Thermal History Dependent Al Distribution in Aluminum Substituted Strontium Hexaferrite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
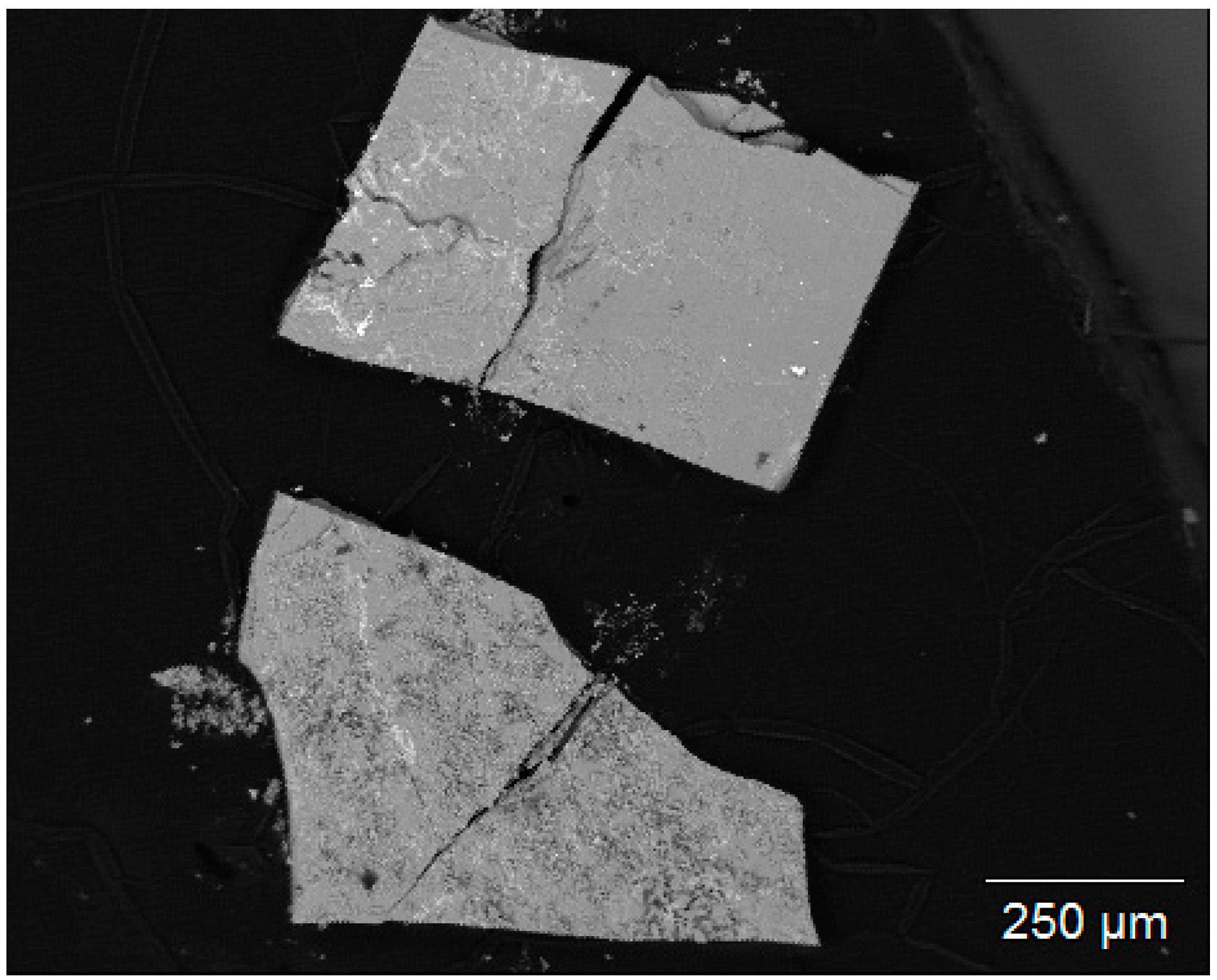
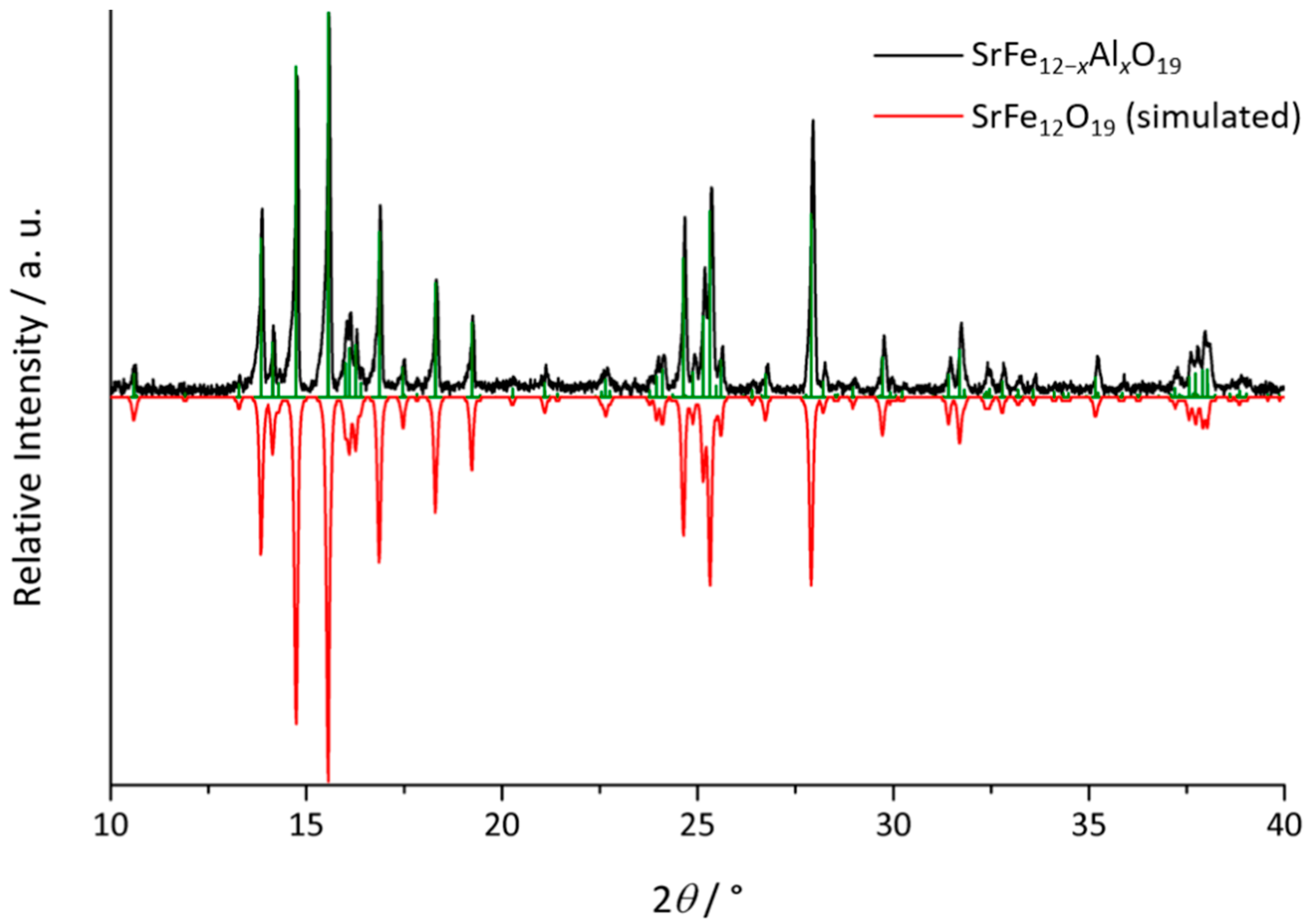
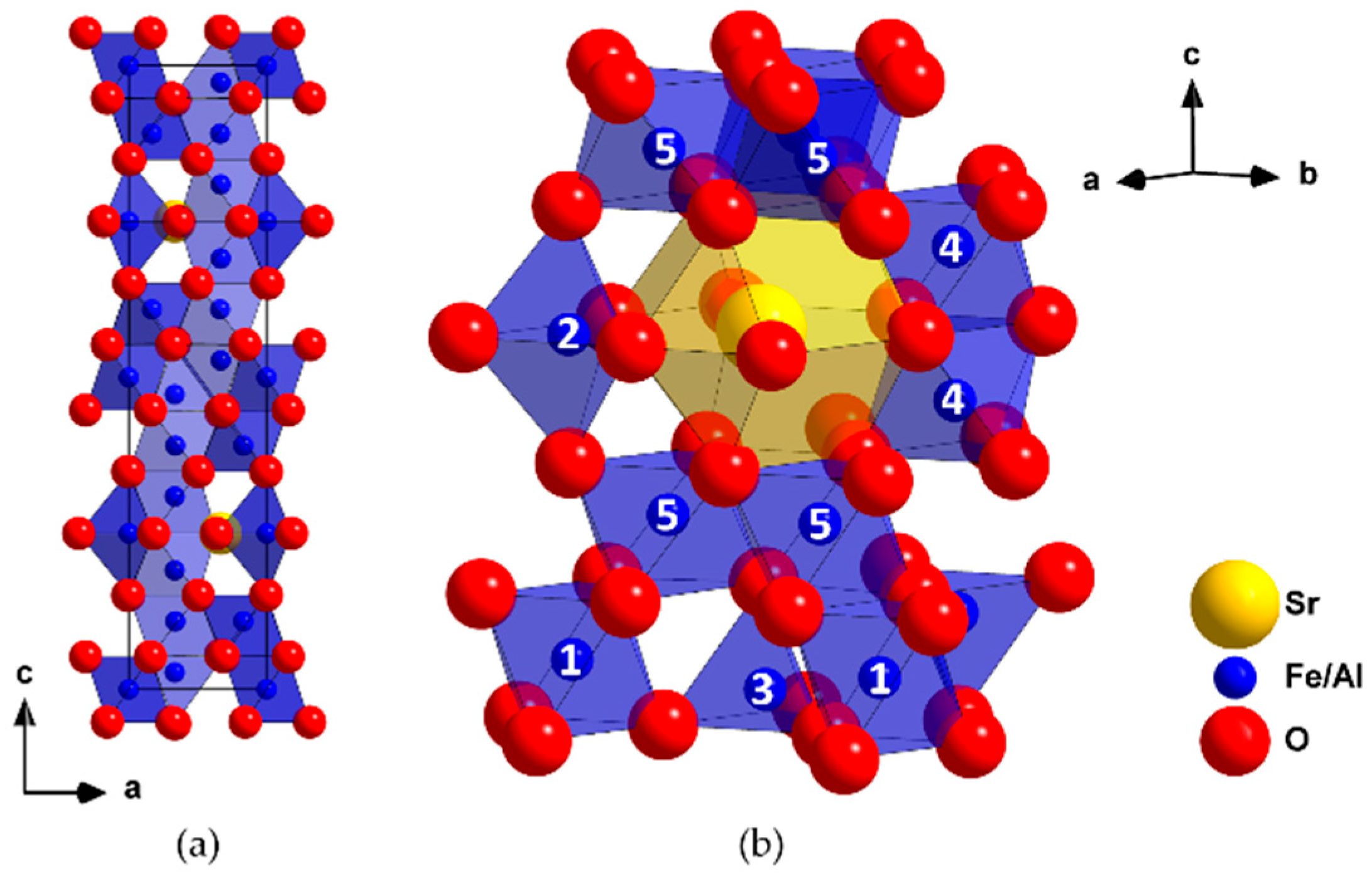
3.1. Structure and Composition
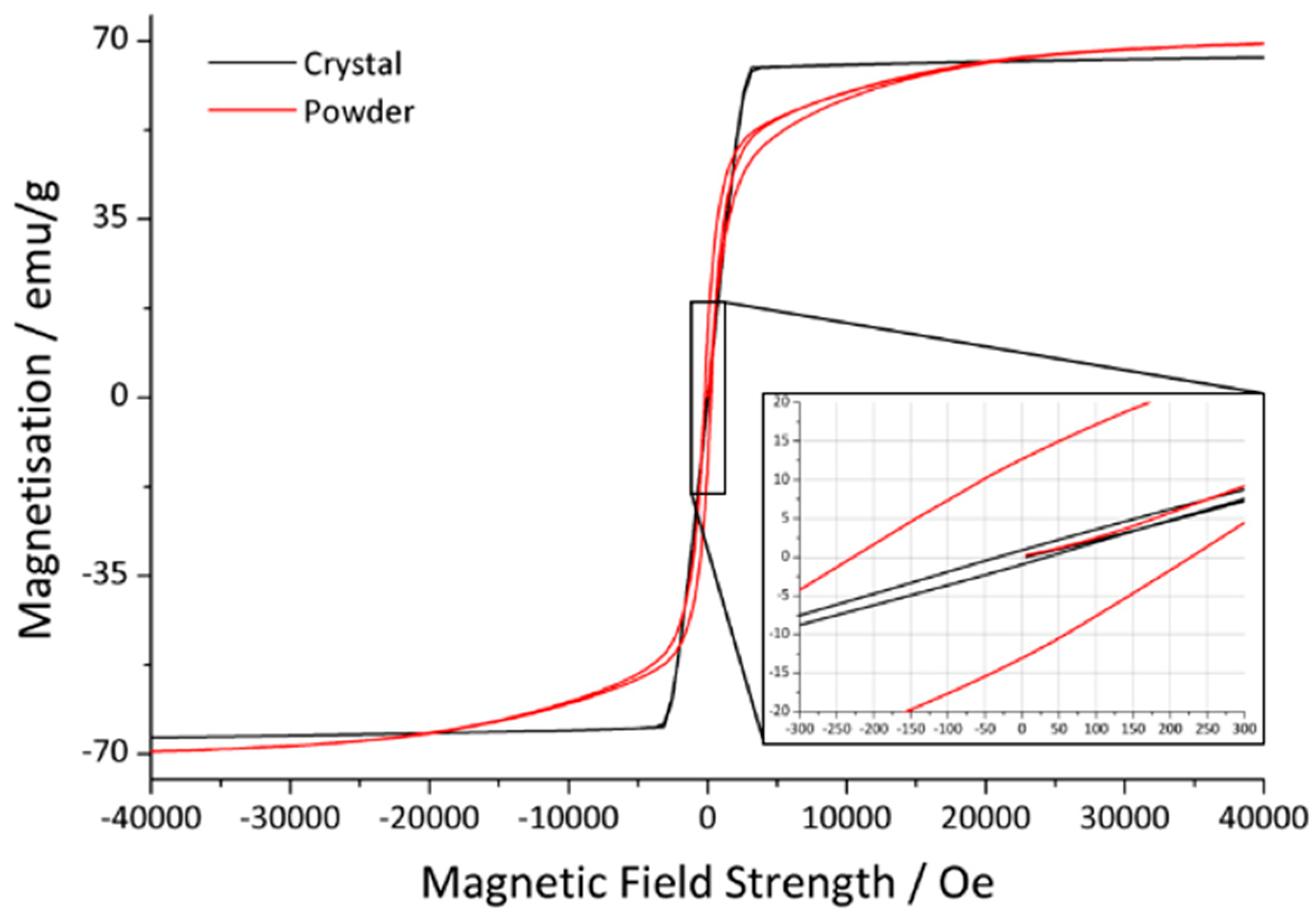
3.2. Magnetism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561. [Google Scholar] [CrossRef]
- Bragg, W.H. XXX. The Structure of the Spinel Group of Crystals. Philos. Mag. 1915, 30, 305–315. [Google Scholar] [CrossRef]
- Obradors, X.; Collomb, A.; Pernet, M. X-Ray Analysis of the Structural and Dynamic Properties of BaFe12O19 Hexagonal Ferrite at Room Temperature. J. Solid State Chem. 1985, 56, 171–181. [Google Scholar] [CrossRef]
- Morisako, A.; Matsumoto, M.; Naoe, M. Ba-ferrite thin film rigid disk for high density perpendicular magnetic recording. IEEE Trans. Magn. 1986, 22, 1146–1148. [Google Scholar] [CrossRef]
- Ferrites - Physical Properties of Ferrimagnetic Oxides in Relation to their Technical Applications; Smit, J., Wijn, H.P.J., Eds.; Philips’ Technical Library: Eindhoven, The Netherlands, 1959. [Google Scholar]
- Primc, D.; Makovec, D.; Lisjak, D.; Drofenik, M. Hydrothermal synthesis of ultrafine barium hexaferrite nanoparticles and the preparation of their stable suspensions. Nanotechnology 2009, 20, 315605–315613. [Google Scholar] [CrossRef]
- Drofenik, M.; Kristl, M.; Žnidaršič, A.; Hanžel, D.; Lisjak, D. Hydrothermal Synthesis of Ba-Hexaferrite Nanoparticles. J. Am. Ceram. Soc. 2007, 90, 2057–2061. [Google Scholar] [CrossRef]
- Sui, X.; Scherge, M.; Kryder, M.H.; Snyder, J.E.; Harris, V.G.; Koon, N.C. Barium ferrite thin-film recording media. J. Magn. Magn. Mater. 1996, 155, 132–139. [Google Scholar] [CrossRef]
- Barb, D.; Diamandescu, L.; Rusi, A.; Tӑrӑbӑsanu-Mihӑilӑ, D.; Morariu, M.; Teodorescu, V. Preparation of barium hexaferrite by a hydrothermal method: Structure and magnetic properties. J. Mater. Sci. 1986, 21, 1118–1122. [Google Scholar] [CrossRef]
- Mali, A.; Ataie, A. Structural characterization of nano-crystalline BaFe12O19 powders synthesized by sol–gel combustion route. Scr. Mater. 2005, 53, 1065–1070. [Google Scholar] [CrossRef]
- Sürig, C.; Hempel, K.A.; Bonnenberg, D. Hexaferrite particles prepared by sol-gel technique. IEEE Trans. Magn. 1994, 30, 4092–4094. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Tarasova, A.Y.; Zherebtsov, D.A.; Gudkova, S.A.; Galimov, D.M.; Zhivulin, V.E.; Trofimov, E.A.; Nemrava, S.; Perov, N.S.; Isaenko, L.I.; et al. Magnetic and Structural Properties of Barium Hexaferrite BaFe12O19 from Various Growth Techniques. Materials 2017, 10. [Google Scholar] [CrossRef]
- Obradors, X.; Solans, X.; Collomb, A.; Samaras, D.; Rodriguez, J.; Pernet, M.; Font-Altaba, M. Crystal Structure of Strontium Hexaferrite SrFe12O19. J. Solid State Chem. 1988, 72, 218–224. [Google Scholar] [CrossRef]
- Blix, R. On the chemical composition of the magnetoplumbite: (With a new analysis.). Geol. Foeren. Stockholm Foerh. 1937, 59, 300–302. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Nemrava, S.; Bischoff, M.; Perov, N.S.; Semisalova, A.S.; Krivtsov, I.V.; Isaenko, L.I.; Mikhailov, G.G.; et al. Growth, structural and magnetic characterization of Al-substituted barium hexaferrite single crystals. J. Alloys Compd. 2014, 615, 1043–1046. [Google Scholar] [CrossRef]
- Shlyk, L.; Vinnik, D.A.; Zherebtsov, D.A.; Hu, Z.; Kuo, C.-Y.; Chang, C.-F.; Lin, H.-J.; Yang, L.-Y.; Semisalova, A.S.; Perov, N.S.; et al. Single crystal growth, structural characteristics and magnetic properties of chromium substituted M-type ferrites. Solid State Sci. 2015, 50, 23–31. [Google Scholar] [CrossRef]
- Wang, C.-A.; Lu, H.; Huang, Z.; Xie, H. Enhanced anti-deliquescent property and ultralow thermal conductivity of magnetoplumbite-type LnMeAl11O19 materials for thermal barrier coating. J. Am. Ceram. Soc. 2018, 101, 1095–1104. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Nemrava, S.; Yakushechkina, A.K.; Semisalova, A.S.; Gudkova, S.A.; Anikeev, A.N.; Perov, N.S.; Isaenko, L.I.; et al. Tungsten substituted BaFe12O19 single crystal growth and characterization. Mater. Chem. Phys. 2015, 155, 99–103. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Nemrava, S.; Perov, N.S.; Semisalova, A.S.; Krivtsov, I.V.; Isaenko, L.I.; Mikhailov, G.G.; Niewa, R. Ti-Substituted BaFe12O19 Single Crystal Growth and Characterization. Cryst. Growth Des. 2014, 14, 5834–5839. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Tarasova, A.Y.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Gudkova, S.A.; Nemrava, S.; Yakushechkina, A.K.; Semisalova, A.S.; Isaenko, L.I.; Niewa, R. Cu-substituted barium hexaferrite crystal growth and characterization. Ceram. Int. 2015, 41, 9172–9176. [Google Scholar] [CrossRef]
- Nemrava, S.; Vinnik, D.A.; Hu, Z.; Valldor, M.; Kuo, C.-Y.; Zherebtsov, D.A.; Gudkova, S.A.; Chen, C.-T.; Tjeng, L.H.; Niewa, R. Three Oxidation States of Manganese in the Barium Hexaferrite BaFe12-xMnxO19. Inorg. Chem. 2017, 56, 3861–3866. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.W.; Jeon, K.W.; Kim, J. Synthesis and Magnetic Properties of BaFe12-xAlxO19 Nanopowders. IEEE Trans. Magn. 2009, 45, 4405–4408. [Google Scholar] [CrossRef]
- Gambino, R.J.; Leonhard, F. Growth of Barium Ferrite Single Crystals. J. Am. Ceram. Soc. 1961, 44, 221–224. [Google Scholar] [CrossRef]
- Albanese, G. Mössbauer investigation of aluminium substituted barium hexaferrite in the paramagnetic state. J. Magn. Magn. Mater. 1995, 147, 421–426. [Google Scholar] [CrossRef]
- Choi, D.H.; An, S.Y.; Lee, S.W.; Shim, I.-B.; Kim, C.S. Site occupancy and anisotropy distribution of Al substituted Ba-ferrite with high coercivity. Phys. Stat. Solidi B 2004, 241, 1736–1739. [Google Scholar] [CrossRef]
- Albanese, G.; Carbucicchio, M.; Deriu, A. Substitution of Fe3+ by Al3+ in the Trigonal Sites of M-Type Hexagonal Ferrites. Nuovo Cimento 1973, 15B, 147–158. [Google Scholar] [CrossRef]
- Awawdeh, M.; Bsoul, I.; Mahmood, S.H. Magnetic properties and Mössbauer spectroscopy on Ga, Al and Cr substituted hexaferrites. J. Alloys Compd. 2014, 585, 465–473. [Google Scholar] [CrossRef]
- Pauling, L.; Hendricks, S.B. The crystal structures of hematite and corundum. J. Am. Chem. Soc. 1925, 47, 781–790. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Meaz, T.M.; Amer, M.A.; El Shersaby, H.A. Magnetic behavior and dielectric properties of aluminum substituted M-type barium hexaferrite. Physica B 2013, 426, 137–143. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N.; Hernandez-Gomez, P.; Munoz, J.M. Synthesis, physical, magnetic and electrical properties of Al–Ga substituted co-precipitated nanocrystalline strontium hexaferrite. J. Magn. Magn. Mater. 2008, 320, 881–886. [Google Scholar] [CrossRef]

| Crystal | 1 | 2 | 3 |
|---|---|---|---|
| Refined composition | SrFe11.2(1)Al0.8(1)O19 | SrFe11.2(2)Al0.8(2)O19 | SrFe11.0(1)Al1.0(1)O19 |
| Crystal system | Hexagonal | ||
| Space group type | P63/mmc (No. 194) | ||
| a/pm | 588.37(1) | 587.39(1) | 587.41(1) |
| c/pm | 2304.34(7) | 2300.42(6) | 2300.62(6) |
| Z | 2 | 2 | 2 |
| Density ρ (calculated)/ g/cm³ | 4.966 | 4.991 | 4.990 |
| Volume V/108·pm³ | 6.9084(3) | 6.8737(2) | 6.8748(2) |
| Diffractometer | κ-CCD (Bruker-Nonius) | ||
| Wavelength | Mo-Kα, λ = 71.073 pm | ||
| Index ranges hkl | h = ±8; k = ±9; l = ±36 | –8 ≤ h ≤ 7; k = ±9; –21 ≤ l ≤ 36 | h = ±9; k = ±9; –34 ≤ l ≤ 36 |
| Measurement range θmax/° | 69.88 | 69.87 | 69.87 |
| F(000) | 978.0 | 978.0 | 978.0 |
| µ(Mo Kα)/mm–1 | 15.18 | 15.26 | 15.25 |
| Reflections collected/independent | 15718/645 | 14639/645 | 16598/645 |
| Rint/Rσ | 0.0445/0.0151 | 0.0620/0.0193 | 0.0733/0.0225 |
| R1/R1 with F0 ≥ 4σ(F0) | 0.0297/0.0245 | 0.0371/0.0310 | 0.0443/0.0324 |
| wR2/GooF | 0.0576/1.090 | 0.0785/1.155 | 0.0872/1.125 |
| Largest e− difference peak/hole | 0.89/–0.82 | 0.85/–1.49 | 1.27/–1.53 |
| Atom | Site | x/a | y/b | z/c | Occupation | Uiso/pm2 |
|---|---|---|---|---|---|---|
| Sr | 2d | 2/3 | 1/3 | 1/4 | 1 | 0.0144(2) |
| M(1) | 2a | 0 | 0 | 0 | 88/12(1) | 0.0070(3) |
| M(2) | 2b | 0 | 0 | 1/4 | 92/8(2) | 0.0138(3) |
| M(3) | 4f | 1/3 | 2/3 | 0.02729(3) | 96/4(1) | 0.0065(2) |
| M(4) | 4f | 1/3 | 2/3 | 0.19075(3) | 93/7(1) | 0.0070(2) |
| M(5) | 12k | 0.33750(8) | x/2 | 0.10914(2) | 93/6(1) | 0.0071(2) |
| O(1) | 4e | 0 | 0 | 0.1511(2) | 1 | 0.0087(6) |
| O(2) | 4f | 2/3 | 1/3 | 0.0553(2) | 1 | 0.0099(6) |
| O(3) | 6h | 0.1816(3) | 2x | 1/4 | 1 | 0.0119(6) |
| O(4) | 12k | 0.15616 | 2x | 0.05231(8) | 1 | 0.0085(4) |
| O(5) | 12k | 0.546(2) | 0.15080(8) | 1 | 0.0097(4) |
| Atom | Site | x/a | y/b | z/c | Occupation | Uiso/pm2 |
|---|---|---|---|---|---|---|
| Sr | 2d | 2/3 | 1/3 | 1/4 | 1 | 0.0152(3) |
| M(1) | 2a | 0 | 0 | 0 | 78/22(2) | 0.0070(4) |
| M(2) | 2b | 0 | 0 | 1/4 | 93/7(2) | 0.0151(4) |
| Fe(3) | 4f | 1/3 | 2/3 | 0.02729(3) | 1 | 0.0071(3) |
| M(4) | 4f | 1/3 | 2/3 | 0.19056(4) | 95/5(2) | 0.0076(3) |
| M(5) | 12k | 0.33725(9) | x/2 | 0.10901(2) | 94/6(1) | 0.0077(2) |
| O(1) | 4e | 0 | 0 | 0.1507(2) | 1 | 0.0082(7) |
| O(2) | 4f | 2/3 | 1/3 | 0.0558(2) | 1 | 0.0092(8) |
| O(3) | 6h | 0.1816(4) | 2x | 1/4 | 1 | 0.0128(7) |
| O(4) | 12k | 0.1558(3) | 2x | 0.0521(1) | 1 | 0.0084(5) |
| O(5) | 12k | 0.5043(3) | 0.1502(1) | 1 | 0.0091(4) |
| Atom | Site | x/a | y/b | z/c | Occupation | Uiso/pm2 |
|---|---|---|---|---|---|---|
| Sr | 2d | 2/3 | 1/3 | 1/4 | 1 | 0.0167(3) |
| M(1) | 2a | 0 | 0 | 0 | 76/24(2) | 0.0077(4) |
| M(2) | 2b | 0 | 0 | 1/4 | 92/8(2) | 0.0159(5) |
| M(3) | 4f | 1/3 | 2/3 | 0.02731(4) | 98/2(1) | 0.0079(3) |
| M(4) | 4f | 1/3 | 2/3 | 0.19058(4) | 94/6(1) | 0.0083(3) |
| M(5) | 12k | 0.33715(9) | x/2 | 0.10902(2) | 92/8(1) | 0.0083(2) |
| O(1) | 4e | 0 | 0 | 0.1508(2) | 1 | 0.0095(8) |
| O(2) | 4f | 2/3 | 1/3 | 0.0555(2) | 1 | 0.0097(9) |
| O(3) | 6h | 0.1817(4) | 2x | 1/4 | 1 | 0.0129(8) |
| O(4) | 12k | 0.1553(3) | 2x | 0.0521(2) | 1 | 0.0099(5) |
| O(5) | 12k | 0.5040(3) | 0.1502(2) | 1 | 0.0105(5) |
| Sample | Average Aluminum Content/at.-% |
|---|---|
| Crystal 1 from initial synthesis | 1.7 ± 0.2 |
| Crystal 2 from annealing in platinum crucible | 2.0 ± 0.5 |
| Crystal 3 from annealing in corundum crucible | 2.15 ± 0.08 |
| Sample | Ms/emu/g | Mr/emu/g | Hc/Oe |
|---|---|---|---|
| Powder | 71.1 | 12.8 | 226.1 |
| Crystal 1 from initial synthesis | 67.8 | 0.9 | 33.0 |
| Crystal 2 from annealing in platinum crucible | 68.1 | 0.4 | 18.1 |
| Crystal 3 from annealing in corundum crucible | 69.9 | 0.3 | 10.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häßner, M.; Vinnik, D.A.; Niewa, R. Thermal History Dependent Al Distribution in Aluminum Substituted Strontium Hexaferrite. Materials 2020, 13, 858. https://doi.org/10.3390/ma13040858
Häßner M, Vinnik DA, Niewa R. Thermal History Dependent Al Distribution in Aluminum Substituted Strontium Hexaferrite. Materials. 2020; 13(4):858. https://doi.org/10.3390/ma13040858
Chicago/Turabian StyleHäßner, Manuel, Denis A. Vinnik, and Rainer Niewa. 2020. "Thermal History Dependent Al Distribution in Aluminum Substituted Strontium Hexaferrite" Materials 13, no. 4: 858. https://doi.org/10.3390/ma13040858
APA StyleHäßner, M., Vinnik, D. A., & Niewa, R. (2020). Thermal History Dependent Al Distribution in Aluminum Substituted Strontium Hexaferrite. Materials, 13(4), 858. https://doi.org/10.3390/ma13040858






