Synthesis of Multiwall Boron Nitride (BN) Nanotubes by a PVD Method Based on Vapor–Liquid–Solid Growth
Abstract
1. Introduction
2. Methods and Materials
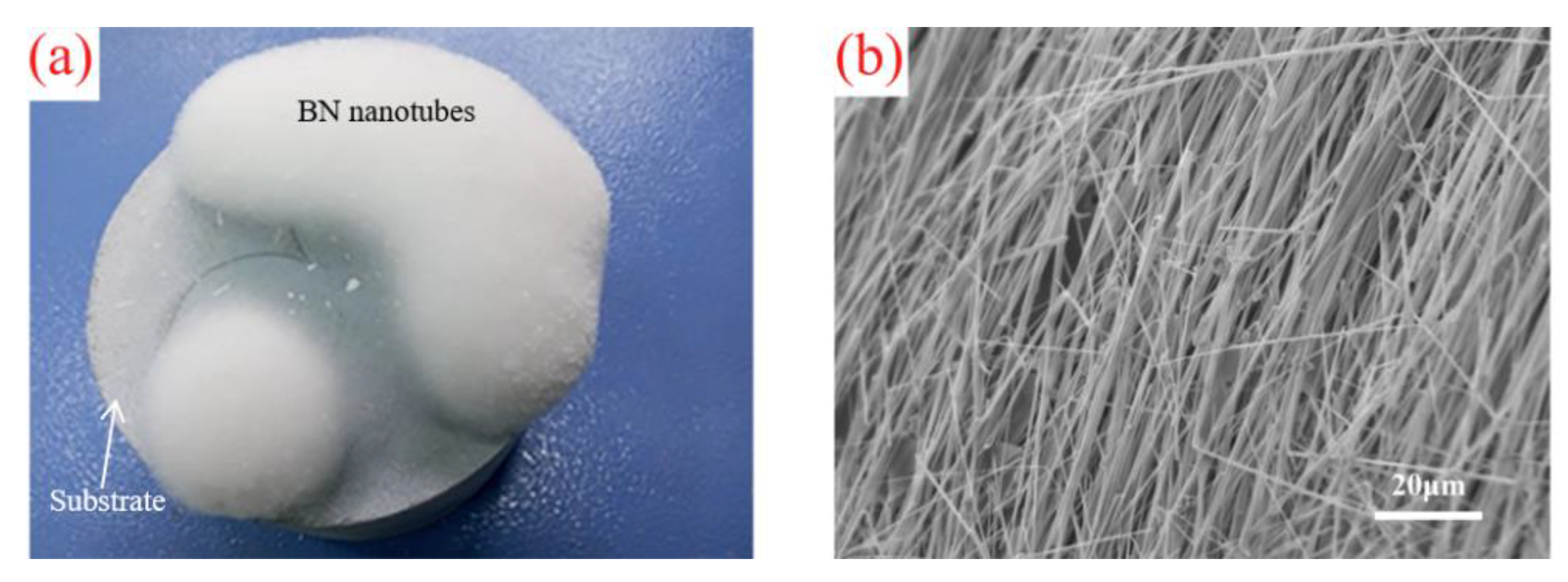
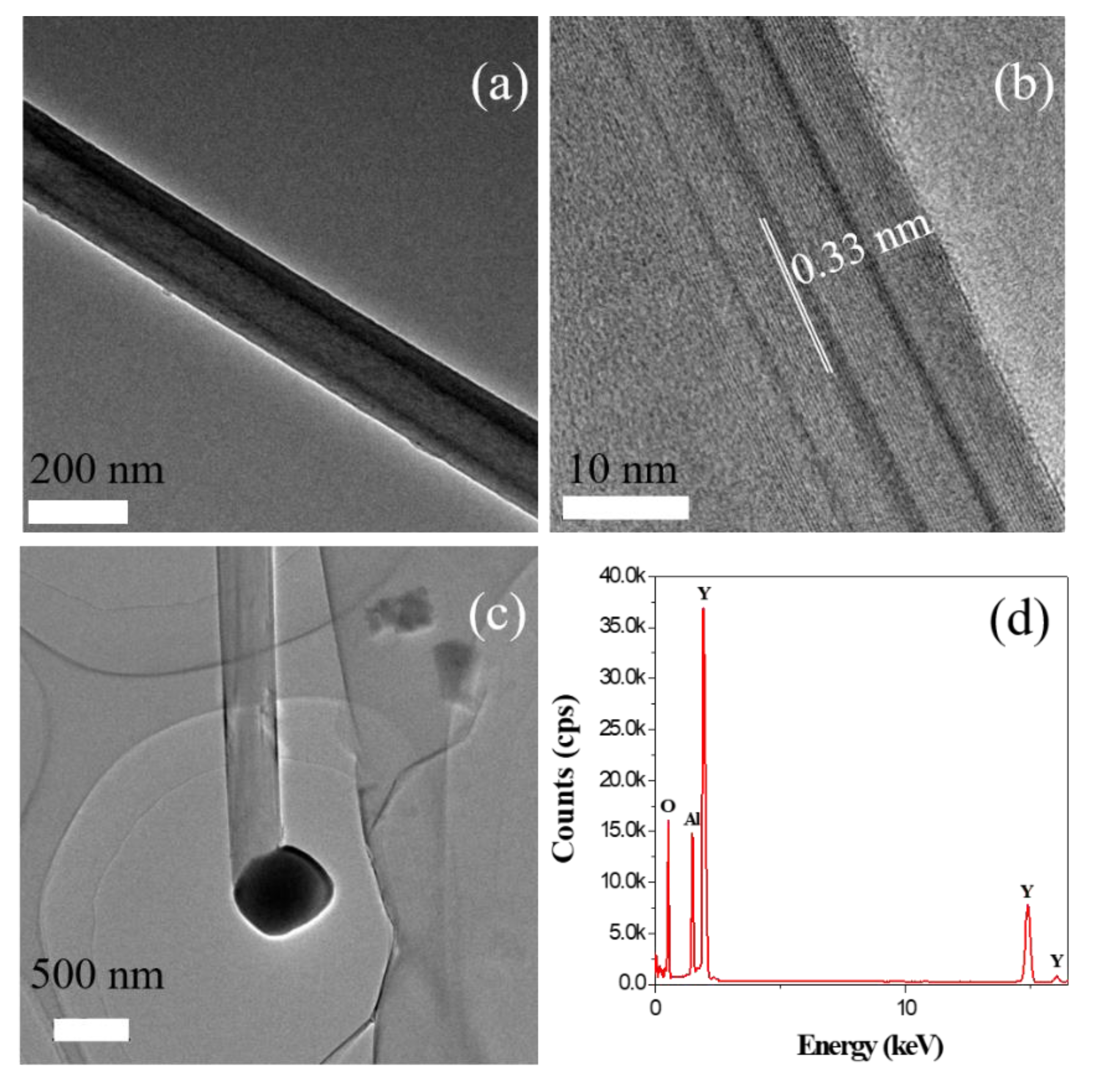
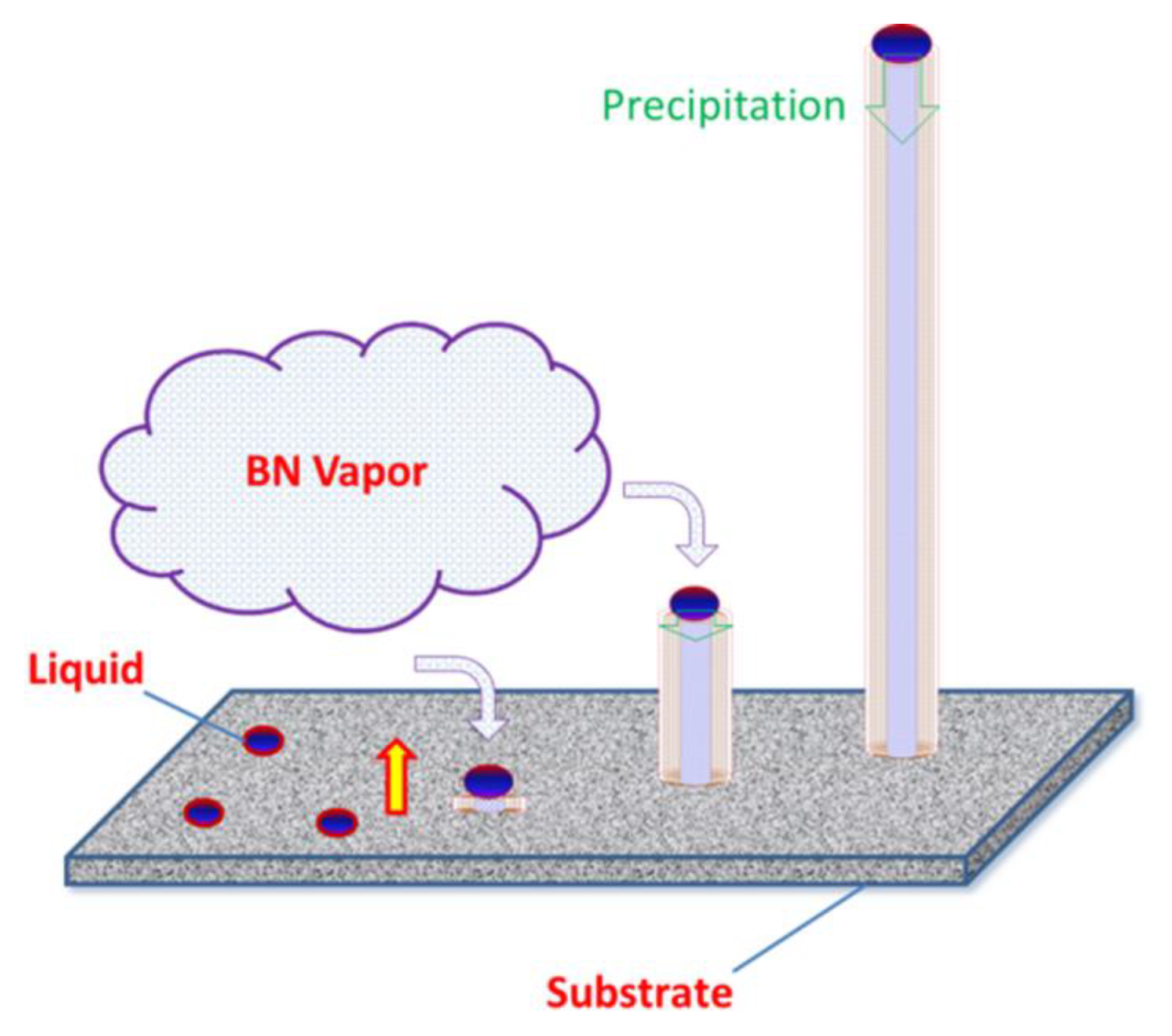
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Terrones, M.; Romo-Herrera, J.M.; Cruz-Silva, E.; Lopez-Urias, F.; Munoz-Sandoval, E.; Velazquez-Salazar, J.J.; Terrones, H.; Bando, Y.; Bolderg, B. Pure and doped boron nitride nanotubes. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Rubio, A.; Corkill, J.L.; Cohen, M.L. Theory of Graphitic Boron-Nitride Nanotubes. Phys. Rev. B 1994, 49, 5081–5084. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Tang, C.C.; Zhi, C.Y. Boron nitride nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Pouch, J.J.; Alterovitz, S.A. Synthesis and Properties of Boron Nitride. J. Mater. Manufact. Proc. 1991, 6, 373–374. [Google Scholar] [CrossRef]
- Lei, Y.P.; Wang, Y.D.; Song, Y.C.; Li, Y.H.; Deng, C.; Wang, H.; Xie, Z. Nearly stoichiometric BN fiber with low dielectric constant derived from poly[(alkylamino)borazine]. Mater. Lett. 2011, 65, 157–159. [Google Scholar] [CrossRef]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron Nitride Nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Self-Assembly of Tubular Fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar] [CrossRef]
- Endo, M.; Takeuchi, K.; Igarashi, S.; Kobori, K.; Shiraishi, M.; Kroto, H.W. The Production and Structure of Pyrolytic Carbon Nanotubes (Pcnts). J. Phys. Chem. Solids 1993, 54, 1841–1848. [Google Scholar] [CrossRef]
- Gleize, P.; Schouler, M.C.; Gadelle, P.; Caillet, M. Growth of Tubular Boron-Nitride Filaments. J. Mater. Sci. 1994, 29, 1575–1580. [Google Scholar] [CrossRef]
- Tang, C.C.; de la Chapelle, M.L.; Li, P.; Liu, Y.M.; Dang, H.Y.; Fan, S.S. Catalytic growth of nanotube and nanobamboo structures of boron nitride. Chem. Phys. Lett. 2001, 342, 492–496. [Google Scholar] [CrossRef]
- Shimizu, Y.; Moriyoshi, Y.; Tanaka, H.; Komatsu, S. Boron nitride nanotubes, webs, and coexisting amorphous phase formed by the plasma jet method. Appl. Phys. Lett. 1999, 75, 929–931. [Google Scholar] [CrossRef]
- Bengu, E.; Marks, L.D. Single-walled BN nanostructures. Phys. Rev. Lett. 2001, 86, 2385–2387. [Google Scholar] [CrossRef] [PubMed]
- Kukovitsky, E.F.; L’vov, S.G.; Sainov, N.A. VLS-growth of carbon nanotubes from the vapor. Chem. Phys. Lett. 2000, 317, 65–70. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Chen, Y.J.; Su, Q.Q.; Huang, Q.M. The effect of iron oxide on the formation of boron nitride nanotubes. Chem. Eng. J. 2011, 174, 687–692. [Google Scholar] [CrossRef]
- Wang, J.L.; Peng, D.J.; Long, F.; Wang, W.M.; Gu, Y.L.; Mo, S.Y.; Zou, Z.; Fu, Z. Glass fabrics self-cracking catalytic growth of boron nitride nanotubes. Solid State Sci. 2017, 64, 23–28. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Turkevich, V.Z.; Holzapfel, W.B. Refined phase diagram of boron nitride. J. Phys. Chem. B 1999, 103, 2903–2905. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Sato, T.; Kurashima, K. A novel precursor for synthesis of pure boron nitride nanotubes. Chem. Commun. 2002, 33, 1290–1291. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.C.P.; Zou, J.; Chen, Y. Influence of nitriding gases on the growth of boron nitride nanotubes. J. Mater. Sci. 2007, 42, 4025–4030. [Google Scholar] [CrossRef]
- Song, X.J.; Gao, J.F.; Nie, Y.F.; Gao, T.; Sun, J.Y.; Ma, D.L.; Li, Q.; Chen, Y.; Jin, C.; Bachmatiuk, A.; et al. Chemical vapor deposition growth of large-scale hexagonal boron nitride with controllable orientation. Nano Res. 2015, 8, 3164–3176. [Google Scholar] [CrossRef]
- Sue, Y.S.; Pan, K.Y.; Wei, D.H. Optoelectronic and photocatalytic properties of zinc sulfide nanowires synthesized by vapor-liquid-solid process. Appl. Surf. Sci. 2019, 471, 435–444. [Google Scholar] [CrossRef]
- Guo, C.C.; Cheng, L.F.; Ye, F.; Li, Z.C.; Xu, Z.S. Synthesis and characterization of carbon-poor SiC nanowires via vapor-liquid-solid growth mechanism. Ceram. Int. 2019, 45, 6440–6446. [Google Scholar] [CrossRef]
- Adylov, G.T.; Voronov, G.V.; Mansurova, E.P.; Sigalov, L.M.; Urazaeva, E.M. Y2O3-Al2O3 system higher 1473-K. Russ. J. Inorg. Chem. 1988, 33, 1867–1869. [Google Scholar]
- Toropov, N.A.; Bondar’, I.A.; Galadhov, F.Y.; Nikogosyan, K.S.; Vinogradova, N.V. Phase equilibria in the yttrium oxide-alumina system. J. Bull. Acad. Sci. USSR Div. Chem. Sci. 1964, 13, 1076–1081. [Google Scholar] [CrossRef]
- Fabrichnaya, O.; Pavlyuchkov, D.; Neher, R.; Herrmann, M.; Seifert, H.J. Liquid phase formation in the system Al2O3-Y2O3-AlN: Part II. Thermodynamic assessment. J. Eur. Ceram. Soc. 2013, 33, 2457–2463. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Xu, Y.; Chen, H.; Wang, Z.; Mao, X.; Zhou, G.; Zhang, J.; Wang, S. Synthesis of Multiwall Boron Nitride (BN) Nanotubes by a PVD Method Based on Vapor–Liquid–Solid Growth. Materials 2020, 13, 915. https://doi.org/10.3390/ma13040915
Guo H, Xu Y, Chen H, Wang Z, Mao X, Zhou G, Zhang J, Wang S. Synthesis of Multiwall Boron Nitride (BN) Nanotubes by a PVD Method Based on Vapor–Liquid–Solid Growth. Materials. 2020; 13(4):915. https://doi.org/10.3390/ma13040915
Chicago/Turabian StyleGuo, Hao, Yonggang Xu, Hetuo Chen, Zhengjuan Wang, Xiaojian Mao, Guohong Zhou, Jian Zhang, and Shiwei Wang. 2020. "Synthesis of Multiwall Boron Nitride (BN) Nanotubes by a PVD Method Based on Vapor–Liquid–Solid Growth" Materials 13, no. 4: 915. https://doi.org/10.3390/ma13040915
APA StyleGuo, H., Xu, Y., Chen, H., Wang, Z., Mao, X., Zhou, G., Zhang, J., & Wang, S. (2020). Synthesis of Multiwall Boron Nitride (BN) Nanotubes by a PVD Method Based on Vapor–Liquid–Solid Growth. Materials, 13(4), 915. https://doi.org/10.3390/ma13040915





