Development of Precipitation Hardening Parameters for High Strength Alloy AA 7068
Abstract
:1. Introduction
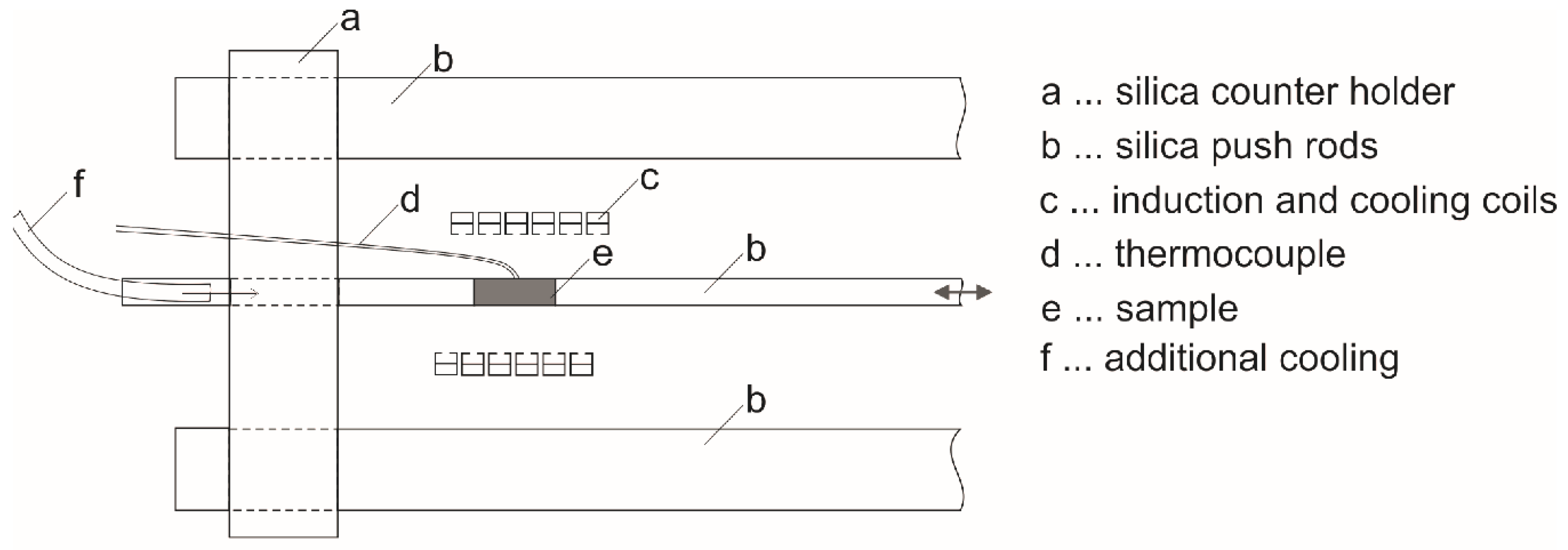
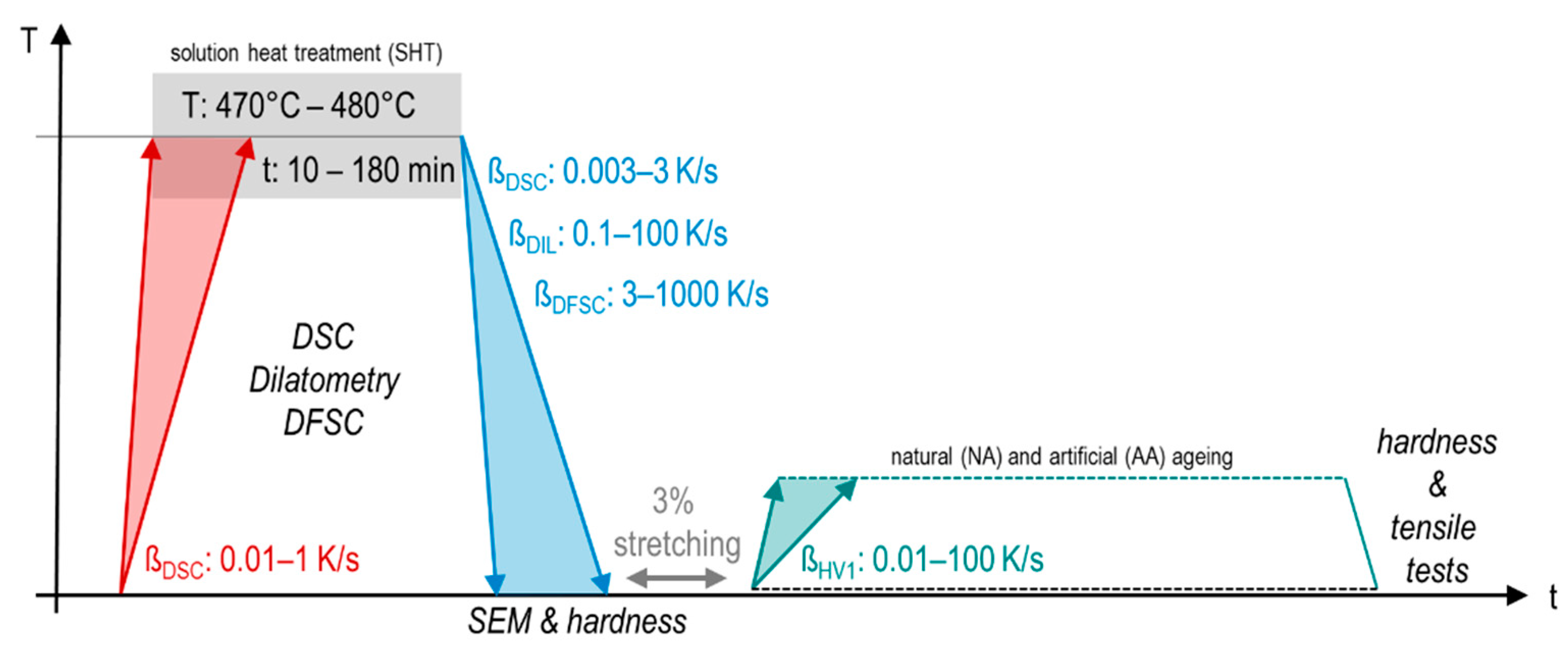
2. Materials and Methods
3. Results and Discussion
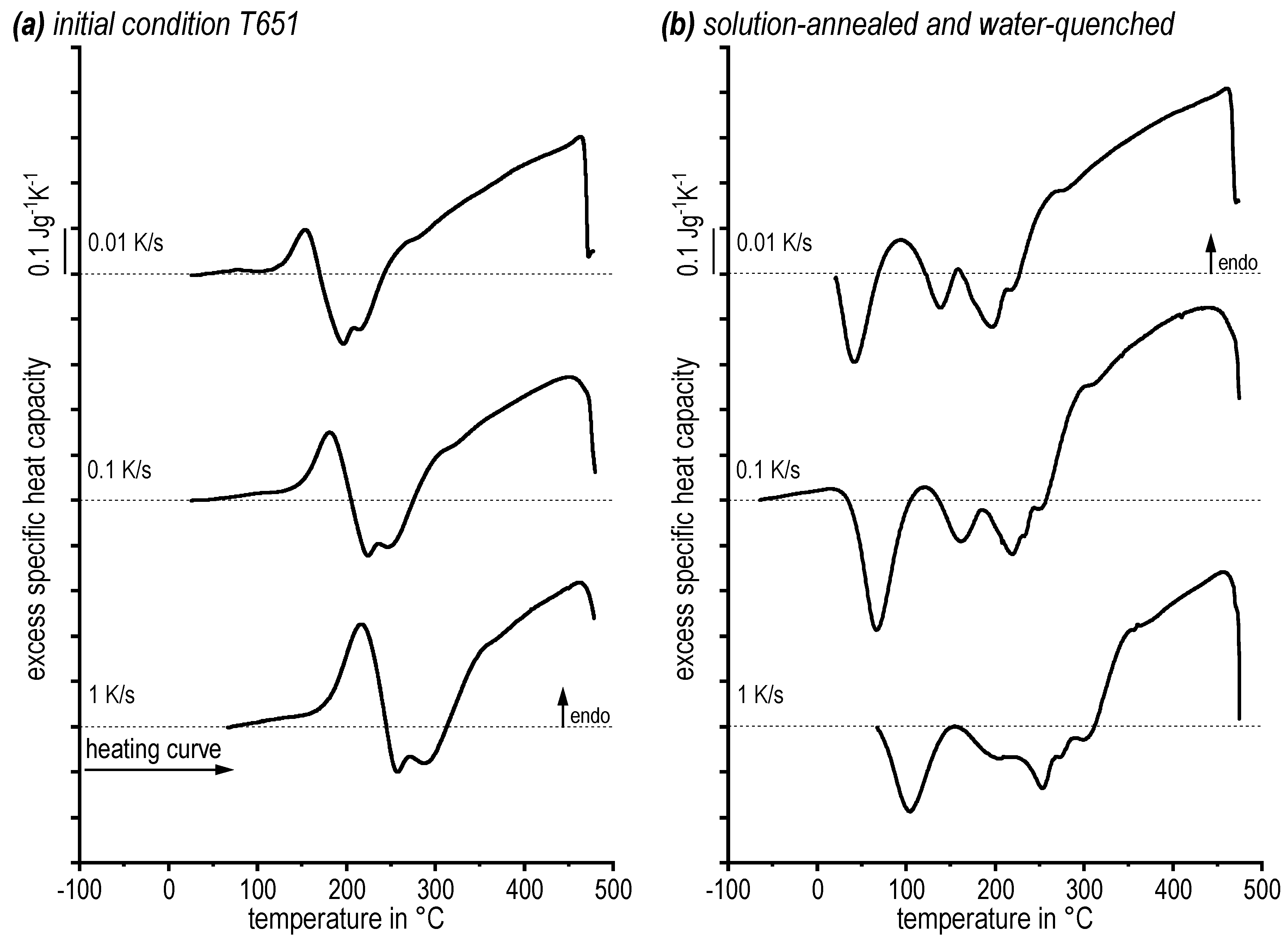
3.1. DSC Heating Curves: Determination of an Appropriate Solution Annealing Temperature
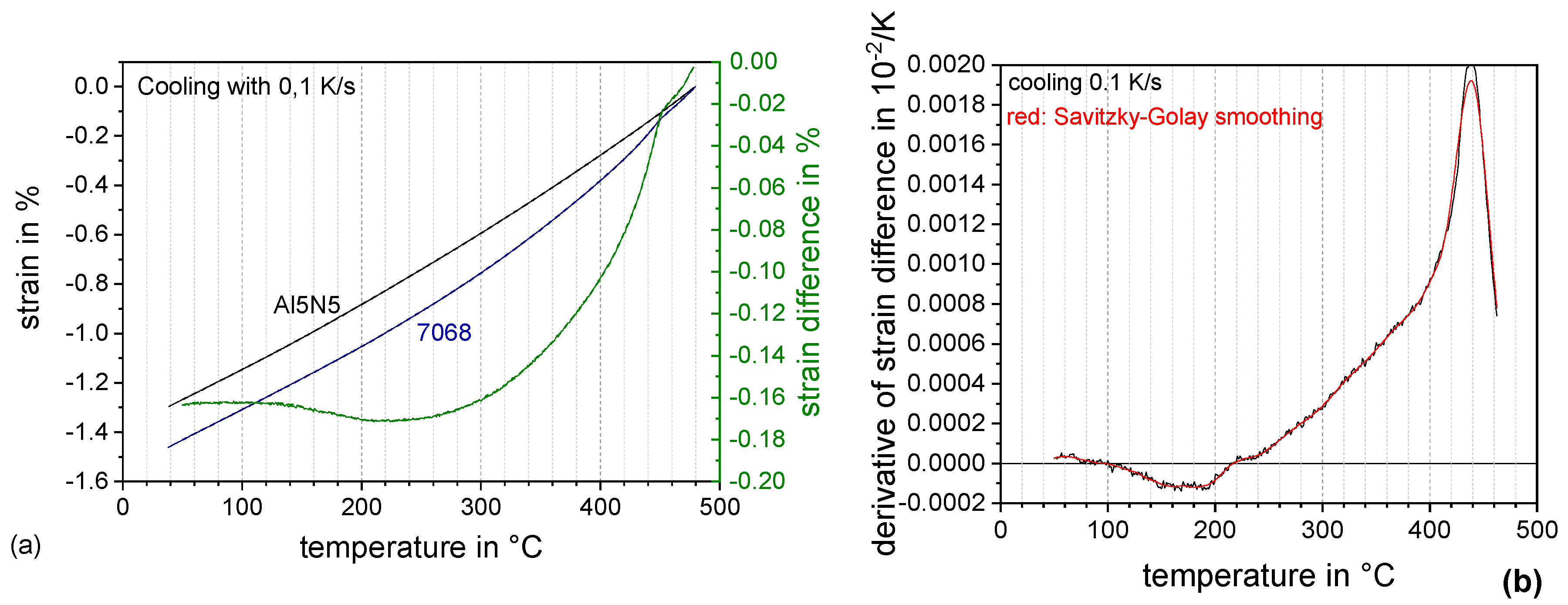
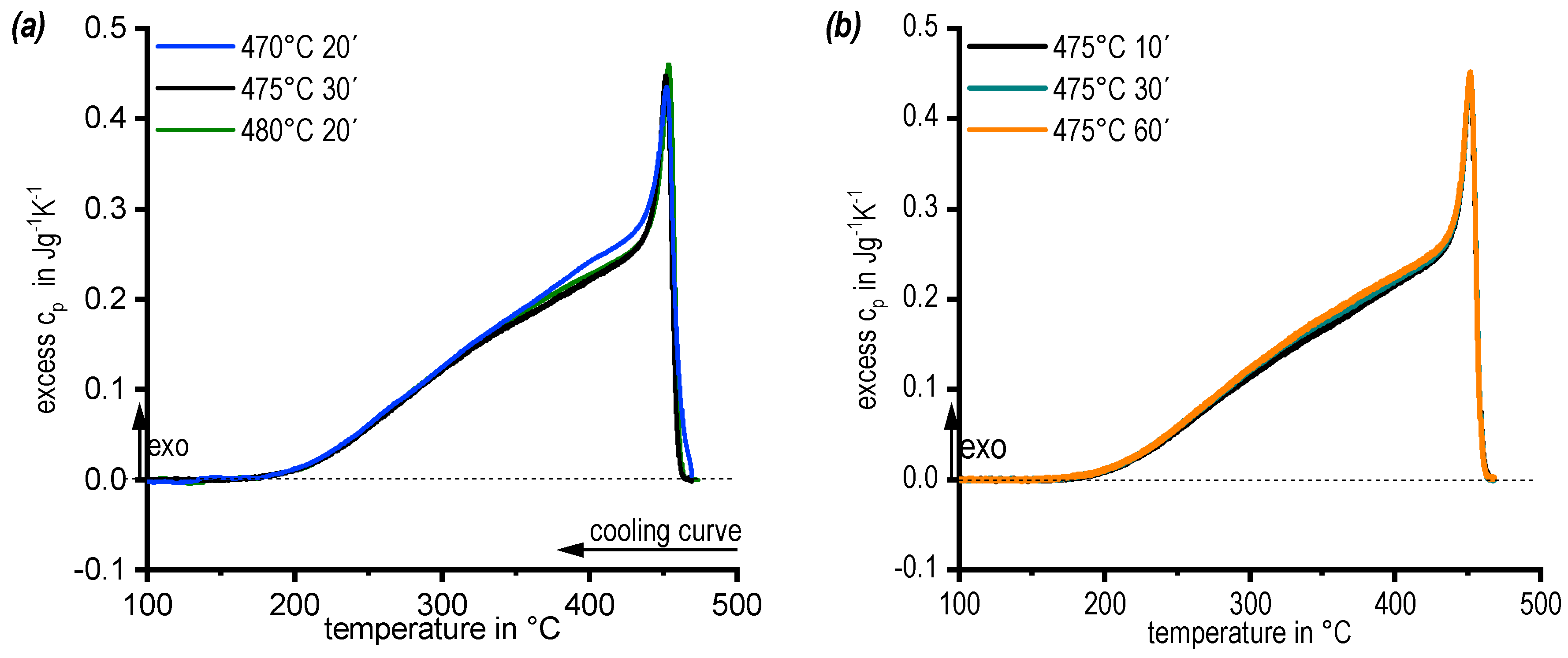
3.2. DSC Cooling Curves: Assessing the Influence of Solution Annealing Temperature and Duration
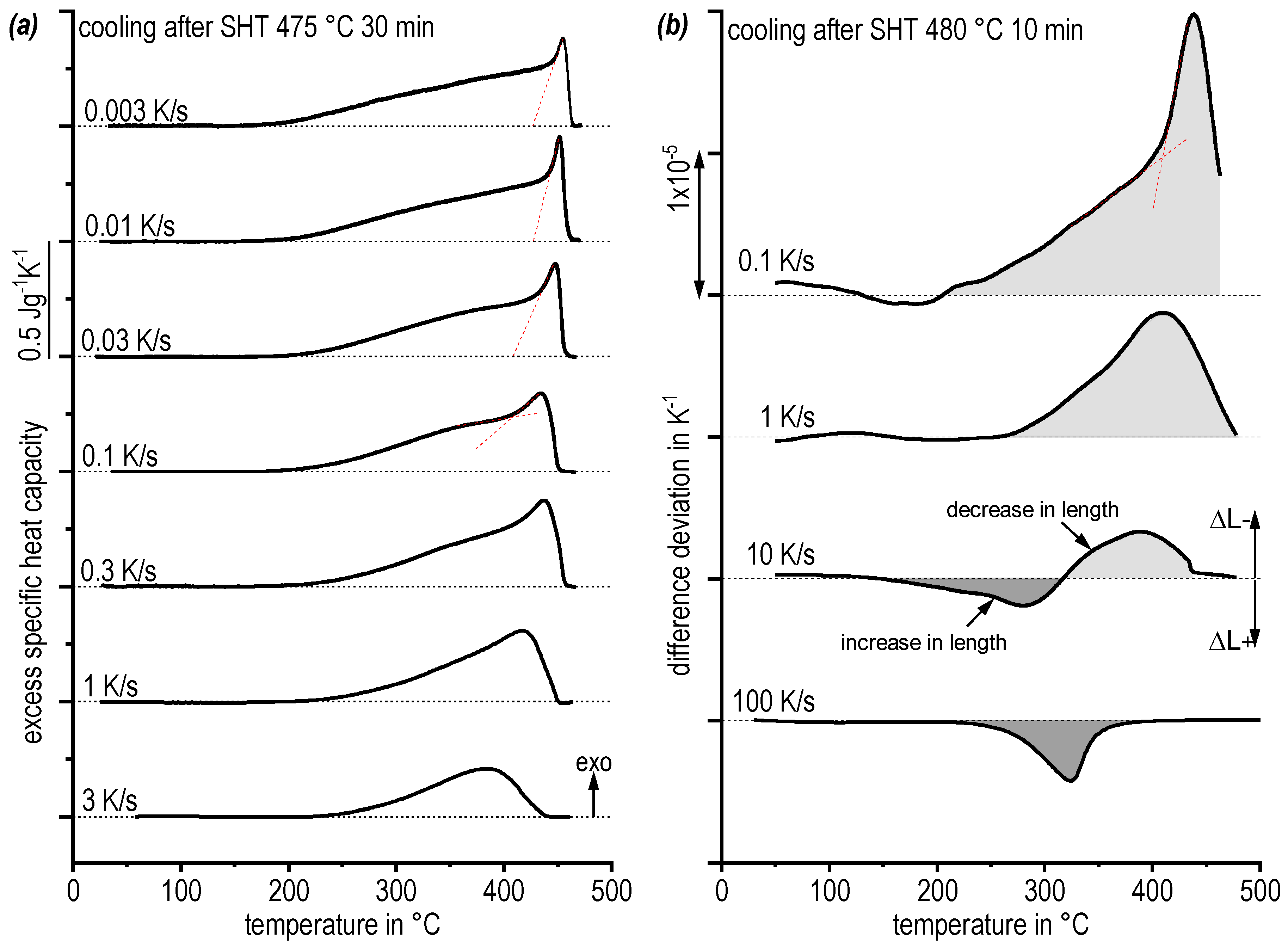
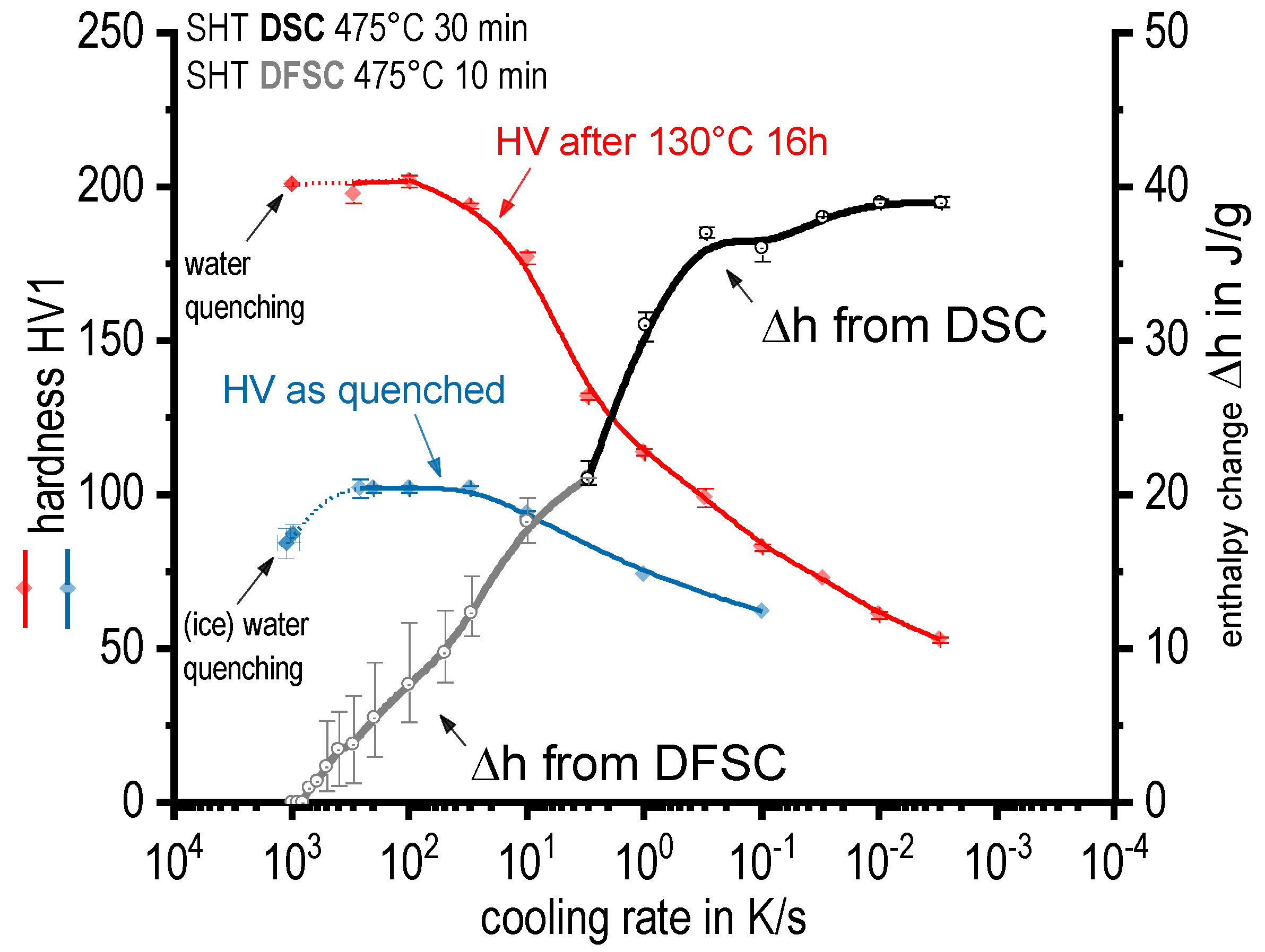
3.3. Kinetic Behaviour of Quench Induced Precipitation Analysed by DSC, DIL, DFSC, and Hardness Testing
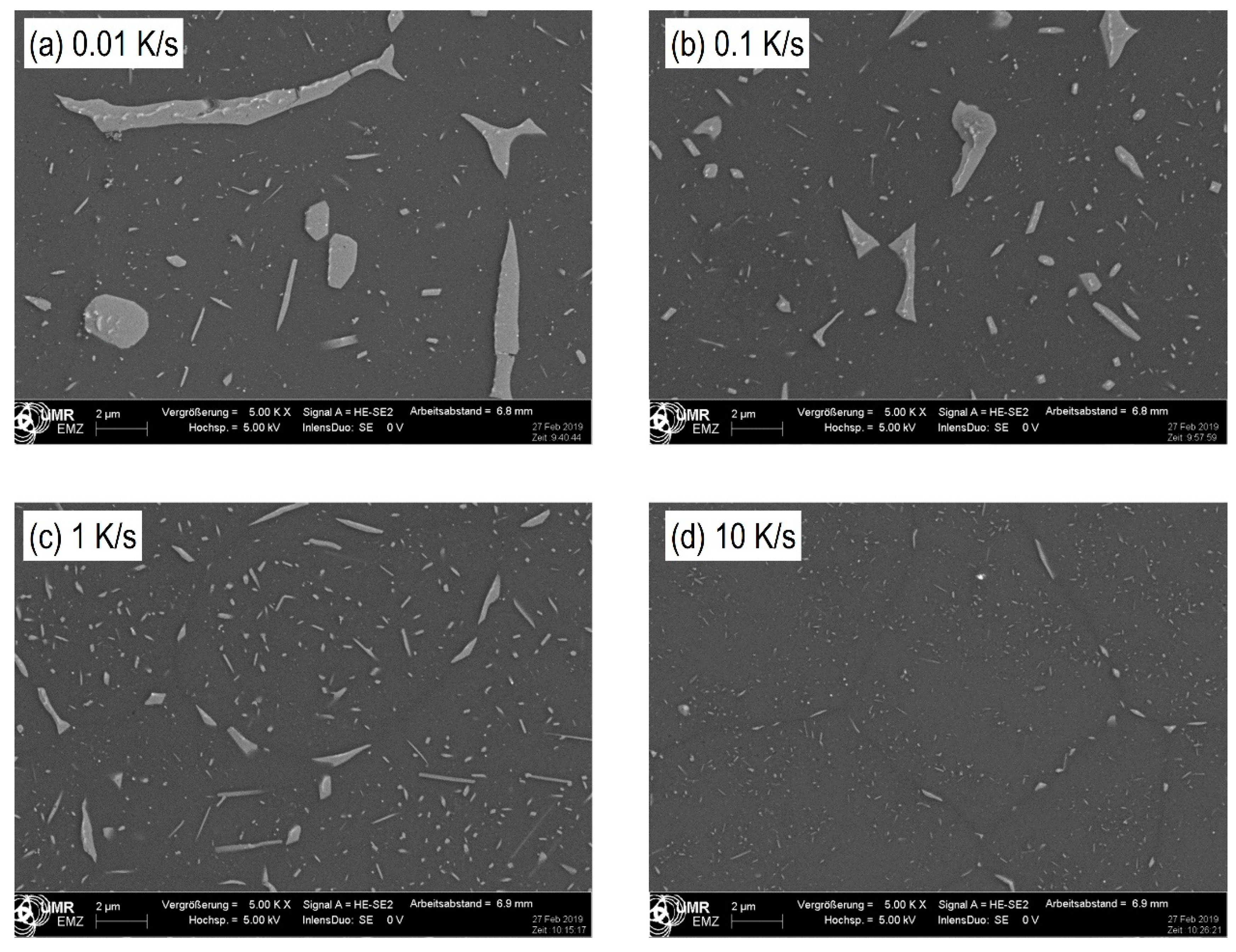
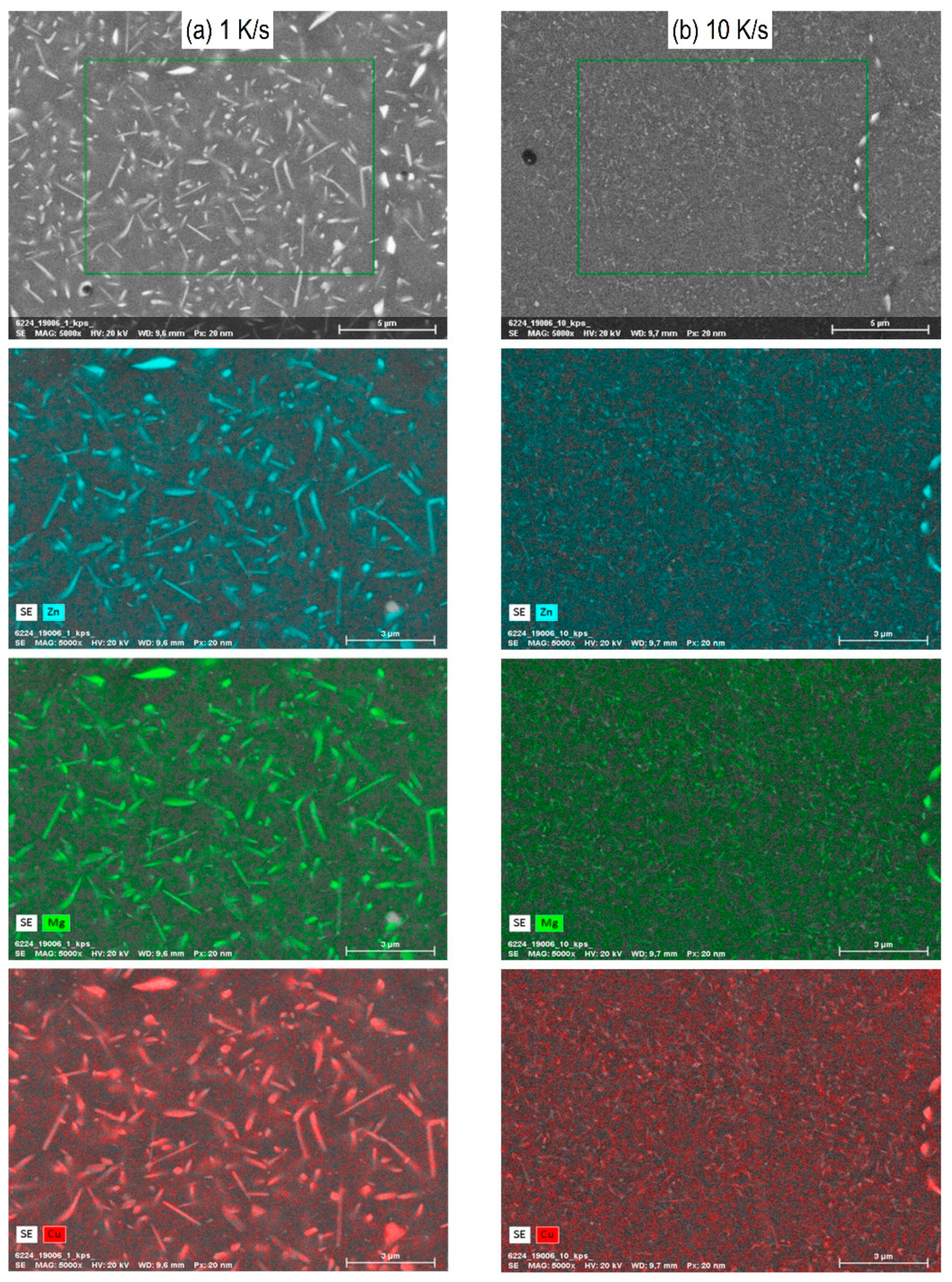
3.4. Microstructure of Different Cooled Samples through Scanning Electron Microscopy (SEM)
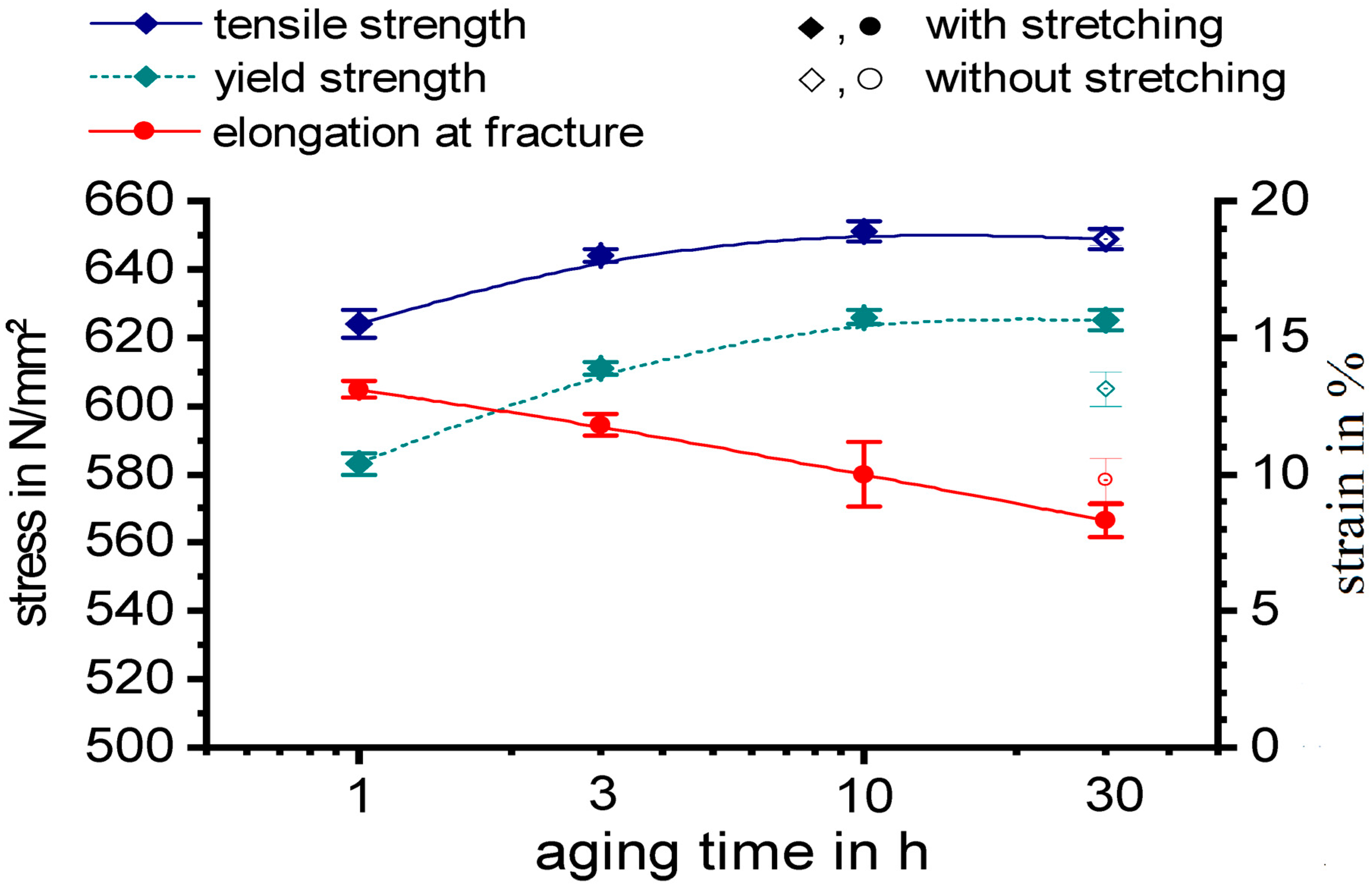
3.5. Mechanical Properties after Ageing: Tensile Tests
4. Conclusions
- An appropriate temperature for solution treatment is determined to be 475 °C (−480 °C), as the heating rate specific solvus at 0.01 K/s is 470 °C;
- Cooling DSC after soaking durations of 10 to 60 min at 475 °C showed no differences, and a soaking duration of 30 min was chosen;
- The application of differential dilatometry allows the in-situ detection of quench induced precipitation in a wide cooling rate range of 0.1 K/s to 100 K/s. At fast cooling of 100 K/s dilatometry still reveals quenched induced precipitation;
- While DSC only shows one very broad precipitation peak for a wide range of cooling rates, dilatometry indicates the overlap of several quench induced precipitation reactions;
- The physical upper critical cooling rate (CCR) at which quench induced precipitation is fully suppressed was determined by DFSC analysis to be about 600 to 800 K/s;
- A technological CCR is found to be ≈ 100 K/s (i.e., hardness after artificial ageing remains on a saturation level for higher cooling rates including WQ). This indicates, that quench induced precipitation at cooling rates faster than 100 K/s contributes to a direct hardening effect;
- For the investigated batch of AA 7068 the age hardening treatment comprising of solution heat treatment 475 °C 30 min, water quenching, stretching 3%, and ageing (130 °C 10 h) results in a yield strength Rp0.2 of 626 N/mm2, a tensile strength Rm of 651 N/mm2, and a fracture elongation of 10%.
Author Contributions
Funding
Conflicts of Interest
References
- Polmear, I.J. Light Alloys. From Traditional Alloys to Nanocrystals, 4th ed.; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2006; ISBN 0750663715. [Google Scholar]
- Hornbogen, E. Hundred years of precipitation hardening. J. Light Met. 2001, 1, 127–132. [Google Scholar] [CrossRef]
- Hornbogen, E. Precipitation hardening—The oldest nanotechnology. Metall-Berlin 2001, 55, 522–526. [Google Scholar]
- Baba, Y. Influence of Additional Elements on the Quench-Sensitivity and Nucleation of Precipitates in Al-Zn-Mg Alloys. Nippon Kinzoku Gakkaishi 1967, 31, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Baba, Y. Quench-Sensitivity and Age-Hardening of Al-Zn-Mg Alloys Containing Trace Elements. Trans. Jpn. Inst. Met. 1968, S9, 356. [Google Scholar]
- Conserva, M.; Fiorini, P. Interpretation of quench-sensitivity in Al-Zn-Mg-Cu alloys. Metall. Trans. A 1973, 4, 857–862. [Google Scholar] [CrossRef]
- Shuey, R.T.; Tiryakioğlu, M. Quenching of Aluminum Alloys. Quenching Theory and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Starink, M.J.; Milkereit, B.; Zhang, Y.; Rometsch, P.A. Predicting the quench sensitivity of Al-Zn-Mg-Cu alloys: A model for linear cooling and strengthening. Mater. Des. 2015, 88, 958–971. [Google Scholar] [CrossRef] [Green Version]
- Milkereit, B.; Reich, M.; Kessler, O. Detection of Quench Induced Precipitation in Al Alloys by Dilatometry. Mater. Sci. Forum. 2016, 877, 147–152. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schick, C. Fast scanning power compensated differential scanning nano-calorimeter: 1. The device. Thermochim. Acta 2010, 50, 1–13. [Google Scholar] [CrossRef]
- Yang, B.; Milkereit, B.; Zhang, Y.; Rometsch, P.A.; Kessler, O.; Schick, C. Continuous cooling precipitation diagram of aluminium alloy AA7150 based on a new fast scanning calorimetry and interrupted quenching method. Mater. Charact. 2016, 120, 30–37. [Google Scholar] [CrossRef]
- Mucha, J. The effect of material properties and joining process parameters on behavior of self-pierce riveting joints made with the solid rivet. Mater. Des. 2013, 52, 932–946. [Google Scholar] [CrossRef]
- Jäckel, M.; Grimm, T.; Niegsch, R.; Drossel, W.-G. Overview of Current Challenges in Self-Pierce Riveting of Lightweight Materials. Proceedings 2018, 2, 384. [Google Scholar] [CrossRef] [Green Version]
- Gröber, D.; Georgi, W.; Sieber, M.; Scharf, I.; Hellmig, R.J.; Leidich, E.; Lampke, T.; Mayr, P. The effect of anodising on the fatigue performance of self-tapping aluminium screws. Int. J. Fatigue 2015, 75, 108–114. [Google Scholar] [CrossRef]
- Milkereit, B.; Kessler, O.; Schick, C. Recording of continuous cooling precipitation diagrams of aluminium alloys. Thermochim. Acta 2009, 492, 73–78. [Google Scholar] [CrossRef]
- Osten, J.; Milkereit, B.; Schick, C.; Kessler, O. Dissolution and precipitation behaviour during continuous heating of Al-Mg-Si alloys in a wide range of heating rates. Materials 2015, 8, 2830–2848. [Google Scholar] [CrossRef] [Green Version]
- Hengge, E.; Enzinger, R.; Luckabauer, M.; Sprengel, W.; Würschum, R. Quantitative volumetric identification of precipitates in dilute alloys using high-precision isothermal dilatometry. Philos. Mag. Lett. 2018, 98, 301–309. [Google Scholar] [CrossRef]
- Luckabauer, M.; Hengge, E.; Klinser, G.; Sprengel, W.; Würschum, R. In Situ Real-Time Monitoring of Aging Processes in an Aluminum Alloy by High-Precision Dilatometry. In Magnesium Technology 2017; The Minerals, Metals & Materials Series; Solanki, K., Orlov, D., Singh, A., Neelameggham, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 669–674. [Google Scholar] [CrossRef]
- Resch, L.; Klinser, G.; Hengge, E.; Enzinger, R.; Luckabauer, M.; Sprengel, W.; Würschum, R. Precipitation processes in Al–Mg–Si extending down to initial clustering revealed by the complementary techniques of positron lifetime spectroscopy and dilatometry. J. Mater. Sci. 2018, 53, 14657–14665. [Google Scholar] [CrossRef] [Green Version]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Zohrabyan, D.; Milkereit, B.; Kessler, O.; Schick, C. Precipitation enthalpy during cooling of aluminum alloys obtained from calorimetric reheating experiments. Thermochim. Acta 2012, 529, 51–58. [Google Scholar] [CrossRef]
- Milkereit, B.; Jonas, L.; Schick, C.; Kessler, O. The continuous cooling precipitation diagram of an aluminum alloy en AW-6005A. HTM J. Heat Treat. Mat. 2010, 65, 159–171. [Google Scholar] [CrossRef]
- Osten, J.; Reich, M.; Milkereit, B.; Kessler, O. Adapting age hardening parameters of high-alloyed 7xxx aluminium alloys through thermal analysis. In Proceedings of the 16th International Conference on Aluminium Alloys, Montreal, QC, Canada, 17–21 June 2018. [Google Scholar]
- Fröck, H.; Milkereit, B.; Wiechmann, P.; Springer, A.; Sander, M.; Kessler, O.; Reich, M. Influence of Solution-Annealing Parameters on the Continuous Cooling Precipitation of Aluminum Alloy 6082. Metals 2018, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Milkereit, B.; Starink, M.J.; Rometsch, P.A.; Schick, C.; Kessler, O. Review of the Quench Sensitivity of Aluminium Alloys: Analysis of the Kinetics and Nature of Quench-Induced Precipitation. Materials 2019, 12, 4083. [Google Scholar] [CrossRef] [Green Version]
- Milkereit, B.; Kessler, O.; Schick, C. Quench Sensitivity and Continuous Cooling Precipitation Diagrams. In Encyclopedia of Aluminum and Its Alloys; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351045636. [Google Scholar]
- Zhang, Y.; Weyland, M.; Milkereit, B.; Reich, M.; Rometsch, P.A. Precipitation of a new platelet phase during the quenching of an Al-Zn-Mg-Cu alloy. Sci. Rep. 2016, 6, 23109. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, Q.; Lin, H.; Sun, L.; Long, T.; Ye, L.; Deng, Y. Effect of quench-induced precipitation on microstructure and mechanical properties of 7085 aluminum alloy. Mater. Des. 2017, 132, 119–128. [Google Scholar] [CrossRef]
- Reich, M.; Kessler, O. Quenching Simulation of Aluminum Alloys Including Mechanical Properties of the Undercooled States. Mater. Perform. Charact. 2012, 1, 104632. [Google Scholar] [CrossRef]
- Deschamps, A.; Texier, G.; Ringeval, S.; Delfaut-Durut, L. Influence of cooling rate on the precipitation microstructure in a medium strength Al-Zn-Mg alloy. Mater. Sci. Eng. A 2009, 501, 133–139. [Google Scholar] [CrossRef]
- Schloth, P. Precipitation in the High Strength AA7449 Aluminium Alloy: Implications on Internal Stresses on Different Length Scales. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2015. [Google Scholar]
- Schloth, P.; Wagner, J.N.; Fife, J.L.; Menzel, A.; Drezet, J.M.; van Swygenhoven, H. Early precipitation during cooling of an Al-Zn-Mg-Cu alloy revealed by in situ small angle X-ray scattering. Appl. Phys. Lett. 2014, 105, 101908. [Google Scholar] [CrossRef] [Green Version]


| Aluminium Alloy | Mass Fraction in % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Zr | |
| AA 7068 | 0.052 | 0.076 | 1.60 | 0.008 | 2.96 | 0.013 | 8.43 | 0.022 | 0.084 |
| AMS 4331 | ≤0.12 | ≤0.15 | 1.6–2.4 | ≤0.10 | 2.2–3.0 | ≤0.05 | 7.3–8.3 | ≤0.10 | 0.05–0.15 |
| T6 475 °C 30 min, Quenching in Water, 130 °C 30 h | T651 475 °C 30 min, Quenching in Water, Stretching 3%, 130 °C 10 h | |
|---|---|---|
| yield strength in N/mm2 | 605 ± 5 | 626 ± 2 |
| tensile strength in N/mm2 | 649 ± 2 | 651 ± 3 |
| elongation at fracture in % | 9.8 ± 0.8 | 10.0 ± 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osten, J.; Milkereit, B.; Reich, M.; Yang, B.; Springer, A.; Nowak, K.; Kessler, O. Development of Precipitation Hardening Parameters for High Strength Alloy AA 7068. Materials 2020, 13, 918. https://doi.org/10.3390/ma13040918
Osten J, Milkereit B, Reich M, Yang B, Springer A, Nowak K, Kessler O. Development of Precipitation Hardening Parameters for High Strength Alloy AA 7068. Materials. 2020; 13(4):918. https://doi.org/10.3390/ma13040918
Chicago/Turabian StyleOsten, Julia, Benjamin Milkereit, Michael Reich, Bin Yang, Armin Springer, Karina Nowak, and Olaf Kessler. 2020. "Development of Precipitation Hardening Parameters for High Strength Alloy AA 7068" Materials 13, no. 4: 918. https://doi.org/10.3390/ma13040918
APA StyleOsten, J., Milkereit, B., Reich, M., Yang, B., Springer, A., Nowak, K., & Kessler, O. (2020). Development of Precipitation Hardening Parameters for High Strength Alloy AA 7068. Materials, 13(4), 918. https://doi.org/10.3390/ma13040918





