CrossRef]
- Olayiwola, S.O.; Dejam, M. A Comprehensive Review on Interaction of Nanoparticles with Low Salinity Water and Surfactant for Enhanced Oil Recovery in Sandstone and Carbonate Reservoirs. Fuel 2019, 241, 1045–1057. [Google Scholar] [CrossRef]
- Wagay, T.A.; Askari, H.; Ismail, K. Synthesis, Aggregation and Adsorption Behavior of Benzyldimethylhexadecylammonium Based Double-Chained Metallosurfactants. J. Mol. Liq. 2019. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Gemini Surfactant Modified Clays: Effect of Surfactant Loading and Spacer Length. Appl. Clay Sci. 2018, 161, 48–56. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Synthesis, Characterization, and Physicochemical Properties of a Series of Quaternary Gemini Surfactants with Different Spacer Lengths. Colloid Polym. Sci. 2017, 295, 2261–2277. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Studies on the Physicochemical Properties of Synthesized Tailor-Made Gemini Surfactants for Application in Enhanced Oil Recovery. J. Mol. Liq. 2018, 258, 211–224. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Kamal, M.S. Effect of Large Spacer on Surface Activity, Thermal, and Rheological Properties of Novel Amido-Amine Cationic Gemini Surfactants. J. Mol. Liq. 2017, 242, 1131–1137. [Google Scholar] [CrossRef]
- Akram, M.; Anwar, S.; Bhat, I.A. Kabir-ud-Din. In Vitro Evaluation of the Non-Covalent Interactions of Hemoglobin with Distinctively Modified Gemini Surfactants: Effect of Structural Variation. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 527, 145–157. [Google Scholar] [CrossRef]
- Hordyjewicz-Baran, Z.; Woch, J.; Kuliszewska, E.; Zimoch, J.; Libera, M.; Dworak, A.; Trzebicka, B. Aggregation Behavior of Anionic Sulfonate Gemini Surfactants with Dodecylphenyl Tails. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 484, 336–344. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S.; Azov, V. Gemini Surfactants with Acetylenic Spacers. Langmuir 2000, 16, 2062–2067. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, Y.; Yan, H.; Li, P.; Thomas, R.K. Effect of the Nature of the Spacer on the Aggregation Properties of Gemini Surfactants in an Aqueous Solution. Langmuir 2004, 20, 53–56. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, X.; Wang, X.; Song, C. Synthesis, Surface Activity and Thermodynamic Properties of Cationic Gemini Surfactants with Diester and Rigid Spacers. J. Mol. Liq. 2017, 230, 505–510. [Google Scholar] [CrossRef]
- Zhou, L.; He, Y.; Gou, S.; Zhang, Q.; Liu, L.; Tang, L.; Zhou, X.; Duan, M. Efficient Inhibition of Montmorillonite Swelling through Controlling Flexibly Structure of Piperazine-Based Polyether Gemini Quaternary Ammonium Salts. Chem. Eng. J. 2019, 123190. [Google Scholar] [CrossRef]
- Mao, J.; Yang, X.; Wang, D.; Li, Y.; Zhao, J. A Novel Gemini Viscoelastic Surfactant (VES) for Fracturing Fluids with Good Temperature Stability. RSC Adv. 2016. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Zhang, H.; Zhang, Z.; Zhao, J. Reutilization of Thickener from Fracturing Flowback Fluid Based on Gemini Cationic Surfactant. Fuel 2019, 235, 670–676. [Google Scholar] [CrossRef]
- Migahed, M.A.; elgendy, A.; EL-Rabiei, M.M.; Nady, H.; Zaki, E.G. Novel Gemini Cationic Surfactants as Anti-Corrosion for X-65 Steel Dissolution in Oilfield Produced Water under Sweet Conditions: Combined Experimental and Computational Investigations. J. Mol. Struct. 2018, 1159, 10–22. [Google Scholar] [CrossRef]
- Cao, X.-L.; Feng, J.; Guo, L.-L.; Zhu, Y.; Zhang, L.; Zhang, L.; Luo, L.; Zhao, S. Dynamic Surface Dilational Properties of Anionic Gemini Surfactants with Polyoxyethylene Spacers. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 490, 41–48. [Google Scholar] [CrossRef]
- Chen, Z.; Han, X.; Kurnia, I.; Yu, J.; Zhang, G.; Li, L. Adoption of Phase Behavior Tests and Negative Salinity Gradient Concept to Optimize Daqing Oilfield Alkaline-Surfactant-Polymer Flooding. Fuel 2018, 232, 71–80. [Google Scholar] [CrossRef]
- Parikh, K.; Singh, S.; Desai, A.; Kumar, S. An Interplay between Spacer Nature and Alkyl Chain Length on Aqueous Micellar Properties of Cationic Gemini Surfactants: A Multi-Technique Approach. J. Mol. Liq. 2019, 278, 290–298. [Google Scholar] [CrossRef]
- Ahel, M.; Giger, W. Aqueous Solubility of Alkylphenols and Alkylphenol Polyethoxylates. Chemosphere 1993, 26, 1461–1470. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y. A Facile Route towards the Preparation of Ultra-Long-Chain Amidosulfobetaine Surfactants. Synlett 2009, 16, 2655–2658. [Google Scholar]
- Devinsky, F.; Masarova, L.; Lacko, I.; Bittererova, F.; Tomeckova, L.; Rozycka-Roszak, B.; Wittek, S.; Przestalski, S. Alkanediyl-CK,v-Bis(Dimethylalkylammonium Bromide) Surfactants. 1. Effect of the Spacer Chain Length on the Critical Micelle Concentration and Micelle Ionization Degree. Langmuir 1991, 7, 1072–1075. [Google Scholar]
- Kaczerewska, O.; Brycki, B.; Ribosa, I.; Comelles, F.; Garcia, M.T. Cationic Gemini Surfactants Containing an O-Substituted Spacer and Hydroxyethyl Moiety in the Polar Heads: Self-Assembly, Biodegradability and Aquatic Toxicity. J. Ind. Eng. Chem. 2018, 59, 141–148. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhang, Z.; Yang, B.; Zhang, Y.; Zhao, J. Study of a Novel Gemini Viscoelastic Surfactant with High Performance in Clean Fracturing Fluid Application. Polymers 2018, 10, 1215. [Google Scholar] [CrossRef]
- Ghumare, A.K.; Pawar, B.V.; Bhagwat, S.S. Synthesis and Antibacterial Activity of Novel Amido-Amine-Based Cationic Gemini Surfactants. J. Surfactants Deterg. 2013, 16, 85–93. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Kamal, M.S.; Fogang, L.T. Effect of Internal Olefin on the Properties of Betaine-Type Zwitterionic Surfactants for Enhanced Oil Recovery. J. Mol. Liq. 2018, 266, 43–50. [Google Scholar] [CrossRef]
- Nessim, M.I.; Osman, M.M.; Ismail, D.A. Surface-Active Properties of New Cationic Gemini Surfactants with Cyclic Spaceractive Properties of New Cationic Gemini Surfactants with Cyclic Spacer Surface-Active Properties of New Cationic Gemini Surfactants with Cyclic Spacer. J. Dispers. Sci. Technol. 2018, 39, 1047–1055. [Google Scholar] [CrossRef]
- Zhao, T.; Dong, Z.; Peng, G.; Xing, J.; He, Y. Synthesis and Properties of Quaternary Ammonium Gemini Surfactants with Hydroxyl Groups. Russ. J. Appl. Chem. 2016, 89, 650–662. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Hydrophilicity and Flexibility of the Spacer as Critical Parameters on the Aggregation Behavior of Long Alkyl Chain Cationic Gemini Surfactants in Aqueous Solution. J. Mol. Liq. 2017, 230, 453–460. [Google Scholar] [CrossRef]




| GSs | Proton NMR (δ in ppm, 500 MHz, CDCl3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lipophilic Tail | Ethoxy & Spacer | Acetic | Amide Group | Amido-Amine Group | Spacer | |||||
| CH3 (a) | CH2 (b) | CH2 (c) | CH2 (d, i) | CH2 (e) | NH (f) | CH3 (j) CH2 (g) | CH2 (h) | CH2 (k) | - | |
| g1 | 0.88 | 1.26 | 1.56 | 3.64 | 4.04 | 7.79 | 3.39 | 2.10 | 4.05 | - |
| g2 | 0.88 | 1.26 | 1.57 | 3.68 | 4.00 | 7.82 | 3.44 | 2.11 | 3.44 | 2.91 (NH) |
| g3 | 0.87 | 1.26 | 1.56 | 3.65 | 4.00 | 7.93 | 3.41 | 2.07 | 4.04 (CH) | 3.65 (OH) |
| GSs | 13C NMR (ppm, CDCl3, 125 MHz) |
|---|---|
| g1 | 13.9, 22.5, 25.9, 29.1, 29.3, 29.4, 31.7, 35.6, 51.7, 62.9, 63.3, 64.4, 70.3, 170.7 |
| g2 | 14.1, 22.6, 26.0, 29.1, 29.3, 29.4, 31.9, 35.7, 51.7, 60.8, 61.5, 70.2, 170.9 |
| g3 | 13.9, 22.5, 25.9, 29.1, 29.3, 29.4, 31.7, 35.8, 52.1, 60.6, 61.4, 70.3, 170.7 |
| GSs | FTIR Characteristic Values (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N-H | CH2 asym. | CH2 sym. | Amide (I) | Amide (II) | CH2 (Bending) | CH3 (Bending) | Ethoxy Stretch | asym. | |
| g1 | 3424 | 2922 | 2855 | 1656 | 1544 | 1465 | 1349 | 1100 | 947 |
| g2 | 3397 | 2922 | 2855 | 1655 | 1546 | 1466 | 1349 | 1099 | 947 |
| g3 | 3412 | 2922 | 2855 | 1656 | 1544 | 1465 | 1349 | 1098 | 947 |
| GSs | Base Peak | Proposed Structure |
|---|---|---|
| g1 | 813.63 | 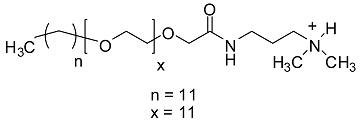 |
| g2 | 725.57 | 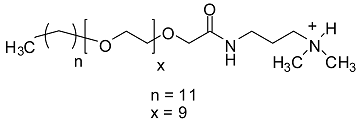 |
| g3 | 681.54 |  |
| Ions | FW (g/L) | SW (g/L) |
|---|---|---|
| Na+ | 59.5 | 18.3 |
| Ca2+ | 19.1 | 0.7 |
| Mg2+ | 2.5 | 2.1 |
| SO42− | 0.4 | 4.3 |
| Cl− | 132.1 | 32.2 |
| HCO3− | 0.4 | 0.1 |
| Total | 214 | 57.7 |
| GSs | Solvent | CMC (mmol/L) | γcmc (mN/m) | гmax × 106 (mol/m2) | Amin (nm2) |
|---|---|---|---|---|---|
| g1 | DW | 0.0049 | 39.64 | 3.66 | 0.45 |
| g1 | NaCl | 0.0036 | 34.13 | 3.31 | 0.50 |
| g1 | SW | 0.0029 | 34.66 | 3.17 | 0.52 |
| g2 | DW | 0.0164 | 33.883 | 3.15 | 0.52 |
| g2 | NaCl | 0.0123 | 32.914 | 2.63 | 0.63 |
| g2 | SW | 0.0118 | 32.963 | 2.61 | 0.64 |
| g3 | DW | 0.0102 | 34.72 | 3.28 | 0.51 |
| g3 | NaCl | 0.0052 | 33.97 | 2.85 | 0.58 |
| g3 | SW | 0.0064 | 33.65 | 3.12 | 0.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.M.S.; Mahboob, A.; Kamal, M.S. Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants for Oilfield Applications: Effect of Hydrophilicity of Spacer Group. Materials 2020, 13, 1046. https://doi.org/10.3390/ma13051046
Hussain SMS, Mahboob A, Kamal MS. Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants for Oilfield Applications: Effect of Hydrophilicity of Spacer Group. Materials. 2020; 13(5):1046. https://doi.org/10.3390/ma13051046
Chicago/Turabian StyleHussain, S. M. Shakil, Ahmad Mahboob, and Muhammad Shahzad Kamal. 2020. "Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants for Oilfield Applications: Effect of Hydrophilicity of Spacer Group" Materials 13, no. 5: 1046. https://doi.org/10.3390/ma13051046
APA StyleHussain, S. M. S., Mahboob, A., & Kamal, M. S. (2020). Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants for Oilfield Applications: Effect of Hydrophilicity of Spacer Group. Materials, 13(5), 1046. https://doi.org/10.3390/ma13051046







