Lanthanum and Manganese Co-Doping Effects on Structural, Morphological, and Magnetic Properties of Sol-Gel Derived BiFeO3
Abstract
:1. Introduction
2. Materials and Methods
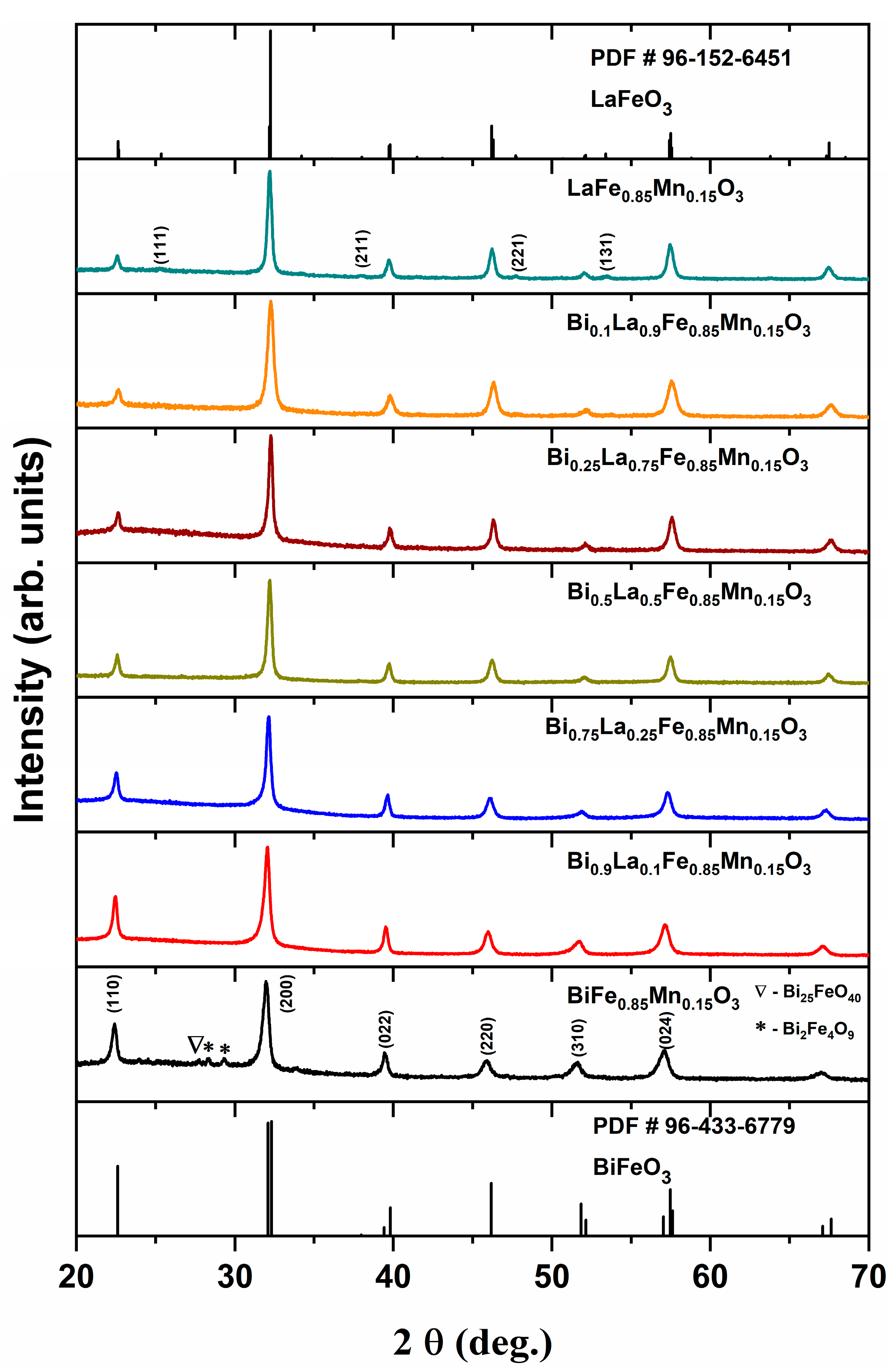
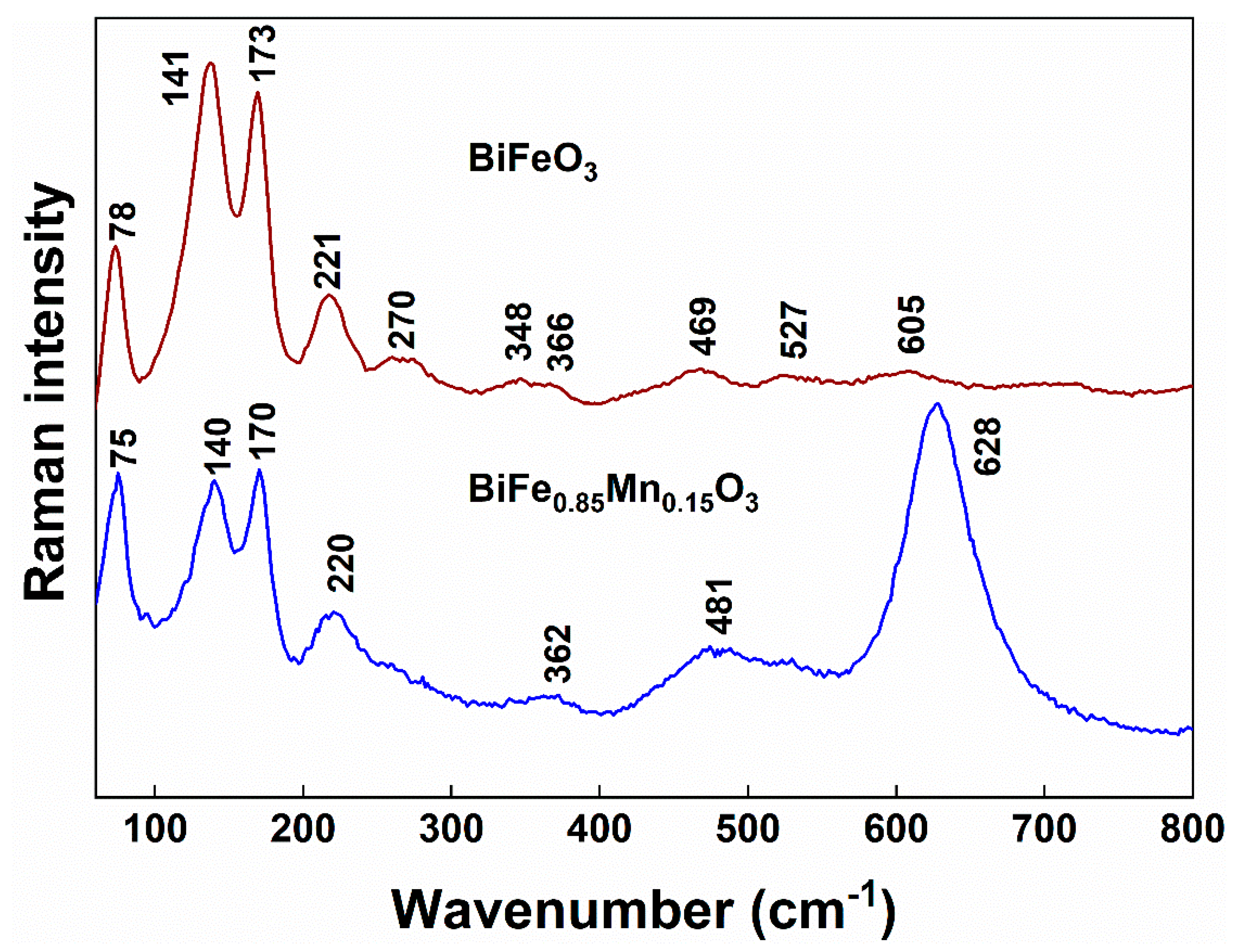
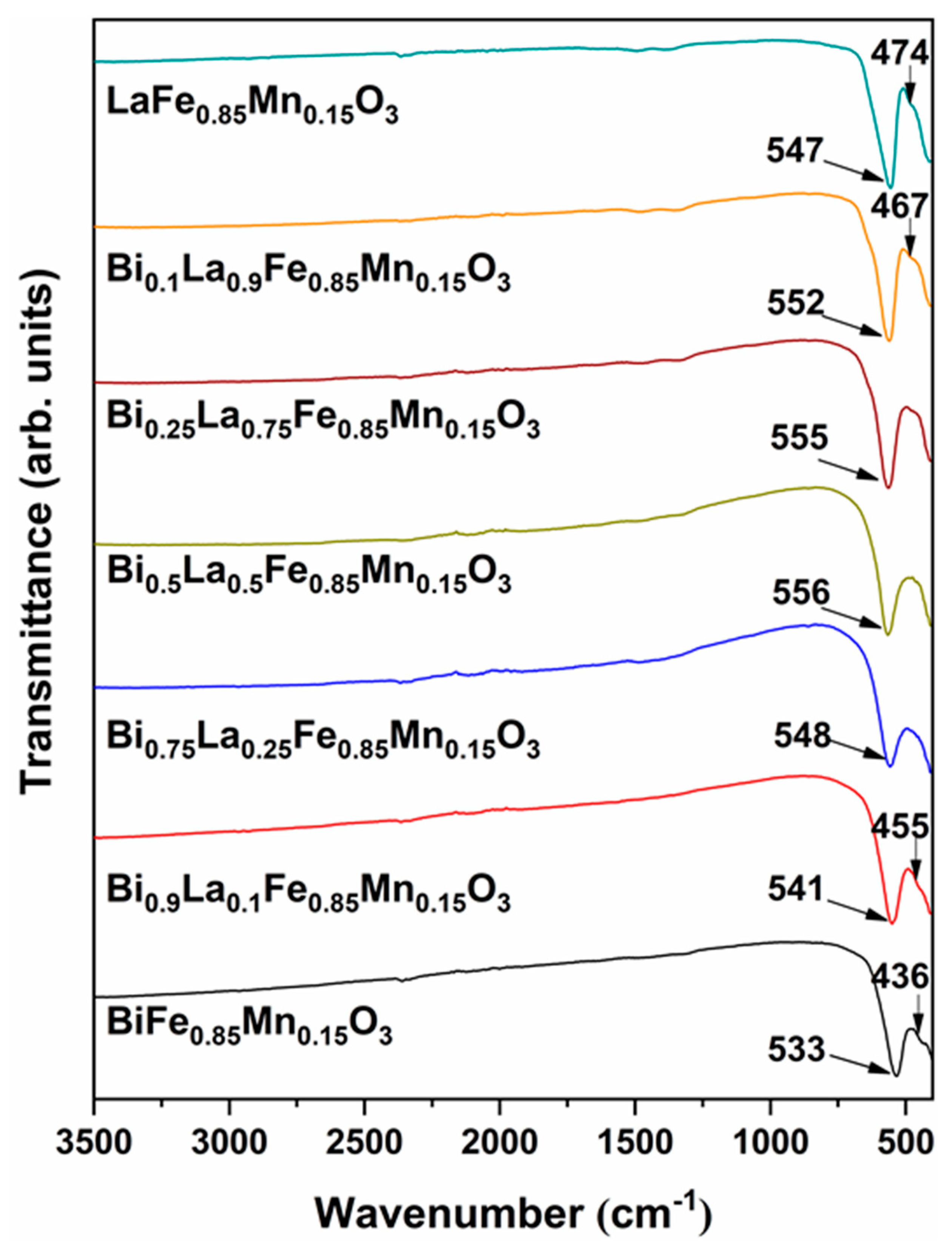
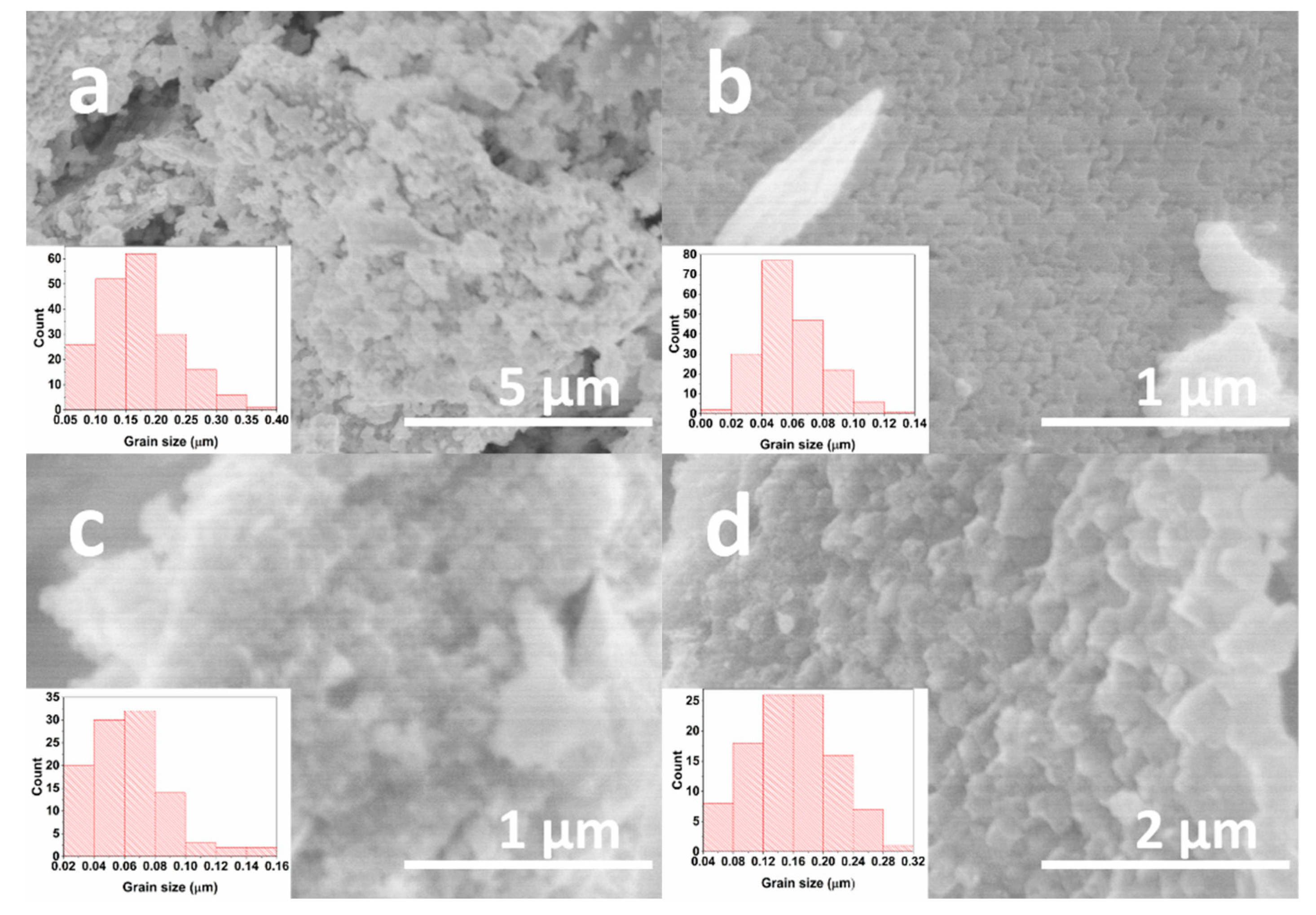
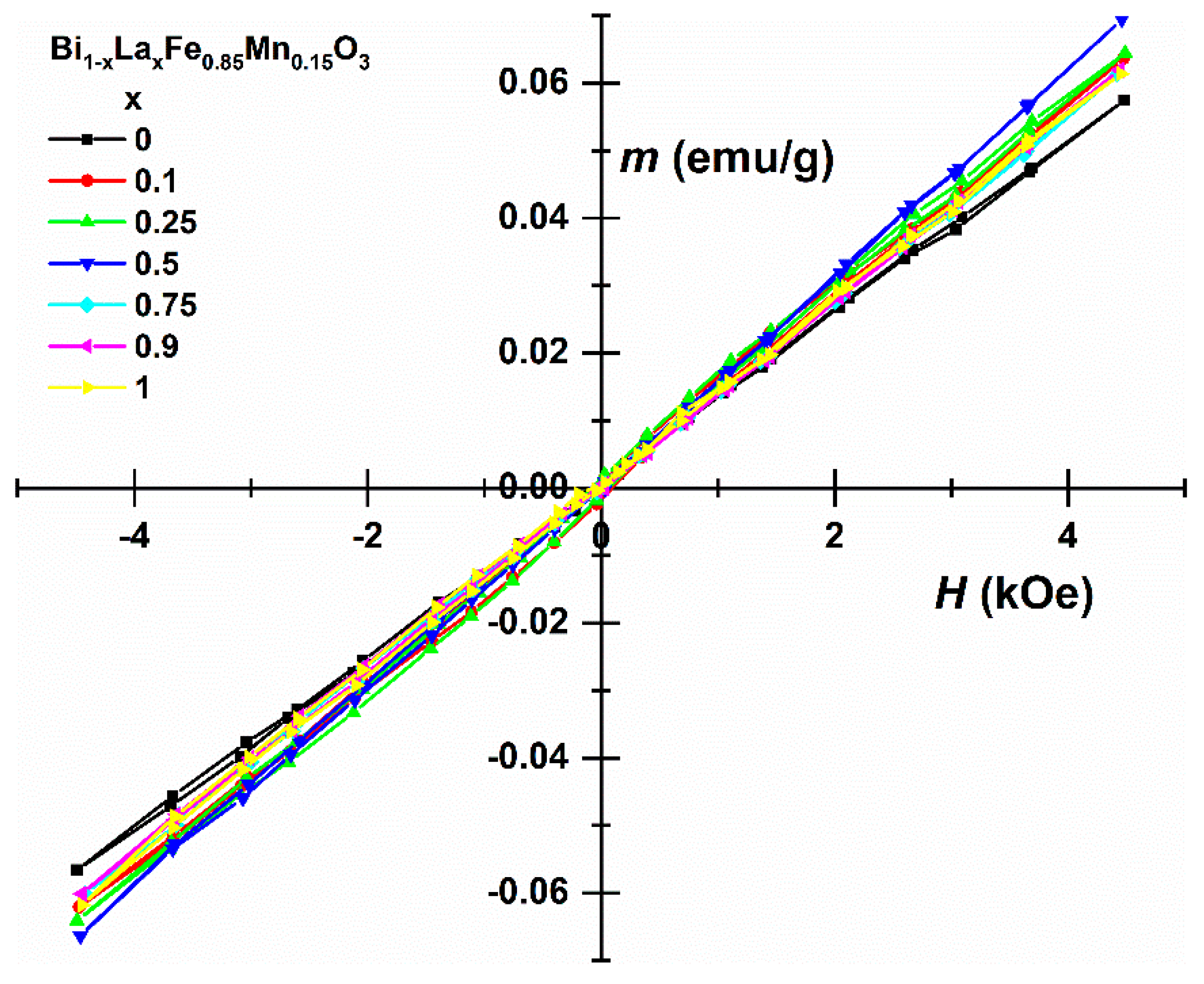
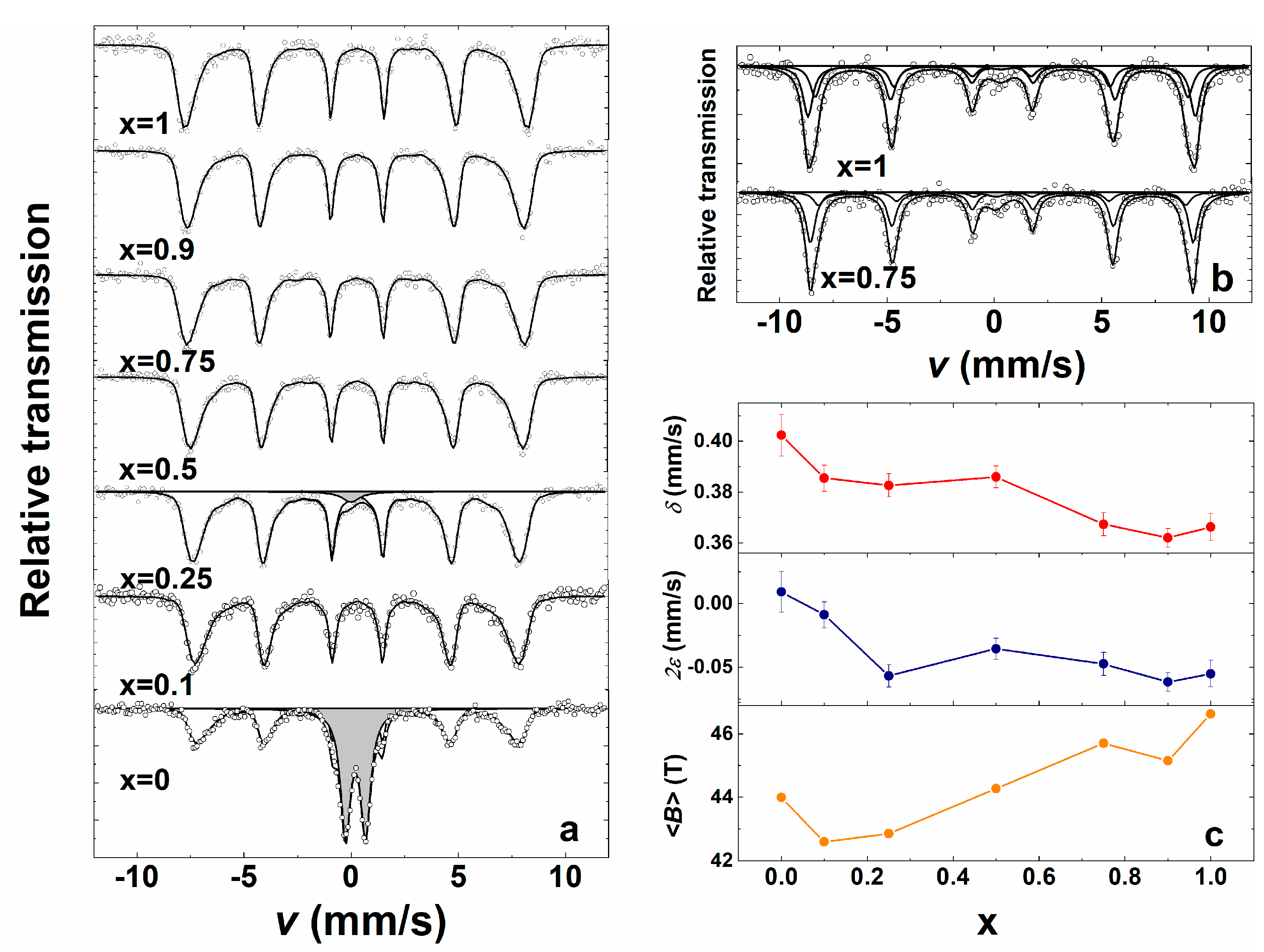
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, N.A. Why are there so few magnetic ferroelectrics? J. Phys. Chem. B 2000, 104, 6694–6709. [Google Scholar] [CrossRef]
- Manipatruni, S.; Nikonov, D.E.; Lin, C.C.; Gosavi, T.A.; Liu, H.; Prasad, B.; Huang, Y.L.; Bonturim, E.; Ramesh, R.; Young, I.A. Scalable energy-efficient magnetoelectric spin–orbit logic. Nature 2019, 565, 35–42. [Google Scholar] [CrossRef]
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Effect of (Nd, Ni) co-doped on the multiferroic and photocatalytic properties of BiFeO3. Mater. Res. Bull. 2015, 72, 299–305. [Google Scholar] [CrossRef]
- Guduru, R.; Liang, P.; Runowicz, C.; Nair, M.; Atluri, V.; Khizroev, S. Magneto-electric nanoparticles to enable field-controlled high-specificity drug delivery to eradicate ovarian cancer cells. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Ustinov, A.B.; Srinivasan, G.; Kalinikos, B.A. Ferrite-ferroelectric hybrid wave phase shifters. Appl. Phys. Lett. 2007, 90, 31913. [Google Scholar] [CrossRef]
- Khomskii, D. Trend: Classifying multiferroics: Mechanisms and effects. Physics 2009, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U. V Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.C.; Xu, J.H.; Ke, H.; Wang, W.; Zhou, Y. Structure and multiferroic properties of BiFeO3 powders. J. Eur. Ceram. Soc. 2009, 29, 3099–3103. [Google Scholar] [CrossRef]
- Shokrollahi, H. Magnetic, electrical and structural characterization of BiFeO3 nanoparticles synthesized by co-precipitation. Powder Technol. 2013, 235, 953–958. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Liu, J. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Acharya, S.; Mondal, J.; Ghosh, S.; Roy, S.K.; Chakrabarti, P.K. Multiferroic behavior of lanthanum orthoferrite (LaFeO3). Mater. Lett. 2010, 64, 415–418. [Google Scholar] [CrossRef]
- Theingi, M.; Tun, K.T.; Aung, N.N. Preparation, characterization and optical property of LaFeO3 nanoparticles via sol-gel combustion method. SciMedicine J. 2019, 1, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Sazelee, N.A.; Idris, N.H.; Din, M.F.M.; Yahya, M.S.; Ali, N.A.; Ismail, M. LaFeO3 synthesised by solid-state method for enhanced sorption properties of MgH2. Results Phys. 2020, 16, 102844. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, R.; Peng, D.; Meng, G. Hydrothermal synthesis of LaFeO3 under carbonate-containing medium. Mater. Lett. 2000, 43, 19–22. [Google Scholar] [CrossRef]
- Thuy, N.T.; Minh, D. Le Size effect on the structural and magnetic properties of nanosized perovskite LaFeO3 prepared by different methods. Adv. Mater. Sci. Eng. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Wu, J.M.; Lai, C.H. Influence of La doping in multiferroic properties of BiFeO3 thin films. Appl. Phys. Lett. 2006, 88, 42903. [Google Scholar] [CrossRef]
- Pandit, P.; Satapathy, S.; Gupta, P.K. Effect of La substitution on conductivity and dielectric properties of Bi1− xLaxFeO3 ceramics: An impedance spectroscopy analysis. Phys. B Condens. Matter. 2011, 406, 2669–2677. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.; Qi, Y.; Cheng, J.; Meng, Z. Hydrothermal synthesis and characterization of multiferroic Bi1−xLaxFeO3 crystallites. J. Eur. Ceram. Soc. 2010, 30, 265–269. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.D.; Zhu, L.; Wang, Y.G. Structural transition and its effect on magnetoelectric coupling in the BiFe1− xMnxO3 ceramics prepared by sol–gel method. J. Magn. Magn. Mater. 2018, 465, 784–788. [Google Scholar] [CrossRef]
- Pálová, L.; Chandra, P.; Rabe, K.M. Magnetostructural Effect in the Multiferroic BiFeO3− BiMnO3 Checkerboard from First Principles. Phys. Rev. Lett. 2010, 104, 37202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diliautas, R.; Beganskiene, A.; Karoblis, D.; Mazeika, K.; Baltrunas, D.; Zarkov, A.; Raudonis, R.; Kareiva, A. Reinspection of formation of BiFe1-xMnxO3 solid solutions via low temperature sol-gel synthesis route. Solid State Sci. 2021, 111, 106458. [Google Scholar] [CrossRef]
- Kolte, J.; Daryapurkar, A.S.; Agarwal, M.; Gulwade, D.D.; Gopalan, P. Effect of substrate temperature on the structural and electrical properties of La and Mn co-doped BiFeO3 thin films. Thin Solid Films 2016, 619, 308–316. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Ali, S.I.; Amin, F.; Tariq, A.; Iqbal, M.Z.; Rizwan, S. La-and Mn-codoped Bismuth Ferrite/Ti3C2 MXene composites for efficient photocatalytic degradation of Congo Red dye. ACS Omega 2019, 4, 8661–8668. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kar, M. Effect of structural transition on magnetic and dielectric properties of La and Mn co-substituted BiFeO3 ceramics. Mater. Chem. Phys. 2014, 148, 968–977. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H.; Chiu, K.C.; Jean, J.H. Preparation and electrical properties of LaFeO3 compacts using chemically synthesized powders. Jpn. J. Appl. Phys. 2008, 47, 8498. [Google Scholar] [CrossRef]
- Köferstein, R.; Ebbinghaus, S.G. Synthesis and characterization of nano-LaFeO3 powders by a soft-chemistry method and corresponding ceramics. Solid State Ionics 2013, 231, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Xu, Y. Synthesis of nanocrystalline LaFeO3 powders via glucose sol–gel route. Mater. Chem. Phys. 2011, 129, 1047–1050. [Google Scholar] [CrossRef]
- Awasthi, R.R.; Asokan, K.; Das, B. Structural, dielectric and magnetic domains properties of Mn-doped BiFeO3 materials. Int. J. Appl. Ceram. Technol. 2020, 17, 1410–1421. [Google Scholar] [CrossRef]
- Selbach, S.M.; Einarsrud, M.A.; Grande, T. On the thermodynamic stability of BiFeO3. Chem. Mater. 2009, 21, 169–173. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, M.; Chhoker, S.; Katyal, S.C.; Singh, H.; Jewariya, M.; Yadav, K.L. Multiferroic, magnetoelectric and optical properties of Mn doped BiFeO3 nanoparticles. Solid State Commun. 2012, 152, 525–529. [Google Scholar] [CrossRef]
- Gholam, T.; Ablat, A.; Mamat, M.; Wu, R.; Aimidula, A.; Bake, M.A.; Zheng, L.; Wang, J.; Qian, H.; Wu, R. An experimental study of the local electronic structure of B-site gallium doped bismuth ferrite powders. Phys. Lett. A 2017, 381, 2367–2373. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Ibrahim, I.; Salama, T.M.; Elseman, A.M.; Mohamed, M.M. Structural, optical, dielectric and magnetic properties of Bi1− xLaxFeO3 nanoparticles. J. Magn. Magn. Mater. 2018, 465, 309–315. [Google Scholar] [CrossRef]
- Tang, P.; Kuang, D.; Yang, S.; Zhang, Y. Hydrothermal synthesis and the structural, morphologic and magnetic characteristics of Mn doped bismuth ferrite crystallites. J. Mater. Sci. Mater. Electron. 2016, 27, 2594–2600. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Pakalniškis, A.; Niaura, G.; Zhaludkevich, D.V.; Zhaludkevich, A.L.; Latushka, S.I.; Silibin, M.; Serdechnova, M.; Garamus, V.M.; Lukowiak, A. Evolution of the crystal structure and magnetic properties of Sm-doped BiFeO3 ceramics across the phase boundary region. Ceram. Int. 2021, 47, 5399–5406. [Google Scholar] [CrossRef]
- Ahlawat, A.; Satapathy, S.; Sathe, V.G.; Choudhary, R.J.; Singh, M.K.; Kumar, R.; Sharma, T.K.; Gupta, P.K. Modification in structure of La and Nd co-doped epitaxial BiFeO3 thin films probed by micro Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 636–643. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.Y.; Zhu, K.; Liu, Y.L.; Wan, L. Structure properties of BiFeO3 films studied by micro-Raman scattering. J. Appl. Phys. 2008, 103, 93532. [Google Scholar] [CrossRef]
- Hlinka, J.; Pokorny, J.; Karimi, S.; Reaney, I.M. Angular dispersion of oblique phonon modes in BiFeO3 from micro-Raman scattering. Phys. Rev. B 2011, 83, 20101. [Google Scholar] [CrossRef]
- Sharma, P.; Satapathy, S.; Varshney, D.; Gupta, P.K. Effect of sintering temperature on structure and multiferroic properties of Bi0. 825Sm0. 175FeO3 ceramics. Mater. Chem. Phys. 2015, 162, 469–476. [Google Scholar] [CrossRef]
- Thang, D.V.; Hung, N.M.; Khang, N.C.; Oanh, L.T.M. Structural and multiferroic properties of (Sm, Mn) co-doped BiFeO3 materials. AIMS Mater. Sci. 2020, 7, 160–169. [Google Scholar] [CrossRef]
- Yuan, G.L.; Or, S.W.; Chan, H.L.W. Raman scattering spectra and ferroelectric properties of Bi1−xNdxFeO3 (x= 0–0.2) multiferroic ceramics. J. Appl. Phys. 2007, 101, 64101. [Google Scholar] [CrossRef]
- Pandey, R.; Pradhan, L.K.; Kumar, P.; Kar, M. Double crystal symmetries and magnetic orderings in co-substituted (Y and Mn) bismuth ferrite. Ceram. Int. 2018, 44, 18609–18616. [Google Scholar] [CrossRef]
- Martín-Carrón, L.; De Andres, A.; Martínez-Lope, M.J.; Casais, M.T.; Alonso, J.A. Raman phonons as a probe of disorder, fluctuations, and local structure in doped and undoped orthorhombic and rhombohedral manganites. Phys. Rev. B 2002, 66, 174303. [Google Scholar] [CrossRef] [Green Version]
- Rao, G.S.; Rao, C.N.R.; Ferraro, J.R. Infrared and Electronic Spectra of Rare Earth Perovskites. Ortho- Chromites, -Manganites and—Ferrites. Appl. Spectrosc. 1970, 24, 436–444. [Google Scholar] [CrossRef]
- Karoblis, D.; Mazeika, K.; Baltrunas, D.; Lukowiak, A.; Strek, W.; Zarkov, A.; Kareiva, A. Novel synthetic approach to the preparation of single-phase BixLa1−xMnO3+δ solid solutions. J. Sol-Gel Sci. Technol. 2020, 93, 650–656. [Google Scholar] [CrossRef]
- Lebeugle, D.; Colson, D.; Forget, A.; Viret, M.; Bonville, P.; Marucco, J.-F.; Fusil, S. Room-temperature coexistence of large electric polarization and magnetic order in BiFeO3 single crystals. Phys. Rev. B 2007, 76, 24116. [Google Scholar] [CrossRef] [Green Version]
- Karoblis, D.; Griesiute, D.; Mazeika, K.; Baltrunas, D.; Karpinsky, D.V.; Lukowiak, A.; Gluchowski, P.; Raudonis, R.; Katelnikovas, A.; Zarkov, A. A Facile Synthesis and Characterization of Highly Crystalline Submicro-Sized BiFeO3. Materials 2020, 13, 3035. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Z.; Lu, X.; Zhang, J.; Min, K.; Lin, W.; Ti, R.; Xu, T.; He, J.; Yue, C. Peculiar magnetism of BiFeO3 nanoparticles with size approaching the period of the spiral spin structure. Sci. Rep. 2013, 3, 2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.Y.; Yun, H.J.; Yoon, J.W. Characterization and magnetic properties of LaFeO3 nanofibers synthesized by electrospinning. J. Alloys Compd. 2014, 583, 320–324. [Google Scholar] [CrossRef]
- Sendil Kumar, A.; Manivel Raja, M.; Bhatnagar, A.K. Surface driven effects on magnetic properties of antiferromagnetic LaFeO3 nanocrystalline ferrite. J. Appl. Phys. 2014, 116, 113912. [Google Scholar] [CrossRef]
- Papaefthymiou, G.C.; Viescas, A.J.; Le Breton, J.M.; Chiron, H.; Juraszek, J.; Park, T.J.; Wong, S.S. Magnetic and Mössbauer characterization of the magnetic properties of single-crystalline sub-micron sized Bi2Fe4O9 cubes. Curr. Appl. Phys. 2015, 15, 417–422. [Google Scholar] [CrossRef] [Green Version]

| Formula | D, nm |
|---|---|
| BiFe0.85Mn0.15O3 | 19.18 |
| Bi0.9La0.1Fe0.85Mn0.15O3 | 20.39 |
| Bi0.75La0.25Fe0.85Mn0.15O3 | 25.18 |
| Bi0.5La0.5Fe0.85Mn0.15O3 | 28.72 |
| Bi0.25La0.75Fe0.85Mn0.15O3 | 29.39 |
| Bi0.1La0.9Fe0.85Mn0.15O3 | 19.60 |
| LaFe0.85Mn0.15O3 | 29.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoblis, D.; Diliautas, R.; Mazeika, K.; Baltrunas, D.; Niaura, G.; Talaikis, M.; Beganskiene, A.; Zarkov, A.; Kareiva, A. Lanthanum and Manganese Co-Doping Effects on Structural, Morphological, and Magnetic Properties of Sol-Gel Derived BiFeO3. Materials 2021, 14, 4844. https://doi.org/10.3390/ma14174844
Karoblis D, Diliautas R, Mazeika K, Baltrunas D, Niaura G, Talaikis M, Beganskiene A, Zarkov A, Kareiva A. Lanthanum and Manganese Co-Doping Effects on Structural, Morphological, and Magnetic Properties of Sol-Gel Derived BiFeO3. Materials. 2021; 14(17):4844. https://doi.org/10.3390/ma14174844
Chicago/Turabian StyleKaroblis, Dovydas, Ramunas Diliautas, Kestutis Mazeika, Dalis Baltrunas, Gediminas Niaura, Martynas Talaikis, Aldona Beganskiene, Aleksej Zarkov, and Aivaras Kareiva. 2021. "Lanthanum and Manganese Co-Doping Effects on Structural, Morphological, and Magnetic Properties of Sol-Gel Derived BiFeO3" Materials 14, no. 17: 4844. https://doi.org/10.3390/ma14174844
APA StyleKaroblis, D., Diliautas, R., Mazeika, K., Baltrunas, D., Niaura, G., Talaikis, M., Beganskiene, A., Zarkov, A., & Kareiva, A. (2021). Lanthanum and Manganese Co-Doping Effects on Structural, Morphological, and Magnetic Properties of Sol-Gel Derived BiFeO3. Materials, 14(17), 4844. https://doi.org/10.3390/ma14174844









