Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Spread Flow Test
2.4. Penetration Test with a Multipurpose Incremental Loading Device (MILD)
2.5. In-Situ X-ray Diffraction (XRD)
2.6. Heat Flow Calorimetry
2.7. Ultrasonic Measurements
2.8. Scanning Electron Microscope (SEM)
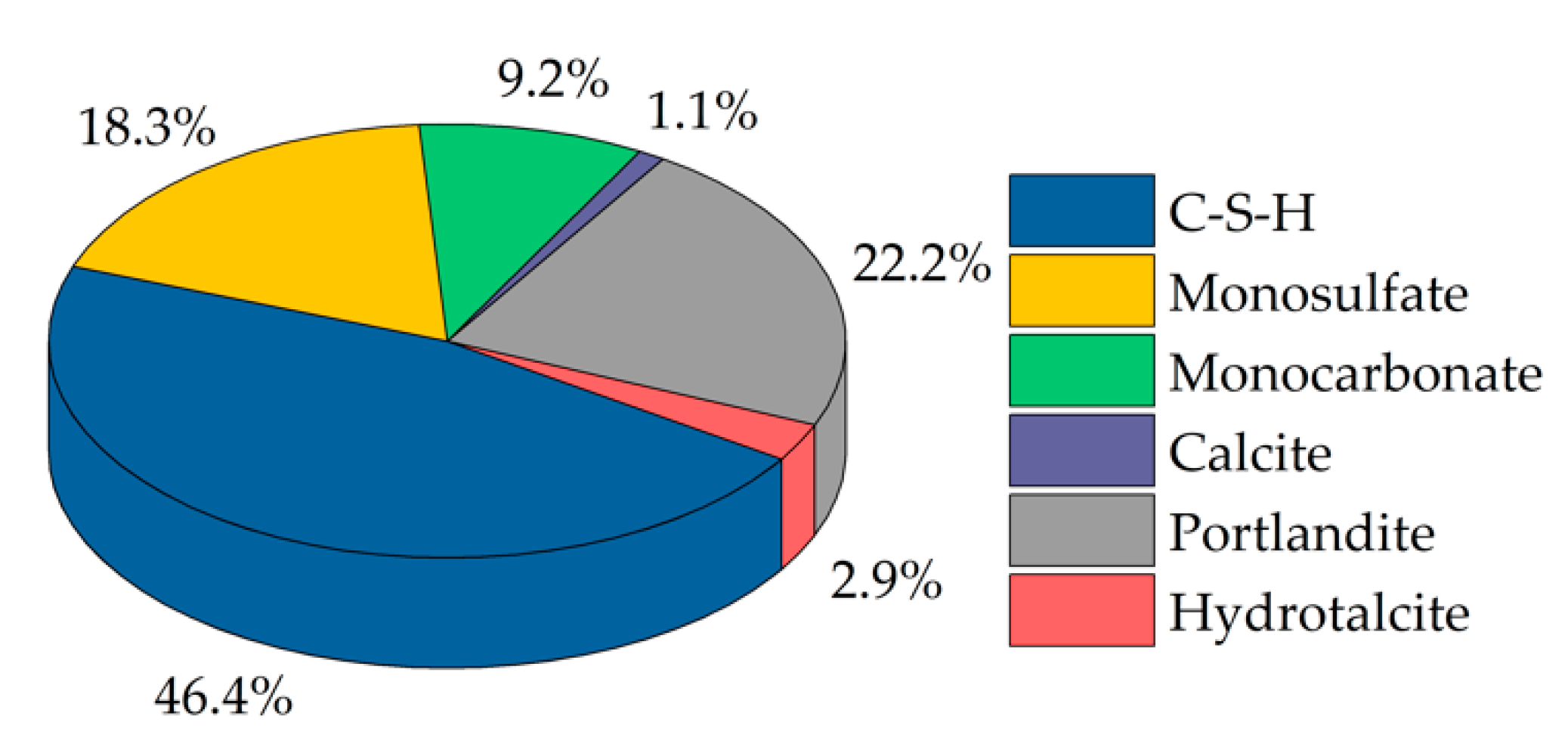
2.9. Thermodynamic Modeling
3. Results
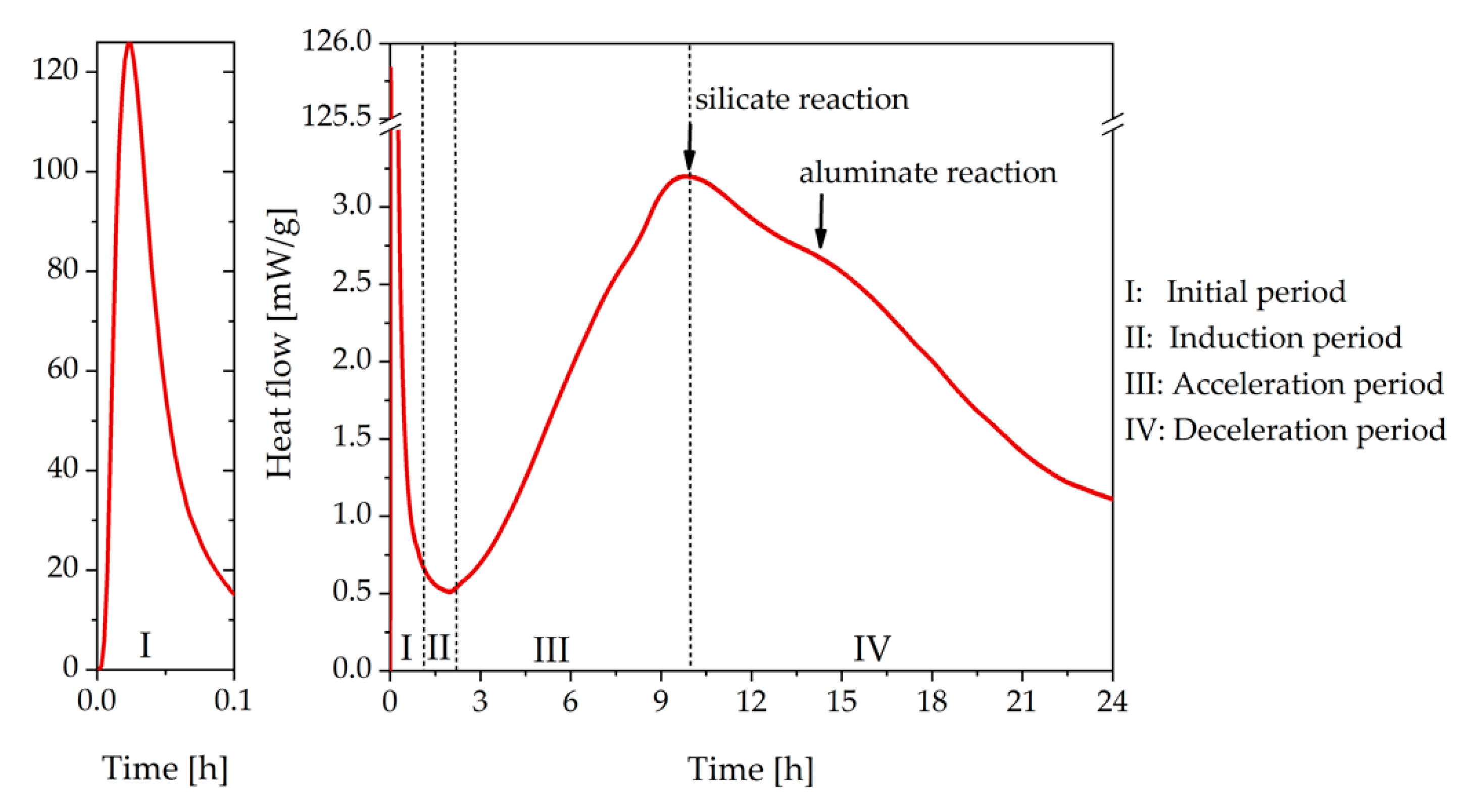
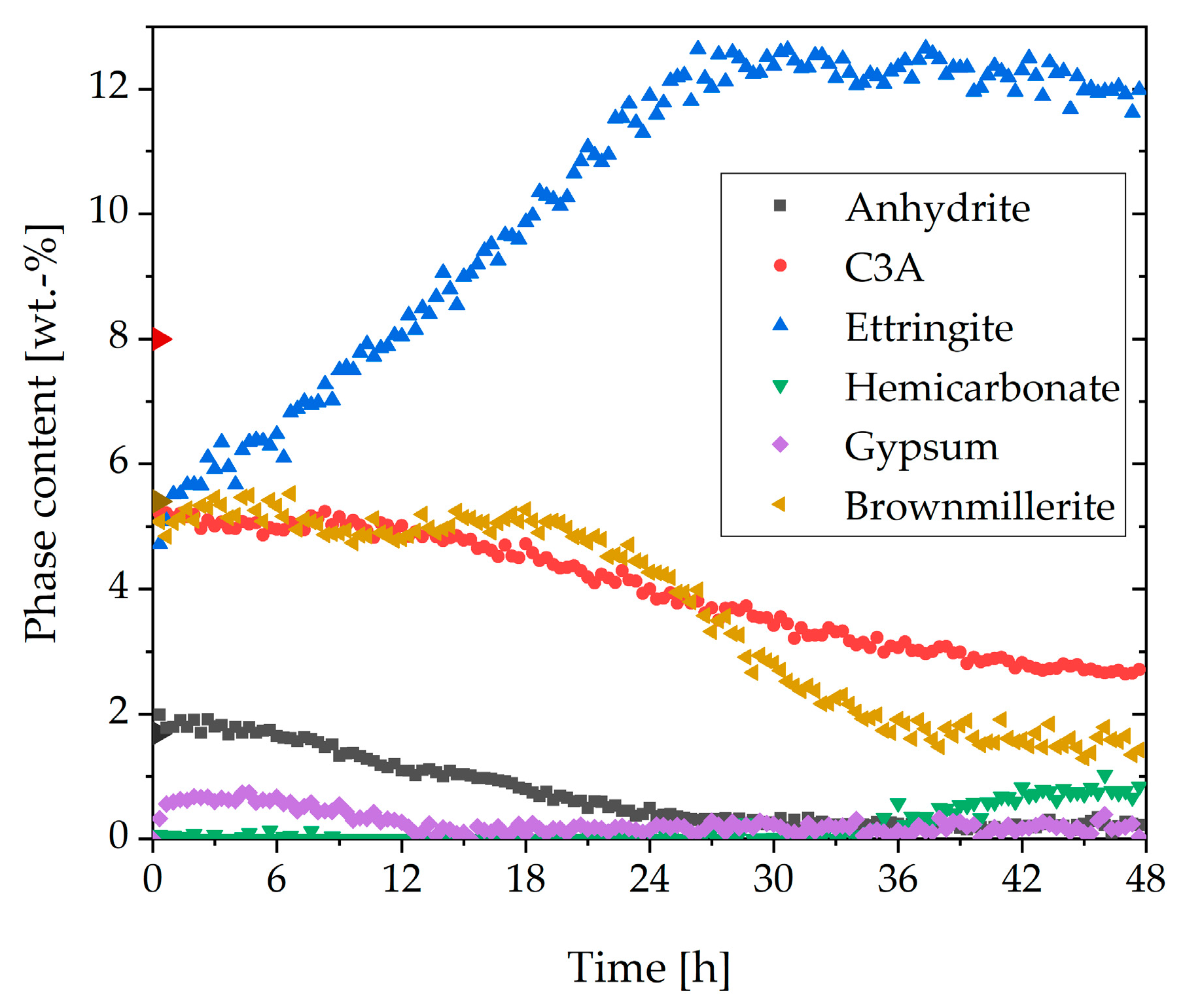
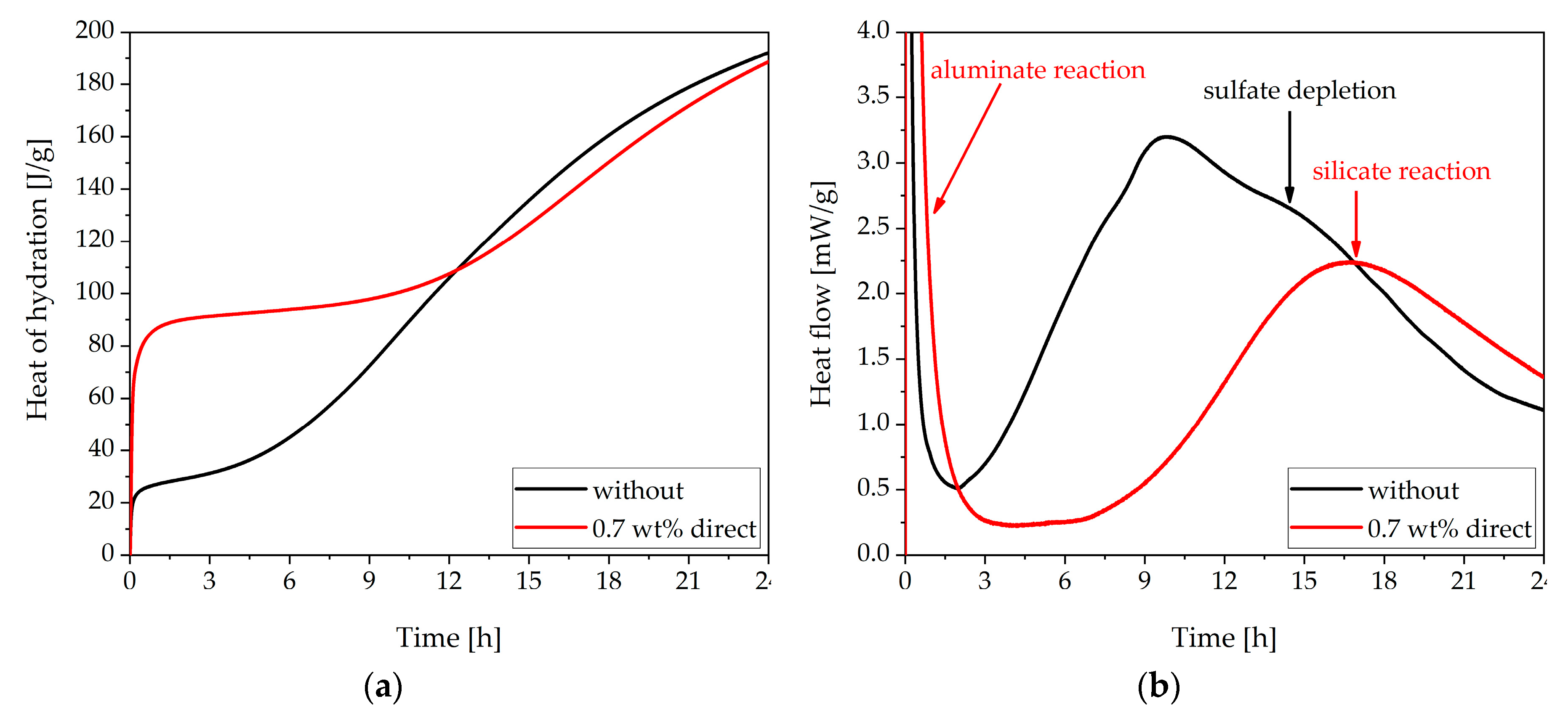
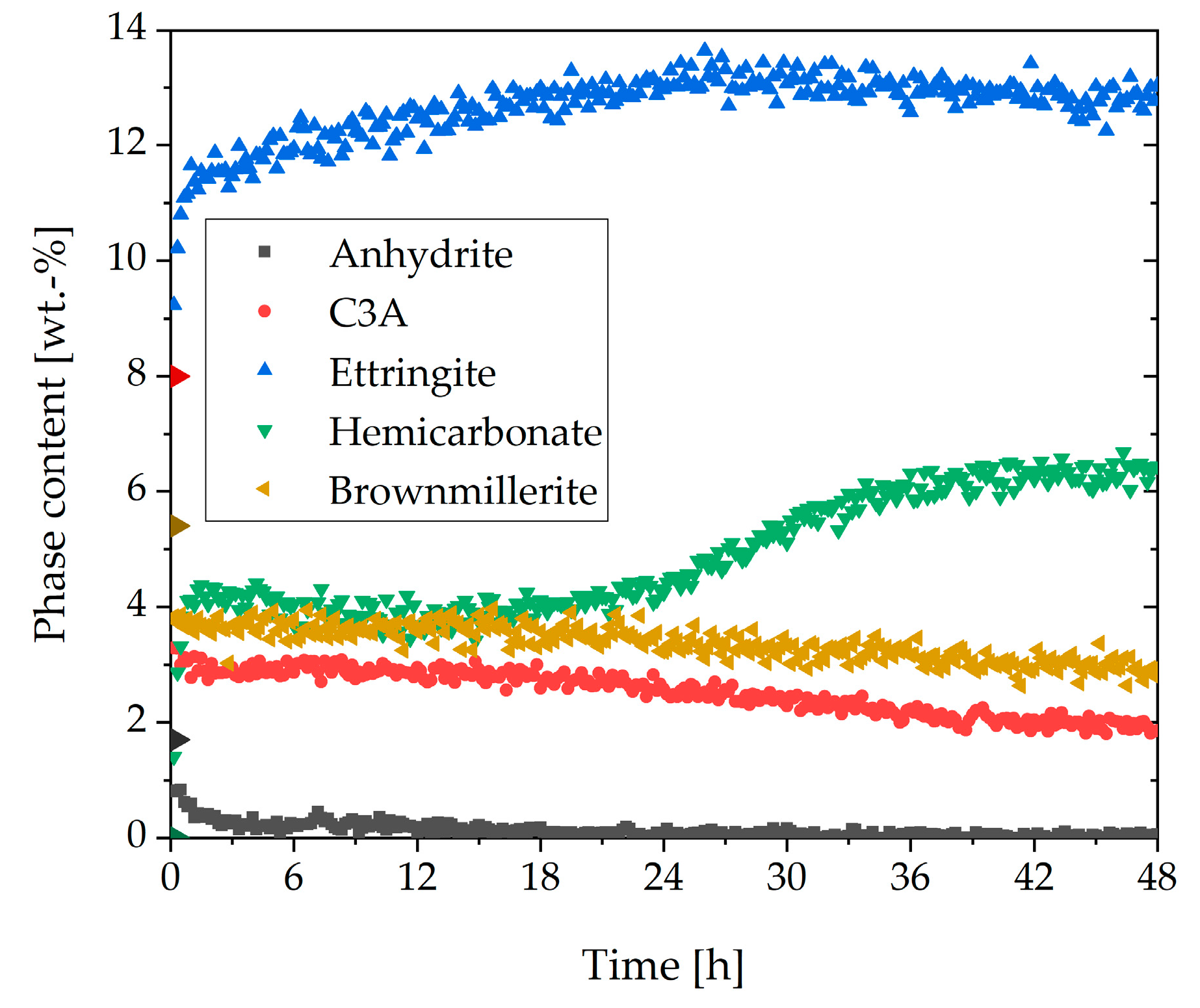
3.1. Cement Paste Without Superplasticizer
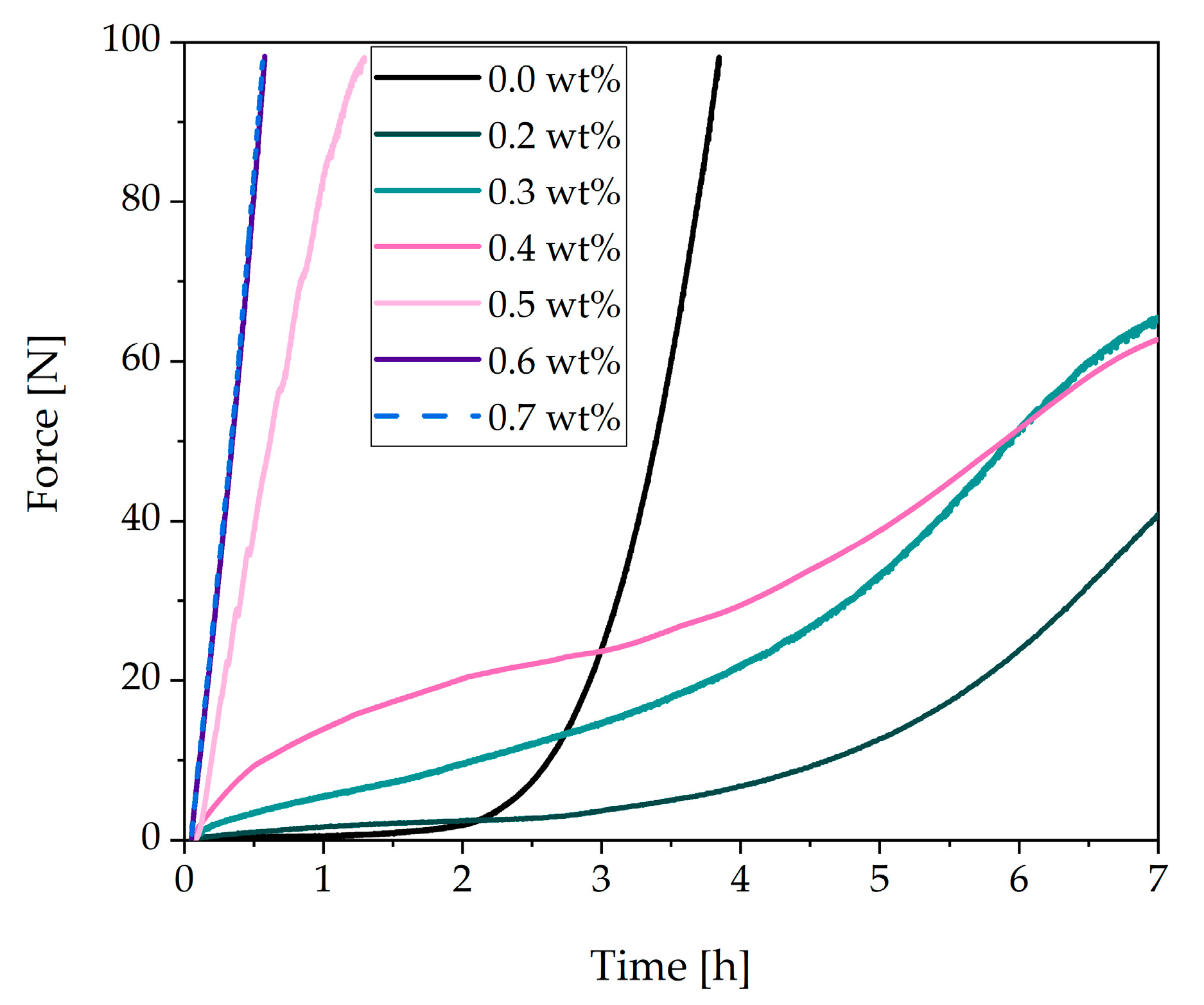
3.2. Rheological Tests of a Cement Paste with Different Dosages of Superplasticizer
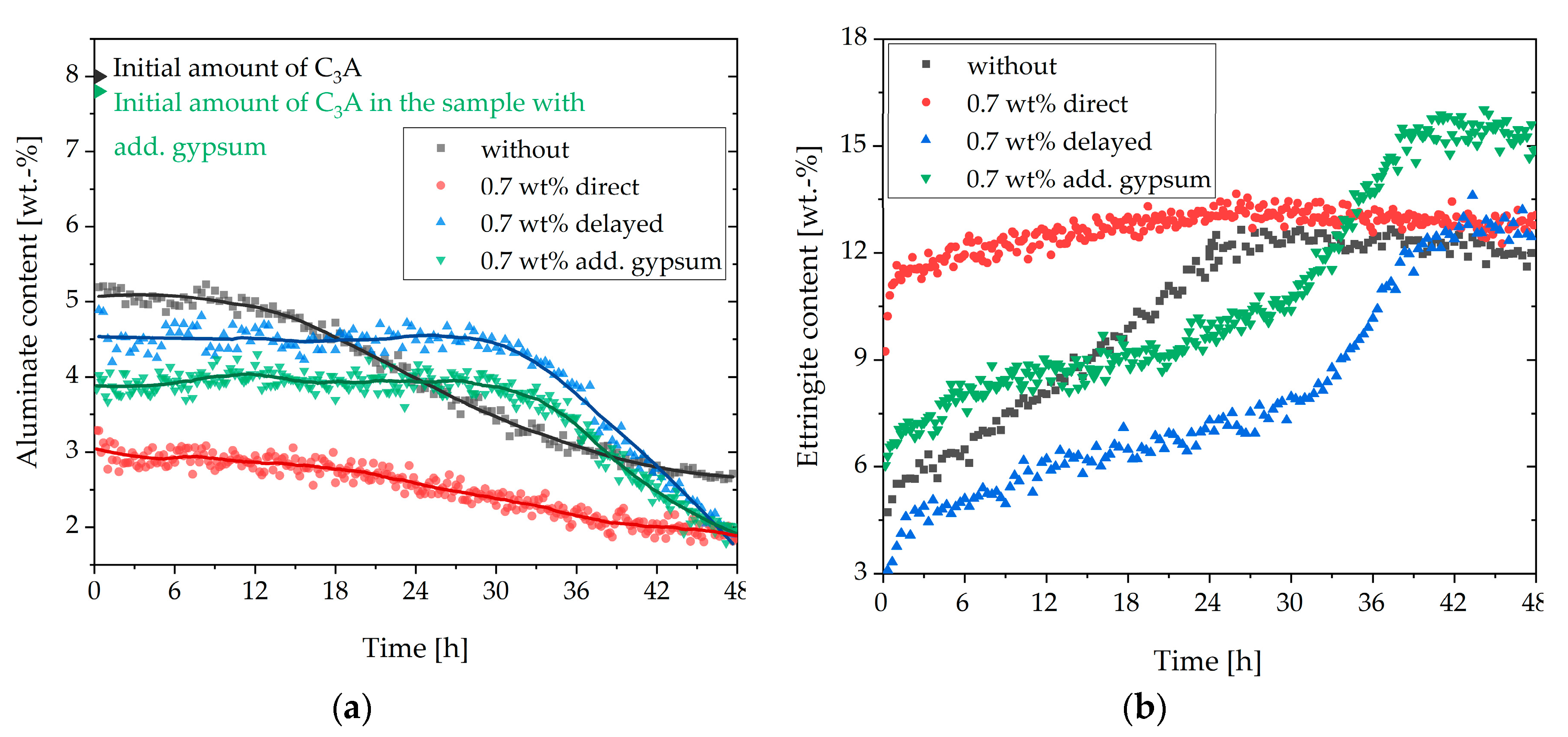
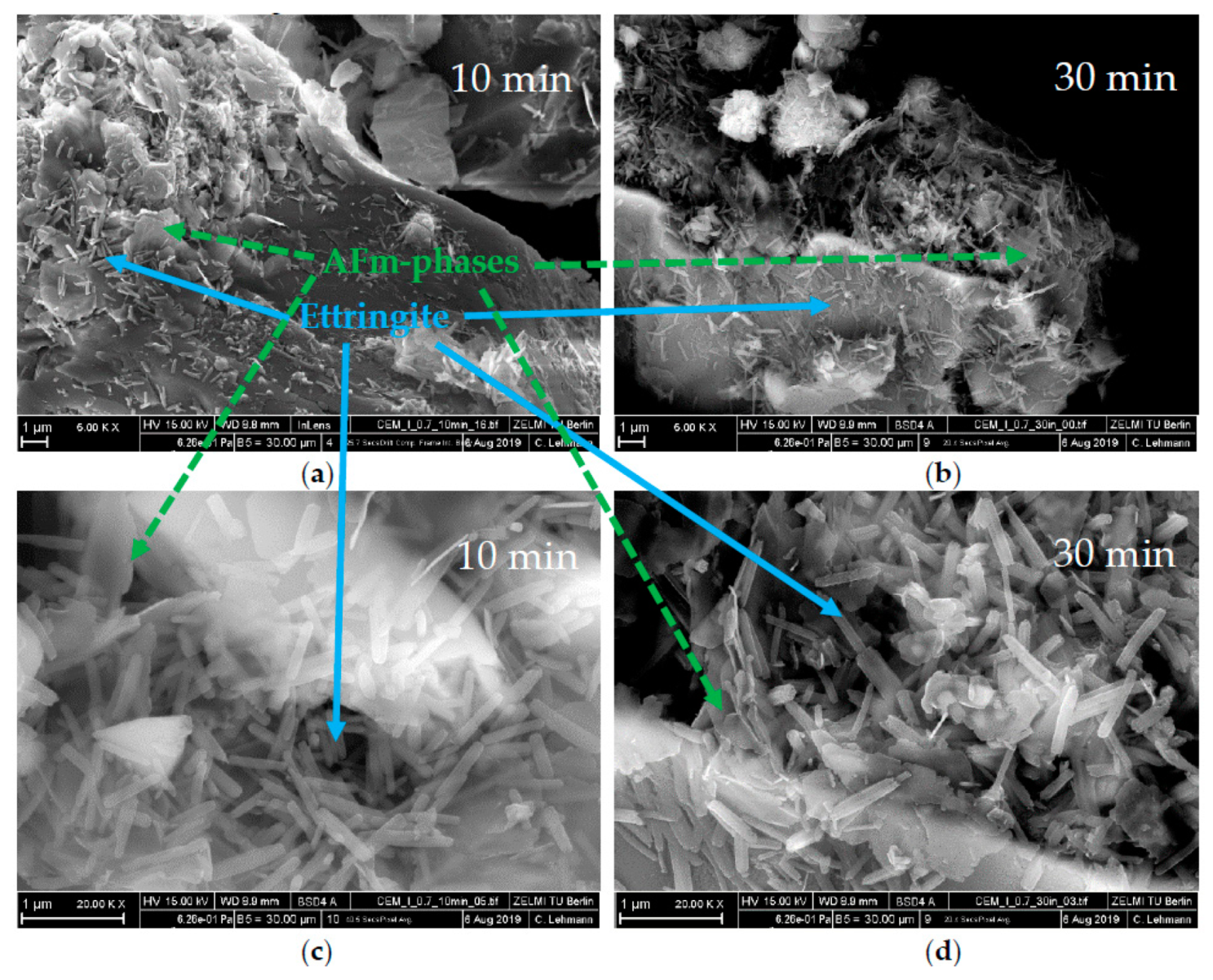
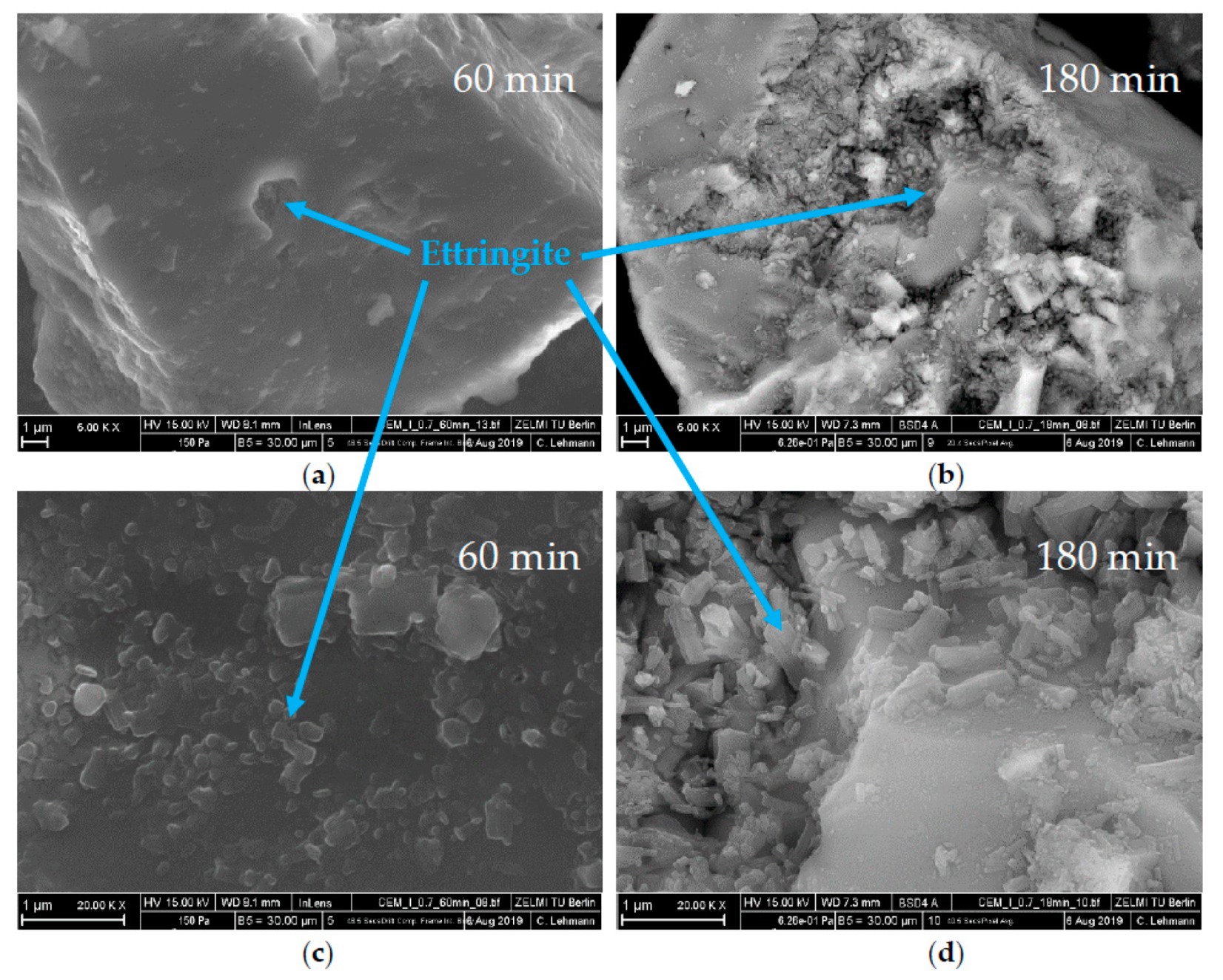
3.3. Analysis of the Incompatible Behavior Caused by Direct Addition of 0.7 wt% of SP
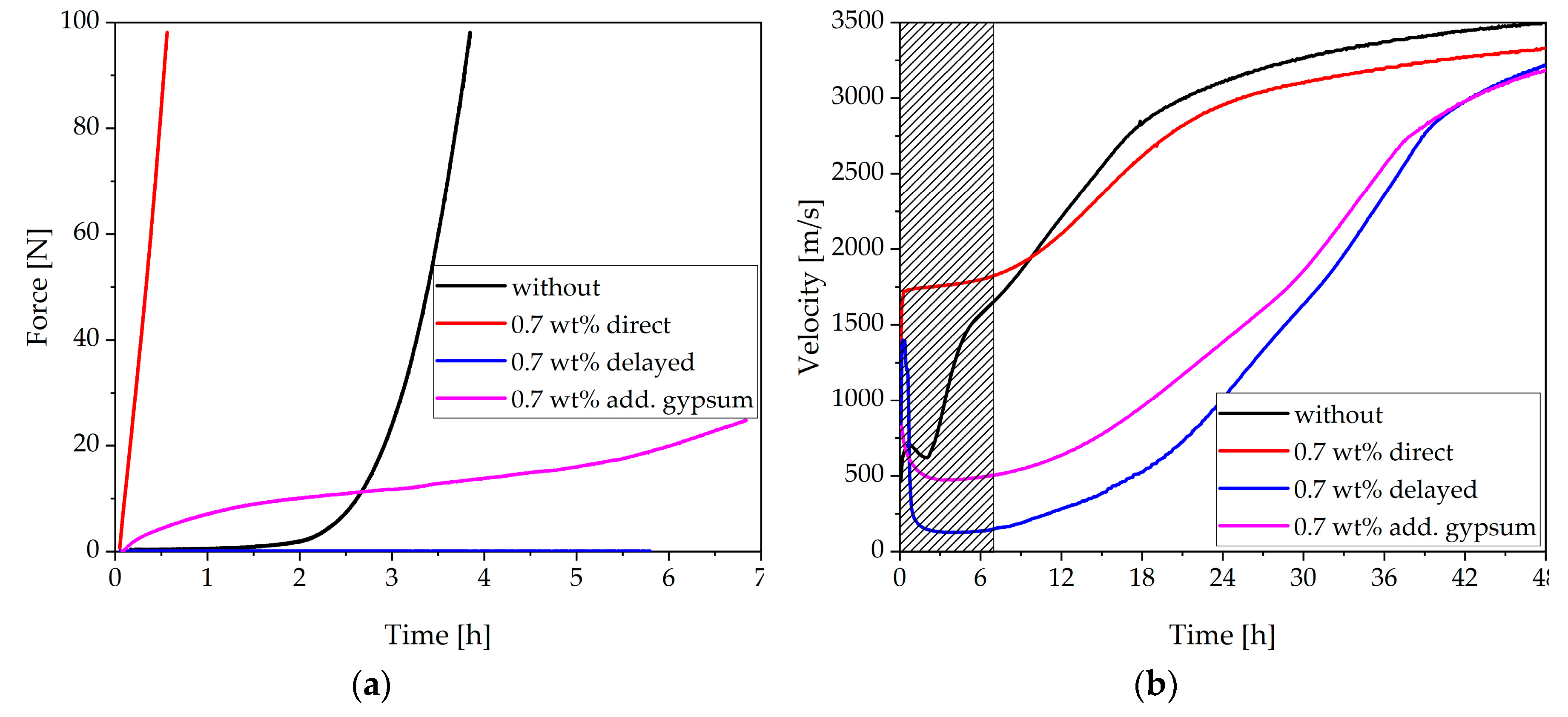
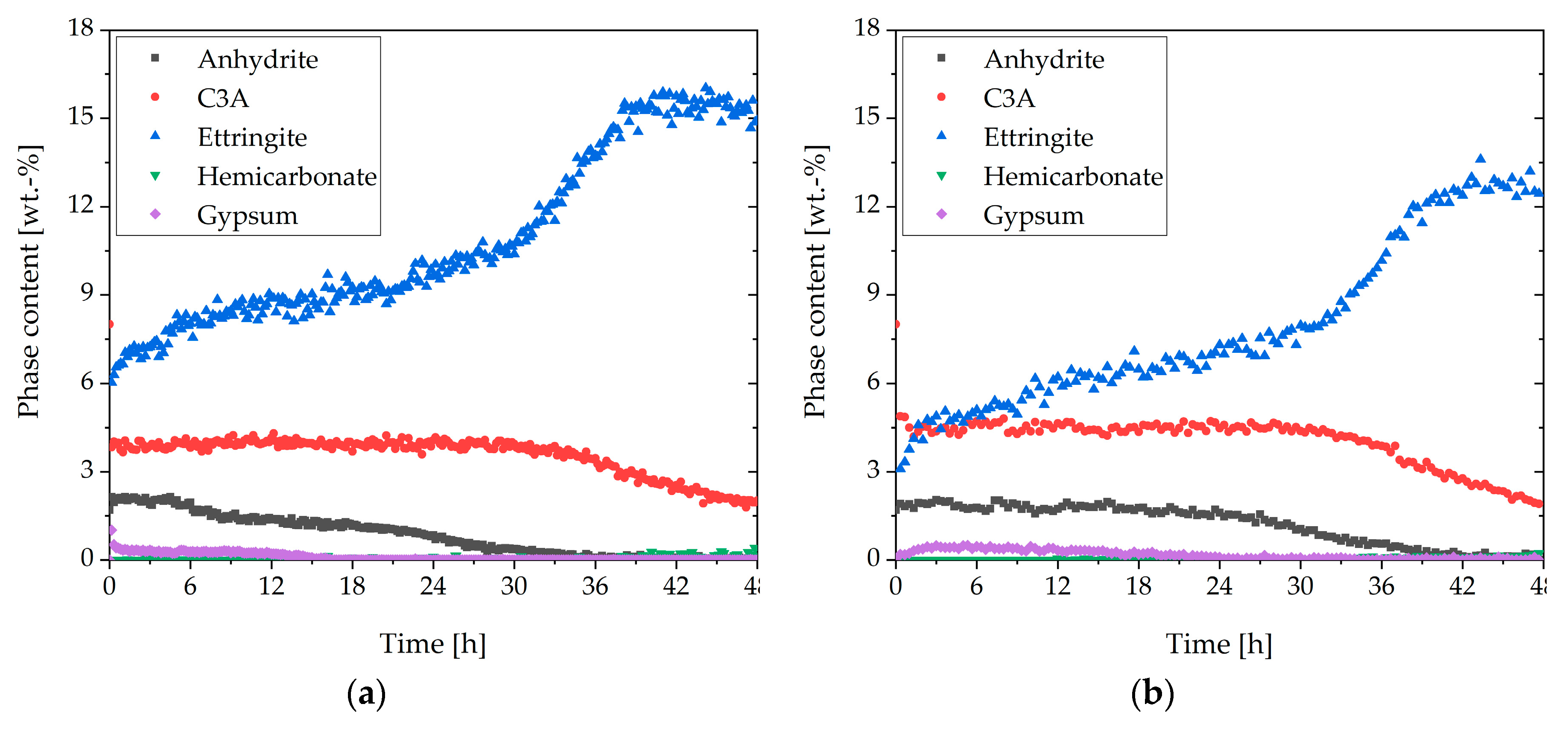
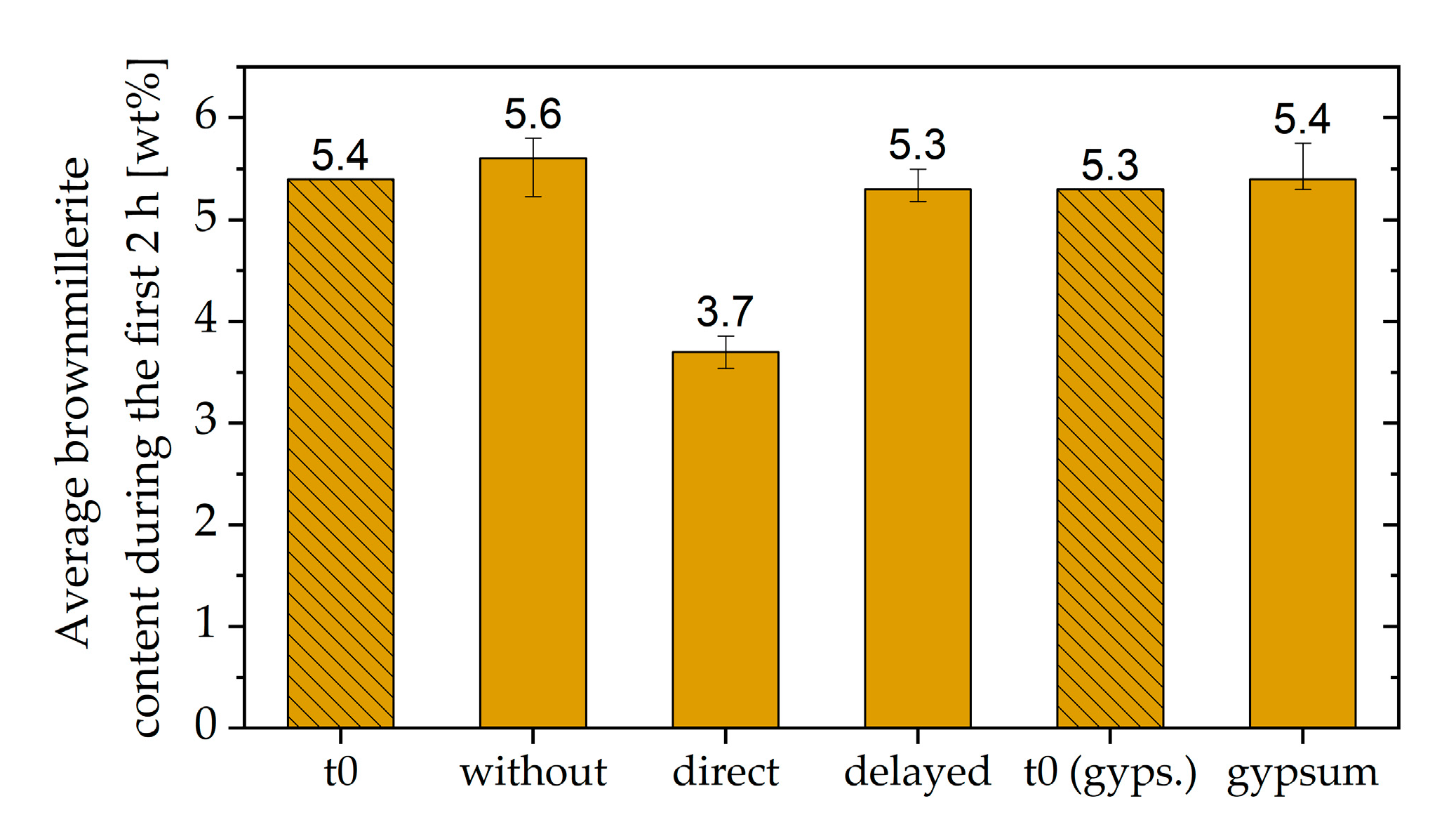
3.4. Two Approaches to Avoiding Incompatible Behavior
4. Discussion
5. Conclusions
- Above a critical dosage, the addition of SP led to a fast stiffening
- This was caused by the formation of vast amounts of ettringite and hemicarbonate
- These two phases were formed because the SP in the initial period prevented the passivation of aluminate-containing phases
- The fast stiffening could be attenuated by adding gypsum and prevented completely by delayed addition of the SP
- Adding gypsum reduced the amount of initially reacted aluminate-containing phases, and no hemicarbonate was formed
- A delayed addition of the SP allowed the passivation of the aluminate-containing phases to develop before the SP was added
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jansen, D.; Naber, C.; Ectors, D.; Lu, Z.; Kong, X.-M.; Goetz-Neunhoeffer, F.; Neubauer, J. The early hydration of OPC investigated by in-situ XRD, heat flow calorimetry, pore water analysis and 1H NMR: Learning about adsorbed ions from a complete mass balance approach. Cem. Concr. Res. 2018, 109, 230–242. [Google Scholar] [CrossRef]
- Bergold, S.T.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Gartner, E.M.; Young, J.F.; Damidot, D.; Jawed, I. Structure and Performance of Cements. Hydration of Portland Cement, 2nd ed.; Taylor & Francis: Abingdon, UK, 2001. [Google Scholar]
- Merlini, M.; Artioli, G.; Cerulli, T.; Cella, F.; Bravo, A. Tricalcium aluminate hydration in additivated systems. A crystallographic study by SR-XRPD. Cem. Concr. Res. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Rößler, C.; Eberhardt, A.; Kučerová, H.; Möser, B. Influence of hydration on the fluidity of normal Portland cement pastes. Cem. Concr. Res. 2008, 38, 897–906. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd: London, UK, 1997; ISBN 978-0727725929. [Google Scholar]
- Jakob, C.; Jansen, D.; Ukrainczyk, N.; Koenders, E.; Pott, U.; Stephan, D.; Neubauer, J. Relating Ettringite Formation and Rheological Changes during the Initial Cement Hydration: A Comparative Study Applying XRD Analysis, Rheological Measurements and Modeling. Materials 2019, 12, 2957. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Aitcin, P.-C. Science and Technology of Concrete Admixtures. Portland Cement; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Ectors, D.; Neubauer, J.; Goetz-Neunhoeffer, F. The hydration of synthetic brownmillerite in presence of low Ca-sulfate content and calcite monitored by quantitative in-situ-XRD and heat flow calorimetry. Cem. Concr. Res. 2013, 54, 61–68. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Lothenbach, B.; Neubauer, J. The early hydration of Ordinary Portland Cement (OPC): An approach comparing measured heat flow with calculated heat flow from QXRD. Cem. Concr. Res. 2012, 42, 134–138. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Stabler, C.; Neubauer, J. A remastered external standard method applied to the quantification of early OPC hydration. Cem. Concr. Res. 2011, 41, 602–608. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Quzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Juilland, P.; Gallucci, E.; Flatt, R.J.; Scrivener, K.L. Dissolution theory applied to the induction period in alite hydration. Cem. Concr. Res. 2010, 40, 831–844. [Google Scholar] [CrossRef]
- Collepardi, M.; Baldini, G.; Pauri, M.; Corradi, M. Tricalcium aluminate hydration in the presence of lime, gypsum or sodium sulfate. Cem. Concr. Res. 1978, 8, 571–580. [Google Scholar] [CrossRef]
- Myers, R.; Geng, G.; Rodriguez, E.D.; Rosa, P.d.; Kirchheim, A.P.; Monteiro, P.J.M. Solution chemistry of cubic and orthorhombic, tricalcium aluminate hydration. Cem. Concr. Res. 2017, 100, 176–185. [Google Scholar] [CrossRef]
- Stein, H.N.; Stevels, J.M. Influence of silica on the hydration of 3 CaO, SiO2. J. Appl. Chem. 1964, 14, 338–346. [Google Scholar] [CrossRef]
- Bellmann, F.; Sowoidnich, T.; Ludwig, H.-M.; Damidot, D. Analysis of the surface of tricalcium silicate during the induction period by X-ray photoelectron spectroscopy. Cem. Concr. Res. 2012, 42, 1189–1198. [Google Scholar] [CrossRef]
- Barret, P.; Menetrier, D.; Bertrandie, D. Mechanism of C3S dissolution and problem of the congruency in the very initial period and later on. Cem. Concr. Res. 1983, 13, 728–738. [Google Scholar] [CrossRef]
- Flatt, R.J.; Schober, I. Understanding the Rheology of Concrete. Superplasticizers and the Rheology of Concrete; Woodhead Publishing Limited: Cambridge, UK, 2012; ISBN 978-0-85709-028-7. [Google Scholar]
- Marchon, D.; Juilland, P.; Gallucci, E.; Frunz, L.; Flatt, R.J. Molecular and submolecular scale effects of comb-copolymers on tri-calcium silicate reactivity: Toward molecular design. J. Am. Ceram. Soc. 2016, 817–841. [Google Scholar] [CrossRef]
- Gelardi, G.; Mantellato, S.; Marchon, D.; Palacios, M.; Eberhardt, A.; Flatt, R.J. Science and Technology of Concrete Admixtures. Chemistry of Chemical Admixtures; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Gelardi, G.; Flatt, R.J. Science and Technology of Concrete Admixtures. Working Mechanisms of Water Reducers and Superplasticizers; Woodhead Publishing: Cambridge, UK, 2015; ISBN 978-0-08-100693-1. [Google Scholar]
- Winnefeld, F.; Becker, S.; Pakusch, J.; Götz, T. Effects of the molecular architecture of comb-shaped superplasticizers on their performance in cementitious systems. Cem. Concr. Compos. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Nkinamubanzi, P.-C.; Mantellato, S.; Flatt, R.J. Science and Technology of Concrete Admixtures. Superplasticizers in Practice; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- von Daake, H.; Stephan, D. Setting of cement with controlled superplasticizer addition monitored by ultrasonic measurements and calorimetry. Cem. Concr. Compos. 2016, 66, 24–37. [Google Scholar] [CrossRef]
- Flatt, R.J.; Houst, Y.F. A simplified view on chemical effects perturbing the action of superplasticizers. Cem. Concr. Res. 2001, 31, 1169–1176. [Google Scholar] [CrossRef]
- Lu, Z.; Haist, M.; Ivanov, D.; Jakob, C.; Jansen, D.; Leinitz, S.; Link, J.; Mechtcherine, V.; Neubauer, J.; Plank, J.; et al. Characterization data of reference cement CEM I 42.5 R used for Priority Program DFG SPP 2005 Opus Fluidum Futurum—Rheology of reactive, multiscale, multiphase construction materials. Data Brief 2019, 27, 104699. [Google Scholar] [CrossRef] [PubMed]
- Hertel, T.; Neubauer, J.; Goetz-Neunhoeffer, F. Study of hydration potential and kinetics of the ferrite phase in iron-rich CAC. Cem. Concr. Res. 2016, 83, 79–85. [Google Scholar] [CrossRef]
- DIN EN 1015-3. Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table); Deutsches Institut für Normung e.V.: Berlin, Germany, 2007; (1015-3:2007-05). [Google Scholar]
- Roussel, N.; Coussot, P. Fifty-cent rheometer for yield stress measurements: From slump to spreading flow. J. Rheol. 2005, 49, 705–718. [Google Scholar] [CrossRef]
- Roussel, N. Understanding the Rheology of Concrete. From Industrial Testing to Rheological Parameters for Concrete; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Roussel, N.; Stefani, c.; Leroy, R. From mini-cone test to Abrams cone test: Measurement of cement-based materials yield stress using slump tests. Cem. Concr. Res. 2005, 35, 817–822. [Google Scholar] [CrossRef]
- Pott, U.; Ehm, C.; Jakob, C.; Stephan, D. Investigation of the early cement hydration with a new penetration test, rheometry and in-situ XRD: In: Rheology and Processing of Construction Materials. RILEM Bookseries 2020, 23. [Google Scholar] [CrossRef]
- Lootens, D.; Jousset, P.; Martinie, L.; Roussel, N.; Flatt, R.J. Yield stress during setting of cement pastes from penetration tests. Cem. Concr. Res. 2009, 39, 401–408. [Google Scholar] [CrossRef]
- Wadsö, L. Operational issues in isothermal calorimetry. Cem. Concr. Res. 2010, 40, 1129–1137. [Google Scholar] [CrossRef]
- Desmet, B.; Atitung, K.C.; Abril Sanchez, M.A.; Vantomme, J.; Feys, D.; Robeyst, N.; Audenaert, K.; Schutter, G.d.; Boel, V.; Heirman, G.; et al. Monitoring the early-age hydration of self-compacting concrete using ultrasonic p-wave transmission and isothermal calorimetry. Mater. Struct. 2011, 44, 1537–1558. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.; Myers, R. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Wagner, T.; Kulik, D.; Hingerl, F.; Dmytrieva, S. Gem-selektor geochemical modeling package: TSolMod library and data interface for multicomponent phase models. Can. Mineral. 2012, 50, 1173–1195. [Google Scholar] [CrossRef]
- Kulik, D.; Wagner, T.; Dmytrieva, S.; Kosakowski, G.; Hingerl, F.; Chudnenko, K.; Berner, U. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Myers, R.; Geng, G.; Li, J.; Rodriguez, E.D.; Ha, J.; Kidkhunthod, P.; Sposito, G.; Lammers, L.N.; Kirchheim, A.P.; Monteiro, P.J.M. Role of Adsorption Phenomena in Cubic tricalcium Aluminate Dissolution. 33 Langmuir ACS Publ. 2017, 33, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sowoidnich, T.; Rachowski, T.; Rößler, C.; Völkel, A.; Ludwig, H.-M. Calcium complexation and cluster formation as principal modes of action of polymers used as superplasticizer in cement systems. Cem. Concr. Res. 2015, 73, 42–50. [Google Scholar] [CrossRef]
- Hirsch, C. Untersuchungen zur Wechselwirkung Zwischen Polymeren Fliessmitteln und Zementen Bzw. Mineralphasen Der Frühen Zementhydratation; unv. Diss., Technischen Universität München: München, Germany, 2005. [Google Scholar]
- Wangler, T.; Lloret, E.; Reiter, L.; Hack, N.; Gramazio, F.; Kohler, M.; Bernhard, M.; Dillenburger, B.; Buchli, J.; Roussel, N.; et al. Digital Concrete: Opportunities and Challenges. RILEM Tech. Lett. 2016, 1, 67–75. [Google Scholar] [CrossRef]
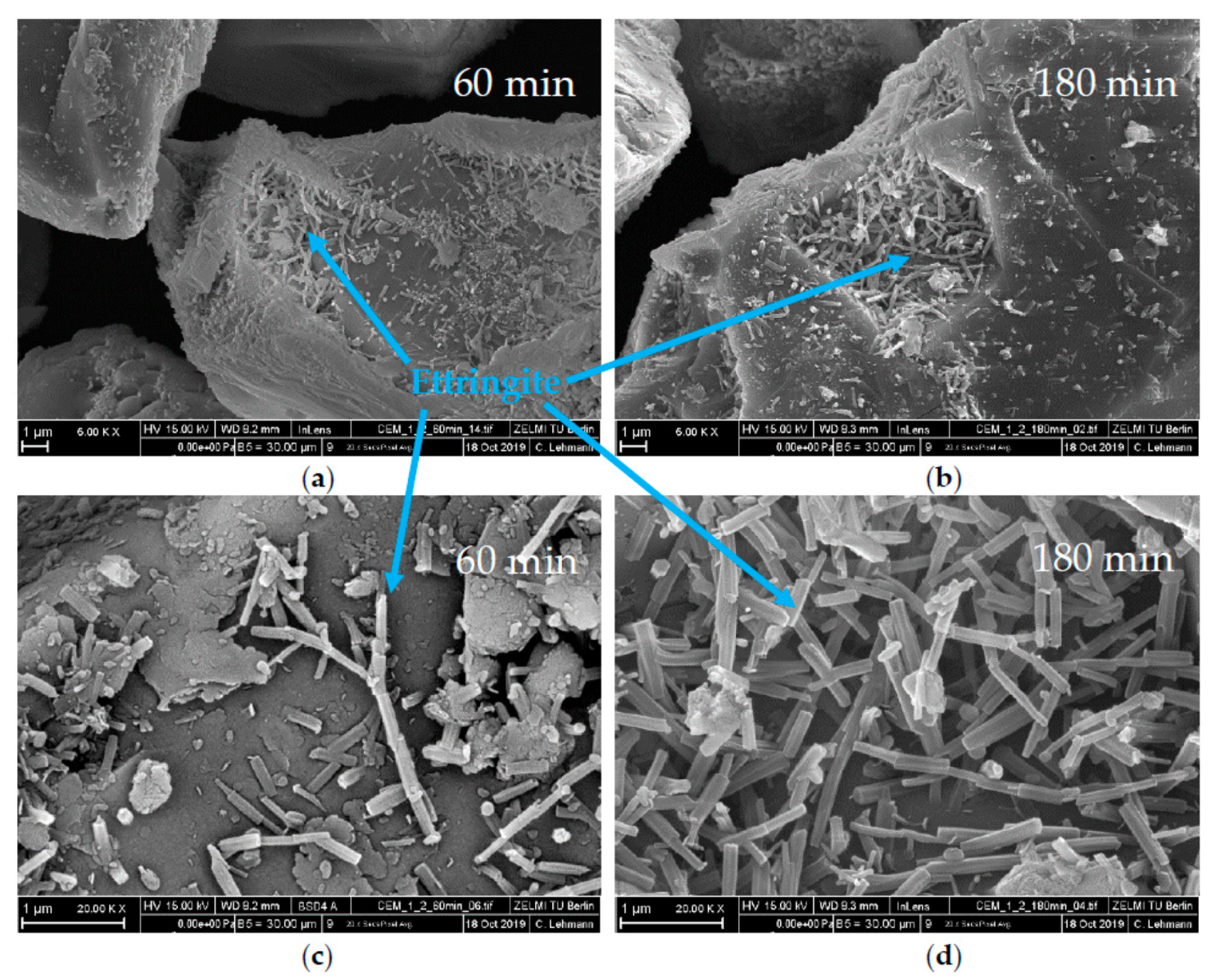
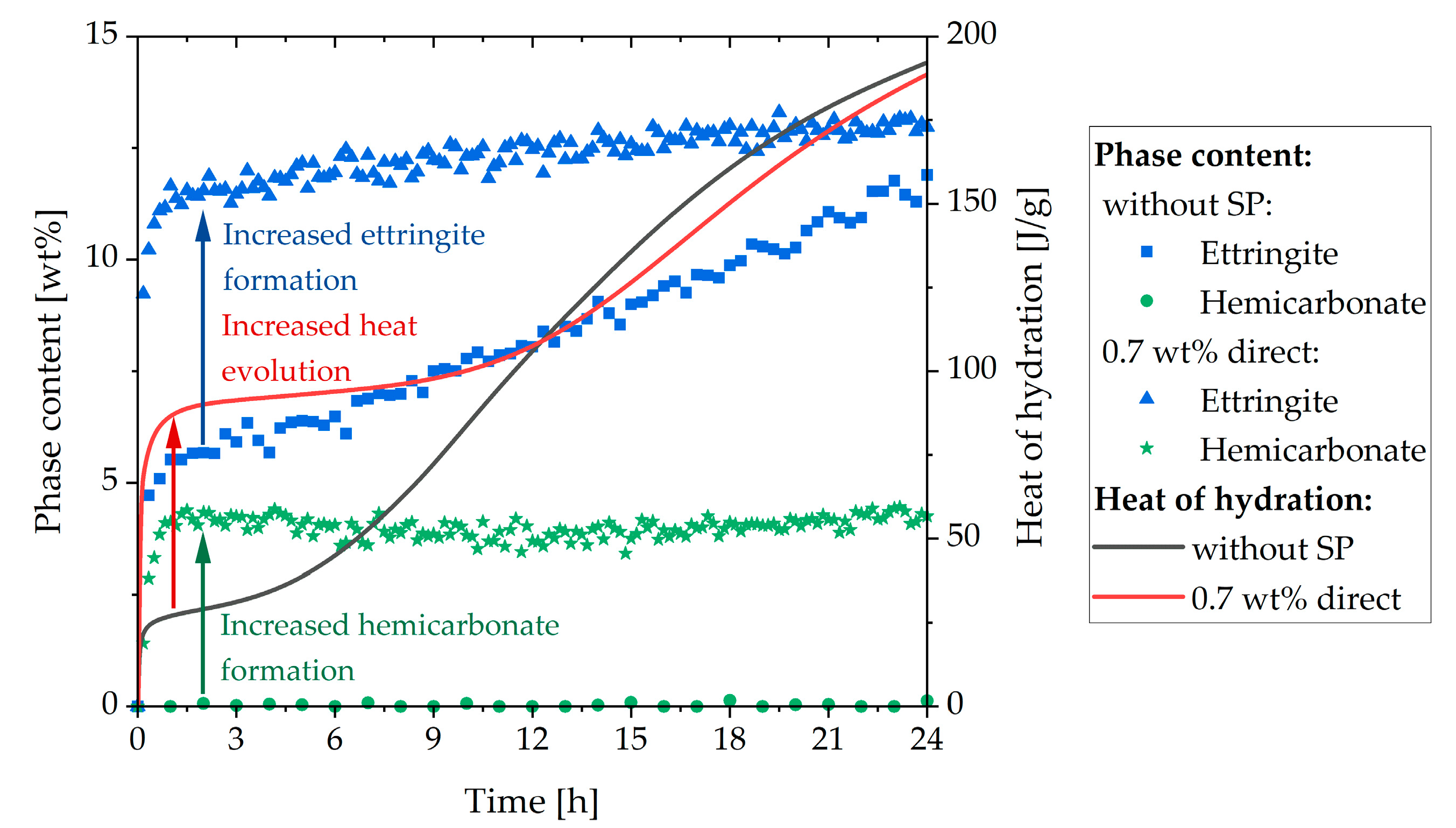
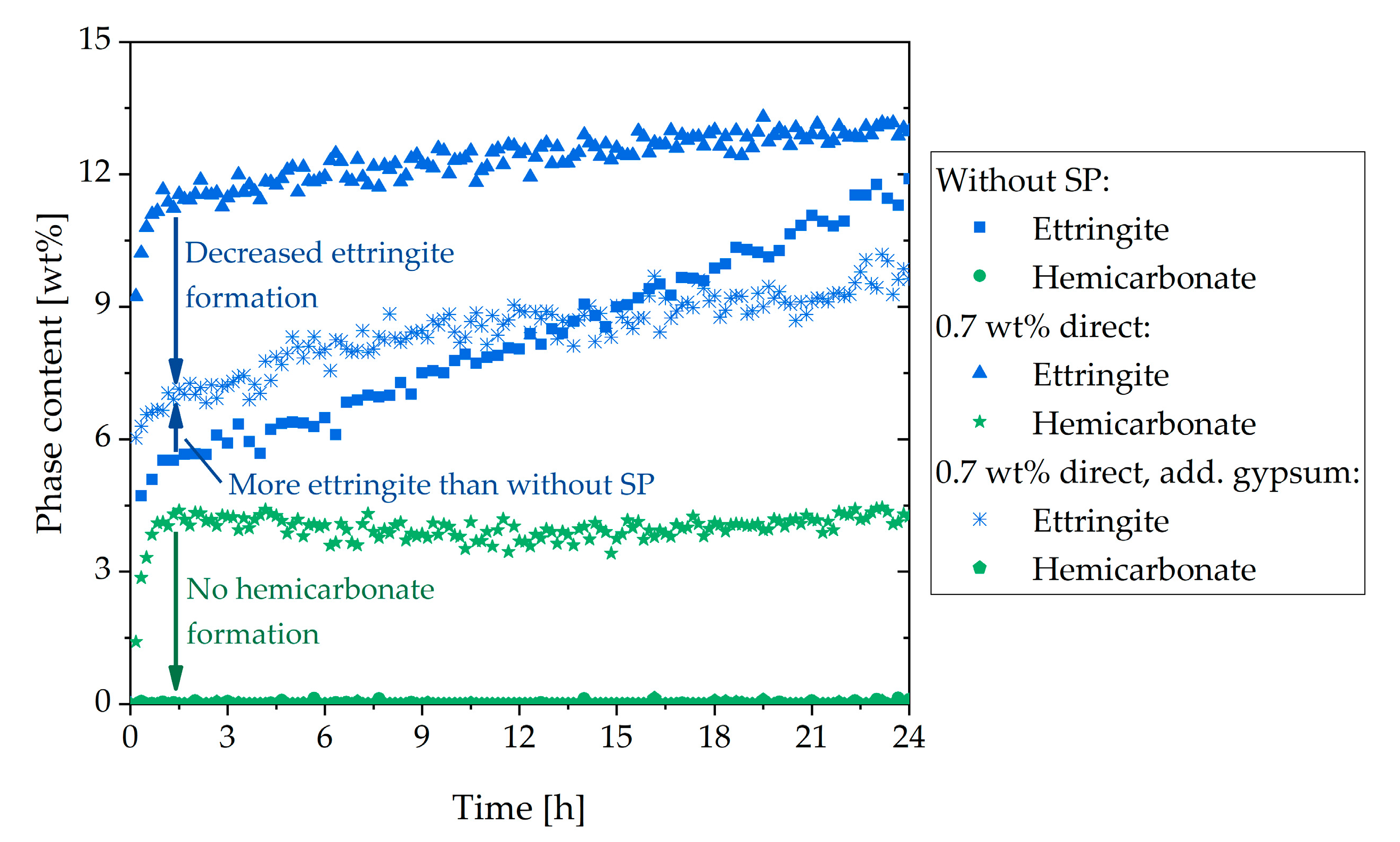
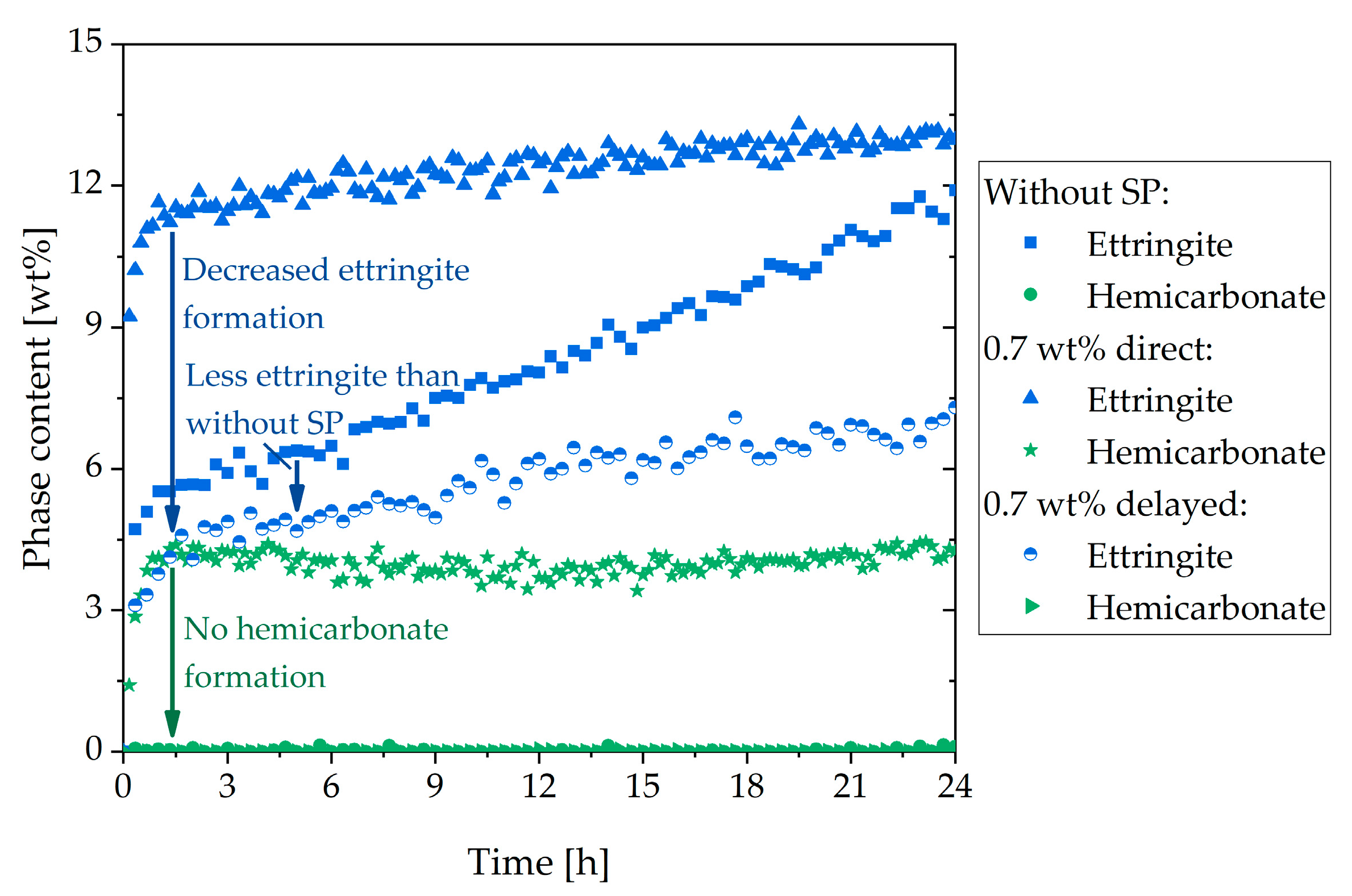
| Phase | wt% | Phase | wt% | Oxide | wt% | Oxide | wt% |
|---|---|---|---|---|---|---|---|
| Alite | 55.8 | Anhydrite | 2.2 | CaO | 64.4 | Na2O | 0.2 |
| Belite | 14.6 | Bassanite | 2.7 | SiO2 | 20.4 | K2O | 0.8 |
| C3Acubic | 7.3 | Gypsum | - | Al2O3 | 5.4 | SO3 | 3.1 |
| C3Aorth. | 3.6 | Calcite | 3.7 | Fe2O3 | 2.6 | TiO2 | 0.3 |
| Arcanite | 0.5 | Brownmillerite | 7.4 | MgO | 1.4 |
| Solid content (M%) | MN 1 (g/mol) | MW 2 (g/mol) | PDI 3 | Anionic Charge Density (µeg/g) | Side Chain Length (PEO 4) |
|---|---|---|---|---|---|
| 23 | 14800 | 25000 | 1.69 | 1530 | 18 |
| Dosage of the SP (wt%) | Spread Flow (cm) | Yield Stress (Pa) |
|---|---|---|
| 0.0 | 10.75 | 467.4 |
| 0.2 | 23.50 | 9.4 |
| 0.3 | 27.00 | 5.0 |
| 0.4 | 20.75 | 17.4 |
| 0.5 | 29.75 | 2.9 |
| 0.6 | 10.00 | 632.0 |
| 0.7 | 10.00 | 671.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pott, U.; Jakob, C.; Jansen, D.; Neubauer, J.; Stephan, D. Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior. Materials 2020, 13, 977. https://doi.org/10.3390/ma13040977
Pott U, Jakob C, Jansen D, Neubauer J, Stephan D. Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior. Materials. 2020; 13(4):977. https://doi.org/10.3390/ma13040977
Chicago/Turabian StylePott, Ursula, Cordula Jakob, Daniel Jansen, Jürgen Neubauer, and Dietmar Stephan. 2020. "Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior" Materials 13, no. 4: 977. https://doi.org/10.3390/ma13040977
APA StylePott, U., Jakob, C., Jansen, D., Neubauer, J., & Stephan, D. (2020). Investigation of the Incompatibilities of Cement and Superplasticizers and Their Influence on the Rheological Behavior. Materials, 13(4), 977. https://doi.org/10.3390/ma13040977





