Optimization of Hybrid Sol-Gel Coating for Dropwise Condensation of Pure Steam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Coating Characterizations
2.4. Condensation Tests
3. Results and Discussion
3.1. Layers Characterization
3.1.1. Evaluation of Wettability Proprieties
3.1.2. Surface Chemistry Investigation
3.1.3. Thickness Measurement
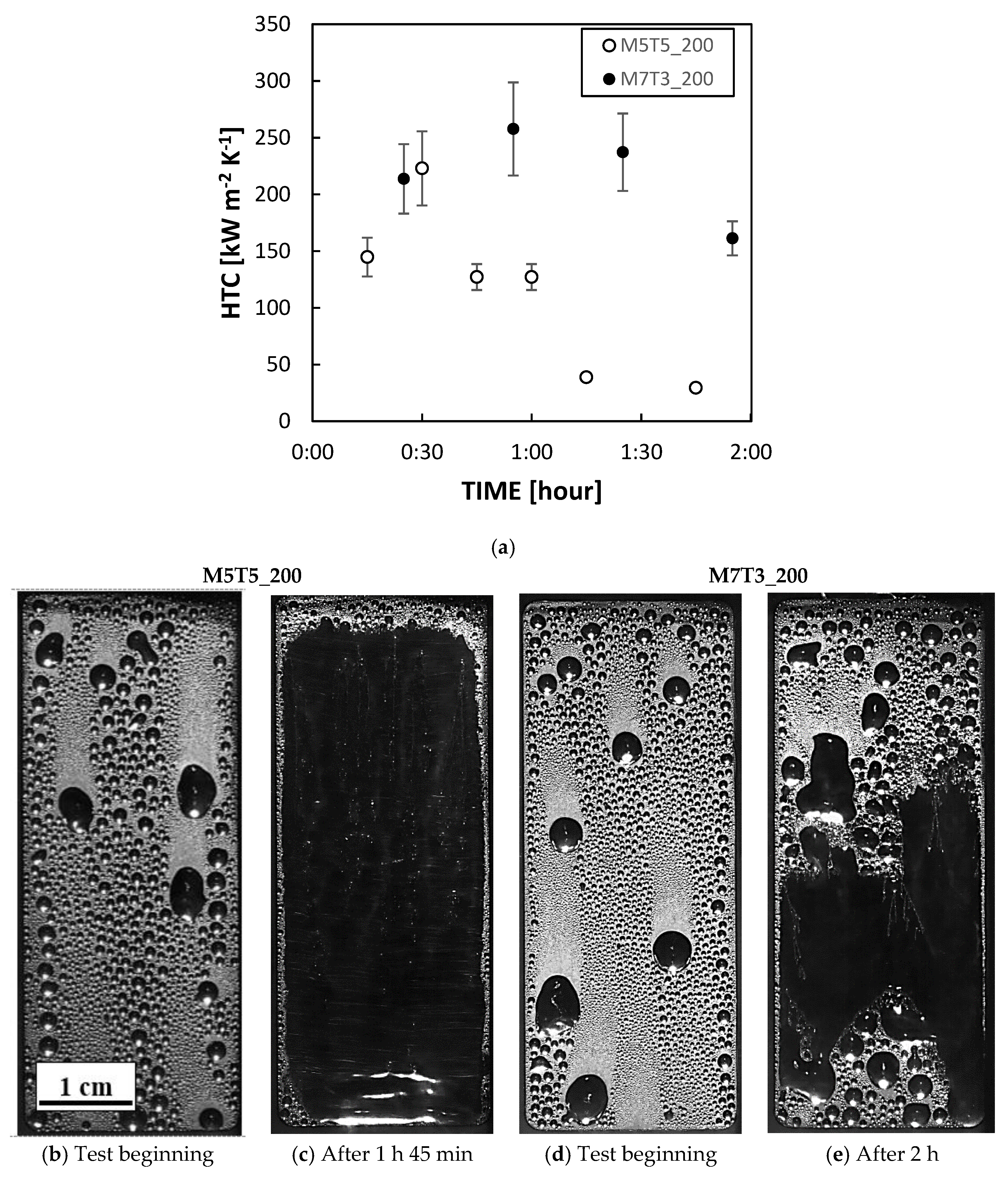
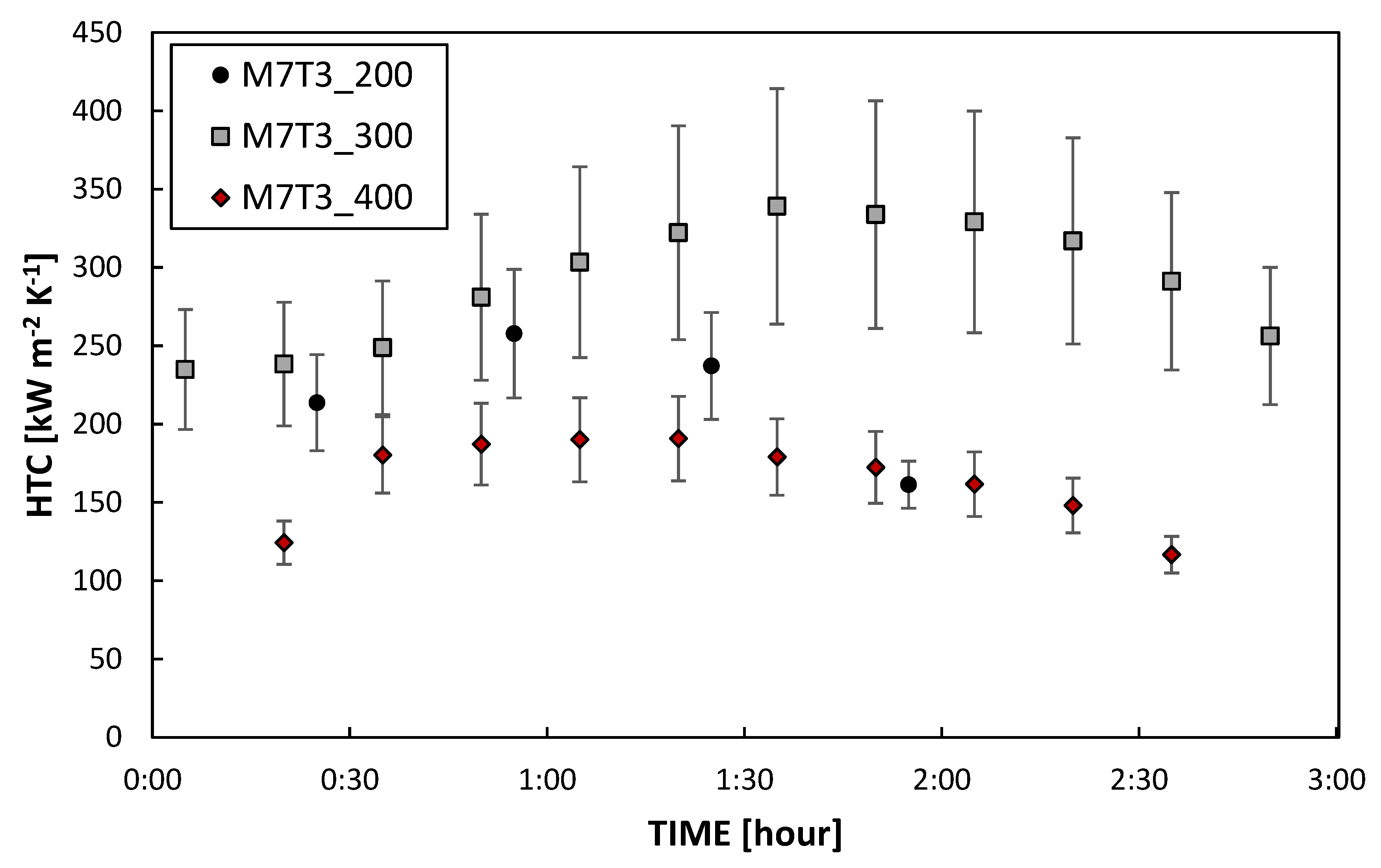
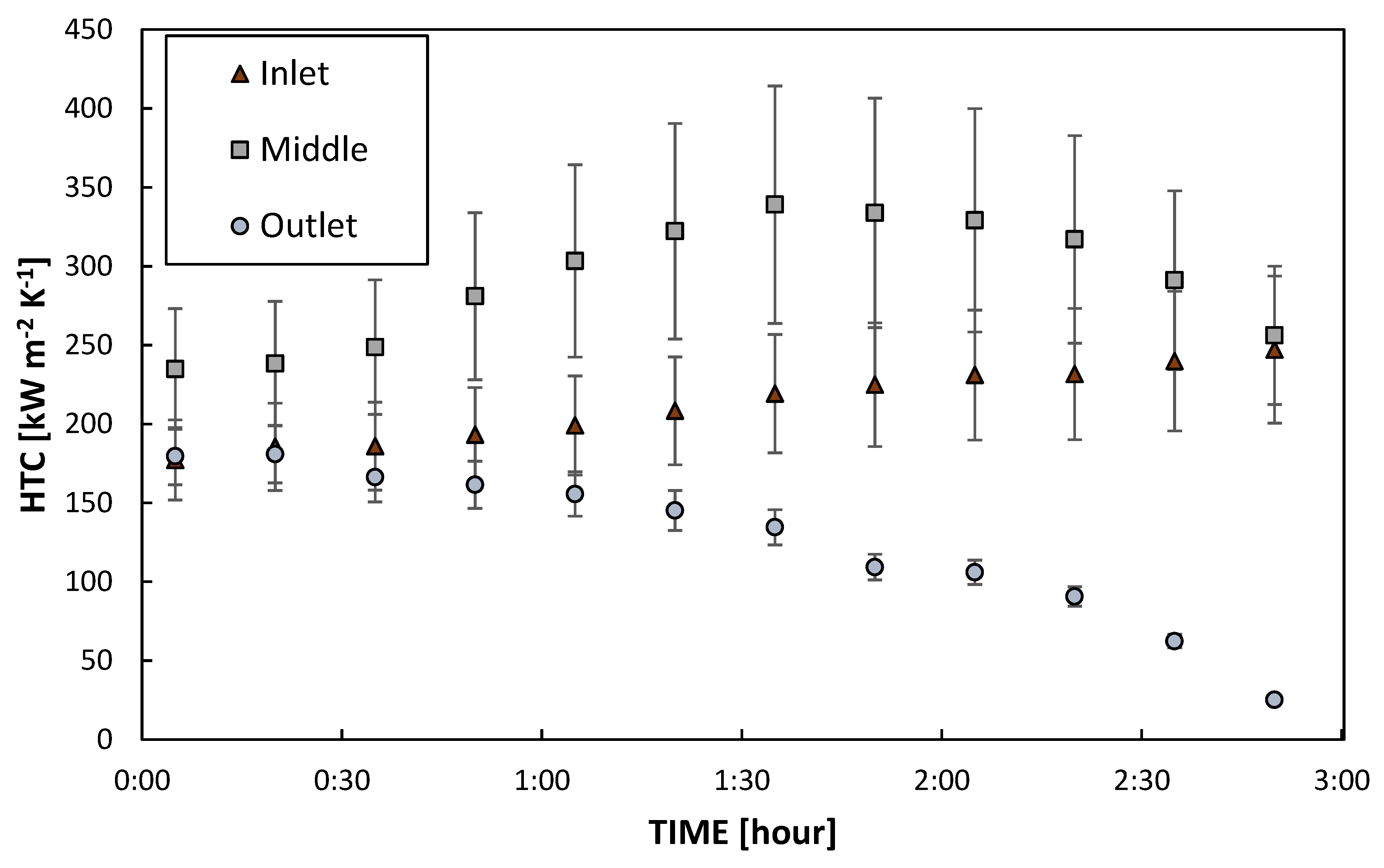
3.2. Heat Exchange Tests
3.2.1. DWC Measurements
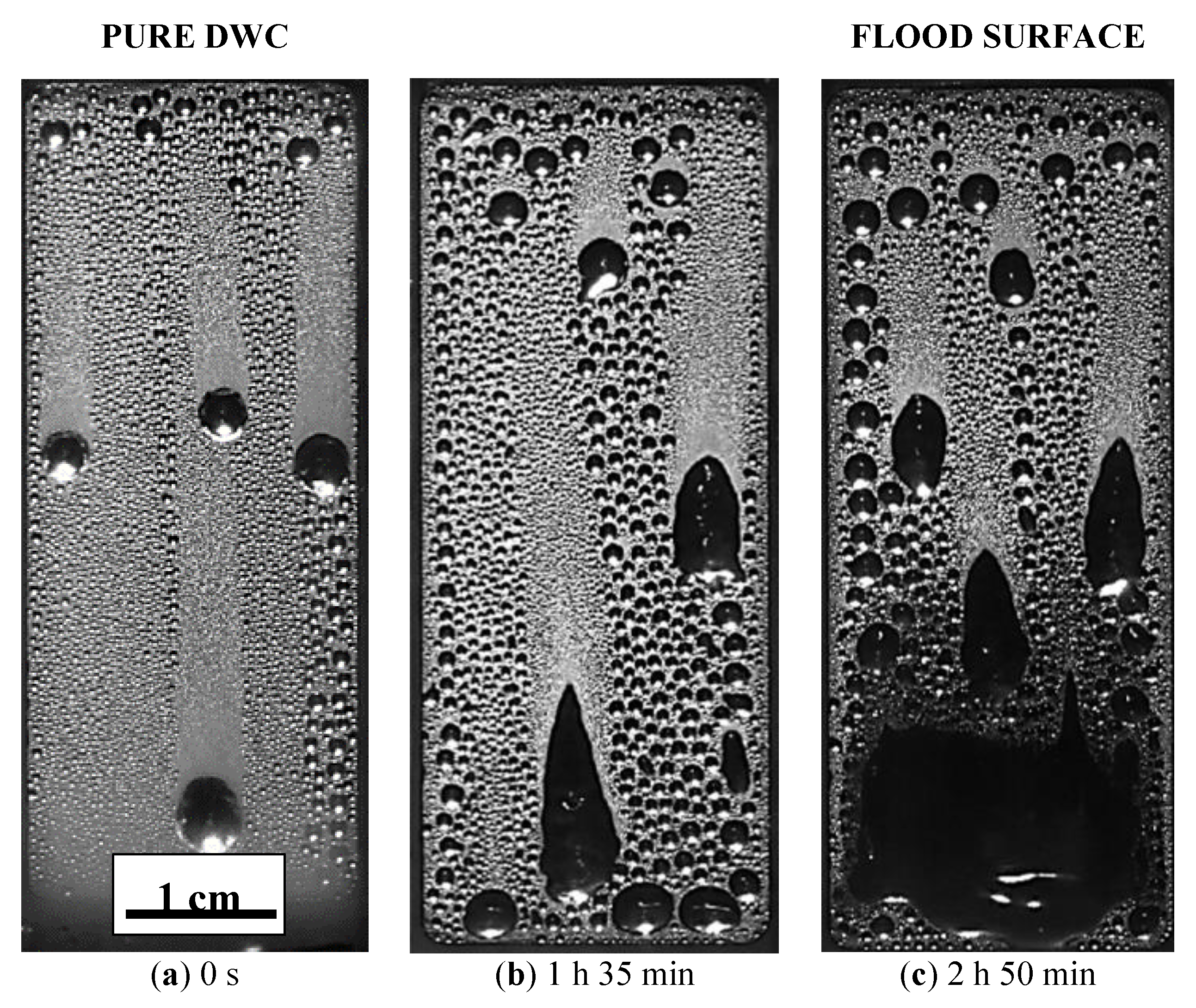
3.2.2. DWC Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| HTC | Heat transfer coefficient (between steam and metallic substrate), kW m−2 K−1 |
| k | coverage factor, - |
| q | Heat flux, kW m−2 |
| T | Temperature, K |
| z | Orthogonal axis of the sample, m |
| Greek symbols | |
| ΔT | Temperature difference, K |
| λ | Thermal conductivity, W m−1 K−1 |
| θ | Contact angle, ° |
| Subscripts | |
| a | Advancing |
| r | Receding |
| sat | Saturation |
| wall | Surface |
References
- Ijsseling, F.P. General guidelines for corrosion testing of materials for marine applications: Literature review on sea water as test environment. Br. Corros. J. 1989, 24, 53–78. [Google Scholar] [CrossRef]
- Tiwari, A. A topical review on hybrid quasi-ceramic coatings for corrosion protection. Corros. Rev. 2018, 36, 117–125. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press Inc.: San Diego, CA, USA, 1990; ISBN 978-0121349707. [Google Scholar]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Enright, R.; Miljkovic, N.; Alvarado, J.L.; Kim, K.; Rose, J.W. Dropwise Condensation on Micro- and Nanostructured Surfaces. Nanoscale Microscale Thermophys. Eng. 2014, 18, 223–250. [Google Scholar] [CrossRef]
- Rose, J.W. Dropwise condensation theory and experiment: A review. Proc. Inst. Mech. Eng. Part A J. Power Energy 2002, 216, 115–128. [Google Scholar] [CrossRef]
- Parin, R.; Sturaro, M.; Bortolin, S.; Martucci, A.; Del Col, D. Heat transfer during dropwise condensation of steam over a mirror polished sol-gel coated aluminum substrate. Int. J. Therm. Sci. 2019, 144, 93–106. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Zhang, Y.; Zhao, F.; Zhang, Y. Fabrication of superhydrophobic surface by hierarchical growth of lotus-leaf-like boehmite on aluminum foil. J. Colloid Interface Sci. 2011, 358, 277–283. [Google Scholar] [CrossRef]
- Chavan, S.; Cha, H.; Orejon, D.; Nawaz, K.; Singla, N.; Yeung, Y.F.; Park, D.; Kang, D.H.; Chang, Y.; Takata, Y.; et al. Heat Transfer through a Condensate Droplet on Hydrophobic and Nanostructured Superhydrophobic Surfaces. Langmuir 2016, 32, 7774–7787. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Basu, B.J. Fabrication of superhydrophobic sol–gel composite films using hydrophobically modified colloidal zinc hydroxide. J. Colloid Interface Sci. 2009, 339, 454–460. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically stable and corrosion resistant superhydrophobic sol-gel coatings on copper substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]
- Goswami, D.; Medda, S.K.; De, G. Superhydrophobic films on glass surface derived from trimethylsilanized silica gel nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Gurav, A.B.; Latthe, S.S.; Vhatkar, R.S.; Imai, H.; Kappenstein, C.; Wagh, P.B.; Gupta, S.C. Water repellent porous silica films by sol–gel dip coating method. J. Colloid Interface Sci. 2010, 352, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, Y.; Xu, W.; Liu, W. Controlled growth of superhydrophobic films by sol–gel method on aluminum substrate. Appl. Surf. Sci. 2010, 256, 6072–6075. [Google Scholar] [CrossRef]
- Liang, J.; Hu, Y.; Wu, Y.; Chen, H. Facile formation of superhydrophobic silica-based surface on aluminum substrate with tetraethylorthosilicate and vinyltriethoxysilane as co-precursor and its corrosion resistant performance in corrosive NaCl aqueous solution. Surf. Coat. Technol. 2014, 240, 145–153. [Google Scholar] [CrossRef]
- Parin, R.; Martucci, A.; Sturaro, M.; Bortolin, S.; Bersani, M.; Carraro, F.; Del, D. Nano-structured aluminum surfaces for dropwise condensation. Surf. Coat. Technol. 2018, 348, 1–12. [Google Scholar] [CrossRef]
- Parin, R.; Del Col, D.; Bortolin, S.; Martucci, A. Dropwise condensation over superhydrophobic aluminium surfaces. J. Phys. Conf. Ser. 2016, 745, 032134. [Google Scholar] [CrossRef]
- Paxson, A.T.; Yagüe, J.L.; Gleason, K.K.; Varanasi, K.K. Stable dropwise condensation for enhancing heat transfer via the initiated chemical vapor deposition (iCVD) of grafted polymer films. Adv. Mater. 2014, 26, 418–423. [Google Scholar] [CrossRef]
- Kananeh, A.B.; Rausch, M.H.; Leipertz, A.; Fröba, A.P. Dropwise Condensation Heat Transfer on Plasma- Ion-Implanted Small Horizontal Tube Bundles. Heat Transf. Eng. 2010, 31. [Google Scholar] [CrossRef]
- Innocenzi, P.; Abdirashid, M.O.; Guglielmi, M. Structure and properties of sol-gel coatings from methyltriethoxysilane and tetraethoxysilane. J. Sol-Gel Sci. Technol. 1994, 3, 47–55. [Google Scholar] [CrossRef]
- Del Col, D.; Parin, R.; Bisetto, A.; Bortolin, S.; Martucci, A. Film condensation of steam flowing on a hydrophobic surface. Int. J. Heat Mass Transf. 2017, 107, 307–318. [Google Scholar] [CrossRef]
- ISO; IEC & OIML; BIPM. Guide to the Expression of Uncertainty in Measurement; ISO – International Organization for Standardization: Geneva, Switzerland, 1995; Volume 122. [Google Scholar]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dabalà, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol-gel derived silica-based films: A spectra-microstructure overwiev. J. Non Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Yu, S.; Wong, T.K.S.; Hu, X.; Yong, M.S. Dielectric and mechanical properties of surface modified organosilicate films. J. Sol-Gel Sci. Technol. 2005, 35, 69–75. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol-gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Song, J. Studies of the surface wettability and hydrothermal stability of methyl-modified silica films by FT-IR and Raman spectra. Vib. Spectrosc. 2009, 50, 178–184. [Google Scholar] [CrossRef]
- Colusso, E.; Martucci, A.; Neto, C. Fabrication of Biomimetic Micropatterned Surfaces by Sol–Gel Dewetting. Adv. Mater. Interfaces 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Chomel, A.D.; Dempsey, P.; Latournerie, J.; Hourlier-Bahloul, D.; Jayasooriya, U.A. Gel to Glass Transformation of Methyltriethoxysilane: A Silicon Oxycarbide Glass Precursor Investigated Using Vibrational Spectroscopy. Chem. Mater. 2005, 17, 4468–4473. [Google Scholar] [CrossRef]
- Usami, K.I.; Sugahara, S.; Kobayashi, M.; Sumimura, K.; Hattori, T.; Matsumura, M. Preparation and properties of silica films with higher-alkyl groups. J. Non Cryst. Solids 1999, 260, 199–207. [Google Scholar] [CrossRef]
- Imai, H.; Hirashima, H.; Awazu, K. Alternative modification methods for sol-gel coatings of silica, titania and silica-titania using ultraviolet irradiation and water vapor. Thin Solid Films 1999, 351, 91–94. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.J. Dropwise Condensation Modeling Suitable for Superhydrophobic Surfaces. J. Heat Transf. 2011, 133, 081502. [Google Scholar] [CrossRef]
- Miljkovic, N.; Enright, R.; Wang, E.N. Modeling and Optimization of Superhydrophobic Condensation. J. Heat Transf. 2013, 135, 111004. [Google Scholar] [CrossRef]
- Allred, T.P.; Weibel, J.A.; Garimella, S.V. The petal effect of parahydrophobic surfaces offers low receding contact angles that promote effective boiling. Int. J. Heat Mass Transf. 2019, 135, 403–412. [Google Scholar] [CrossRef]
- Antonini, C.; Villa, F.; Bernagozzi, I.; Amirfazli, A.; Marengo, M. Drop rebound after impact: The role of the receding contact angle. Langmuir 2013, 29, 16045–16050. [Google Scholar] [CrossRef] [PubMed]
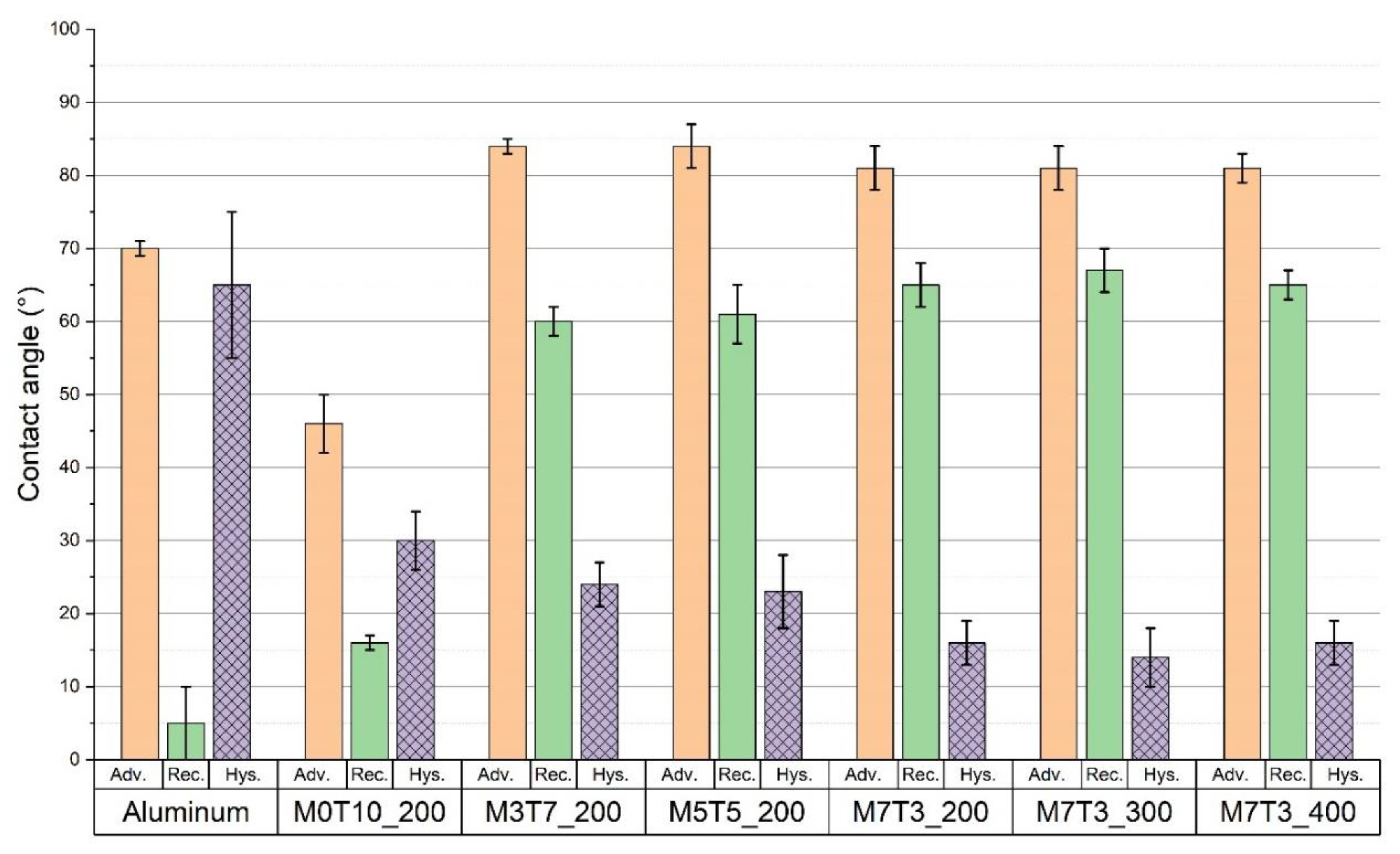
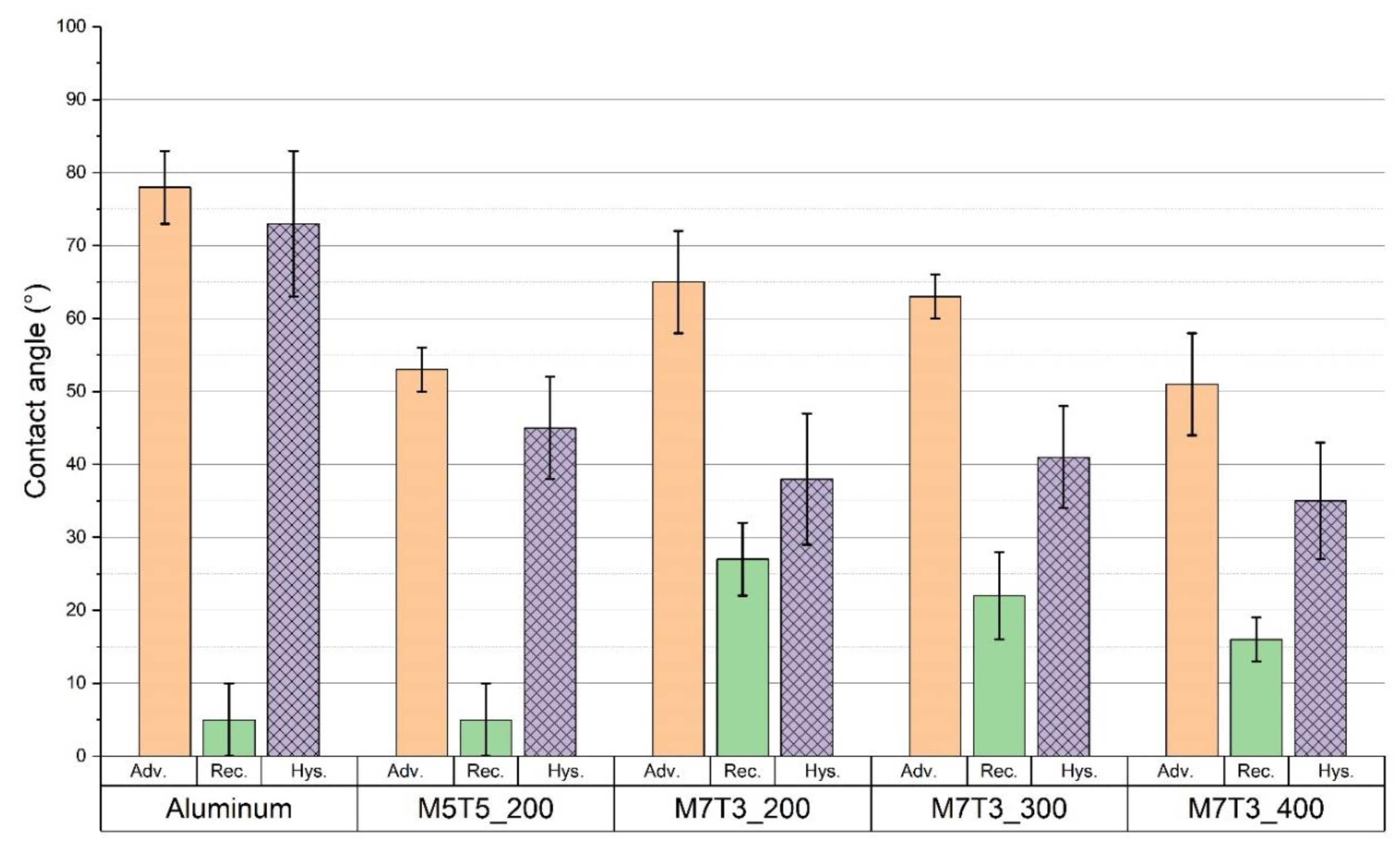
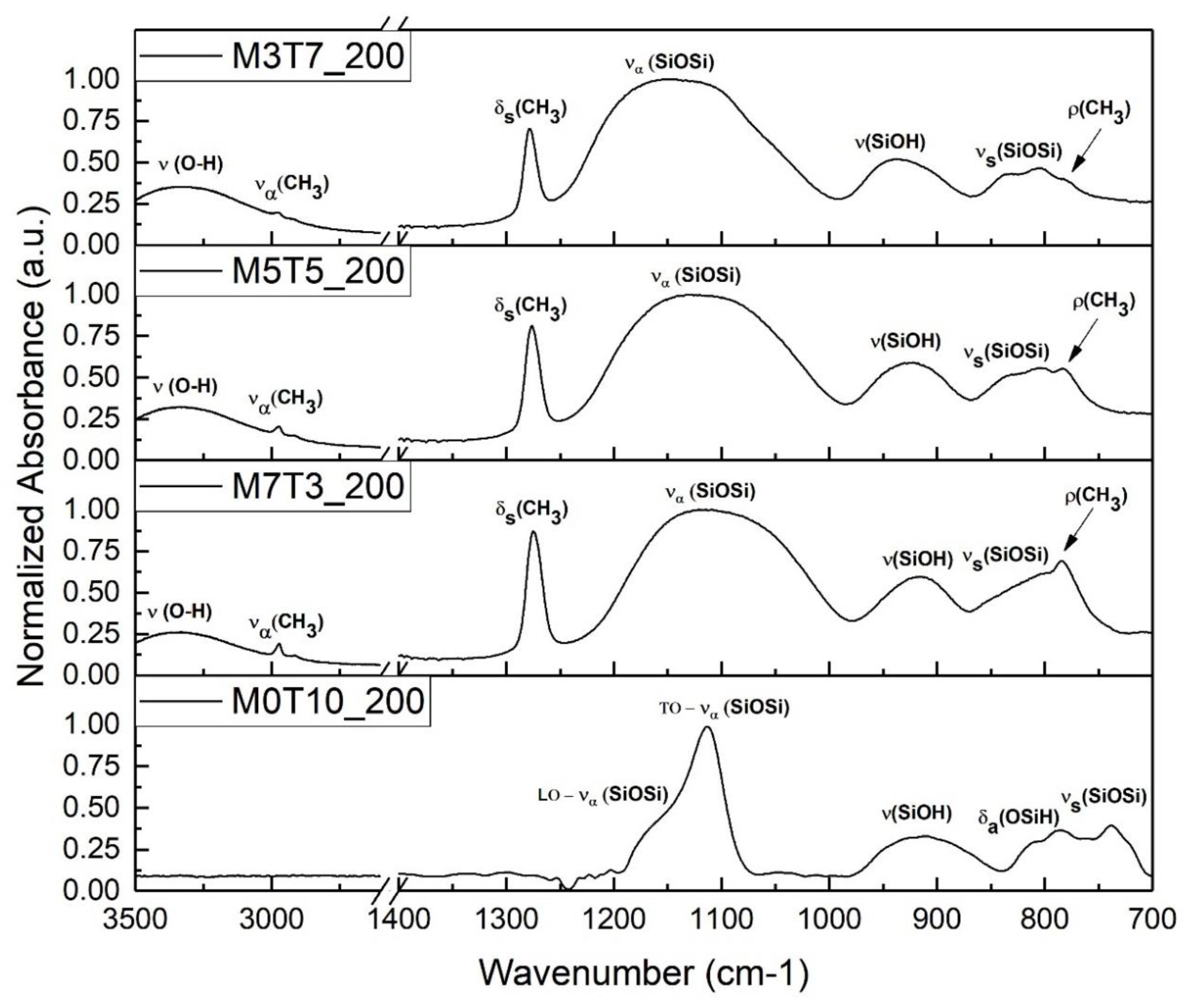
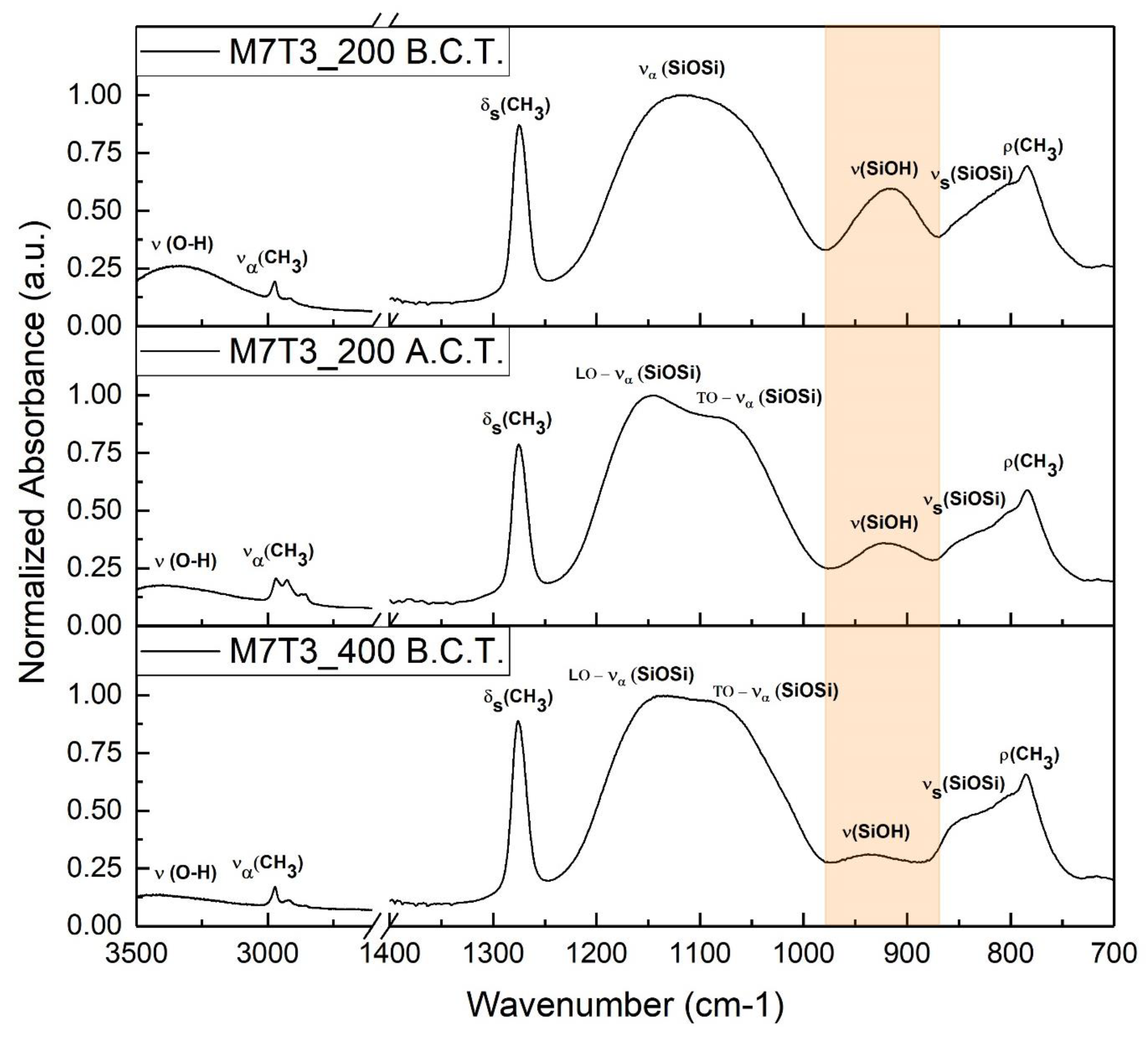
| Name | MTES Molar Percentage | TEOS Molar Percentage | Baking Temperature |
|---|---|---|---|
| M0T10_200 | 0% | 100% | 200 °C |
| M3T7_200 | 30% | 70% | 200 °C |
| M5T5_200 | 50% | 50% | 200 °C |
| M7T3_200 | 70% | 30% | 200 °C |
| M7T3_300 | 70% | 30% | 300 °C |
| M7T3_400 | 70% | 30% | 400 °C |
| Before Condensation Test | After Condensation Test | |||||
|---|---|---|---|---|---|---|
| ID | n (640 nm) | Thickness | M.S.E. | n (640 nm) | Thickness | M.S.E. |
| M0T10_200 | 1.4372 | 173.2 nm | 1.96 | / | / | / |
| M3T7_200 | 1.4226 | 196.4 nm | 3.38 | / | / | / |
| M5T5_200 | 1.4445 | 226.0 nm | 1.45 | 1.4088 | 154.78 nm | 5.10 |
| M7T3_200 | 1.4252 | 252.60 nm | 2.74 | 1.3832 | 115.65 nm | 11.11 |
| M7T3_300 | 1.4216 | 229.36 nm | 1.71 | 1.3920 | 125.32 nm | 13.12 |
| M7T3_400 | 1.3945 | 199.27 nm | 2.90 | 1.3896 | 120.12 nm | 12.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parin, R.; Rigon, M.; Bortolin, S.; Martucci, A.; Del Col, D. Optimization of Hybrid Sol-Gel Coating for Dropwise Condensation of Pure Steam. Materials 2020, 13, 878. https://doi.org/10.3390/ma13040878
Parin R, Rigon M, Bortolin S, Martucci A, Del Col D. Optimization of Hybrid Sol-Gel Coating for Dropwise Condensation of Pure Steam. Materials. 2020; 13(4):878. https://doi.org/10.3390/ma13040878
Chicago/Turabian StyleParin, Riccardo, Michele Rigon, Stefano Bortolin, Alessandro Martucci, and Davide Del Col. 2020. "Optimization of Hybrid Sol-Gel Coating for Dropwise Condensation of Pure Steam" Materials 13, no. 4: 878. https://doi.org/10.3390/ma13040878
APA StyleParin, R., Rigon, M., Bortolin, S., Martucci, A., & Del Col, D. (2020). Optimization of Hybrid Sol-Gel Coating for Dropwise Condensation of Pure Steam. Materials, 13(4), 878. https://doi.org/10.3390/ma13040878






