Abstract
Sewage sludge (SS) recycling is an important part of the proposed ‘circular economy’ concept. SS can be valorized via torrefaction (also known as ‘low-temperature pyrolysis’ or ‘roasting’). SS can, therefore, be considered a low-quality fuel or a source of nutrients essential for plant growth. Biochar produced by torrefaction of SS is a form of carbonized fuel or fertilizer. In this research, for the first time, we tested the feasibility of torrefaction of SS with high ash content for either fuel or organic fertilizer production. The research was conducted in 18 variants (six torrefaction temperatures between 200~300 °C, and three process residence times of 20, 40, 60 min) in 5 repetitions. Fuel and fertilizer properties and multiple regression analysis of produced biochar were conducted. The higher heating value (HHV) of raw SS was 21.2 MJ·kg−1. Produced biochar was characterized by HHV up to 12.85 MJ·kg−1 and lower H/C and O/C molar ratio. Therefore, torrefaction of SS with high ash content should not be considered as a method for improving the fuel properties. Instead, the production of fertilizer appears to be favorable. The torrefaction increased C, N, Mg, Ca, K, Na concentration in relation to raw SS. No significant (p < 0.05) influence of the increase of temperature and residence time on the increase of biogenic elements in biochar was found, however the highest biogenic element content, were found in biochar produced for 60 min, under the temperature ranging from 200 to 240 °C. Obtained biochars met the Polish regulatory criteria for mineral-organic fertilizer. Therefore SS torrefaction may be considered a feasible waste recycling technology. The calculation of torrefaction energy and the mass balance shows energy demand <2.5 GJ∙Mg−1 w.m., and the expected mass yield of the product, organic fertilizer, is ~178 kg∙Mg−1 w.m of SS. Further investigation should consider the scaling-up of the SS torrefaction process, with the application of other types of SSs.
1. Introduction
Municipal sewage sludge (SS) is a ‘by-product’ produced during the municipal wastewater treatment process. It is estimated that the volume of generated SS constitutes about 2% of treated wastewater and contains more than half of the initial load of pollutants flowing with sewage to the treatment plant. Both the quantity and composition of the SS from wastewater treatment plants (WWTP) depends on the origin of treated sewage, the applied method of wastewater and sludge treatment, as well as the amount and properties of (e.g., industrial) sewage [1]. SS consists of ~55%–80% of water and up to 20%~45% of solids. The varied composition of SS from different sources and WWTPs processes implies a local, site-specific solution to the problem of SS disposal and utilization [2,3].
The main SS treatment methods are agricultural use, land reclamation, thermal treatment, and storage. SS management methods differ significantly between countries. Countries with high gross domestic product (GDP) prefer combustion as the primary method of processing, while countries with lower GDP are forced to landfilling. EU countries thermally treat ~22% of the total weight of the SS [4]. The most common method of SS treatment in Poland is agricultural usage (19%), followed by thermal treatment (14%) [5]. Between 2000 and 2015, Poland significantly increased the use of thermal methods and decreased the amount of landfilled SS and sludge stored in WWTP for future utilization. This trend is similar to treatment in countries with higher GDP than Poland’s, where the amount of thermally treated SS has significantly increased over the past years, and the amount of SS sent for storage, or landfilling has decreased [6]. Agricultural usage of SS is relatively inexpensive, and the mass of SS utilized in this manner in Poland has not increased significantly in recent years [5]. This is due to stricter requirements regulating agricultural sewage deposits and concerns about accumulated toxins in SS.
Direct agricultural applications of untreated SS may pose an environmental risk [7]. Therefore, SS pre-treatment methods are developed. One of the directions is thermochemical treatment. Thermochemical methods comprise many processes that lead to the utilization of a large amount of waste and create value-added products by reducing the volume of water by 80%~90% [8]. The main criteria to use thermal and thermochemical methods are the production of energy from locally available raw materials and the reduction of environmental contaminants and concentration of nutrients in biochar [9]. Combustion, pyrolysis, fast pyrolysis, slow pyrolysis, gasification [10], and torrefaction are in the center of thermochemical methods and lead to creating biochar [11,12]. Other thermal processes for biomass depolymerization are solvolysis [13,14,15]. All types of thermal conversion of municipal SS lead to the production of one or more ash char fractions. The quality and quantity of these products can vary and depends on the sludge’s source and the design of the thermal process. Thus, addressing the same question as in the manuscript title (fuel or fertilizer?) require further investigation and discussion on the suitability of other thermochemical conversion methods to produce either solid fuels or fertilizers.
Torrefaction is a biomass thermal decomposition process that produces a C-rich product—biochar [12,16,17,18,19,20,21,22,23,24,25]. Even though there is no exact definition and the term ‘biochar’ is loosely used for all char, which is thermochemically or hydrothermally produced out of biomass or waste products with high organic content, there are some limitations. Sometimes the term biochar is referring to material produced in temperature above 350 °C [26]. Other references are limiting the usage of the term depending on application or quality; for example, C content between 50% and 80% [27]. According to Lehmann et al. [28] and Sohi et al. [29], the term biochar implies the usage of the char for amelioration or C-sequestration. Polish regulations [30] prohibit the usage of terms ‘biocarbon’ and ‘toryfikat’ (from Polish: ‘torrefied biomass’) when it originated from SS. Considering that SS is regarded as ‘biomass’ in Polish legislation [30], the term ‘biochar’ will be used in this study.
During torrefaction, biomass partly decomposes, generating both condensable and non-condensable gases resulting in higher C content [28]. Torrefaction may also be referred to as ‘roasting,’ ‘slow and mild pyrolysis’, ‘wood-cooking’, and ‘high-temperature drying’ [31]. Apart from treated biomass, temperature and retention time are the main influencing factors on process efficiency [32]. The torrefaction is usually carried out in the temperature range between 200~300 °C, and the processing time of 15~20 min depending on the material properties [33]. Torrefaction is most commonly used for lignocellulosic biomass [34,35].
Torrefaction of SS has been initially characterized by Poudel et al. [36]. As demonstrated by research conducted by Poudel et al. [36], the SS is a suitable substrate for the torrefaction process. The increase in the C density was observed in the resulting product [36]. On the other hand, produced biochar contained a smaller amount of O and H in its structure. This was caused by the release of volatile compounds during the torrefaction included in the gaseous products. Poudel et al. [36] studies were conducted in material with low ash content. However, the high ash content in SS is site/source and treatment technology-specific. Other research on SS torrefaction was performed on materials characterized by ash content between 12 to 43%. Produced biochars calorific value ranged between 11.43 to 19.86 MJ∙kg−1 [36,37,38,39,40], with one research indicate extremely high devolatilization in the temperature range between 260~300 °C resulting in biochars with the lower heating value (LHV) from 6.50 to 3.88 MJ∙kg−1 [41].
Due to improved combustion properties, torrefaction products can be used as a fuel source for torrefaction of lignocellulosic biomass. In the case of SS, due to high degradability organic matter and relatively high ash content, torrefaction does not improve the fuel properties of SS [12] and can even decrease the calorific value [41]. Therefore, SS torrefaction may be considered as pre-treatment before SS biochar application in agriculture.
Lignocellulosic biochar agricultural use is being researched in the context of biorenewable fuels. It has been proven that biochar application significantly improves soil quality and an essential C and other nutrients balance [42,43]. The positive effect of biochar on the soil is caused by the improvement of texture and porosity, which reflects changes in the physical, chemical and biological parameters. Nutrients in the biochar are more easily absorbed into their cell matrix. The ability to exchange cationic biochar, for example, allows the retention of K ions available to plants [44].
SS biochar produced in the pyrolysis process was characterized by a significant increase of K and P but increasing temperature above 300 °C caused rapid N loss via volatilization [45]. Yue et al. [46] tested pyrolysis SS biochar influence on poor quality urban soil. A positive effect of increased nutrient content in biochar co-composted with poultry manure was shown [47]. Addition of biochar from SS in the amount of 10 Mg∙ha−1 in a pot experiment, resulted in an increased yield of tomatoes by 64%, and a synergistic effect of increased availability of nutrients along with the improvement of soil quality. Biochar has a heterogeneous and highly porous structure. Both its internal and external surface is very extensive and has a different character both hydrophilic and hydrophobic. This makes biochar a material with high water retention capacity, which can be important for example, during the reclamation of poor soils [48].
Most publications on SS biochar agricultural properties is based on the pyrolysis process, that occurs in higher temperature, compared to torrefaction. Therefore, biochars generated from both processes will differ significantly. To date, the only research on the influence of torrefaction on the nutrient properties of SS biochar was published by Wesenbeeck et al. [49]. This is in contrast to a greater number of studies focused on the SS biochar agricultural application after pyrolysis [44,45,46,47,48]. Lessons learned from pyrolysis can be useful but are not necessarily applicable to SS torrefaction biochar.
This study aimed to evaluate the influence of torrefaction process residence time and temperature on essential fuel and fertilizer properties of SS with high initial ash content. The research was designed to perform analysis of both raw SS as reference samples as well as those generated in 18 variants of biochars (6 temperatures, 3 retention times). The scope of analyses included the characterization of fuel and fertilizer properties. The results of the analyses allowed us to develop multiple regression models describing the influence of torrefaction parameters on the properties of the generated torrefied product. Models can be used to identify torrefaction parameters with the highest efficiency and to propose the optimal use of the biochar as a fuel or fertilizer. The energy and mass balance of SS torrefaction was performed to analyze the applicability of this solution.
2. Materials and Methods
2.1. Sewage Sludge Characterization
Sewage sludge originated from municipal WWTP in Jastrzębie-Zdrój, Poland. The facility description and sample collection methods were described in Pulka et al. [41]. Raw SS properties used in this research are summarized in Table 1.

Table 1.
Raw sewage sludge properties.
2.2. Experimental Design
Torrefaction, with the generation of biochar products, was performed using the method described in Białowiec et al. [50] in the muffle furnace 8.1/1100, SNOL, Utena, Lithuania. SS was dried for 24 h at 105 °C before the experiment in accordance with Stępień et al. [24]. The drying was because the dewatered (raw) SS had the moisture content of 80.2%. Thus, in order to avoid the influence of a long period of heat transfer to the sample, dry sludge was used with dry matter content of 96.3% (Table 1). Two independent factors were applied: torrefaction temperature and torrefaction duration. The target temperatures of 200, 220, 240, 260, 280, and 300 °C were maintained for 20-, 40- or 60-min. The reaction temperature was measured throughout the experiment. The total number of variants was 18. Each variant was performed in 5 repetitions.
The experimental procedure was the same as [24]. One crucible for a single torrefaction variant, contained between 250~300 g d.m. of SS, was put into the cold muffle furnace. CO2 was supplied at 10 dm3∙h−1 to ensure inert conditions during torrefaction. CO2 was introduced into the reactor by the steel 0.25-inch tube inserted through the muffle furnace chimney. The end of the tube was placed in the central point of the reactor chamber, above the crucible with the SS sample. CO2 and process gases were outflowing thought the reactor chimney. The heating of the reactor began 5 min after CO2 was introduced into the reactor. Heating always started at ambient temperature and took 5 to 10 min, depending on the target temperature. After torrefaction, the samples were left in the furnace to cool down for 60 to 90 min, depending on the initial process temperature. CO2 flow was shut off when the temperature inside the reactor dropped below 100 °C during cooling. The cooled sample was moved from the muffle and weighed with 0.1 mg accuracy to determine the mass loss. After that, the sample was analyzed. The cooling period was incorporated because the reactor could not be opened until the temperature drops down to a certain point. Without this procedure, we would expose the biochar to atmospheric oxygen and possible self-ignition could occur.
2.3. Analyses of the Biochar Properties
Torrefied and dry SS samples were characterized by the analysis described below:
- Moisture content using the KBC65W (WAMED, Warsaw, Poland) laboratory dryer with Radwag PS 3500.R2 (Radwag, Radom, Poland) analytical balance following the PN-EN 14346:2011 standard [51],
- Losses on ignition (LOI) by means of model 8.1/1100, SNOL, Utena, Lithuania muffle furnace with Radwag PS 3500.R2 analytical balance following the PN-EN 15169:2011 standard [52],
- Ash content using the SNOL 8.1/1100 muffle furnace with Radwag PS 3500.R2 analytical balance following the PN-G-04516:1998 standard [53],
- Elementary C, H, N, and O composition using Perkin Elmer 2400 Series CHNS/O (Waltham, MA, USA) with Radwag, MYA 2.4 Y analyzer following PN-EN ISO 16948:2015-07 [54]
- HHV and LHV using the IKA C2000 Basic calorimeter (IKA® Poland Sp. z o.o., Warsaw, Poland) following the PN-G-04513:1981 standard [55],
- Mg, Ca, K, Na total content in solid samples were analyzed with atomic absorption spectroscopy (AAS) after dry mineralization using Varian Spektra AA 240 FS following PN-EN 14082: 2004 standard [56] (Agilent Technologies, Santa Clara, CA, USA). Dry mineralization was carried out with the procedure described below. The homogeneous sample (10 g) was incinerated on the heating plate; then the samples were mineralized in a muffle furnace for 8 h, the ash was burned for 2 h after dissolving in 2 cm3 HNO3. The mineralization was transferred quantitatively into 10 cm3 vessels using 2M HNO3.
2.4. Data Analysis
The following parameters were calculated based on the experiment results:
Mass yield,% [57,58]:
where M0 and Mt is mass before and after process, respectively, g.
Energy yield,% [57,58]:
where HHVt and HHVo is energy after and before the process, respectively, MJ·kg−1 d.m.
H/C ratio [36]:
where H and C is the percentage of hydrogen and carbon content.
O/C ratio [36]:
where O is the percentage of oxygen content.
The LHV was estimated based on HHV, moisture content (W), and the H content:
where W is moisture content,%; 8.94 is the H-to-H2O conversion, H-hydrogen content,%
HHVdaf (on dry and on ash-free bases) was estimated as [59]:
where HHV—high heating value (on dry basis), MJ·kg−1; Mf—fuel mass (on dry basis), mass, g; Mash—the mass of ash in fuel, g.
Multiple regression analysis at the significance level p < 0.05 was then performed to elucidate the influence of process temperature and residence time on biochars fuel and fertilizer properties. Finally, the polynomial models of process temperature and residence time influence on biochars fuel properties were used according to the previous study [60] with regression modeling:
where v is modeled dependent variable; T and t are independent variables of temperature and time, respectively; a2–a9 are regression coefficients; a1 is an intercept.
Statistica 13 software (StatSoft, Inc., TIBCO Software Inc., Palo Alto, CA, USA) was used to conduct statistical analysis. The detail regression coefficients values and statistical evaluation of determined coefficients describing the influence of torrefaction temperature and process duration on fuel and fertilizer parameters of SS biochars are given in Table A1, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7, Table A8, Table A9, Table A10, Table A11, Table A12, Table A13, Table A14, Table A15, Table A16, Table A17 and Table A18 in the Appendix A.
Torrefaction energy demand was calculated following [61]. Estimated price for covering the energy demand using pelletized biomass incineration was calculated with assumption (HHV = 20 MJ·kg−1, 1 Mg price = 999 PLN (1 USD ~ 4 PLN), incineration efficiency = 90%) [62]. Prices were given in EURO at the exchange rate from National Polish Bank in December 2019 (EURO = 4.261 PLN). The economic analysis of the biochar was based on the unit prices of mineral fertilizers used in Poland. For this purpose, the N fertilizer prices have been converted into the cost of 1 kg of a pure component following [63].
3. Results
3.1. The Influence of Torrefaction Temperature and Residence Time on Fuel Properties of Biochars
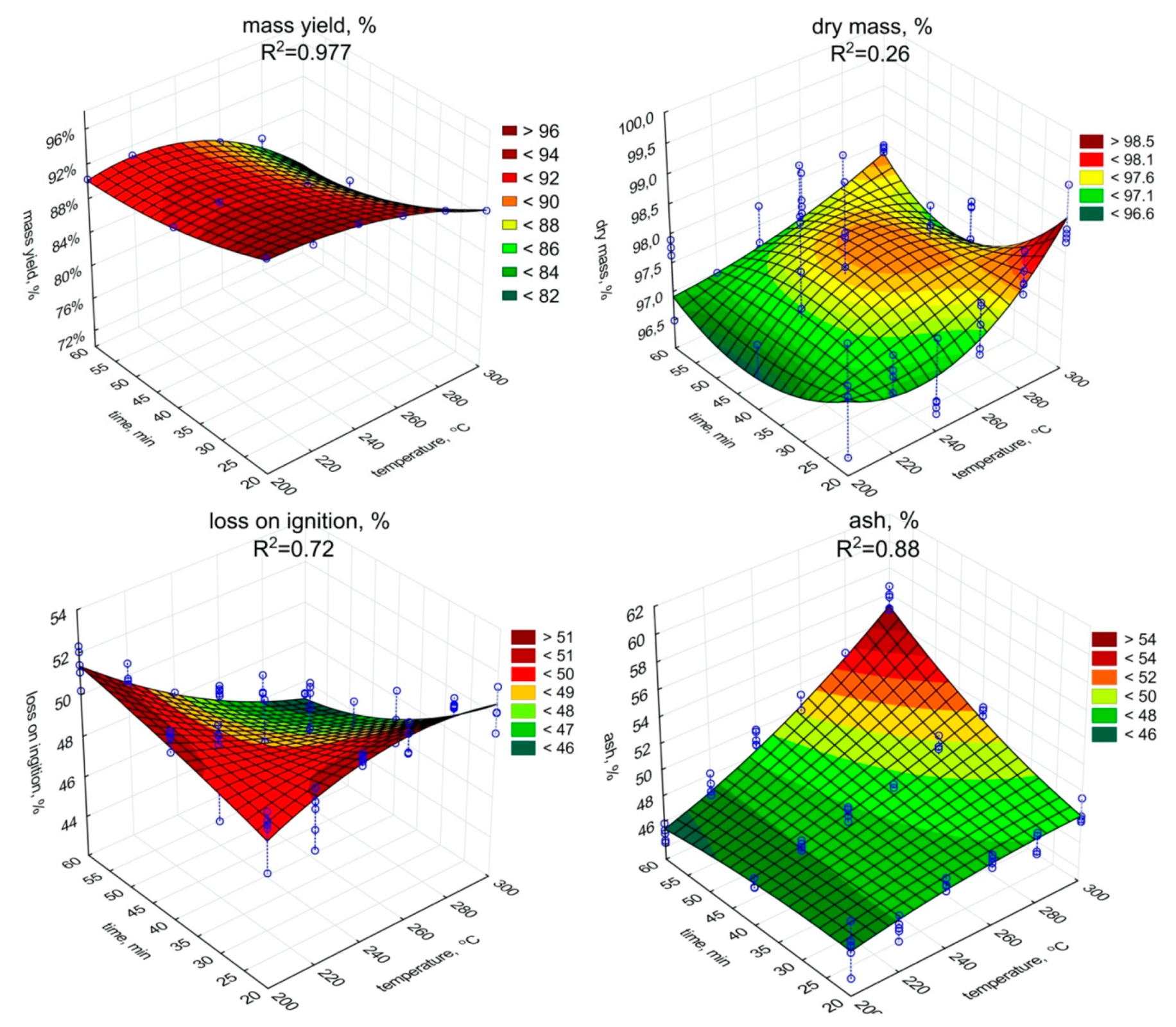
The significant (p < 0.05) decrease in the mass yield value with the increase of the temperature and retention time was observed (Figure 1, Table A1). The low moisture content in biochar samples was observed. It could be caused due to hygroscopic condensation of air vapor, and indirectly may indicate the degree of hydrophobicity of biochars. The dry matter content ranged from 97.4% to 98.5%. The increase in torrefaction temperature and retention time resulted in a significant (p < 0.05) dry matter increase (and decrease of water content) (Figure 1, Table A2). The lowest content of dry matter (97.4%) was observed in the biochar generated at 200 °C with a retention time of 20 min. Whereas the highest dry matter values were obtained for a retention time of 20 min at 300 °C (98.5%), and 97.8% at 300 °C with a retention time of 60 min.
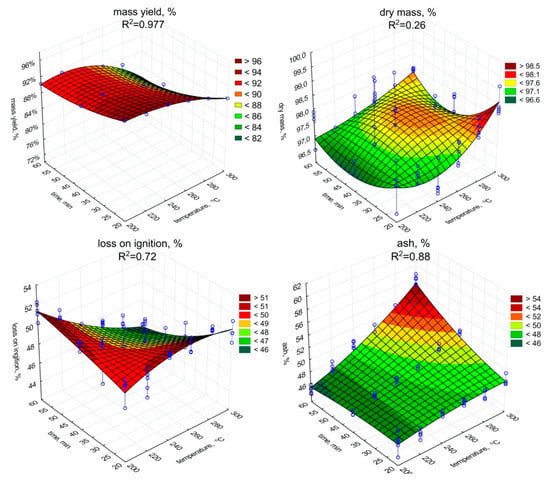
Figure 1.
Mass yield, dry mass, loss on ignition, the ash content in torrefied SS in relation to process temperature, and residence time.
The LOI content in biochars (Figure 1) decreased due to the torrefaction in relation to raw SS (Table 1), while ash content (Figure 1) increased. The lowest LOI content, 44.4% d.m., was recorded for biochar produced during 60 min and under 300 °C, while the highest LOI content, 51.6% d.m., for 20 min of torrefaction under 200 °C. An opposite trend between LOI and ash content were observed. A significant (p < 0.05) increase in temperature and residence time resulted in LOI decrease during SS torrefaction (Figure 1, Table A3). The increase of temperature and retention time increased (p < 0.05) the ash content (Figure 1, Table A4). The highest ash content value 55.6% was obtained in the most robust variant of SS torrefaction (300 °C, 60 min).
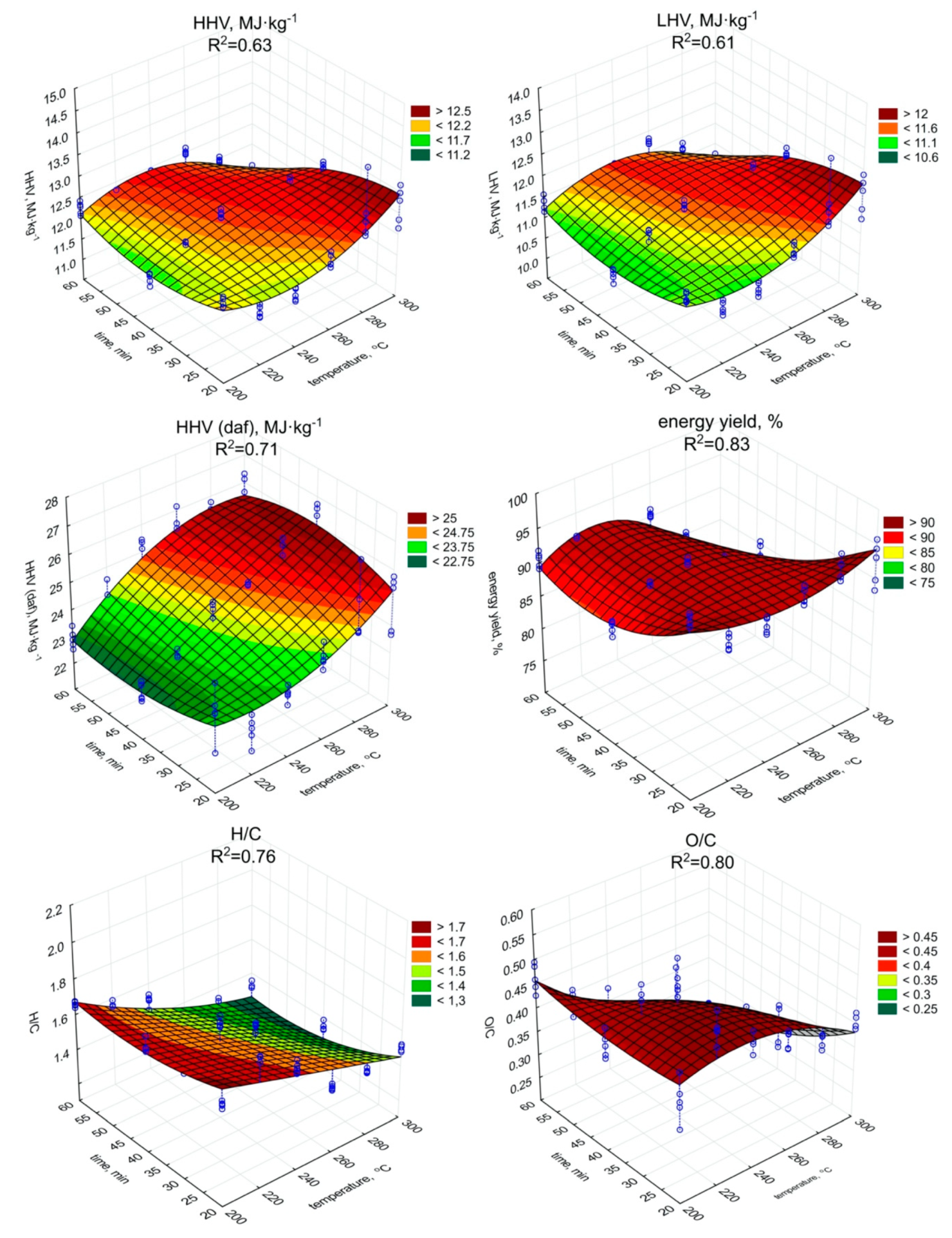
The torrefaction improved the fuel properties of the biochar (Figure 2 and Figure 3) in comparison to raw SS (Table 1), but only in individual cases. The highest HHV value was observed in the case of the biochar produced during the 60 min process under 260 °C (12.52 MJ·kg−1) (Figure 2). However, (surprisingly) the lowest observed HHV was found in the case of the biochar produced during the 60 min at 300 °C (11.09 MJ·kg−1). As the HHV depends on C content, the similar trend of the increase (p < 0.05) of HHV (Figure 2, Table A5), and C content in biochars (Figure 3, Table A6) with the temperature and residence time can be observed.
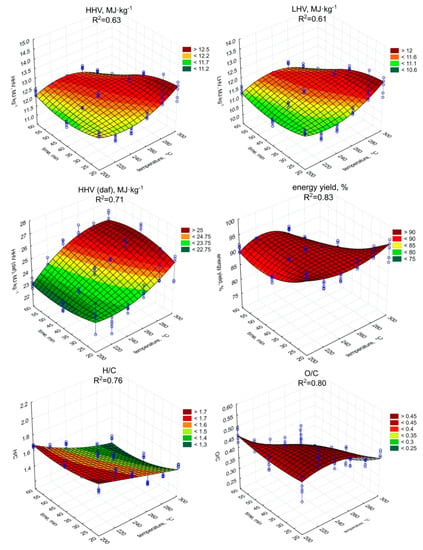
Figure 2.
HHV, LHV, HHV daf., energy yield, and H/C, O/C molar ratios of torrefied SS in relation to process temperature, and residence time.
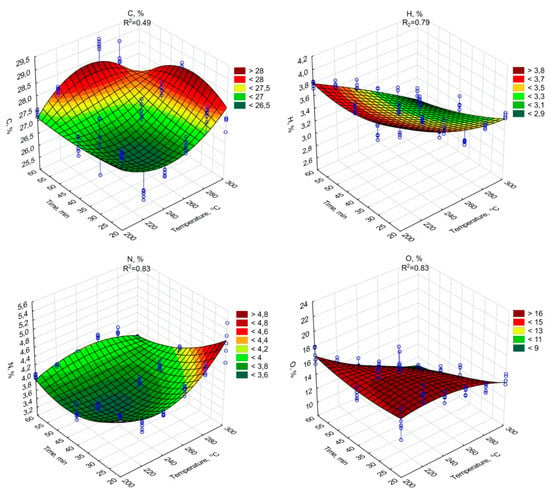
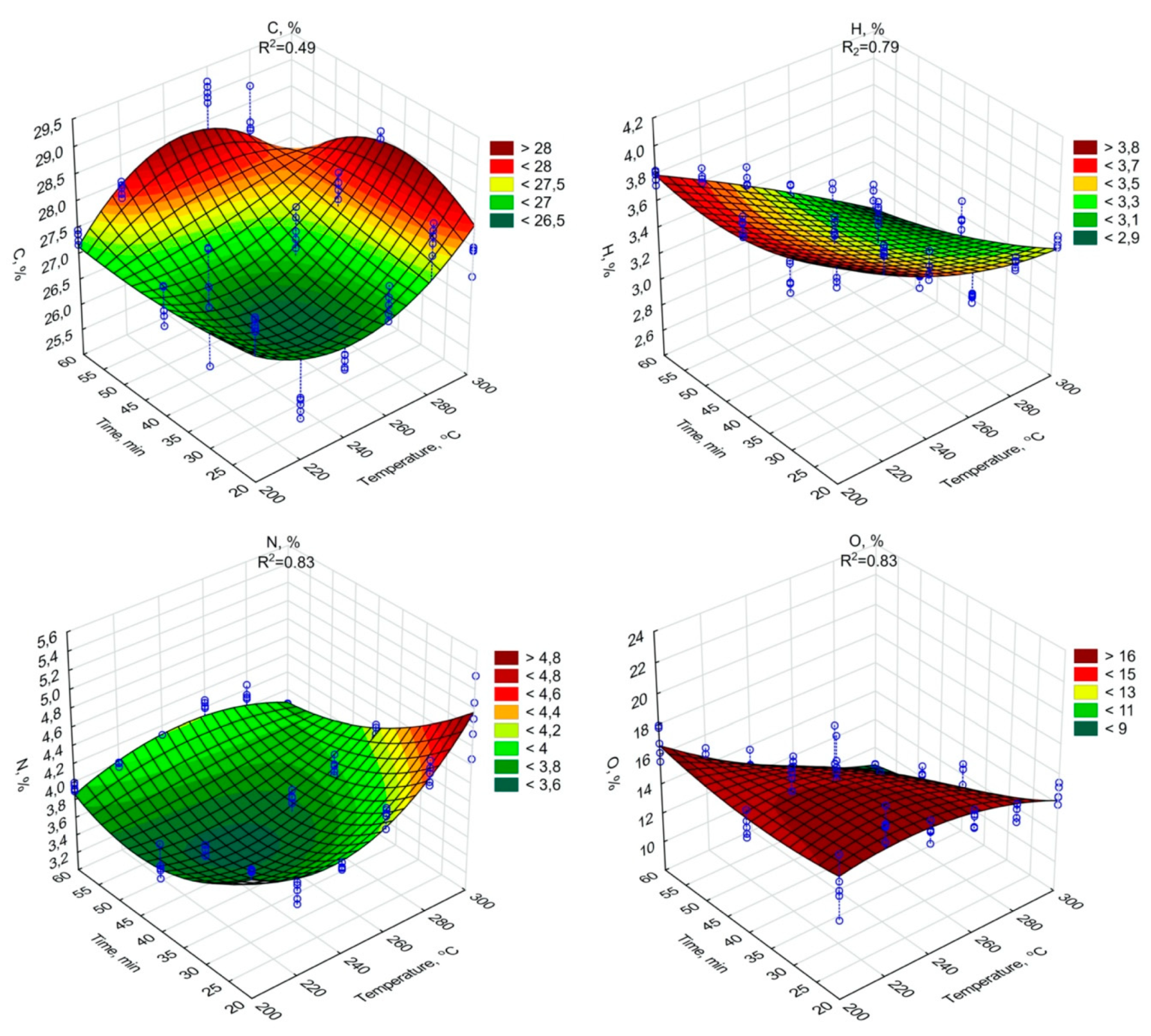
Figure 3.
C, H, N, O percentage content in torrefied SS in relation to process temperature and residence time.
The highest C content, 29.02% d.m., (similarly as in the case of HHV), was found in biochar generated during 60 min of torrefaction under 260 °C (Figure 3). The increase of ash content with temperature and retention time did not negatively influence the HHV, while the C content in biochars increased significantly (p < 0.05). Additionally, the significant decrease of H (Figure 3, Table A7), and O (Figure 3, Table A8) content due to the increase in the temperature and duration of the torrefaction was observed. The H content was 3.74% d.m. in raw SS (Table 1). The lowest content of H 2.85% d.m. was in the case of 300 °C and 60 min (Figure 3). The H removal efficiency was 23.9%. The lowest O content was in the variant of 300 °C and 60 min (Figure 3). The removal efficiency in comparison to O content of 23.97% d.m. (Table 1) in raw SS was 64.9% in the case of the variant of 300 °C and 60 min (Figure 3). It shows that significant removal of O due to torrefaction offset the negative influence of the ash content increase on HHV values. However, the importance of the ash on fuel properties was demonstrated by the calculations of dry ash-free HHV daf. Simulation of ash removal made the trend of the significant (p < 0.05) increase in HHV daf with an increase in both temperature and residence time (Figure 2, Table A9) more apparent. The HHV daf. values were much higher than HHV. The highest HHV daf. was obtained for the SS torrefied at 300 °C, with a retention time of 40 min (26.2 MJ·kg−1).
The LHV is a function of HHV but additionally depends on moisture and H content. Although the changes in moisture content and H content in biochars obtained under different torrefaction conditions were statistically significant, they did not affect the pattern of LHV (Figure 2) depending both on process duration and temperature, which was similar to HHV. The LHV showed an upward trend (p < 0.05) as the time and temperature of the process increased (Figure 2, Table A10). The highest LHV (12.11 MJ·kg−1) value was associated with the biochar torrefied at 260 °C with 60 min residence time (Figure 2). Regression analyses indicated that temperature increase affects HHV and LHV values, causing an apparent rise when short residence times were used (Figure 2). For torrefaction above 20 min at higher temperatures, devolatilization was too robust, which is confirmed by significant (p < 0.05) decrease in LOI values, hence HHV and LHV values were declining.
The energy yield showed a trend of significant (p < 0.05) decreasing value along with the increase of the process temperature (Figure 2, Table A11). At the retention time of 20 min, the percentage of energy yield ranged from 96.2% (200 °C) to 90.5% (240 °C). The results in the above retention time showed distinct fluctuation. With the 40 min process duration, the highest value was obtained at 240 °C (92.2%) and the lowest at 300 °C (84.5%). Two lowest results were obtained for biochar generated at the 60 min residence time at 280 and 300 °C with 84.2% and 73.3%, respectively. For the remaining lower process temperatures (200~260 °C), the highest value was obtained at 220 °C (91.5%). Regression analysis indicated significant (p < 0.05) energy yield drop at higher temperatures, due to substantial mass loss (Figure 1) despite the increase in HHV values (Figure 2).
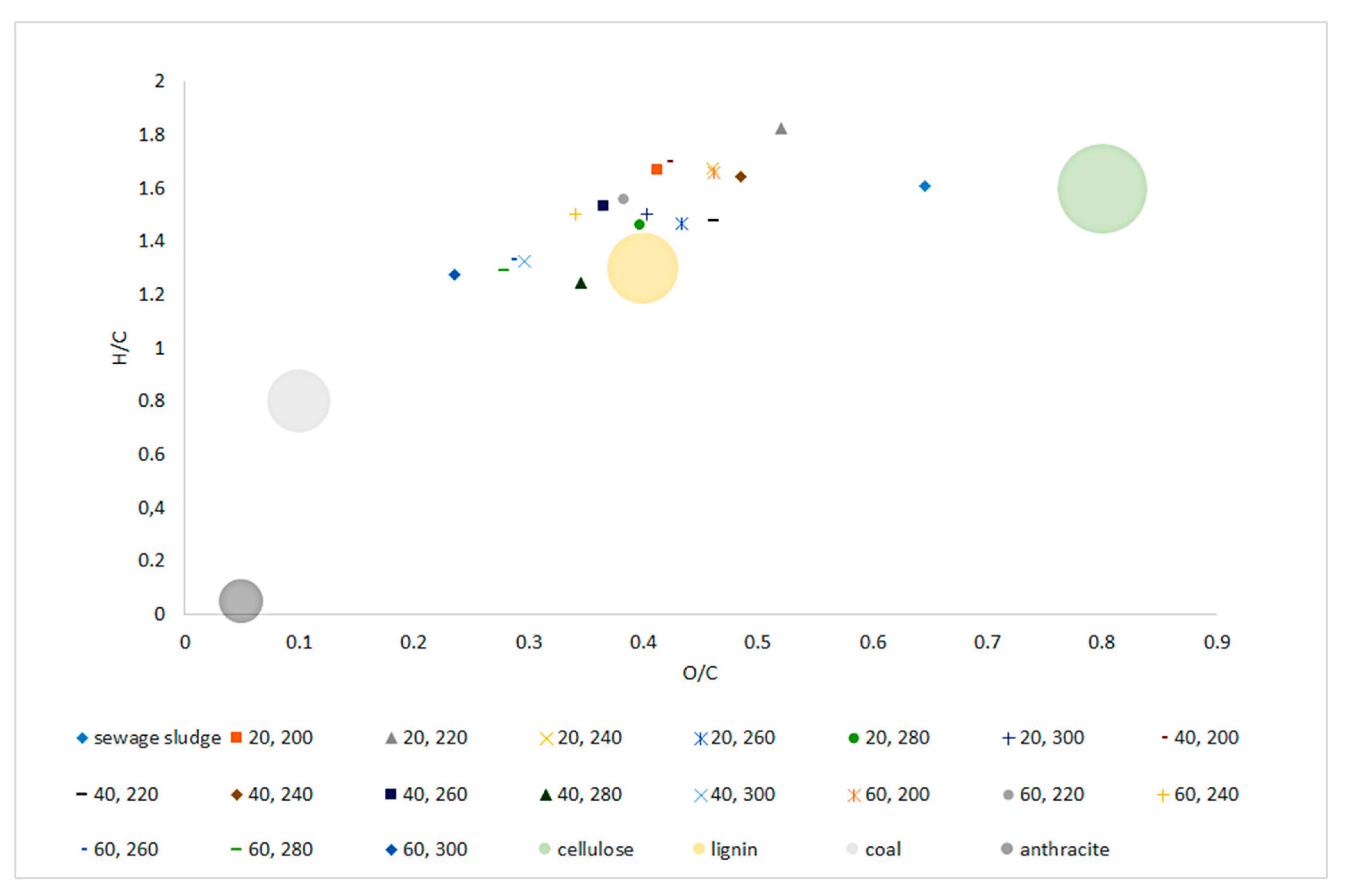
The van Krevelen diagram (Figure 4) shows the material orientation relative to the molar O/C and H/C ratios. The tested SS was in the range characteristic for biomass between areas typical for cellulose and lignin. The apparent decrease in the ratio of both O/C and H/C, along with the increase in temperature and process retention time was significant (p < 0.05) and clearly visible (Figure 4, Table A12 and Table A13). Biochar samples H/C and O/C values obtained at 240~260 °C placed those materials in the area reserved for ‘lignin.’ Higher temperatures (280~300 °C) caused further decrease in H/C, O/C ratio, allowing biochars generated at those conditions to be placed outside of the ‘biomass area’, and trending into areas reserved for ‘coal.’ Regression models confirmed this trend indicating significant (p < 0.05) drops in H/C and O/C molar ratio with the increase of both temperature and process time with a high determination coefficient of 0.76 and 0.79, respectively.
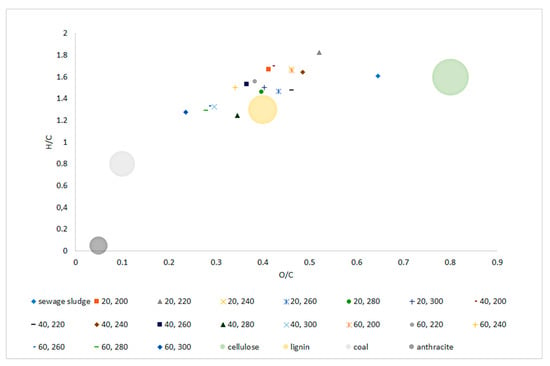
Figure 4.
Van Krevelen diagram of dry sludge and torrefied sewage sludge generated at three retention times (20, 40, 60 min), in six temperature variants (200, 220, 240, 260, 280, 300 °C), in relation to coal (anthracite) and biomass (cellulose and lignin) reference.
3.2. The Influence of Torrefaction Temperature and Residence Time on Fertilizer Properties of Biochars
The N content in the raw SS was 4.3% d.m. (Table 1). The percentage of N content significantly (p < 0.05) increased with the process temperature (ranging from 3.4% to 4.9%) and was inversely proportional to the processing time (Figure 3, Table A14).
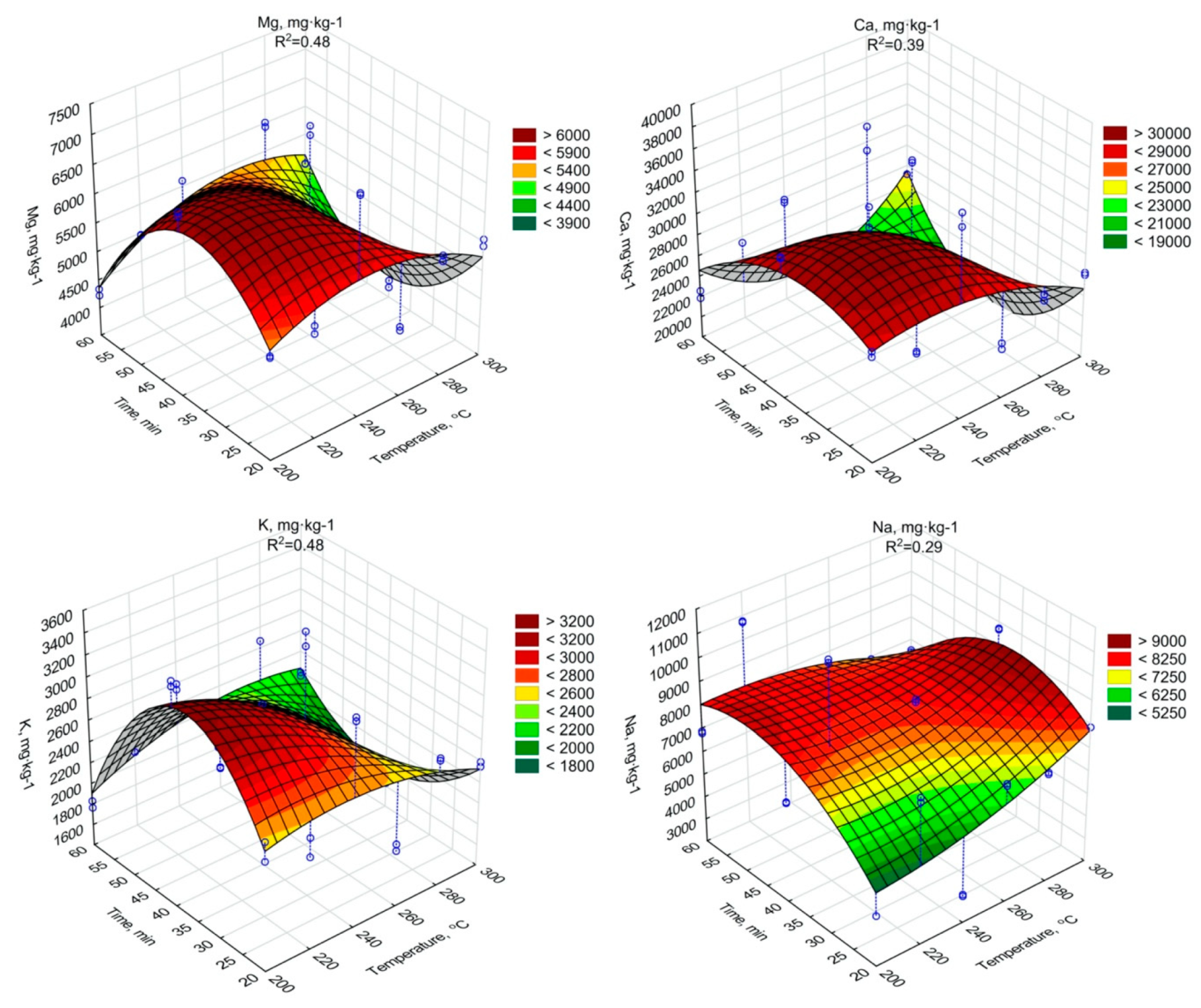
The regression analysis showed a significant decrease of the Mg, Ca, K content due to an increase of the torrefaction temperature and duration (Figure 5, Table A15, Table A16 and Table A17). In the case of Na similar tendency was confirmed, but with some fluctuations (Figure 5, Table A18). It should be noted, however, that all Mg, Ca, K, and Na concentrations in biochars were higher than in raw SS. It indicates that the torrefaction improved SS fertilizer properties, due to the densification of biogenic elements.
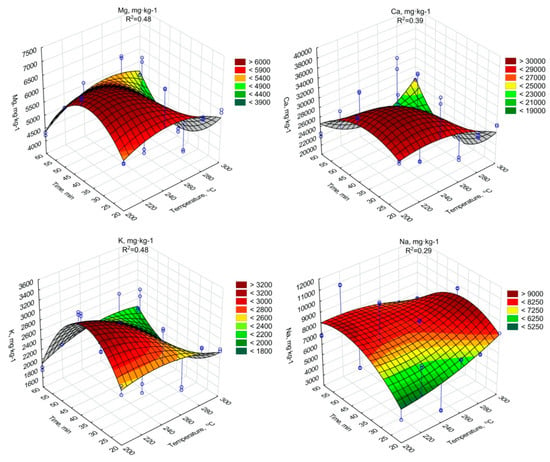
Figure 5.
Mg, Ca, K, Na content in torrefied SS in relation to process residence time and temperature.
4. Discussion
The results were compared to biochars obtained from other SSs to discuss the differences or similarities obtained using similar feedstock and, in some cases, to lignocellulosic materials as a useful comparison showing that some of the observed trends are similar between vastly different substrates.
4.1. Proximate Analysis
SS dry matter values can be compared to a dry matter content ranging from 96.4% to 98.4% obtained in the work of Pulka et al. [12], or up to 99.8% described in the work of Atienza-Martinez et al. [64] and Dhungana [65]. The observed drop in the dry matter content, along with the increase of the process temperature may be related to the greater mass of condensates in the reactor during the cooling phase or be related to potentially higher hydrophobicity of the biochars obtained in higher temperatures.
The results of LOI were lower than in the work of Dhungana, [65]. This is due to the significantly lower initial volatile matter content of 57.2% d.m. compared to 76% d.m. reported by Dhungana [65]. In the work of Pulka et al. [12], the LOI results in biochars from SS obtained from the wastewater treatment plant in Olsztyn, Poland, ranged from 35.0% to 53.8% d.m., with an initial content of 54.4% d.m. The results obtained in this work are characterized by significantly lower LOI values, which may be related to a smaller torrefaction crucible; consequently, easier heat transfer into the sample. However, the trend of decrease in the LOI as the process temperature increases is apparent in all cited research including our recent study [41].
The ash content is the sum of the solid residue after the thermal decomposition of organic matter and non-flammable parts. A clear trend of ash content rise with the increase of temperature and the process duration is visible. This is due to the reduction of organic matter that is decomposed and degassed. Results of ash content in biochars obtained from SS originating from WWTP in Olsztyn presented in the work of Pulka et al. [12] ranging from 33% to 45% d.m. are lower relative to the results in this study. This indicates a significant impact of the source of SS on the obtained results and the economics of the possible implementation of SS torrefaction that needs to be site-specific. Generally, due to the high level of non-flammable fraction in SS, the use of torrefaction for SS processing should be counted with the increase of ash content during processing, which adversely affects fuel parameters. It also has a very unfavorable impact on the mass balance of the further thermal conversion of the obtained biochars, because a significant part of the mass (ash) will remain after the process as a waste requiring further utilization.
4.2. Fuel Properties
Higher heating value is a parameter that indicates the amount of energy that can be generated from the mass of the substrate during combustion, including the energy generated during gas condensation [66]. The HHV results obtained indicate that the torrefaction of the tested SS in more than 20 min may result in lower HHV values. Confirmation of this theory is in the results described elsewhere [12,41,50], where the torrefaction of two non-lignocellulosic materials, SS and RDF respectively, in 60 min residence time, did not result in statistically significant higher HHV values in relation to the substrate. In the work of Poudel et al. [36], a similar relationship occurred. The increase in the duration of the process above 30 min for the tested temperatures of 250 and 300 °C caused a decrease in the HHV in relation to the values obtained in shorter process times. A similar trend was visible in the work of Atienza-Martinez et al. [64], where lengthening of the process resulted in lowering the product HHV. Obtained HHV results are in line with the influence of temperature increase and residence time on the reduction of organic matter content (LOI). The HHV did not decrease as the organic C content increased (mainly compounds with high H and O concentration decomposed and degassed). The opposite tendency in comparison with lignocellulosic biomass may result from lower torrefaction activation energy of SS [12,41] and, consequently, the higher susceptibility of organic substances contained in SS to decomposition by degassing than organic substances from wood biomass.
The LHV increased significantly in relation to the raw SS and the dry SS. Such an increase in calorific value is associated with evaporation of 95% of water. For comparison, in research on SS torrefaction from the wastewater treatment plant in Olsztyn [12], the LHV increased from 0.5 MJ·kg−1 to 14.4 MJ·kg−1 generated at 200 °C. There was also an increase in the calorific value in relation to the dry SS. The maximum LHV value in the present study was 7% higher than the values characterizing dry SS. Such a small increase in LHV is associated with increased ash content, hence lowering volatile matter percentage in the biochars in relation to the dry SS.
The parameter allowing to assess the impact of the valorization process on energy value in relation to the organic matter is the HHV daf. (dry ash-free). There was a visible trend of increasing calorific value together with the increase of temperature and duration of the process. A similar tendency was confirmed in the work of Atienza-Martinez et al. [64], where the increase in both retention time and temperature resulted in higher HHV daf. values for biochars. In the work of Pulka et al. [12], temperature rise caused a similar effect.
The energy yield is a parameter strongly correlated with the percentage mass yield. As in the case of mass yield, the decrease in the energy yield is associated with both the increase of the process temperature and the increase of the retention time. It should be noted that the energy yield dropped below 80% only with the longest retention time and the highest process temperature. This is likely caused by a relatively small decrease in mass and an increase in the HHV. Much higher residual energy values were obtained compared with the biochars obtained from SS by Dhungana [65]. The results in Poudel et al. [36], where the values obtained at 300 °C amounted to approx. 55%. It is related to the lower initial percentage of volatile content in SS used in this research, compared to the works of Dhungana [65] and Poudel et al. [36]. Similar values of residual energy for SS were observed in Atienza-Martinez et al. [64].
The content of C in the biochar increased with the increase in temperature and process residence time. A similar trend is visible for the previous work done by Pulka et al. [12]. It should be noted, however, that the increases in the percentage of C are lower than in the cited publication.
An opposite trend was observed for the H and O content in biochars. The H and O content decreased as the temperature and duration of the process increased. A similar trend was reported in the works of Mock et al. [67] and Pulka et al. [12]. In both cases, the H content decreases were observed as the temperature increased, and also with the increase of the retention time in the case of Mock et al. [67]. It is worth emphasizing that in the work of Mock et al. [67], much higher H concentrations were observed, which may be associated with almost 2× higher content of this element in the raw material than in the presented studies. The decrease in O content is most strongly associated with degassing, in particular, CO2, CO, and O, and VOCs containing carboxyl, carbonyl, and hydroxyl groups. Confirmation of the described trend is reported, among others, in [12,36], where the increase in temperature caused a significant reduction in the O content in the processed SS. A similar trend, but not as apparent, is visible in the work of Huang et al. [68], where despite the higher range values of the analyzed temperatures, the decreases in O content were not as high.
Van Krevelen diagram allows the material to be positioned in relation to O/C and H/C molar ratios, allows for quick determination of material potential when considering thermal processing. Increasing the temperature of the SS torrefaction process resulted in a decrease in H/C and O/C ratios, and thus a shift in biochars positions towards higher calorific materials. The raw SS was located in the area characteristic for cellulose, while the biochars were located in the area characteristic for lignin’s and were approaching the area reserved for hard coal. Such shaping of the van Krevelen diagram along with the increase in the temperature and retention time of torrefaction is typical and confirmed in the works of others [36,69].
4.3. Fertilizer Properties
Similarly, to this research, the increase in N content with increasing temperature can be observed in Poudel et al. [36]. However, it should be noted that the relative increase in N content in the cited work was significantly lower. The results obtained for the biochars in Huang et al. [68] were different, due to a decrease in N content with the rise of the process temperature.
The increase of other biogenic elements Mg, Ca, K, and Na with the increase of the temperature and torrefaction residence time was observed. The increase of Mg content during the SS torrefaction process at 300 °C was obtained in the work of Lu et al. [70], where increase occurred compared to three different SS samples from 4.1 to 8.1 g·kg−1 d.m., from 6.7 to 11.6 g·kg−1 d.m. and from 4.1 to 5.4 g·kg−1 d.m. A similar trend, although with lower growths, was presented by Hossain et al. [71], where the Mg content increased from 3.3 to 3.5 g·kg−1 d.m. In the case of Ca, Hossain et al. [71] observed that Ca content increased from 30.2 to 34.7 g·kg−1 d.m., while in the work of Lu et al. [70] where values for 3 different SS sample increased from 4.8 to 8.1 g·kg-1 d.m., 6.7 to 11.6 g·kg−1 d.m. and from 1.5 to 1.8 g·kg−1 d.m. The changes of K content in biochars had similar character as results obtained in the work of Lu et al. [70], where the K content in biochars from 3 different samples of dry SS ranged from 1.2 to 2.1 g·kg−1 d.m., 0.8 to 1.6 g·kg−1 d.m. and 1.3 to 1.8 g·kg−1 d.m. Additionally, in the case of Na, Lu et al. [70] showed a similar tendency indicating an increase in the value of generated SS biochars in relation to the dry sludge.
The obtained results for N, Ca, Mg, K, Na content indicate that torrefaction of SS can be considered as a method of improving the SS fertilizing properties, and thus enabling its recycling in agriculture. The amount of organic matter, the content of total N and K (as K2O), allows qualifying of the obtained biochars as solid mineral-organic fertilizers following Polish regulations [72]. Examples of effective usage of biochar from SS as fertilizers for agriculture are described in the works of Waqas et al. [73], Hossain et al. [74], Song et al. [75], and Zornoza et al. [76], where the biochars were generated from SS under the pyrolytic conditions, and their high agricultural suitability was found. However, the manuscript did not address the question concerning which of the direction of the biochars from SS utilization (fuel or fertilizer) is more preferable.
Hossain et al. [74] verified the hypothesis about the agro-technical properties of pyrolyzed biochar in relation to the improvement of soil quality, plant growth, yield, and slight bioavailability of metals in tomatoes. The results showed an improvement in vegetable growth by 64% as a result of the increased availability of P and N, as well as the improvement of chemical soil conditions. The yield reached its maximum following the application of char with fertilizer. Shinogi and Kanri [77] reported other positive aspects of biochar from animal and human waste pyrolysis applications to the soil. It was noted that due to its lightness, it is a potential material for improving water permeability. However, the authors pointed to the need for monitoring the changes in the content of Zn in the biochars obtained in the low-temperature pyrolysis processes. The varied impact of biochar on the development of indicator organisms was caused by the inhomogeneous composition of waste sent to the reactor.
The simplified economic evaluation of the SS torrefaction for fertilization purposes was added as the results indicate that biochar produced from the SS has a higher potential for application in agriculture than for energy purposes. The energy demand of 1 Mg of raw SS drying and torrefaction amounted to 2.43 GJ, with only 2% of the overall value was needed for torrefaction purposes. Most of the energy was required for water evaporation during the heating stage of the process. The price of energy necessary for raw SS torrefaction was calculated using the pelletized biomass incineration process and amounted for 32 EURO∙Mg−1 of raw SS. Taking into account the percentage of N in the torrefied samples (240 °C, 1 h), the price of N considered as pure fertilizer was evaluated. The analysis indicates that 178.4 kg of biochar is produced from 1 Mg of raw SS, with a fertilization value of 5 EURO. It is worth mentioning that with decreasing moisture, less energy will be required for drying and torrefaction.
Taking into account that torrefaction did significantly increase N, Ca, Mg, K, and Na compounds content torrefied SS and that many researchers pointed out that SS biochars had a positive influence on plant growth, this mode of SS processing appears to be favored. Considering this research as an initial study, further evaluation of torrefaction biochar from SS for agricultural purposes should be conducted. Future research should include bioavailability and leachability of biogenic compounds, the influence of the SS biochar on soil texture and porosity, as well as availability of cationic part to the plants. Next, the biochar fertilization field tests and toxicological assessment should be conducted.
5. Conclusions
In this research, for the first time, we tested the feasibility of torrefaction of SS with high ash content for either fuel or organic fertilizer production. This research indicated that a better solution is to consider the biochar produced from torrefied SS as a fertilizer. The concentration of biogenic compounds increased as a result of SS torrefaction. The obtained biochars may be classified as a solid mineral-organic fertilizer according to the Polish regulations. The best biochars (i.e., with the highest biogenic element content, were the biochars produced from 200 to 240 °C and 60 min. Considering the high agricultural potential of SS biochar from the torrefaction process in this initial research, future tests including bioavailability and leachability of biogenic compounds and heavy metals, biochar fertilization field tests, and toxicological assays should be conducted. Additionally, the influence of SS biochar on soil physical properties should be studied. The selected fuel properties are also worth highlighting. Particularly interesting are the results of HHV and H/C and O/C molar ratios, demonstrating a slight improvement of the biochar fuel parameters. Unfortunately, the increase in the ash content along with the increase in temperature and the retention time-limited the feasibility of further improvement to fuel parameters. The best variant of SS torrefaction (from the fuel as an end-use scenario) were biochars generated at 280 °C and 20 min. The increase of the H/C and O/C ratios, with a slight increase in the ash content, resulting in high values of both the HHV and the LHV was observed along with the increase of the torrefaction temperature. Obtained results indicate that torrefaction of SS with high ash content did not increase fuel properties (especially HHV and LHV). Additionally, the biochars were characterized by much higher ash content than the raw SS.
Author Contributions
A.B., P.M.: methodology; A.B., J.P., P.M., J.A.K.: formal analysis; A.B., J.P., J.A.K.: validation; J.P., A.B., M.S.: investigation; J.P.: resources; J.P., A.B., P.M., P.S., M.S.: data curation; A.B., J.P.: writing—original draft preparation; J.P., A.B., P.M. J.A.K.: writing—review and editing; A.B., J.P., P.S., J.A.K.: visualization; A.B., P.M.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Preludium 6 Program, the National Research Center, Poland, grant #UMO-2013/11/N/NZ9/04587 titled “The influence of sewage sludge torrefaction on biocarbon phytotoxicity, leachability of heavy metals from biocarbon and sorption parameters of biocarbon.” Authors would like to thank the Fulbright Poland Foundation for funding the project titled “Research on pollutants emission from Carbonized Refuse Derived Fuel into the environment,” completed at the Iowa State University. Partial funding of this paper came from the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa. Project no. IOW05556 (Future Challenges in Animal Production Systems: Seeking Solutions through Focused Facilitation) sponsored by Hatch Act and State of Iowa funds.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on process mass yield.
Table A1.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on process mass yield.
| Mass Yield, % | ||||||
|---|---|---|---|---|---|---|
| Model | Mass Yield, % = (1.44809) + (7.03892 × 10-6)·T2 + (−0.00364092)·T + (0.000414299)·t2+ (−0.0460416)·t + (0.000374584)·T·t + (−8.04432 × 10-7)·T2·t + (−3.39151 × 10−6)·T·t2 + (7.27706 × 10−9)·T2·t2 R2 = 0.98 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 1.448091 | 0.285892 | 0.00 | 0.00 | 0.879256 | 2.016927 |
| a2 | 0.000007 | 0.000000 | 0.00 | 0.00 | 0.000007 | 0.000007 |
| a3 | −0.003641 | 0.002318 | 0.00 | 0.00 | −0.008253 | 0.000971 |
| a4 | 0.000414 | 0.000201 | 0.00 | 0.00 | 0.000015 | 0.000814 |
| a5 | −0.046042 | 0.016232 | 0.00 | 0.00 | −0.078339 | −0.013745 |
| a6 | 0.000375 | 0.000132 | 0.00 | 0.00 | 0.000113 | 0.000636 |
| a7 | −0.000001 | 0.000000 | 0.00 | 0.00 | −0.000001 | −0.000001 |
| a8 | −0.000003 | 0.000000 | 0.00 | 0.00 | −0.000003 | −0.000003 |
| a9 | 0.000000 | 0.000000 | 0.00 | 0.00 | 0.000000 | 0.000000 |

Table A2.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on dry mass content in biochars.
Table A2.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on dry mass content in biochars.
| dry mass, % | ||||||
|---|---|---|---|---|---|---|
| Model | d.m., % = (223.1) + (0.002)·T2 + (−1.053)·T + (0.083)·t2 + (−7.035)·t + (0.0583)·T·t + (−0.0001)·T2· t + (−0.0007)·T·t2 + (1.393 × 10−6)·T2·t2 R2 = 0.26 | |||||
| Function Parameters | value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 223.1321 | 39.68104 | 0.00 | 0.00 | 144.1793 | 302.0849 |
| a2 | 0.0022 | 0.00064 | 0.00 | 0.00 | 0.0009 | 0.0034 |
| a3 | −1.0528 | 0.32172 | 0.00 | 0.00 | −1.6929 | −0.4127 |
| a4 | 0.0828 | 0.02787 | 0.00 | 0.00 | 0.0273 | 0.1382 |
| a5 | −7.0352 | 2.25299 | 0.00 | 0.00 | −11.5179 | −2.5524 |
| a6 | 0.0583 | 0.01827 | 0.00 | 0.00 | 0.0219 | 0.0946 |
| a7 | −0.0001 | 0.00004 | 0.00 | 0.00 | −0.0002 | −0.0000 |
| a8 | −0.0007 | 0.00023 | 0.00 | 0.00 | −0.0011 | −0.0002 |
| a9 | 0.0000 | 0.00000 | 0.00 | 0.00 | 0.0000 | 0.0000 |

Table A3.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on loss on ignition content in biochars.
Table A3.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on loss on ignition content in biochars.
| Loss on Ignition, % d.m. | ||||||
|---|---|---|---|---|---|---|
| Model | LOI, % = (−43.517) + (−0.0012353)·T2 + (0.702014)·t + (−0.0291921)·T2 + (3.83877)·T + (−0.0285369)·t·T + (4.89634 × 10−5)·t2·T + (0.000221984)·T·t2 + (−3.90553 × 10−7)·T2·t2 R2 = 0.72 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | −43.5170 | 58.73534 | 0.00 | 0.00 | −160.382 | 73.34794 |
| a2 | −0.0012 | 0.00095 | 0.00 | 0.00 | −0.003 | 0.00066 |
| a3 | 0.7020 | 0.47621 | 0.00 | 0.00 | −0.245 | 1.64952 |
| a4 | −0.0292 | 0.04126 | 0.00 | 0.00 | −0.111 | 0.05290 |
| a5 | 3.8388 | 3.33485 | 0.00 | 0.00 | −2.797 | 10.47407 |
| a6 | −0.0285 | 0.02704 | 0.00 | 0.00 | −0.082 | 0.02526 |
| a7 | 0.0000 | 0.00005 | 0.00 | 0.00 | −0.000 | 0.00016 |
| a8 | 0.0002 | 0.00033 | 0.00 | 0.00 | −0.000 | 0.00089 |
| a9 | −0.0000 | 0.00000 | 0.00 | 0.00 | −0.000 | −0.00000 |

Table A4.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on ash content in biochars.
Table A4.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on ash content in biochars.
| Ash Content, % d.m. | ||||||
|---|---|---|---|---|---|---|
| Model | ash, % = (14.0912) + (−0.000486683)·T2 + (0.256399)·t + (−0.00923204)·t2 + (1.13906)·t + (−0.00882318)·T·t + (1.67301 × 10−5)·T2·t + (4.9036 × 10−5)·t·T2 + (−3.61091 × 10−8)·t2·T2 R2 = 0.88 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 14.09118 | 43.69689 | 0.00 | 0.00 | −72.8519 | 101.0343 |
| a2 | −0.00049 | 0.00071 | 0.00 | 0.00 | −0.0019 | 0.0009 |
| a3 | 0.25640 | 0.35428 | 0.00 | 0.00 | −0.4485 | 0.9613 |
| a4 | −0.00923 | 0.03069 | 0.00 | 0.00 | −0.0703 | 0.0518 |
| a5 | 1.13906 | 2.48099 | 0.00 | 0.00 | −3.7973 | 6.0755 |
| a6 | −0.00882 | 0.02012 | 0.00 | 0.00 | −0.0488 | 0.0312 |
| a7 | 0.00002 | 0.00004 | 0.00 | 0.00 | −0.0001 | 0.0001 |
| a8 | 0.00005 | 0.00025 | 0.00 | 0.00 | −0.0004 | 0.0005 |
| a9 | −0.00000 | 0.00000 | 0.00 | 0.00 | −0.0000 | −0.0000 |

Table A5.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on HHV of the biochars.
Table A5.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on HHV of the biochars.
| HHV, MJ·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | HHV, MJ·kg−1 = (56.7993) + (0.000704123)·T2 + (−0.35836)·T + (0.0154298)·t2 + (−1.93107)·t + (0.0147992)·T·t + (−2.75086 × 10−5)·T2·t + (−0.000109331)·T·t2 + (1.82382 × 10−7)·T2·t2 R2 = 0.63 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 56.79931 | 14.38158 | 0.00 | 0.00 | 28.18447 | 85.41415 |
| a2 | 0.00070 | 0.00023 | 0.00 | 0.00 | 0.00024 | 0.00117 |
| a3 | −0.35836 | 0.11660 | 0.00 | 0.00 | −0.59036 | −0.12636 |
| a4 | 0.01543 | 0.01010 | 0.00 | 0.00 | −0.00467 | 0.03553 |
| a5 | −1.93107 | 0.81655 | 0.00 | 0.00 | −3.55574 | −0.30640 |
| a6 | 0.01480 | 0.00662 | 0.00 | 0.00 | 0.00163 | 0.02797 |
| a7 | −0.00003 | 0.00001 | 0.00 | 0.00 | −0.00005 | −0.00000 |
| a8 | −0.00011 | 0.00008 | 0.00 | 0.00 | −0.00027 | 0.00005 |
| a9 | 0.00000 | 0.00000 | 0.00 | 0.00 | 0.00000 | 0.00000 |

Table A6.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the C content in biochars.
Table A6.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the C content in biochars.
| C, % d.m. | ||||||
|---|---|---|---|---|---|---|
| Model | C, % = (−6.51131) + (−0.000660336)·T2 + (0.304374)·T + (−0.0668472)·t2 + (4.04284)·t + (−0.0353957)·T·t + (7.50162 × 10−5)·T2·t + (0.000579037)·T·t2 + (−1.21488 × 10−6)·T2·t2 R2 = 0.49 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | −6.51131 | 30.69734 | 0.00 | 0.00 | −67.5894 | 54.56676 |
| a2 | −0.00066 | 0.00050 | 0.00 | 0.00 | −0.0016 | 0.00033 |
| a3 | 0.30437 | 0.24889 | 0.00 | 0.00 | −0.1908 | 0.79958 |
| a4 | −0.06685 | 0.02156 | 0.00 | 0.00 | −0.1098 | −0.02394 |
| a5 | 4.04284 | 1.74291 | 0.00 | 0.00 | 0.5750 | 7.51068 |
| a6 | −0.03540 | 0.01413 | 0.00 | 0.00 | −0.0635 | −0.00728 |
| a7 | 0.00008 | 0.00003 | 0.00 | 0.00 | 0.0000 | 0.00013 |
| a8 | 0.00058 | 0.00017 | 0.00 | 0.00 | 0.0002 | 0.00093 |
| a9 | −0.00000 | 0.00000 | 0.00 | 0.00 | −0.0000 | −0.00000 |

Table A7.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the H content in biochars.
Table A7.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the H content in biochars.
| H, % d.m. | ||||||
|---|---|---|---|---|---|---|
| Model | H, % = (11.1242) + (8.72369 × 10−5)·T2 + (−0.0504356)·T + (0.00103414)·t2 + (−0.162667)·t + (0.00102235)·T·t + (−1.89313 ×·10−6)·T2·t + (−4.26597 × 10−6)·T·t2 + (4.46763 × 10−9)·T2·t2 R2 = 0.79 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 11.12415 | 7.851332 | 0.00 | 0.00 | −4.49753 | 26.74584 |
| a2 | 0.00009 | 0.000127 | 0.00 | 0.00 | −0.00017 | 0.00034 |
| a3 | −0.05044 | 0.063657 | 0.00 | 0.00 | −0.17709 | 0.07622 |
| a4 | 0.00103 | 0.005515 | 0.00 | 0.00 | −0.00994 | 0.01201 |
| a5 | −0.16267 | 0.445779 | 0.00 | 0.00 | −1.04963 | 0.72429 |
| a6 | 0.00102 | 0.003614 | 0.00 | 0.00 | −0.00617 | 0.00821 |
| a7 | −0.00000 | 0.000000 | 0.00 | 0.00 | −0.00000 | −0.00000 |
| a8 | −0.00000 | 0.000045 | 0.00 | 0.00 | −0.00009 | 0.00008 |
| a9 | 0.00000 | 0.000000 | 0.00 | 0.00 | 0.00000 | 0.00000 |

Table A8.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the O content in biochars.
Table A8.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the O content in biochars.
| O, % d.m. | ||||||
|---|---|---|---|---|---|---|
| Model | O, % = (94.3211) + (0.00132001)·T2 + (−0.646472)·T + (0.110223)·t2 + (−7.32599)·t + (0.0612511)·T·t + (−0.000125945)·T2·t + (−0.000907679)·T·t2 + (1.82155 × 10−6)·T2·t2 R2 = 0.83 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 1051.470 | 55631.35 | 0.02 | 0.99 | −113095 | 115197.6 |
| a2 | 0.248 | 0.90 | 0.27 | 0.79 | −2 | 2.1 |
| a3 | −47.137 | 450.67 | −0.105 | 0.92 | −972 | 877.6 |
| a4 | 5.535 | 38.52 | 0.14 | 0.88 | −74 | 84.6 |
| a5 | −538.670 | 3116.91 | −0.17 | 0.86 | −6934 | 5856.7 |
| a6 | 10.229 | 25.24 | 0.40 | 0.69 | −42 | 62.0 |
| a7 | −0.031 | 0.05 | −0.61 | 0.54 | −0 | 0.1 |
| a8 | −0.122 | 0.31 | −0.39 | 0.70 | −1 | 0.5 |
| a9 | 0.000 | 0.00 | 0.61 | 0.5 | −0 | 0.0 |

Table A9.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on HHV daf of the biochars.
Table A9.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on HHV daf of the biochars.
| HHV (daf), MJ·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | HHV (daf), MJ·kg−1 = (64.4828) + (0.000608875)·T2 + (−0.319667)·T + (0.00555782)·t2 + (−1.47697)·t + (0.0100922)·T·t + (−1.58506 × 10−5)·T2·t + (−2.18888 × 10−5)·T·t2 + (−5.1846 × 10−9)·T2·t2 R2 = 0.71 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 64.48283 | 32.93082 | 0.00 | 0.00 | −1.03918 | 130.0048 |
| a2 | 0.00061 | 0.00053 | 0.00 | 0.00 | −0.00045 | 0.0017 |
| a3 | −0.31967 | 0.26700 | 0.00 | 0.00 | −0.85090 | 0.2116 |
| a4 | 0.00556 | 0.02313 | 0.00 | 0.00 | −0.04047 | 0.0516 |
| a5 | −1.47697 | 1.86972 | 0.00 | 0.00 | −5.19713 | 2.2432 |
| a6 | 0.01009 | 0.01516 | 0.00 | 0.00 | −0.02007 | 0.0403 |
| a7 | −0.00002 | 0.00003 | 0.00 | 0.00 | −0.00008 | 0.0000 |
| a8 | −0.00002 | 0.00019 | 0.00 | 0.00 | −0.00040 | 0.0004 |
| a9 | −0.00000 | 0.00000 | 0.00 | 0.00 | −0.00000 | −0.0000 |

Table A10.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on LHV of the biochars.
Table A10.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on LHV of the biochars.
| LHV, MJ·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | LHV, MJ·kg−1 = (57.3765) + (0.000737877)·T2 + (−0.373049)·T + (0.0172241)·t2 + (−2.06729)·t + (0.015999)·T·t + (−2.99897 × 10−5)·T2·t + (−0.000125133)·T·t2 + (2.15415 × 10−7)·T2·t2 R2 = 0.61 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 57.37645 | 14.96197 | 0.00 | 0.00 | 27.60682 | 87.14608 |
| a2 | 0.00074 | 0.00024 | 0.00 | 0.00 | 0.00026 | 0.00122 |
| a3 | −0.37305 | 0.12131 | 0.00 | 0.00 | −0.61441 | −0.13168 |
| a4 | 0.01722 | 0.01051 | 0.00 | 0.00 | −0.00369 | 0.03814 |
| a5 | −2.06729 | 0.84950 | 0.00 | 0.00 | −3.75753 | −0.37704 |
| a6 | 0.01600 | 0.00689 | 0.00 | 0.00 | 0.00229 | 0.02970 |
| a7 | −0.00003 | 0.00001 | 0.00 | 0.00 | −0.00006 | −0.00000 |
| a8 | −0.00013 | 0.00009 | 0.00 | 0.00 | −0.00029 | 0.00004 |
| a9 | 0.00000 | 0.00000 | 0.00 | 0.00 | 0.00000 | 0.00000 |

Table A11.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the energy yield of the process.
Table A11.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the energy yield of the process.
| Energy Yield, % | ||||||
|---|---|---|---|---|---|---|
| Model | energy yield, % = (500.253) + (0.00637136)·T2 + (−3.23363)·T + (0.179189)·t2 + (−20.5477)·t + (0.161123)·T·t + (−0.00031386)·T2·t + (−0.00135047)·T·t2 + (2.50551 × 10−6)·T2·t2 R2 = 0.83 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 500.2532 | 109.4272 | 0.00 | 0.00 | 282.5274 | 717.9791 |
| a2 | 0.0064 | 0.0018 | 0.00 | 0.00 | 0.0028 | 0.0099 |
| a3 | −3.2336 | 0.8872 | 0.00 | 0.00 | −4.9989 | −1.4684 |
| a4 | 0.1792 | 0.0769 | 0.00 | 0.00 | 0.0262 | 0.3321 |
| a5 | −20.5477 | 6.2130 | 0.00 | 0.00 | −32.9097 | −8.1858 |
| a6 | 0.1611 | 0.0504 | 0.00 | 0.00 | 0.0609 | 0.2614 |
| a7 | −0.0003 | 0.0001 | 0.00 | 0.00 | −0.0005 | −0.0001 |
| a8 | −0.0014 | 0.0006 | 0.00 | 0.00 | −0.0026 | −0.0001 |
| a9 | 0.0000 | 0.0000 | 0.00 | 0.00 | 0.0000 | 0.0000 |

Table A12.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the H/C ratio in biochars.
Table A12.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the H/C ratio in biochars.
| H/C | ||||||
|---|---|---|---|---|---|---|
| Model | H/C = (6.15786) + (6.41009 × 10−5)·T2 + (−0.0338503)·T + (0.00373692)·t2 + (−0.263384)·t + (0.0021366)·T·t + (−4.4153 × 10−6)·T2·t + (−3.03887 × 10−5)·T·t2 + (6.18517 × 10−8)·T2·t2 R2 = 0.76 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 6.1579 | 4.1683 | 0 | 0 | −2.358 | 14.4515 |
| a2 | 0.0001 | 0.0001 | 0 | 0 | −0.0001 | 0.0002 |
| a3 | −0.0339 | 0.0338 | 0 | 0 | −0.1011 | 0.0334 |
| a4 | 0.0037 | 0.0029 | 0 | 0 | −0.0021 | 0.0096 |
| a5 | −0.2634 | 0.2367 | 0 | 0 | −0.7343 | 0.2075 |
| a6 | 0.0021 | 0.0019 | 0 | 0 | −0.0017 | 0.0060 |
| a7 | 0.0000 | 0.0000 | 0 | 0 | 0.0000 | 0.0000 |
| a8 | 0.0000 | 0.0000 | 0 | 0 | −0.0001 | 0.0000 |
| a9 | 0.0000 | 0.0000 | 0 | 0 | 0.0000 | 0.0000 |

Table A13.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the O/C ratio in biochars.
Table A13.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the O/C ratio in biochars.
| O/C | ||||||
|---|---|---|---|---|---|---|
| Model | O/C = (2.85356) + (4.1663 × 10−5)·T2 + (−0.0202011)·T + (0.0038009)·t2 + (−0.246543)·t + (0.00208228)·T·t + (−4.3088 × 10−6)·T2·t + (−3.16778 × 10−7)·T·t2 + (6.4223 × 10−8)·T2·t2 R2 = 0.80 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 2.8536 | 1.8909 | 0 | 0 | −0.9087 | 6.6159 |
| a2 | 0.0000 | 0.0000 | 0 | 0 | 0.0000 | 0.0001 |
| a3 | −0.0202 | 0.0153 | 0 | 0 | −0.0507 | 0.0103 |
| a4 | 0.0038 | 0.0013 | 0 | 0 | 0.0012 | 0.0064 |
| a5 | −0.2465 | 0.1074 | 0 | 0 | −0.4602 | −0.0329 |
| a6 | 0.0021 | 0.0009 | 0 | 0 | 0.0004 | 0.0038 |
| a7 | 0.0000 | 0.0000 | 0 | 0 | 0.0000 | 0.0000 |
| a8 | 0.0000 | 0.0000 | 0 | 0 | −0.0001 | 0.0000 |
| a9 | 0.0000 | 0.0000 | 0 | 0 | 0.0000 | 0.0000 |

Table A14.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the N content in biochars.
Table A14.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the N content in biochars.
| N, % d.m. | ||||||
|---|---|---|---|---|---|---|
| Model | N, % = (13.737) + (0.000154911)·T2 + (−0.0716041)·T + (−0.00987428)·t2 + (0.322928)·t + (−0.00346963)·T·t + (7.1607×10−6)·T2·t + (9.22803 × 10−5)·T·t2 + (−1.91518 × 10−7)·T2·t2 R2 = 0.83 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 13.73703 | 7.673398 | 0.00 | 0.00 | −1.53063 | 29.00468 |
| a2 | 0.00015 | 0.000124 | 0.00 | 0.00 | −0.00009 | 0.00040 |
| a3 | −0.07160 | 0.062214 | 0.00 | 0.00 | −0.19539 | 0.05218 |
| a4 | −0.00987 | 0.005390 | 0.00 | 0.00 | −0.02060 | 0.00085 |
| a5 | 0.32293 | 0.435677 | 0.00 | 0.00 | −0.54393 | 1.18979 |
| a6 | −0.00347 | 0.003532 | 0.00 | 0.00 | −0.01050 | 0.00356 |
| a7 | 0.00001 | 0.000000 | 0.00 | 0.00 | 0.00001 | 0.00001 |
| a8 | 0.00009 | 0.000044 | 0.00 | 0.00 | 0.00001 | 0.00018 |
| a9 | −0.00000 | 0.000000 | 0.00 | 0.00 | −0.00000 | −0.00000 |

Table A15.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the Mg content in biochars.
Table A15.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the Mg content in biochars.
| Mg, mg·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | Mg, mg·kg−1 = (1051.47) + (0.247677)·T2 + (−47.1368)·T + (5.53494)·t2 + (−538.67)·t + (10.2288)·T·t + (−0.0310345)·T2·t + (−0.122493)·T·t2 + (0.000382962)·T2·t2 R2 = 0.50 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 94.32113 | 57.65115 | 0.00 | 0.00 | −20.3866 | 209.0288 |
| a2 | 0.00132 | 0.00093 | 0.00 | 0.00 | −0.0005 | 0.0032 |
| a3 | −0.64647 | 0.46742 | 0.00 | 0.00 | −1.5765 | 0.2835 |
| a4 | 0.11022 | 0.04050 | 0.00 | 0.00 | 0.0296 | 0.1908 |
| a5 | −7.32599 | 3.27329 | 0.00 | 0.00 | −13.8388 | −0.8132 |
| a6 | 0.06125 | 0.02654 | 0.00 | 0.00 | 0.0084 | 0.1141 |
| a7 | −0.00013 | 0.00005 | 0.00 | 0.00 | −0.0002 | −0.0000 |
| a8 | −0.00091 | 0.00033 | 0.00 | 0.00 | −0.0016 | −0.0003 |
| a9 | 0.00000 | 0.00000 | 0.00 | 0.00 | 0.0000 | 0.0000 |

Table A16.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the Ca content in biochars.
Table A16.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the Ca content in biochars.
| Ca, mg·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | Ca, mg·kg−1 = (316658) + (5.82666)·T2 + (−2648.24)·T + (341.228)·t2 + (−23391.2)·t + (213.523)·T·t + (−0.468153)·T2·t + (−3.09572)·T·t2 + (0.00673435)·T2·t2 R2 = 0.32 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | 316658.0 | 485972.1 | 0.65 | 0.52 | −680474 | 1313790 |
| a2 | 5.8 | 7.9 | 0.74 | 0.47 | −10 | 22 |
| a3 | −2648.2 | 3936.8 | −0.67 | 0.51 | −10726 | 5429 |
| a4 | 341.2 | 336.5 | 1.01 | 0.32 | −349 | 1032 |
| a5 | −23391.2 | 27228.4 | −0.86 | 0.40 | −79259 | 32477 |
| a6 | 213.5 | 220.5 | 0.97 | 0.34 | −239 | 666 |
| a7 | −0.5 | 0.4 | −1.06 | 0.30 | −1 | 0 |
| a8 | −3.1 | 2.7 | −1.14 | 0.27 | −9 | 2 |
| a9 | 0.0 | 0.0 | 1.24 | 0.23 | 0 | 0 |

Table A17.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the K content in biochars.
Table A17.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the K content in biochars.
| K, mg·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | K, mg·kg−1 = (−7811.08) + (0.0132002)·T2 + (35.0213)·T + (−5.9302)·t2 + (432.779)·t + (−0.0448789)·T·t + (−0.00580631)·T2·t + (0.002207)·T·t2 + (7.25633 × 10−5)·T2·t2 R2 = 0.48 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | −7811.08 | 33686.23 | −0.23 | 0.82 | −76929.5 | 61307.36 |
| a2 | 0.01 | 0.55 | 0.02 | 0.98 | −1.1 | 1.13 |
| a3 | 35.02 | 272.89 | 0.13 | 0.90 | −524.9 | 594.95 |
| a4 | −5.93 | 23.33 | −0.25 | 0.80 | −53.8 | 41.93 |
| a5 | 432.78 | 1887.38 | 0.23 | 0.82 | −3439.8 | 4305.37 |
| a6 | −0.04 | 15.28 | 0.00 | 1.00 | −31.4 | 31.32 |
| a7 | −0.01 | 0.03 | −0.19 | 0.85 | −0.1 | 0.06 |
| a8 | 0.00 | 0.19 | 0.01 | 0.99 | −0.4 | 0.39 |
| a9 | 0.00 | 0.00 | 0.19 | 0.85 | 0.0 | 0.00 |

Table A18.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the Na content in biochars.
Table A18.
The statistical parameters of polynomial regression analysis of the influence of SS torrefaction temperature and residence time on the Na content in biochars.
| Na, mg·kg−1 | ||||||
|---|---|---|---|---|---|---|
| Model | Na, mg·kg−1 = (−41466.8) + (−0.577248)·T2 + (318.433)·T + (−49.4931)·t2 + (3620.2)·t + (−27.0631)·T·t + (0.0542841)·T2·t + (0.390176)·T·t2 + (−0.000809744)·T2·t2 R2 = 0.29 | |||||
| Function Parameters | Value | Standard Error | t Value | p Value | Lower Confidence Limit | Upper Confidence Limit |
| a1 | −41466.8 | 141846.8 | −0.29 | 0.77 | −332512 | 249,578.7 |
| a2 | −0.6 | 2.3 | −0.25 | 0.80 | −5 | 4.1 |
| a3 | 318.4 | 1149.1 | 0.28 | 0.78 | −2039 | 2676.2 |
| a4 | −49.5 | 98.2 | −0.50 | 0.62 | −251 | 152.0 |
| a5 | 3620.2 | 7947.5 | 0.46 | 0.65 | −12,687 | 19,927.1 |
| a6 | −27.1 | 64.4 | −0.42 | 0.68 | −159 | 105.0 |
| a7 | 0.1 | 0.1 | 0.42 | 0.68 | 0 | 0.3 |
| a8 | 0.4 | 0.8 | 0.49 | 0.63 | −1 | 2.0 |
| a9 | 0.0 | 0.0 | −0.51 | 0.61 | 0 | 0.0 |
References
- Heidrich, Z.; Tiunajtis, K. Ilość osadów pochodzących z wiejskich oczyszczalni ścieków i kierunki ich unieszkodliwiania. Infrastruktura i Ekologia Terenów Wiejskich 2008, 5, 191–198. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-95b2f868-8850-49fb-8644-99d72957c1f6/c/Heidrich.pdf (accessed on 29 December 2019).
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Jama-Rodzeńska, A.M.; Bocianowski, J.; Nowak, W.; Ciszek, D.; Nowosad, K. The influence of communal sewage sludge on the content of macroelements in the stem of selected clones of willow (Salix viminalis L.). Ecol. Eng. 2016, 87, 212–217. [Google Scholar] [CrossRef]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage sludge ash—A promising secondary phosphorus source for fertilizer production. Sci. Total. Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Environment. Central Statistical Office of Poland. 2018. Available online: https://stat.gov.pl/en/topics/environment-energy/environment/environment-2018,1,10.html (accessed on 21 December 2019).
- Publications Office of the European Union. EUROSTAT: Regional Yearbook. Statistical Book; Eurostat: Luxembourg, 2017. Available online: https://ec.europa.eu/eurostat/documents/3217494/8222062/KS-HA-17-001-EN-N.pdf (accessed on 19 February 2020).
- Agopsowicz, M.; Białowiec, A.; Pijarczyk, P. Sewage sludge land disposal effects on ground water. Arch. Environ. Prot. 2008, 34, 73–82. [Google Scholar]
- Atienza–Martínez, M.; Gea, G.; Arauzo, J.; Kersten, S.R.; Kootstra, A.M.J. Phosphorus recovery from sewage sludge char ash. Biomass-Bioenergy 2014, 65, 42–50. [Google Scholar] [CrossRef]
- Robinson, J.S.; Baumann, K.; Hu, Y.; Hagemann, P.; Kebelmann, L.; Leinweber, P. Phosphorus transformations in plant-based and bio-waste materials induced by pyrolysis. Ambio 2018, 47, 73–82. [Google Scholar] [CrossRef]
- Mysior, M.; Tomaszewski, M.; Stępień, P.; Koziel, J.A.; Białowiec, A. Valorization of Sewage Sludge via Gasification and Transportation of Compressed Syngas. Process. 2019, 7, 556. [Google Scholar] [CrossRef]
- Wzorek, Z. The phosphorus compounds recovery from thermally treated waste and its use as a substitute of natural phosphorus raw materials. Monogr. 356. Ser. Inz. i Technol. Chem. 2008, 1–159. [Google Scholar]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Kinetic model of homogeneous lignocellulosic biomass solvolysis in glycerol and imidazolium-based ionic liquids with subsequent heterogeneous hydrodeoxygenation over NiMo/Al2O3 catalyst. Catal. Today 2015, 256, 302–314. [Google Scholar] [CrossRef]
- Faussone, G.C.; Grilc, M.; Likozar, B. Removal of inorganics from sludge and digestate: The BiAR process. Water Pr. Technol. 2017, 12, 937–941. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, C. Simultaneous liquefaction and hydrodeoxygenation of lignocellulosic biomass over NiMo/Al2O3, Pd/Al2O3, and zeolite Y catalysts in hydrogen donor solvents. Chem. Cat. Chem. 2016, 8, 180–191. [Google Scholar] [CrossRef]
- Mimmo, T.; Panzacchi, P.; Baratieri, M.; Davies, C.; Tonon, G. Effect of pyrolysis temperature on miscanthus (Miscanthus × giganteus) biochar physical, chemical and functional properties. Biomass-Bioenergy 2014, 62, 149–157. [Google Scholar] [CrossRef]
- Kosov, V.F.; Kuzmina, J.S.; A Sytchev, G.; Zaichenko, V.M. Challenges and opportunities of torrefaction technology. J. Physics: Conf. Ser. 2016, 774, 012139. [Google Scholar] [CrossRef]
- Balogun, A.O.; Lasode, O.; McDonald, A.G. Thermo-physical, Chemical and Structural Modifications in Torrefied Biomass Residues. Waste Biomass-Valorization 2016, 9, 131–138. [Google Scholar] [CrossRef]
- Garba, M.U.; Gambo, S.; Musa, U.; Tauheed, K.; Alhassan, M.; Adeniyi, O. Impact of torrefaction on fuel property of tropical biomass feedstocks. Biofuels 2017, 9, 369–377. [Google Scholar] [CrossRef]
- Białowiec, A.; Micuda, M.; Koziel, J.A. Waste to Carbon: Densification of Torrefied Refuse-Derived Fuel. Energies 2018, 11, 3233. [Google Scholar] [CrossRef]
- Świechowski, K.; Stegenta-Dąbrowska, S.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Process Kinetics. Materials 2019, 12, 3334. [Google Scholar] [CrossRef]
- Stępień, P.; Serowik, M.; Koziel, J.A.; Białowiec, A. Waste to Carbon: Estimating the Energy Demand for Production of Carbonized Refuse-Derived Fuel. Sustainability 2019, 11, 5685. [Google Scholar] [CrossRef]
- Syguła, E.; Koziel, J.A.; Białowiec, A. Proof-of-Concept of Spent Mushrooms Compost Torrefaction—Studying the Process Kinetics and the Influence of Temperature and Duration on the Calorific Value of the Produced Biocoal. Energies 2019, 12, 3060. [Google Scholar] [CrossRef]
- Stępień, P.; Świechowski, K.; Hnat; Kugler, S.; Dąbrowska, S.; Koziel, J.A.; Manczarski, P.; Białowiec, A.; Hnat, M.; Stegenta-Dąbrowska, S. Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel. Energies 2019, 12, 4344. [Google Scholar] [CrossRef]
- Kalus, K.; Koziel, J.A.; Opalinski, S. A Review of Biochar Properties and Their Utilization in Crop Agriculture and Livestock Production. Appl. Sci. 2019, 9, 3494. [Google Scholar] [CrossRef]
- Kammann, C.; Glaser, B.; Schmidt, H.-P. Combining Biochar and Organic Amendments. Biochar in European soils, Science and Practice; Routledge: London, UK, 2016; pp. 136–164. [Google Scholar]
- Glaser, B.; Parr, M.; Braun, C.; Kopolo, G. Biochar is carbon negative. Nat. Geosci. 2009, 2, 2. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.; Crowley, D. Biochar effects on soil biota—A review. Soil Boil. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. In CSIRO Land and Water Science Report; CSIRO: Canberra, Aurtalia, 2009; pp. 1834–6618. [Google Scholar]
- Polish Law on renewable energy source. Pol. J. Laws 2015, 478. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20150000478/U/D20150478Lj.pdf (accessed on 19 February 2020).
- Bergman, P.C.A.; Kiel, J.H.A. Torrefaction for biomass upgrading. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Verhoeff, F.; Arnuelos, A.A.; Boersma, R.; Pels, J.R.; Lensselink, J.; Kiel, J.H.A.; Schukken, H. Torrefaction Technology for the Production of Solid Bioenergy Carriers from Biomass and Waste; ECN-E-11-039; ECN: Petten, The Netherlands, 2011. [Google Scholar]
- Ratte, J.; Fardet, E.; Mateos, D.; Héry, J.-S. Mathematical modelling of a continuous biomass torrefaction reactor: TORSPYD™ column. Biomass-Bioenergy 2011, 35, 3481–3495. [Google Scholar] [CrossRef]
- .Bergman, P.C.A. Combined Torrefaction and Pelletisation—The TOP Process; ECN Report, ECN-C-05-073; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Poudel, J.; Ohm, T.I.; Lee, S.-H.; Oh, S.C. A study on torrefaction of sewage sludge to enhance solid fuel qualities. Waste Manag. 2015, 40, 112–118. [Google Scholar] [CrossRef]
- Karki, S.; Poudel, J.; Oh, S.C. Thermal Pre-Treatment of Sewage Sludge in a Lab-Scale Fluidized Bed for Enhancing Its Solid Fuel Properties. Appl. Sci. 2018, 8, 183. [Google Scholar] [CrossRef]
- Hernández, A.B.; Okonta, F.; Freeman, N. Sewage sludge charcoal production by N2- and CO2-torrefaction. J. Environ. Chem. Eng. 2017, 5, 4406–4414. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Mastral, J.F.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage Sludge Torrefaction in an Auger Reactor. Energy Fuels 2014, 29, 160–170. [Google Scholar] [CrossRef]
- Lim, D.-W.; Poudel, J.; Oh, S.C. A Study on Torrefaction Characteristics of Sewage Sludge. Appl. Chem. Eng. 2014, 25, 510–514. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of Sewage Sludge: Kinetics and Fuel Properties of Biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK , 2009. [Google Scholar]
- Bach, Q.-V.; Tran, Q. Dry and Wet Torrefaction of Woody Biomass—A Comparative Studyon Combustion Kinetics. Energy Procedia 2015, 75, 150–155. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Boil. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Yue, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. Efficiency of sewage sludge biochar in improving urban soil properties and promoting grass growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef]
- Czekała, W.; Malińska, K.; Cáceres, R.; Janczak, D.; Dach, J.; Lewicki, A. Co-composting of poultry manure mixtures amended with biochar—The effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 2016, 200, 921–927. [Google Scholar] [CrossRef]
- Malińska, K. Biowęgiel odpowiedzią na aktualne problemy ochrony środowiska. Inżynieria i Ochrona Środowiska 2012, 15, 387–403. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-848cbc3b-8a45-4906-b46a-a34dcc1812de/c/Malinska__Biowegiel_4_2012.pdf (accessed on 29 December 2019).
- Van Wesenbeeck, S.; Prins, W.; Ronsse, F.; Antal, M.J. Sewage Sludge Carbonization for Biochar Applications. Fate of Heavy Metals. Energy Fuels 2014, 28, 5318–5326. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF torrefaction: An effect of temperature on characterization of the product - Carbonized Refuse Derived Fuel. Waste Manag. 2017, 70, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Solid Biofuels—Determination of moisture content-drier method—Part 1: Total moisture—Reference method; Polish Standard PN-EN 14774-1:2010E; Polski Komitet Normalizacyjny: Warsaw, Poland, 2010.
- Solid Biofuels—Determination of Volatile Compounds Content; Polish Standard PN-EN 15148:2010; Polski Komitet Normalizacyjny: Warsaw, Poland, 2010.
- Solid Fuels. Determination of Total Sulfur and Fly Ash with Automatic Analyzers; Polish Standard PN-G-04584:2001P; Polski Komitet Normalizacyjny: Warsaw, Poland, 2001.
- Solid Biofuels. Total Carbon, Ammonia and Hydrogen Determination; Polish Standard PN-EN ISO 16948:2015-07; Polski Komitet Normalizacyjny: Warsaw, Poland, 2015.
- Solid Fuels. Higher Heating Value Determination; Polish Standard PN-G-04513:1981; Polski Komitet Normalizacyjny: Warsaw, Poland, 1981.
- Foodstuffs—Determination of Trace Elements—Determination of Lead, Cadmium, Zinc, Copper, Iron and Chromium by Atomic Absorption Spectrometry (AAS) after Dry Ashing; Polish Standard PN-EN 14082:2004; Polski Komitet Normalizacyjny: Warsaw, Poland, 2004.
- Keipi, T.I.-M.; Tolvanen, H.; Kokko, L.; Raiko, R. The effect of torrefaction on the chlorine content and heating value of eight woody biomass samples. Biomass-Bioenergy 2014, 66, 232–239. [Google Scholar] [CrossRef]
- Grigiante, M.; Antolini, D. Mass yield as guide parameter of the torrefaction process. An experimental study of the solid fuel properties referred to two types of biomass. Fuel 2015, 153, 499–509. [Google Scholar] [CrossRef]
- Basu, P. Biomass Characteristics. In Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: Amsterdam, The Netherlands, 2013; pp. 49–91. ISBN 978-0-12-396488-5. [Google Scholar]
- Świechowski, K.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Mathematical Models of the Influence of Temperature and Residence Time on Fuel Properties Improvement. Materials 2019, 12, 2228. [Google Scholar] [CrossRef]
- Stepień, P.; Mysior, M.; Bialowiec, A. Problemy techniczne i technologiczne oraz potencjał aplikacyjny toryfikacji odpadów. In Innowacje w Gospodarce Odpadami, Praca Zbiorowa Pod Redakcją Andrzeja Białowca; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2018; ISBN 978-83-7717-278-0. [Google Scholar]
- Pellet Premium DIN Plus. Available online: http://kominkowybrykiet.pl/produkt/brykiet-kominkowy-typu-pinikay/ (accessed on 21 December 2019).
- Czekała, W.; Wojcieszak, D.; Lewicki, A.; Janczak, D.; Waliszewska, H.; Smurzynska, A. Nutrient Value of Digestate from Agricultural Biogas Plant in Poland. In Proceedings of the 2018 2nd International Conference on Green Energy and Applications (ICGEA), Singapore, 24–26 March 2018; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2018; pp. 10–13. [Google Scholar]
- Atienza-Martínez, M.; Fonts, I.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in a fluidized bed reactor. Chem. Eng. J. 2013, 222, 534–545. [Google Scholar] [CrossRef]
- Dhungana, A. Torrefaction of Biomass. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2011. [Google Scholar]
- Lin, X.; Wang, F.; Chi, Y.; Huang, Q.; Yan, J. A simple method for predicting the lower heating value of municipal solid waste in China based on wet physical composition. Waste Manag. 2015, 36, 24–32. [Google Scholar] [CrossRef]
- Mock, C.; Lee, H.; Choi, S.; Manovic, V. Flame structures and ignition characteristics of torrefied and raw sewage sludge particles at rapid heating rates. Fuel 2017, 200, 467–480. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Co-torrefaction of sewage sludge and leucaena by using microwave heating. Energy 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Microwave torrefaction of sewage sludge and leucaena. J. Taiwan Inst. Chem. Eng. 2017, 70, 236–243. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Polish Ministry of Agriculture and Rural Development on the Implementation of Crops. Fertilizers and Fertilization. Pol. J. Laws 2008, 119, 765. [Google Scholar]
- Waqas, M.; Khan, S.; Qing, H.; Reid, B.J.; Chao, C. The effects of sewage sludge and sewage sludge biochar on PAHs and potentially toxic element bioaccumulation in Cucumis sativa L. Chemosphere 2014, 105, 53–61. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Song, X.; Xue, X.; Chen, D.; He, P.; Dai, X. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Shinogi, Y.; Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).