The Effect of Chitosan’s Addition to Resorcinol/Formaldehyde Xerogels on the Characteristics of Resultant Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbonization and Activation Processes
2.3. Characterization
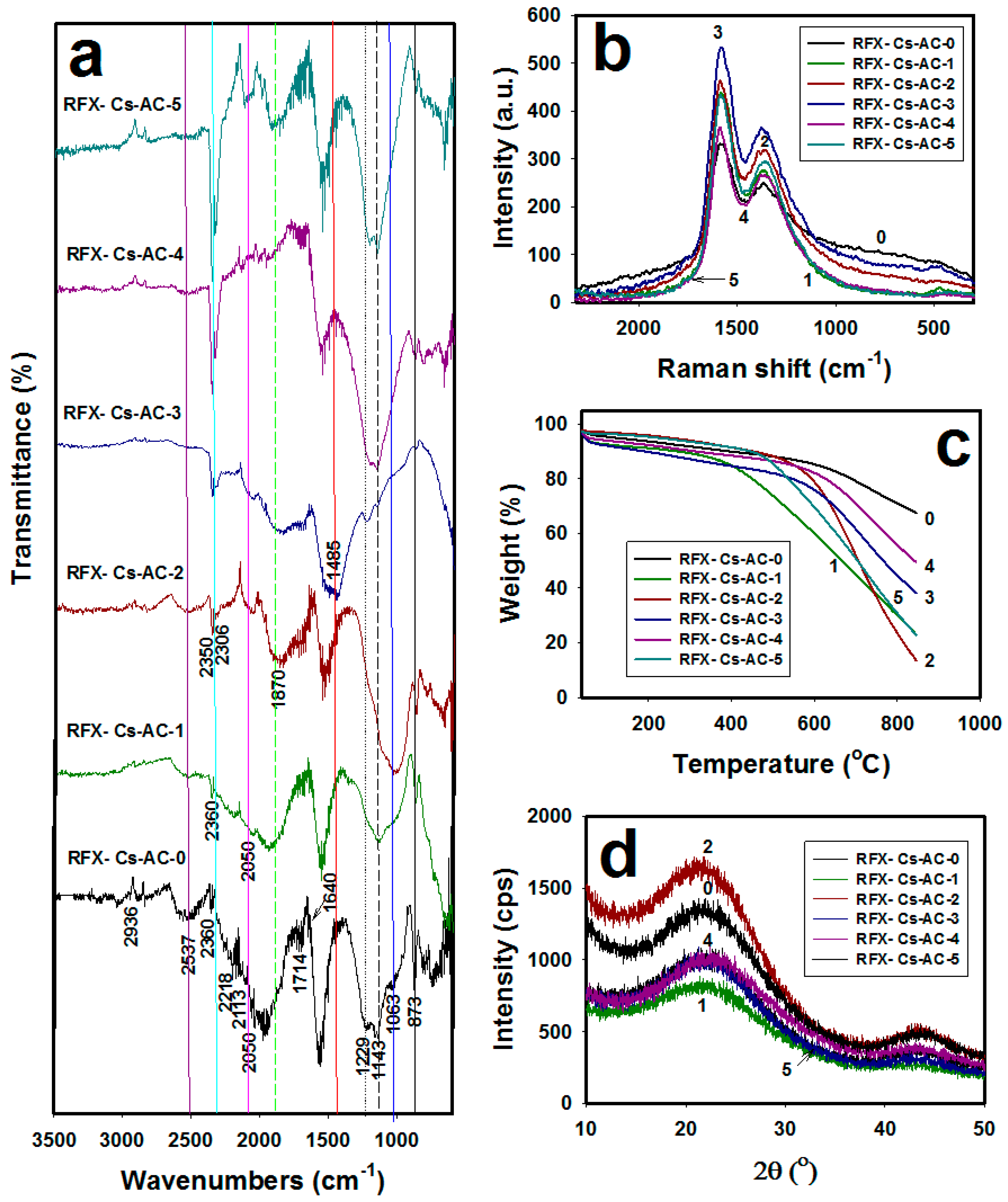
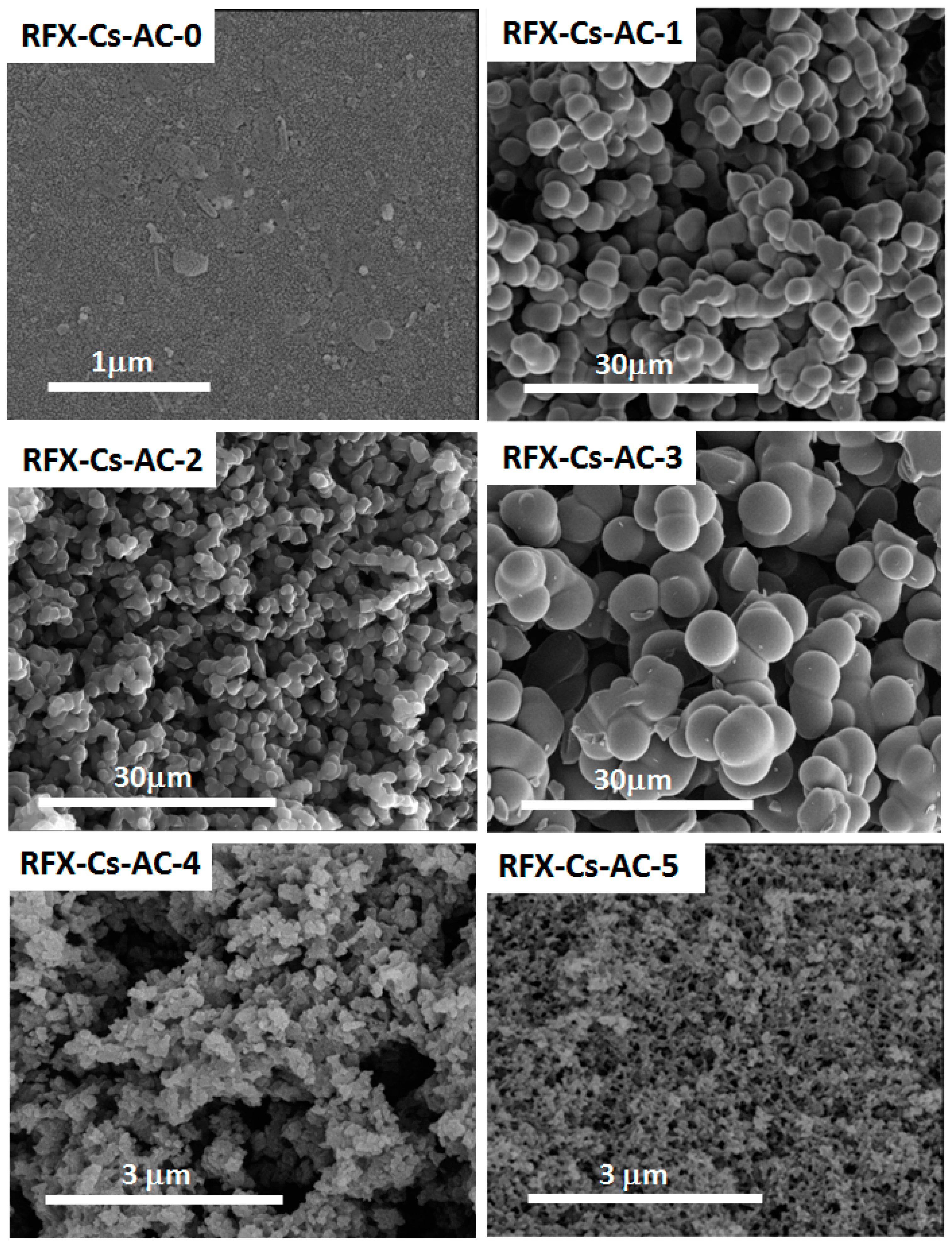
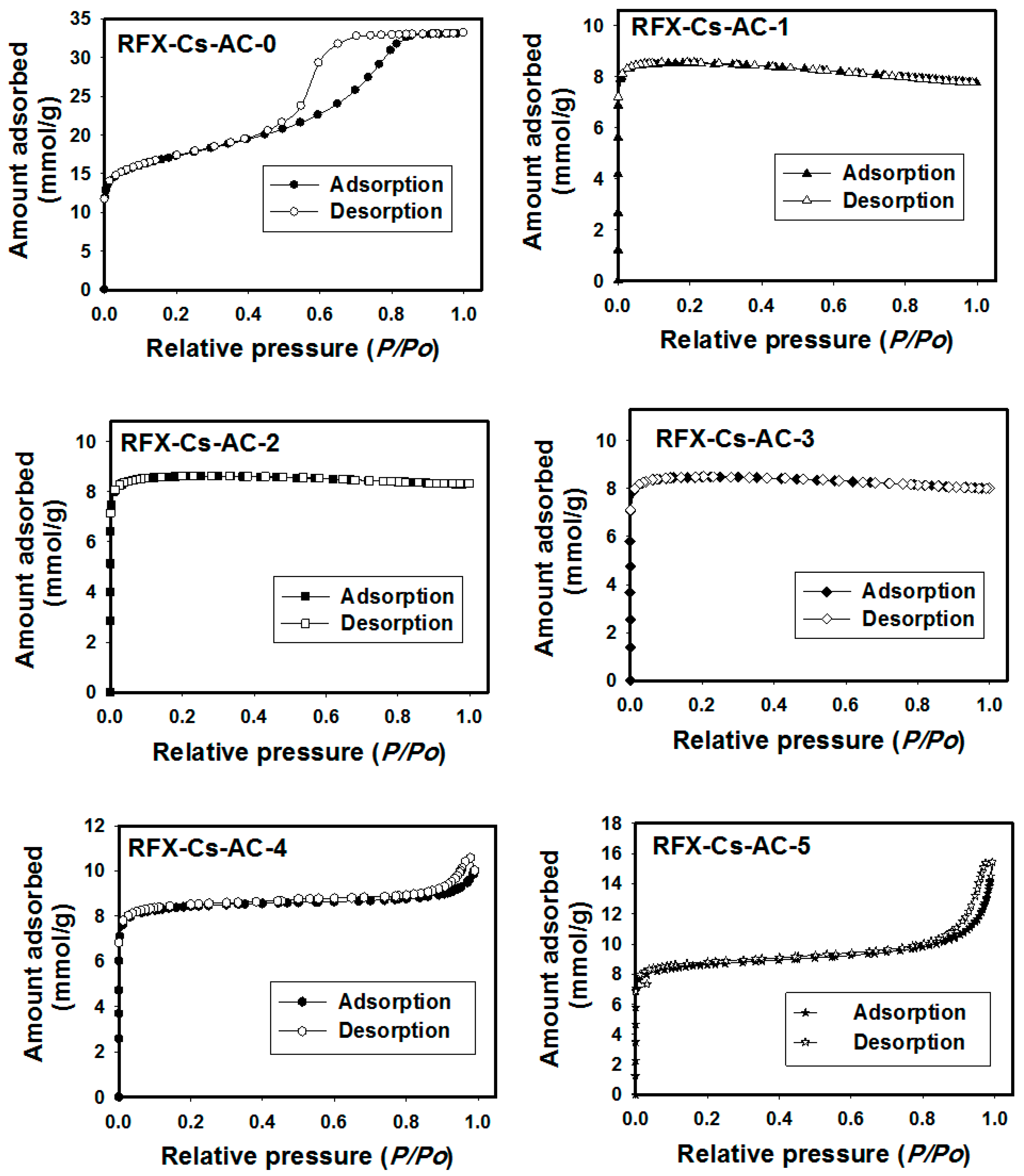
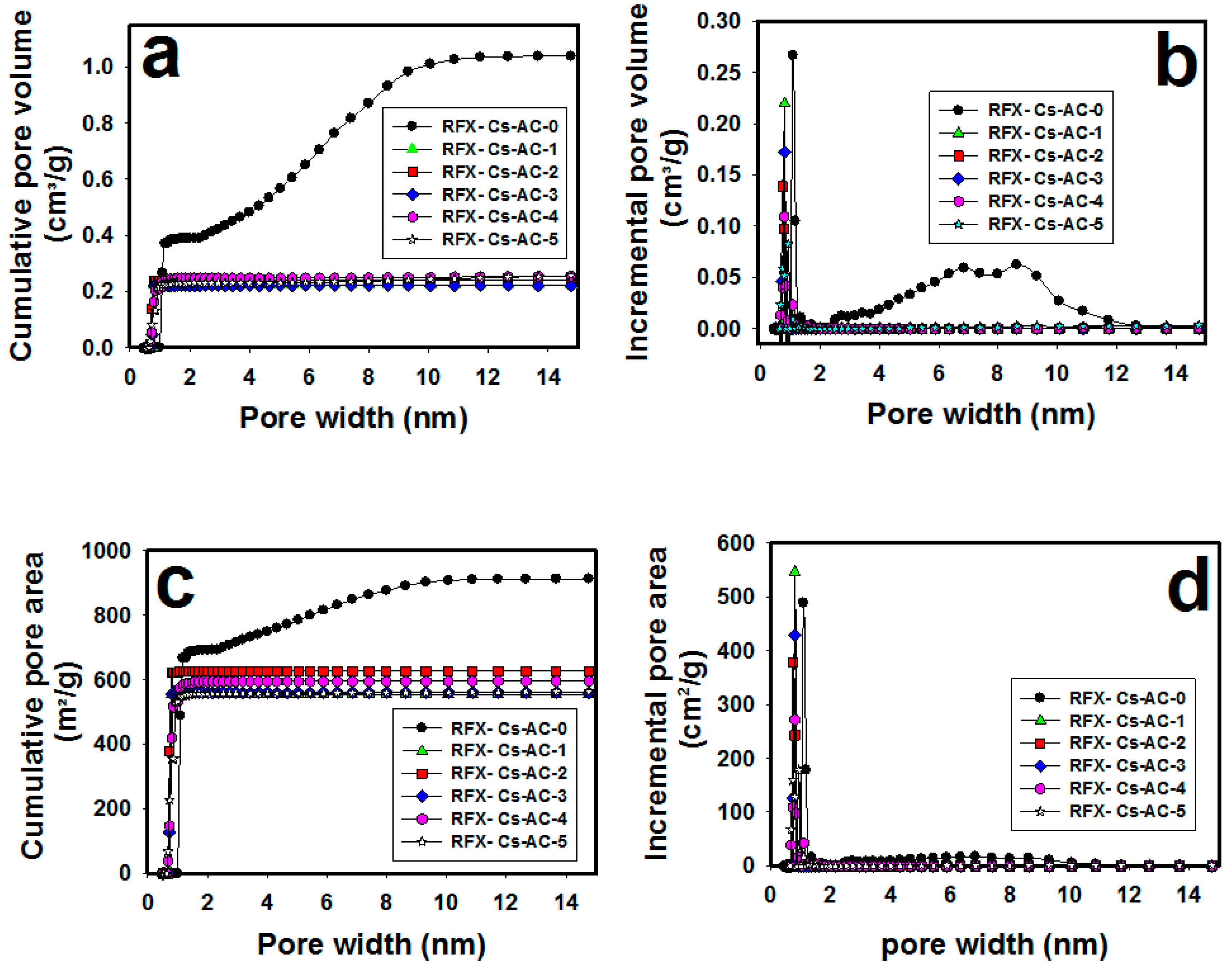
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Zhang, C.; Yang, Z.; Qin, Q.; Zhang, Z.; Wang, X.; Shen, J. Preparation of Carbon Aerogel Electrode for Electrosorption of Copper Ions in Aqueous Solution. Materials 2019, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, D.; Yang, X.; Wang, Y.; Guo, Z.; Xia, Y.; Sun, S.; Guo, S. Double-Helix Structure in Carrageenan-Metal Hydrogels: A General Approach to Porous Metal Sulfides/Carbon Aerogels with Excellent Sodium-Ion Storage. Angew. Chem. Int. Ed. 2016, 55, 15925–15928. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Wang, Z.; Zhang, H.; Hong, F.; Yan, X.; Su, X. Novel synthesis of nitrogen-containing bio-phenol resin and its molten aalt activation of porous carbon for supercapacitor electrode. Materials 2019, 12, 1986. [Google Scholar] [CrossRef]
- Querejeta, N.; Plaza, M.G.; Rubiera, F.; Pevida, C. Water Vapor Adsorption on Biomass Based Carbons under Post-Combustion CO2 Capture Conditions: Effect of Post-Treatment. Materials 2016, 9, 359. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Yao, J. Recent advances in the direct fabrication of millimeter-sized hierarchical porous materials. RSC Adv. 2016, 6, 80840–80846. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, K.-B.; Park, S.-M.; Roh, K.C. Hierarchically structured activated carbon for ultracapacitors. Sci. Rep. 2016, 6, 21182. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liao, Y.; Li, X.; Li, W. Sulfur loaded in micropore-rich carbon aerogel as cathode of lithium-sulfur battery with improved cyclic stability. J. Power Sources 2016, 334, 23–30. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, L.; Wei, X. A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, Y.; Tu, X.; Zhang, Z.; Du, G. Well-dispersed LiFePO 4 nanoparticles anchored on a three-dimensional graphene aerogel as high-performance positive electrode materials for lithium-ion batteries. J. Power Sources 2017, 340, 40–50. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Mei, J.; Lau, W.-M.; Mi, R.; Li, Y.; Liu, H.; Liu, L.-M. From melamine–resorcinol–formaldehyde to nitrogen-doped carbon xerogels with micro- and meso-pores for lithium batteries. J. Mater. Chem. A 2014, 2, 14429–14438. [Google Scholar] [CrossRef]
- Jia, X.; Dai, B.; Zhu, Z.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Strong and machinable carbon aerogel monoliths with low thermal conductivity prepared via ambient pressure drying. Carbon 2016, 108, 551–560. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Luchnikov, V.A. Hierarchical porous nitrogen-doped carbon beads derived from biosourced chitosan polymer. Microporous Mesoporous Mater. 2018, 263, 42–52. [Google Scholar] [CrossRef]
- Wróbel-Iwaniec, I.; Díez, N.; Gryglewicz, G. Chitosan-based highly activated carbons for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 5788–5796. [Google Scholar] [CrossRef]
- Fujiki, J.; Yogo, K. The increased CO 2 adsorption performance of chitosan-derived activated carbons with nitrogen-doping. Chem. Commun. 2016, 52, 186–189. [Google Scholar] [CrossRef]
- Śliwak, A.; Díez, N.; Miniach, E.; Gryglewicz, G. Nitrogen-containing chitosan-based carbon as an electrode material for high-performance supercapacitors. J. Appl. Electrochem. 2016, 46, 667–677. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, C.; Hu, Z.; Liu, K. Synthesis of Se/chitosan-derived hierarchical porous carbon composite as Li–Se battery cathode. Funct. Mater. Lett. 2017, 10, 1650074. [Google Scholar] [CrossRef]
- Prashanth, K.H.; Tharanathan, R. Chitin/chitosan: Modifications and their unlimited application potential—an overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat. Commun. 2015, 6, 8503. [Google Scholar] [CrossRef]
- Chen, L.-F.; Zhang, X.-D.; Liang, H.-W.; Kong, M.; Guan, Q.-F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Bakierska, M.; Świętosławski, M.; Gajewska, M.; Kowalczyk, A.; Piwowarska, Z.; Chmielarz, L.; Dziembaj, R.; Molenda, M. Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S. Materials 2016, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Awadallah-F, A.; Al-Muhtaseb, S.A. Influence of Chitosan Addition on Resorcinol–Formaldehyde Xerogel Structure. Appl. Sci. 2019, 9, 4582. [Google Scholar] [CrossRef]
- Awadallah-F, A.; Al-Muhtaseb, S.A. Nanofeatures of resorcinol–formaldehyde carbon microspheres. Mater. Lett. 2012, 87, 31–34. [Google Scholar] [CrossRef]
- Hebalkar, N.; Arabale, G.; Sainkar, S.R.; Pradhan, S.D.; Mulla, I.S.; Vijayamohanan, K.; Ayyub, P.; Kulkarni, S.K. Study of correlation of structural and surface properties with electrochemical behaviour in carbon aerogels. J. Mater. Sci. 2005, 40, 3777–3782. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry, 3rd ed.; Harcourt College Publishers: Orlando, FL, USA, 2001. [Google Scholar]
- Aramata, A.; Enyo, M.; Koga, O.; Hori, Y. FT-IR Spectrometry of the Reduced CO2 at Pt Electrode and Anomalous Effect of Ca2+ Ions. Chem. Lett. 1991, 5, 749. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Wu, D.; Fu, R.; Yu, Z. Organic and carbon aerogels from the NaOH-catalyzed polycondensation of resorcinol-furfural and supercritical drying in ethanol. J. Appl. Polym. Sci. 2005, 96, 1429–1435. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ryoo, R.; Joo, S.H. Characterization of MCM-48 Silicas with Tailored Pore Sizes Synthesized via a Highly Efficient Procedure. Chem. Mater. 2000, 12, 1414–1421. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
| Sample | Cs in Starting Solution (wt%) | VTotal a (cm3/g) | STotal a at pore ≥ 0.5 nm (m2/g) | Particle Size b (nm) | N2 Adsorption Capacity a (mmol/g) | Pore Width c (nm) | Micro-pores c (%) | Meso-pores c (%) | Macro-pores c (%) | EDXanalysis (wt%) | (ID/IG) d | AC (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | ||||||||||||
| RFX-Cs-AC-0 | 0 | 1.0 at pore ≤ 400.0 nm | 912 | 224 | 33.2 | 2.3 | 0.3 | 11.2 | 88.5 | 98.8 | 1.2 | 0.750 | 38.3 |
| RFX-Cs-AC-1 | 0.007% | 0.2 at pore ≤ 370.7 nm | 554 | 280 | 7.8 | 0.8 | 0.4 | 12.1 | 87.5 | 98.1 | 1.9 | 0.628 | 25.1 |
| RFX-Cs-AC-2 | 0.015% | ~0.2 at pore ≤ 400.3 nm | 627 | 403 | 8.3 | 0.8 | 0.3 | 11.3 | 88.4 | 98.9 | 1.1 | 0.696 | 22.2 |
| RFX-Cs-AC-3 | 0.022% | 0.2 at pore ≤ 400.3 nm | 561 | 795 | 8.6 | 0.8 | 0.3 | 11.3 | 88.4 | 98.0 | 2.0 | 0.683 | 20.0 |
| RFX-Cs-AC-4 | 0.029% | 0.3 at pore ≤ 136.7 nm | 598 | 184 | 10.6 | 1.0 | 1.0 | 32.9 | 66.1 | 98.5 | 1.5 | 0.718 | 21.1 |
| RFX-Cs-AC-5 | 0.037% | 0.4 at pore ≤ 185.8 nm | 592 | 149 | 15.4 | 1.4 | 0.8 | 24.1 | 75.1 | 99.0 | 1.0 | 0.682 | 18.0 |
| Sample | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 845 | |
| RFX-Cs-AC-0 | 4.6 | 6.3 | 8.2 | 10.0 | 11.9 | 14.7 | 20.9 | 29.1 | 32.5 |
| RFX-Cs-AC-1 | 7.4 | 8.8 | 10.6 | 15.2 | 26.2 | 40.1 | 55.1 | 70.0 | 77.2 |
| RFX-Cs-AC-2 | 3.0 | 4.0 | 6.0 | 8.5 | 11.8 | 21.2 | 48.1 | 76.5 | 86.5 |
| RFX-Cs-AC-3 | 7.9 | 10.2 | 12.8 | 15.3 | 17.9 | 24.1 | 38.5 | 55.4 | 61.8 |
| RFX-Cs-AC-4 | 6.0 | 7.6 | 9.7 | 11.5 | 13.5 | 17.8 | 29.1 | 44.3 | 50.5 |
| RFX-Cs-AC-5 | 3.6 | 4.6 | 6.4 | 8.5 | 14.4 | 30.4 | 49.2 | 69.2 | 77.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awadallah-F, A.; Al-Muhtaseb, S.A. The Effect of Chitosan’s Addition to Resorcinol/Formaldehyde Xerogels on the Characteristics of Resultant Activated Carbon. Materials 2019, 12, 3847. https://doi.org/10.3390/ma12233847
Awadallah-F A, Al-Muhtaseb SA. The Effect of Chitosan’s Addition to Resorcinol/Formaldehyde Xerogels on the Characteristics of Resultant Activated Carbon. Materials. 2019; 12(23):3847. https://doi.org/10.3390/ma12233847
Chicago/Turabian StyleAwadallah-F, Ahmed, and Shaheen A. Al-Muhtaseb. 2019. "The Effect of Chitosan’s Addition to Resorcinol/Formaldehyde Xerogels on the Characteristics of Resultant Activated Carbon" Materials 12, no. 23: 3847. https://doi.org/10.3390/ma12233847
APA StyleAwadallah-F, A., & Al-Muhtaseb, S. A. (2019). The Effect of Chitosan’s Addition to Resorcinol/Formaldehyde Xerogels on the Characteristics of Resultant Activated Carbon. Materials, 12(23), 3847. https://doi.org/10.3390/ma12233847