Structural and Optical Characteristics of Highly UV-Blue Luminescent ZnNiO Nanoparticles Prepared by Sol–Gel Method
Abstract
1. Introduction:
2. Experimental Details
2.1. Synthesis of ZnNiO Nanoparticles
2.2. Characterization of ZnNiO Nanoparticles
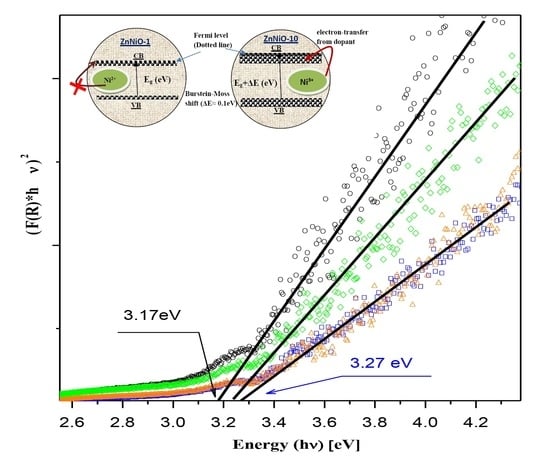
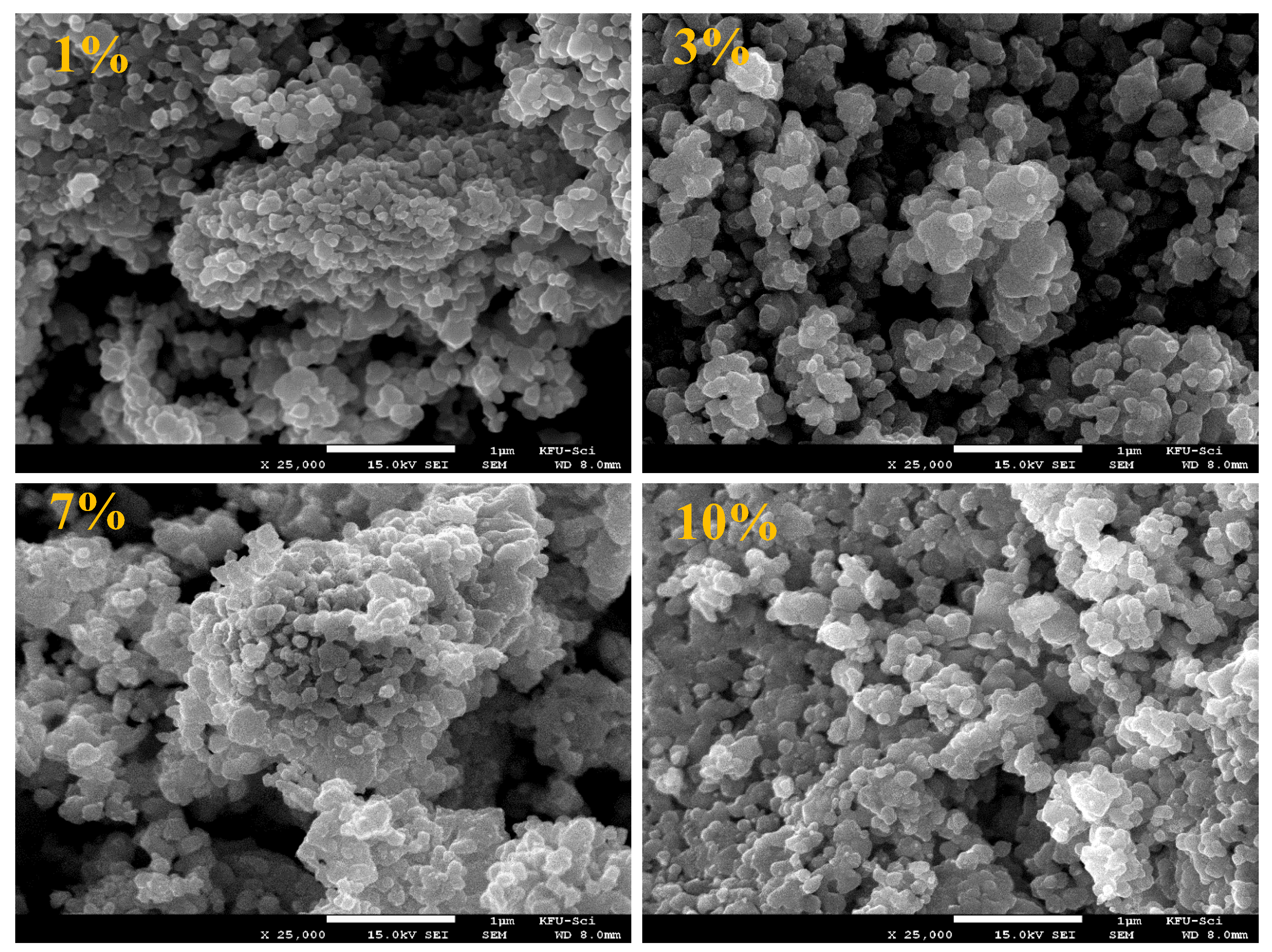
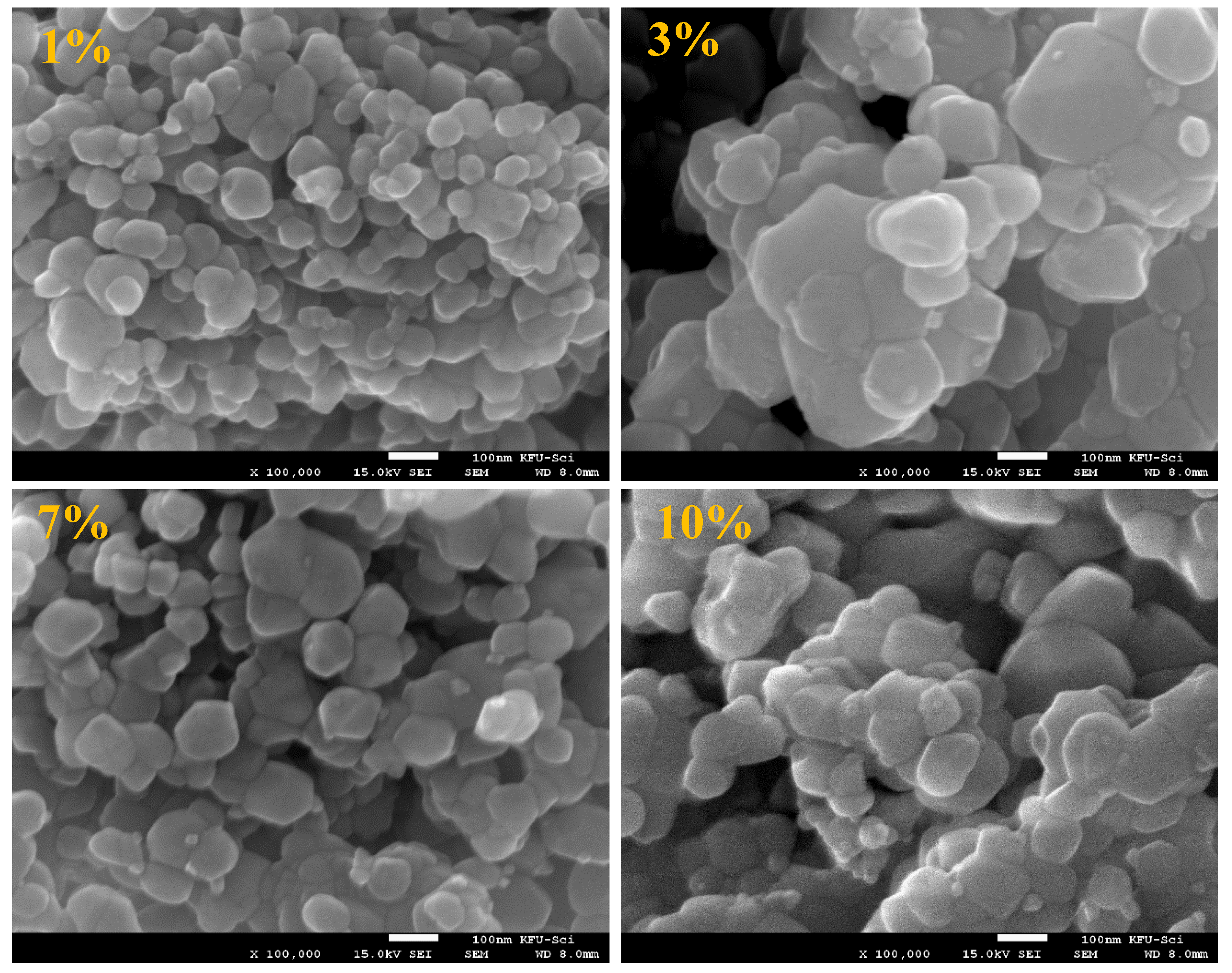
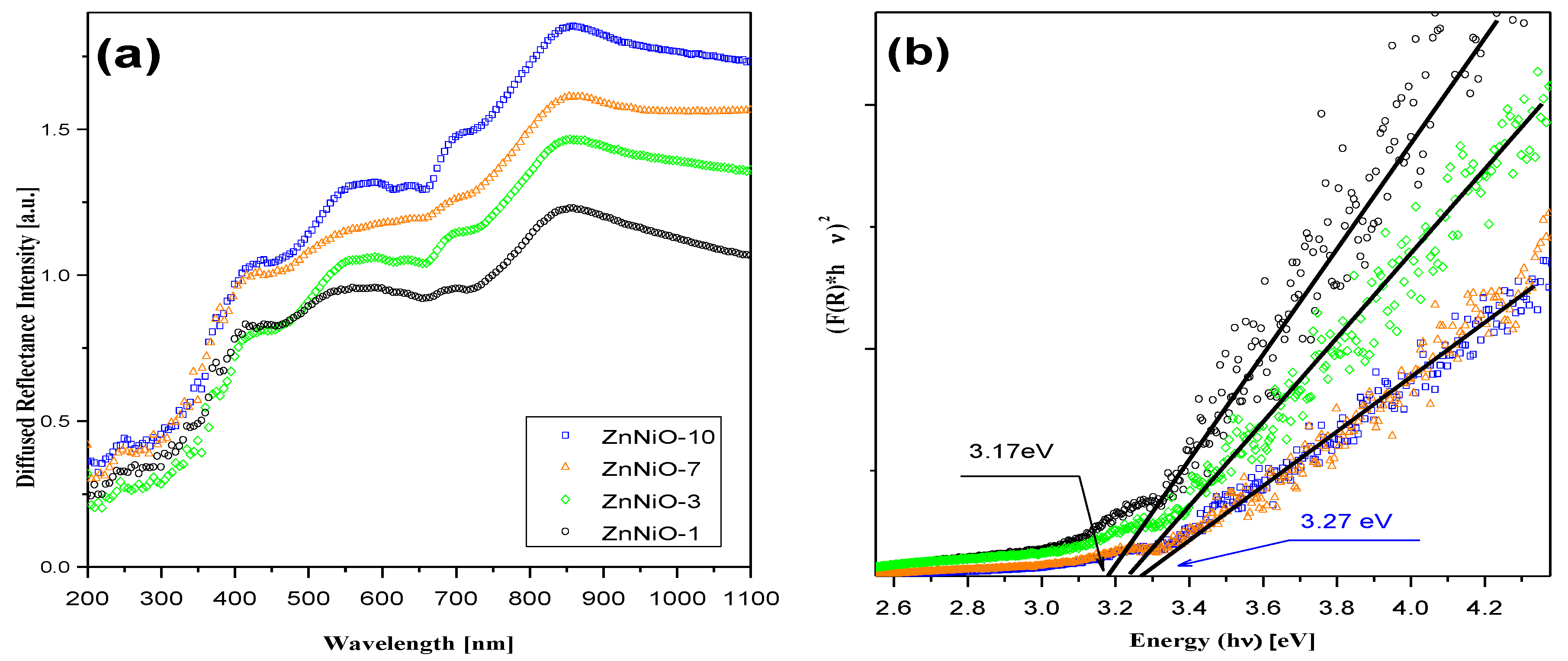
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anandan, S.; Muthukumaran, S. Microstructural, crystallographic and optical characterizations of Cu-doped ZnO nanoparticles co-doped with Ni. J. Mater. Sci. Mater. Electron. 2015, 26, 4298–4307. [Google Scholar] [CrossRef]
- Baruah, J.M.; Narayan, J. Dilute magnetic semiconducting quantum dots: Smart materials for spintronics. Nonmagn. Magn. Quantum Dots 2018, 87–199. [Google Scholar] [CrossRef]
- Li, J.; Haney, P.M. Optical spintronics in organic-inorganic perovskite photovoltaics. Phys. Rev. B 2016, 93, 155432. [Google Scholar] [CrossRef] [PubMed]
- Lalieu, M.L.M.; Lavrijsen, R.; Koopmans, B. Integrating all-optical switching with spintronics. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, R.R.; Zawadzki, W. Magnetoreflection and magnetophotoluminescence in the dilute magnetic semiconductor Zn 1− x Mn x Te. Phys. Rev. B 2018, 97, 214435. [Google Scholar]
- Pradhan, N.; Das Adhikari, S.; Nag, A.; Sarma, D.D. Luminescence, plasmonic, and magnetic properties of doped semiconductor nanocrystals. Angew. Chem. Int. Ed. Engl. 2017, 56, 7038–7054. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.J.; Cui, H.N.; Sun, S.N.; Wang, Z.H.; Wang, H.S. Native Defect Luminescence of Zinc Oxide Films and Its Potential Application as White Light Sources. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 1604–1614. [Google Scholar]
- Tong, L.N.; Cheng, T.; Han, H.B.; Hu, J.L.; He, X.M.; Tong, Y.; Schneider, C.M. Photoluminescence studies on structural defects and room temperature ferromagnetism in Ni and Ni-H doped ZnO nanoparticles. J. Appl. Phys. 2010, 108, 023906. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, W.C.; Yu, C.F.; Lin, C.F.; Kao, P.C. Influence of gallium-doped zinc-oxide thickness on polymer light-emitting diode luminescence efficiency. Microsc. Res. Tech. 2013, 76, 783. [Google Scholar] [CrossRef]
- Song, G.L. Luminescence characteristics of terbium-doped nanocrystalline zinc oxide. Guang Pu Xue Yu Guang Pu Fen Xi 2007, 27, 2409–2412. [Google Scholar]
- Gautam, U.K.; Imura, M.; Rout, C.S.; Bando, Y.; Fang, X.; Dierre, B.; Sakharov, L.; Govindaraj, A.; Sekiguchi, T.; Golberg, D.; et al. Unipolar assembly of zinc oxide rods manifesting polarity-driven collective luminescence. Proc. Natl. Acad. Sci. USA 2010, 107, 13588–13592. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Fashu, S. Effect of annealing on Ni-doped ZnO nanoparticles synthesized by the co-precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 10122–10130. [Google Scholar] [CrossRef]
- Xu, K.; Liu, C.; Chen, R.; Fang, X.X.; Wu, X.L.; Liu, J. Structural and room temperature ferromagnetic properties of Ni doped ZnO nanoparticles via low-temperature hydrothermal method. Phys. B Condens. Matter. 2016, 502, 155–159. [Google Scholar] [CrossRef]
- Mihalache, V.; Negrila, C.; Bercu, V.; Secu, M.; Vasile, E.; Stan, G. Effect of dilute doping and non-equilibrium synthesis on the structural, luminescent and magnetic properties of nanocrystalline Zn1-xNixO (x= 0.0025–0.03). Mater. Res. Bull. 2019, 115, 37–48. [Google Scholar] [CrossRef]
- Muller, S.; Zhou, M.; Li, Q.; Ronning, C. Intra-shell luminescence of transition-metal-implanted zinc oxide nanowires. Nanotechnology 2009, 20, 135704. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Chen, Z.; Kadasala, N.; Mao, C.; Wang, Y.; Zhang, Y.; Liu, H.; Liu, Y.; Yang, J.; et al. Effects of Ni concentration on structural, magnetic and optical properties of Ni-doped ZnO nanoparticles. J. Alloy. Compd. 2014, 604, 281–285. (In English) [Google Scholar] [CrossRef]
- Chacko, L.; Shafeeq, K.M.; Anjana, R.; Jayaraj, M.K.; Aneesh, P.M. Room temperature ferromagnetism in Zn1- x Ni x O nanostructures synthesized by chemical precipitation method. Mater. Res. Express 2017, 4, 105905. [Google Scholar] [CrossRef]
- Aydin, C.; El-sadek, M.S.A.; Zheng, K.B.; Yahia, I.S.; Yakuphanoglu, F. Synthesis, diffused reflectance and electrical properties of nanocrystalline Fe-doped ZnO via sol-gel calcination technique. Opt. Laser Technol. 2013, 48, 447–452. (In English) [Google Scholar] [CrossRef]
- Fabbiyola, S.; Sailaja, V.; Kennedy, L.J.; Bououdina, M.; Vijaya, J.J. Optical and magnetic properties of Ni-doped ZnO nanoparticles. J. Alloy. Compd. 2017, 694, 522–531. (In English) [Google Scholar] [CrossRef]
- Jlassi, M.; Sta, I.; Hajji, M.; Ezzaouia, H. Effect of nickel doping on physical properties of zinc oxide thin films prepared by the spray pyrolysis method. Appl. Surf. Sci. 2014, 301, 216–224. (In English) [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Sreedhar, A.; Shim, J.; Cho, M.; Yoo, K.; Kim, D. Structural, optical, and bifunctional applications: Supercapacitor and photoelectrochemical water splitting of Ni-doped ZnO nanostructures. J. Electroanal. Chem. 2018, 828, 124–136. (In English) [Google Scholar] [CrossRef]
- Rauwel, P.; Salumaa, M.; Aasna, A.; Galeckas, A.; Rauwel, E. A Review of the Synthesis and Photoluminescence Properties of Hybrid ZnO and Carbon Nanomaterials. J. Nanomater. 2016, 2016, 19. (In English) [Google Scholar] [CrossRef]
- Verma, K.C.; Bhatia, R.; Kumar, S.; Kotnala, R.K. Vacancies driven magnetic ordering in ZnO nanoparticles due to low concentrated Co ions. Mater. Res. Express 2016, 3, 076103. (In English) [Google Scholar] [CrossRef]
- Asokan, T.; Freer, R. Characterization of Spinel Particles in Zinc-Oxide Varistors. J. Mater. Sci. 1990, 25, 2447–2453. (In English) [Google Scholar] [CrossRef]
- Wu, M.S.; Chang, H.W. Self-Assembly of NiO-Coated ZnO Nanorod Electrodes with Core-Shell Nanostructures as Anode Materials for Rechargeable Lithium-Ion Batteries. J. Phys. Chem. C 2013, 117, 2590–2599. (In English) [Google Scholar] [CrossRef]
- Carnes, C.L.; Klabunde, K.J. The catalytic methanol synthesis over nanoparticle metal oxide catalysts. J. Mol. Catal. A Chem. 2003, 194, 227–236. (In English) [Google Scholar] [CrossRef]
- Hou, S.; Zhang, G.; Zeng, W.; Zhu, J.; Gong, F.; Li, F.; Duan, H. Hierarchical Core-Shell Structure of ZnO Nanorod@NiO/MoO2 Composite Nanosheet Arrays for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 13564–13570. (In English) [Google Scholar] [CrossRef]
- Mohapatra, J.; Mishra, D.K.; Kamilla, S.K.; Medicherla, V.R.R.; Phase, D.M.; Berma, V.; Singh, S.K. Ni-doped ZnO: Studies on structural and magnetic properties. Phys. Status Solidi B Basic Solid State Phys. 2011, 248, 1352–1359. (In English) [Google Scholar] [CrossRef]
- Saravanan, S.; Silambarasan, M.; Soga, T. Structural, morphological and optical studies of Ag-doped ZnO nanoparticles synthesized by simple solution combustion method. Jpn. J. Appl. Phys. 2014, 53, 11RF01. (In English) [Google Scholar] [CrossRef]
- Sharma, P.K.; Dutta, R.K.; Pandey, A.C. Effect of nickel doping concentration on structural and magnetic properties of ultrafine diluted magnetic semiconductor ZnO nanoparticles. J. Magn. Magn. Mater. 2009, 321, 3457–3461. [Google Scholar] [CrossRef]
- Du, C.L.; Gu, Z.B.; Lu, M.H.; Wang, J.; Zhang, S.T.; Zhao, J.; Cheng, G.X.; Heng, H.; Chen, Y.F. Raman spectroscopy of (Mn, Co)-codoped ZnO films. J. Appl. Phys. 2006, 99, 123515. (In English) [Google Scholar] [CrossRef]
- Wahab, H. Tuning the band gap, electronic polarizability and conduction mechanism of DyxZn1-xO nanostructures: The role of band tail states. Mater. Res. Express 2018, 6, 015034. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Bassi, A.L. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115, 073508. [Google Scholar] [CrossRef]
- Dey, R.; Bhunia, R.; Hussain, S.; Chakraborty, B.R.; Bhar, R.; Pal, A.K. Structural and Optical Studies on Sol-gel Composites of Nickel-Doped Nanocrystalline Zinc Oxide/Polyvinylidene Fluoride. Polym. Plast. Technol. Eng. 2017, 56, 310–320. (In English) [Google Scholar] [CrossRef]
- Dey, R.; Yadav, A.K.; Bhunia, R.; Jha, S.N.; Bhattacharyya, D.; Hussain, S.; Bhar, R.; Pal, A.K. Probing local structure of co doped polyvinylidene fluoride-ZnO thin films using X-ray absorption spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 131, 115–123. (In English) [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. (In English) [Google Scholar] [CrossRef]
- Lv, J.; Xu, J.; Zhao, M.; Sun, Y.; Jiang, Y.; He, G.; Zhang, M.; Sun, Z. Microstructure, surface morphology and optical properties of Na x Cu y Zn 1-x-y O thin films. J. Mater. Sci. Mater. Electron. 2016, 27, 4019–4025. [Google Scholar] [CrossRef]
- Awan, S.U.; Hasanain, S.K.; Mehmood, Z.; Anjum, D.H.; Shah, S.A.; Aftab, M.; Abbas, T.A. Study of room temperature Raman scattering and XPS, high temperature electrical and low temperature magnetic properties of Zn1-yLiyO (0.00 ≤ y ≤ 0.10) nanoparticles. Smart Mater. Struct. 2015, 24, 115025. [Google Scholar] [CrossRef]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: growth, doping & processing. Mater. Today 2004, 7, 34–40. [Google Scholar]
- Thota, S.; Dutta, T.; Kumar, J. On the Sol-Gel Synthesis and Thermal, Structural, and Magnetic Studies of Transition Metal (Ni, Co, Mn) Containing ZnO Powders. J. Phys. Condens. Matter. 2006, 18, 2473. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Mazher, J.; Shrivastav, A.K.; Nandedkar, R.V.; Pandey, R.K. Strained ZnSe nanostructures investigated by x-ray diffraction, atomic force microscopy, transmission electron microscopy and optical absorption and luminescence spectroscopy. Nanotechnology 2004, 15, 572–580. (In English) [Google Scholar] [CrossRef]
- Chen, J.L.; Devi, N.; Li, N.; Fu, D.J.; Ke, X.W. Synthesis of Pr-doped ZnO nanoparticles: Their structural, optical, and photocatalytic properties. Chin. Phys. B 2018, 27, 086102. (In English) [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Jenkins, F.A.; White, H.E. Fundamentals of optics. Am. J. Phys. 1958, 26, 272. [Google Scholar] [CrossRef]
- Lopez, R.; Gomez, R. Band-gap energy estimation from diffuse reflectance measurements on sol--gel and commercial TiO 2: A comparative study. J. Sol-Gel. Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Thien, N.D.; Tu, N.N.; Viet, N.N.; Phuong, N.D.; Long, N.N. Hydrothermal Synthesis and Optical Properties of Undoped and Eu3+-doped Zinc Stannate Nanocrystals. Commun. Phys. 2015, 25, 327. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 2009, 80, 046107. (In English) [Google Scholar] [CrossRef]
- Awan, S.U.; Hasanain, S.; Jaffari, G.H.; Anjum, D.H.; Qurashi, U.S. Defects induced luminescence and tuning of bandgap energy narrowing in ZnO nanoparticles doped with Li ions. J. Appl. Phys. 2014, 116, 083510. [Google Scholar] [CrossRef]
- Srinet, G.; Kumar, R.; Sajal, V. Structural, optical, vibrational, and magnetic properties of sol-gel derived Ni doped ZnO nanoparticles. J. Appl. Phys. 2013, 114, 033912. [Google Scholar] [CrossRef]
- Rajeh, S.; Barhoumi, A.; Mhamdi, A.; Leroy, G.; Duponchel, B.; Amlouk, M.; Guermazi, S. Structural, morphological, optical and opto-thermal properties of Ni-doped ZnO thin films using spray pyrolysis chemical technique. Bull. Mater. Sci. 2016, 39, 177–186. [Google Scholar] [CrossRef]
- Rana, A.K.; Kumar, Y.; S, A.M.; Adarsh, K.; Sen, S.; Shirage, P.M. Enhancement of two photon absorption with Ni doping in the dilute magnetic semiconductor ZnO crystalline nanorods. Appl. Phys. Lett. 2015, 107, 231907. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Kroumova, E.; Aroyo, M.I.; Perez-Mato, J.M.; Kirov, A.; Capillas, C.; Ivantchev, S.; Wondratschek, H. Bilbao crystallographic server: Useful databases and tools for phase-transition studies. Phase Transit. 2003, 76, 155–170. (In English) [Google Scholar] [CrossRef]
- Perry, N.H.; Bishop, S.R.; Tuller, H.L. Tailoring chemical expansion by controlling charge localization: In situ X-ray diffraction and dilatometric study of (La, Sr)(Ga, Ni) O 3- δ perovskite. J. Mater. Chem. A 2014, 2, 18906–18916. [Google Scholar] [CrossRef]
- Lyddane, R.H.; Sachs, R.G.; Teller, E. On the polar vibrations of alkali halides. Phys. Rev. 1941, 59, 673. [Google Scholar] [CrossRef]
- Lu, Q.H.; Huang, R.; Wang, L.S.; Wu, Z.G.; Li, C.; Luo, Q.; Zuo, S.Y.; Li, J.; Peng, D.L.; Han, G.L.; et al. Thermal annealing and magnetic anisotropy of NiFe thin films on n(+)-Si for spintronic device applications. J. Magn. Magn. Mater. 2015, 394, 253–259. (In English) [Google Scholar] [CrossRef]


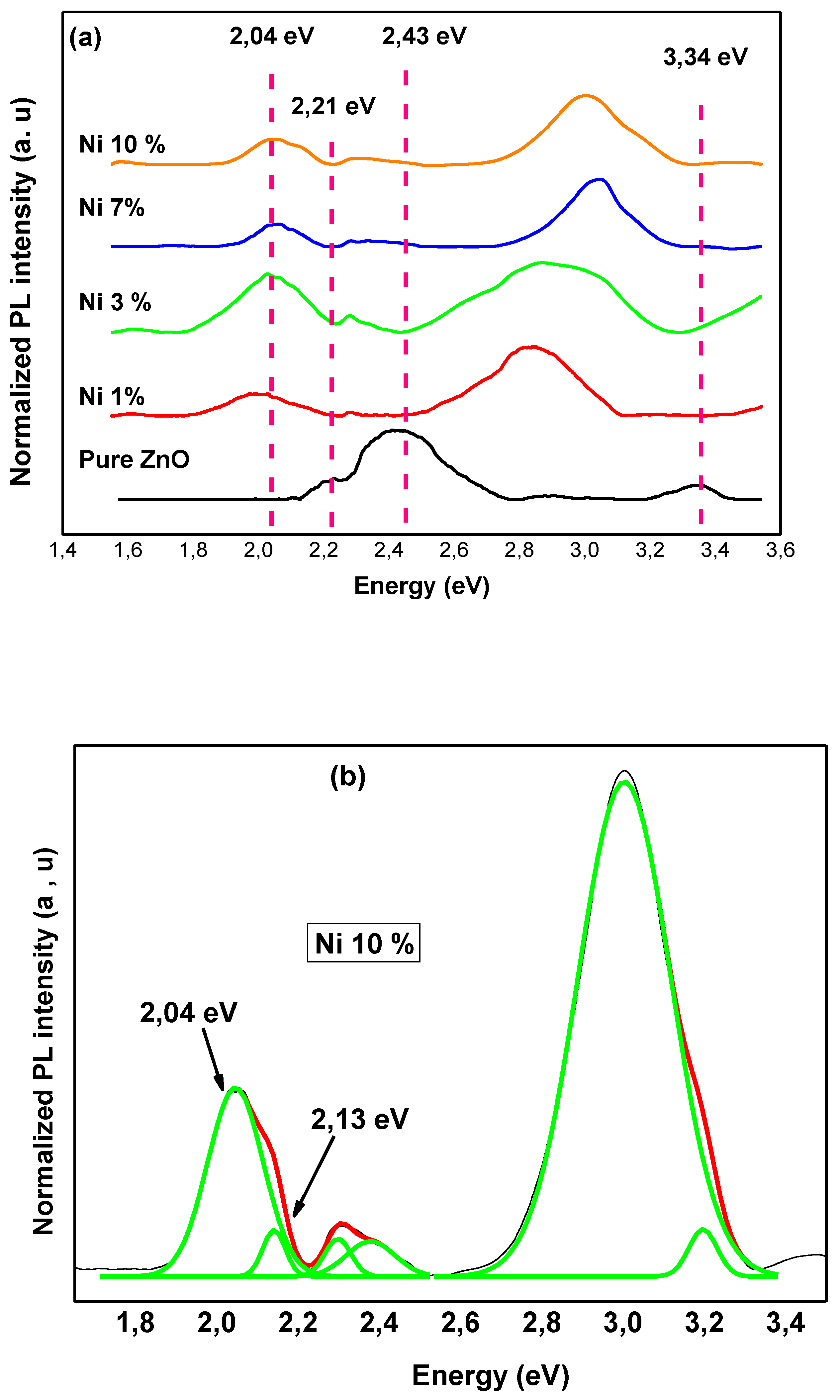

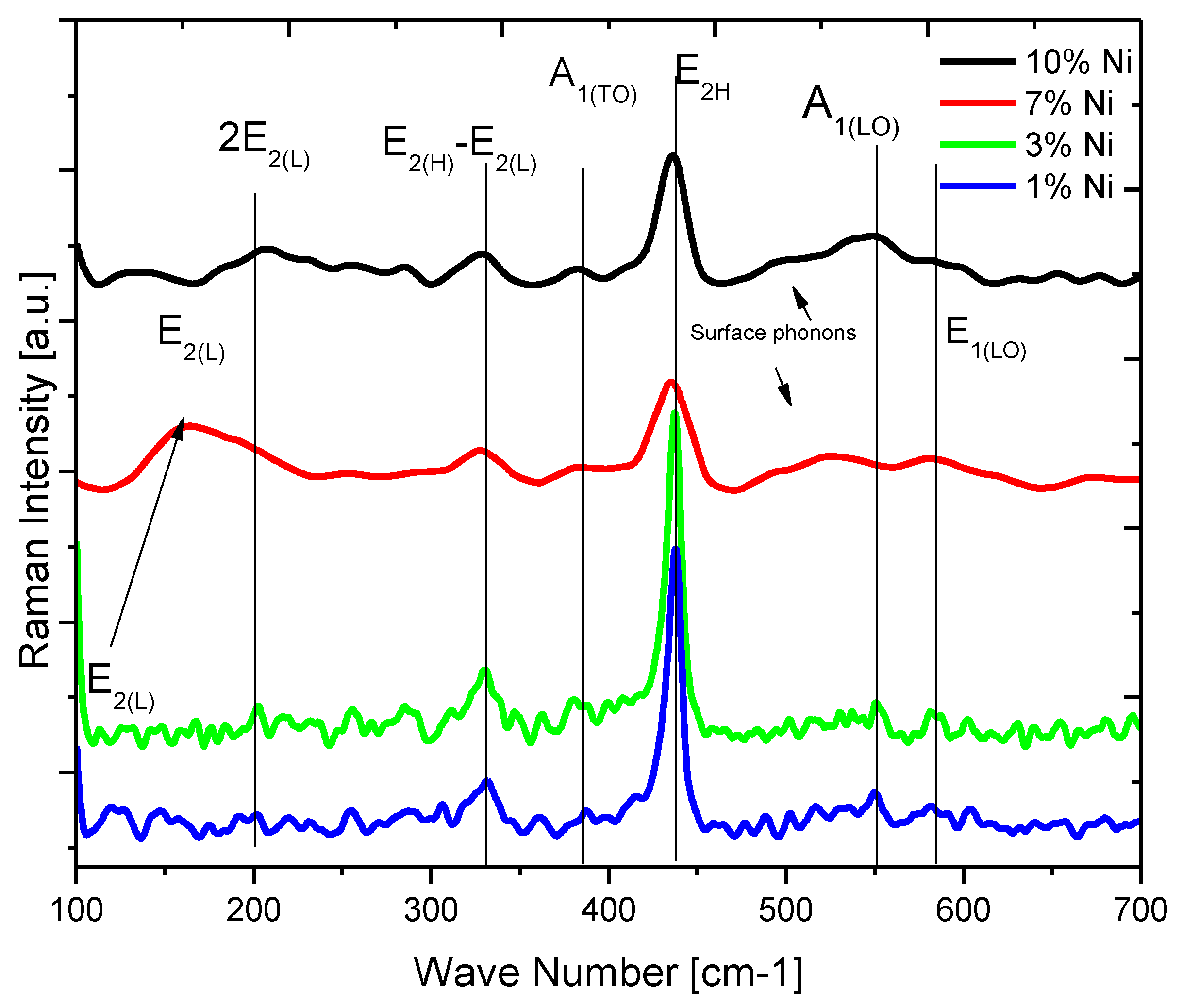
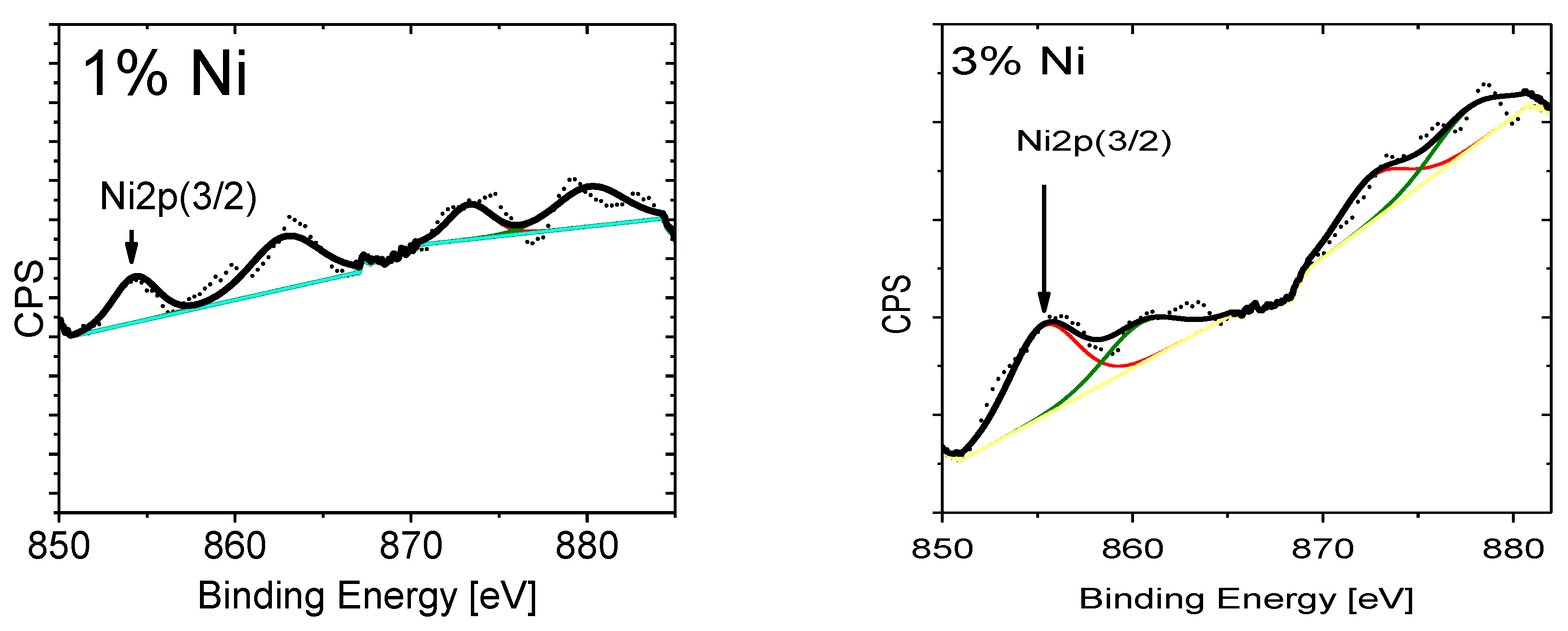
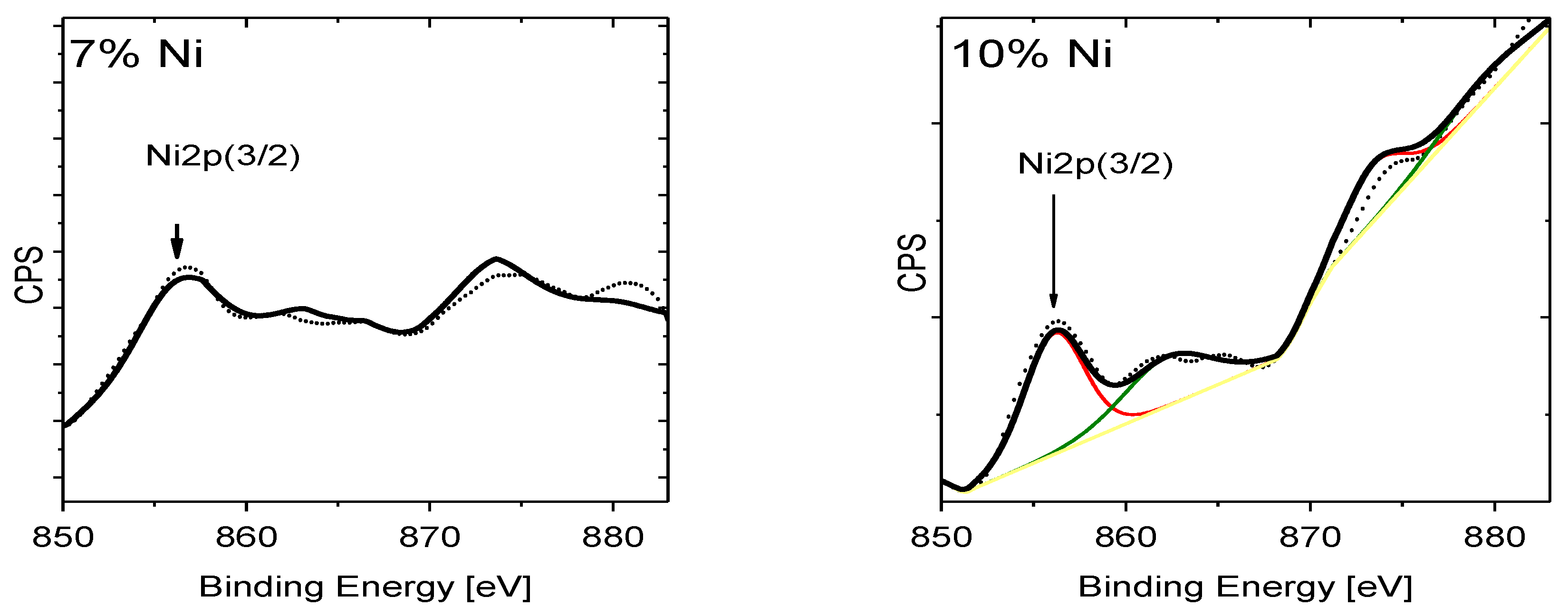
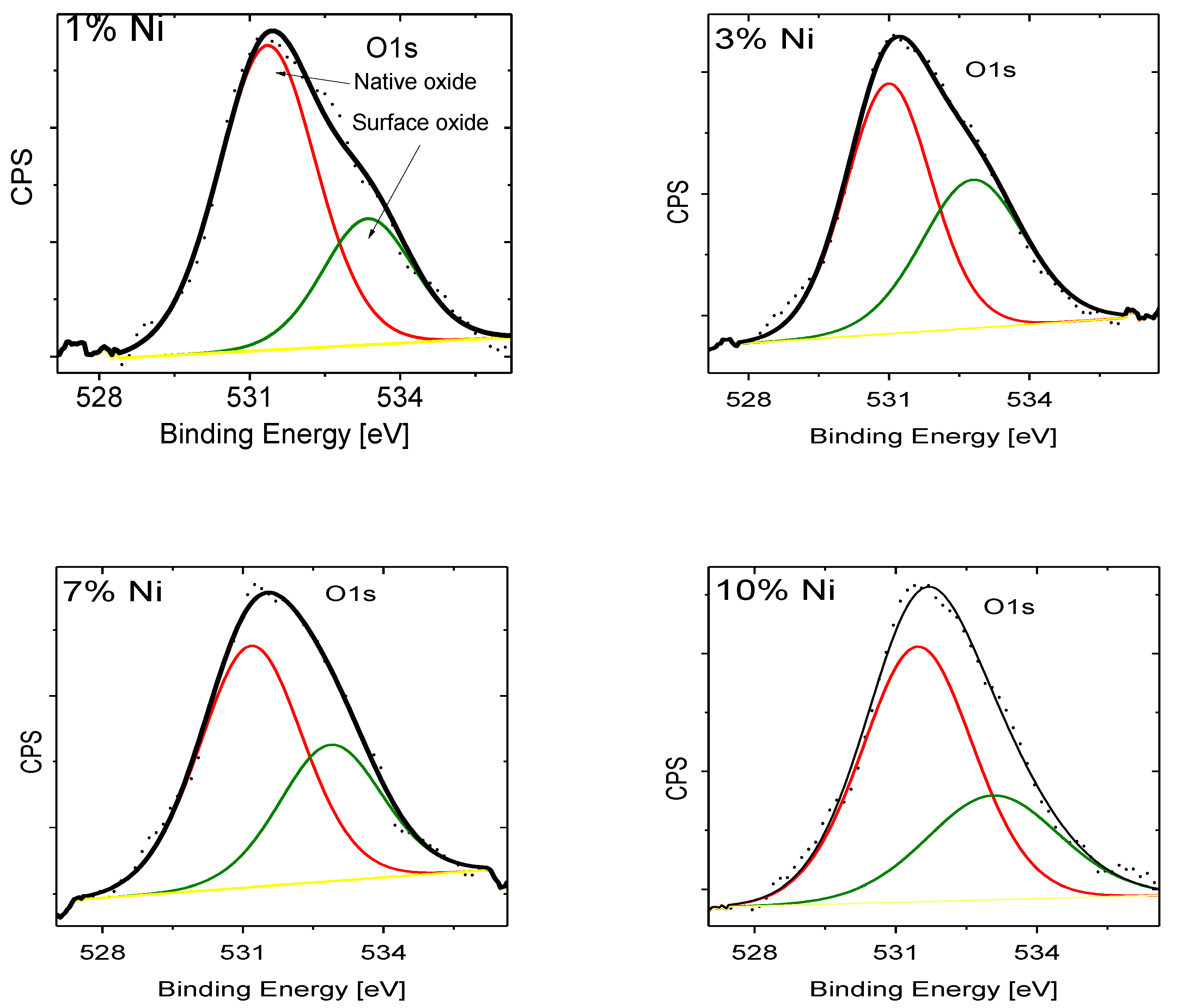
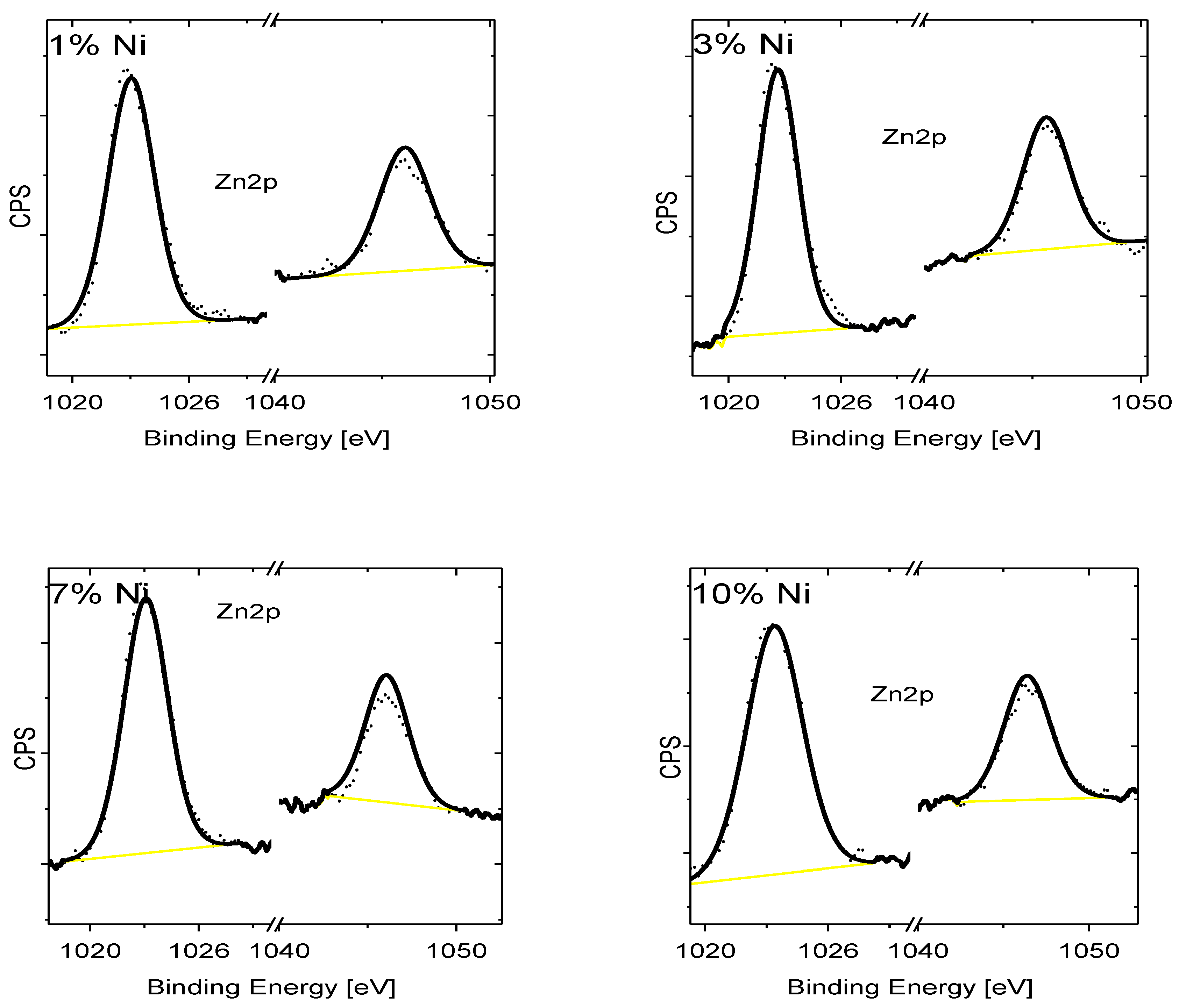
| Doping-Percent | D, nm | a, Å | c, Å | c/a | Strain | V, Å3 |
|---|---|---|---|---|---|---|
| 1% | 29.8 ± 1.57 | 3.23 ± 0.01 | 5.22 ± 0.01 | 1.600 ± 0.01 | 0.19 | 47.99 |
| 3% | 32.2 ± 2.41 | 3.23 ± 0.01 | 5.21 ± 0.01 | 1.597 ± 0.01 | 0.01 | 47.88 |
| 7% | 18.1 ± 1.37 | 3.28 ± 0.02 | 5.22 ± 0.02 | 1.594 ± 0.01 | 0.36 | 48.57 |
| 10% | 14.1 ± 0.80 | 3.30 ± 0.02 | 5.24 ± 0.04 | 1.586 ± 0.02 | 0.68 | 49.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farha, A.H.; Al Naim, A.F.; Mazher, J.; Nasr, O.; Alouane, M.H.H. Structural and Optical Characteristics of Highly UV-Blue Luminescent ZnNiO Nanoparticles Prepared by Sol–Gel Method. Materials 2020, 13, 879. https://doi.org/10.3390/ma13040879
Farha AH, Al Naim AF, Mazher J, Nasr O, Alouane MHH. Structural and Optical Characteristics of Highly UV-Blue Luminescent ZnNiO Nanoparticles Prepared by Sol–Gel Method. Materials. 2020; 13(4):879. https://doi.org/10.3390/ma13040879
Chicago/Turabian StyleFarha, Ashraf H., Abdullah F. Al Naim, Javed Mazher, Olfa Nasr, and Mohamed Helmi Hadj Alouane. 2020. "Structural and Optical Characteristics of Highly UV-Blue Luminescent ZnNiO Nanoparticles Prepared by Sol–Gel Method" Materials 13, no. 4: 879. https://doi.org/10.3390/ma13040879
APA StyleFarha, A. H., Al Naim, A. F., Mazher, J., Nasr, O., & Alouane, M. H. H. (2020). Structural and Optical Characteristics of Highly UV-Blue Luminescent ZnNiO Nanoparticles Prepared by Sol–Gel Method. Materials, 13(4), 879. https://doi.org/10.3390/ma13040879