Effect of Rough Surface Platforms on the Mucosal Attachment and the Marginal Bone Loss of Implants: A Dog Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Sample
2.2. Randomization and Allocation Concealment
2.3. Surgical Preparations
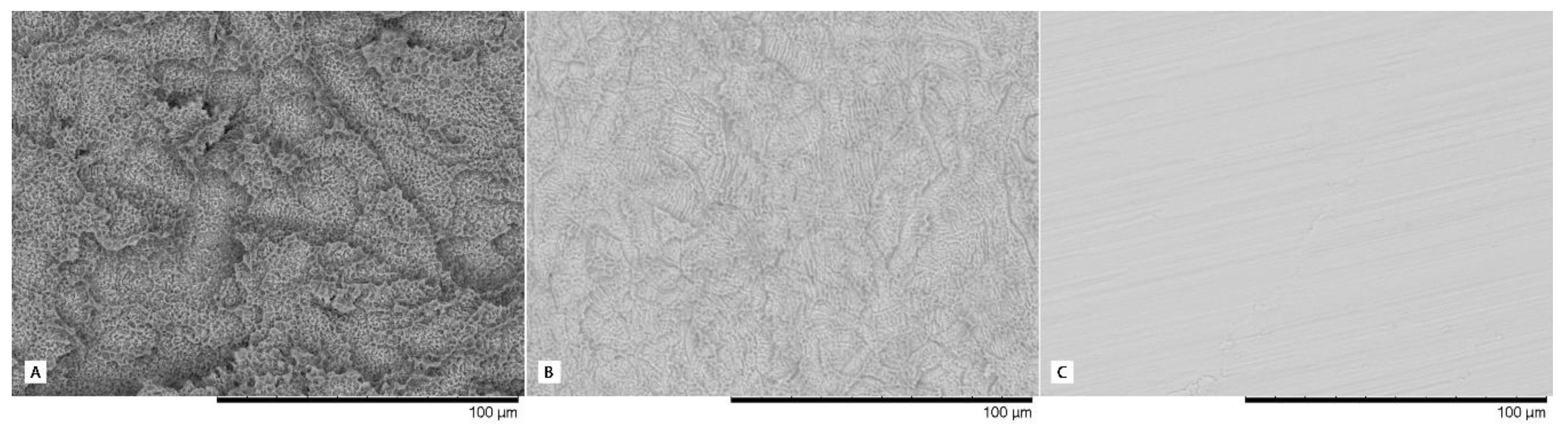
2.4. Implant Characteristics
- Type I: Straumann BL (bone-level) implant with 3.3 mm diameter and 8 mm in length (Institute Straumann AG, Basel, Switzerland), with a body implant treated with SLA® surface (mean Ra value of 1.66 µm) and a machined platform (mean Ra value of 0.27 µm).
- Type II: IPX-Std (standard) with 3.5 mm diameter and 8mm in length (Galimplant SLU, Sarria, Lugo, Spain), with an implant body and neck treated using a Nanoblast Plus® technique (mean Ra value of 1.69 µm) and a fully machined platform (mean Ra value of 0.28 µm).
- Type III: IPX-Half, with 3.5 mm diameter and 8 mm in length (Galimplant SLU, Sarria, Lugo, Spain) with an implant body, neck and the outer half of the platform surface treated with Nanoblast Plus® technique (same Ra value described in Type II treated surface) and the inner half of the platform machined (same Ra value described in Type II machined surface).
- Type IV: IPX-Full, with 3.5 mm diameter and 8 mm in length (Galimplant SLU, Sarria, Lugo, Spain) with the implant body, neck and the whole platform treated with the Nanoblast Plus® technique (mean Ra value of 1.69 µm).
- Type V: IPX-Control, with 3.5 mm diameter and 8 mm in length (Galimplant SLU, Sarria, Lugo, Spain), with implant body, neck, and platform machined (mean Ra value of 0.28 µm).
2.5. Maintenance Procedures
2.6. Euthanasia
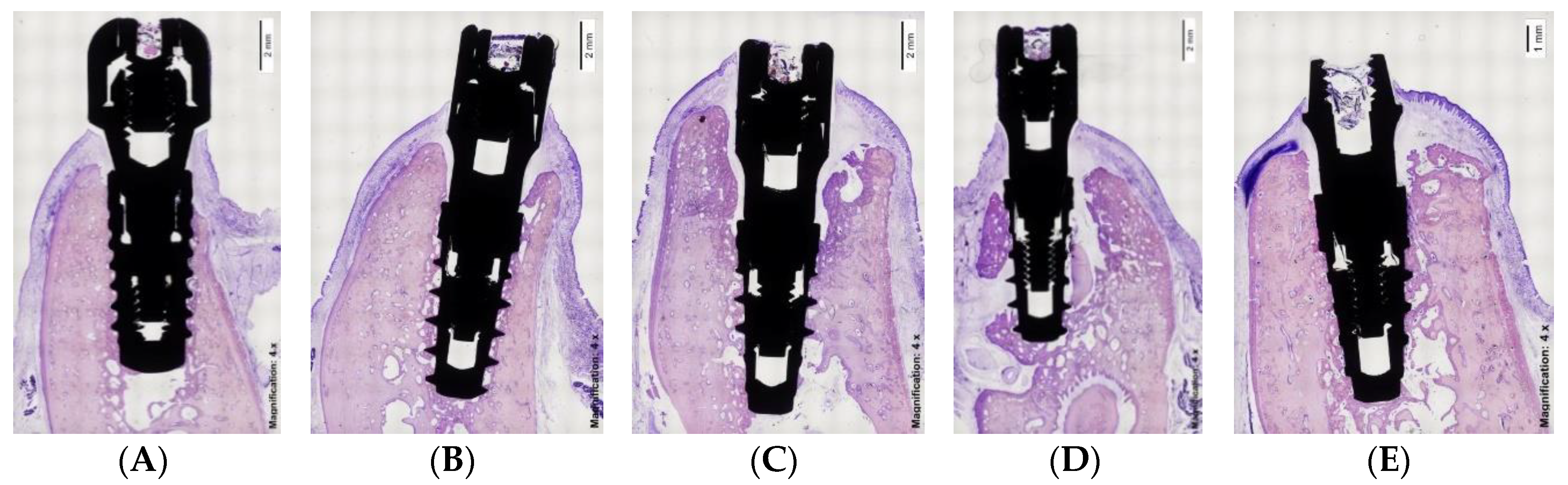
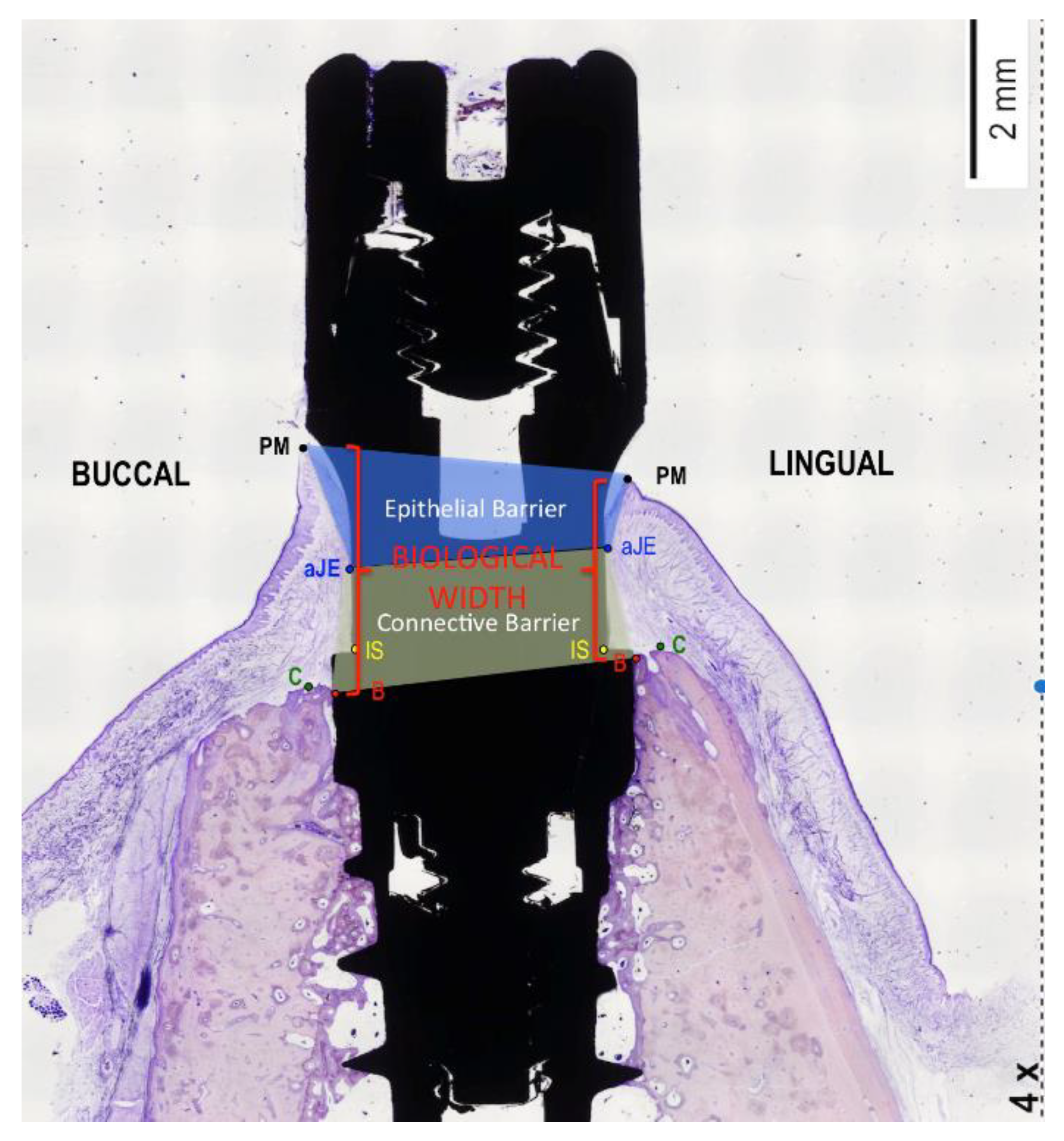
2.7. Histological Preparation
2.8. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implant. Res. 2003, 14, 251–262. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Welander, M.; Lang, N.P.; Lindhe, J. Morphogenesis of the peri-implant mucosa: An experimental study in dogs. Clin. Oral Implant. Res. 2007, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Mainetti, T.; Lang, N.P.; Bengazi, F.; Sbricoli, L.; Soto Cantero, L.; Botticelli, D. Immediate loading of implants installed in a healed alveolar bony ridge or immediately after tooth extraction: An experimental study in dogs. Clin. Oral Implant. Res. 2015, 26, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Mainetti, T.; Lang, N.P.; Bengazi, F.; Favero, G.; Soto Cantero, L.; Botticelli, D. Sequential healing at implants installed immediately into extraction sockets. An experimental study in dogs. Clin. Oral Implant. Res. 2016, 27, 130–138. [Google Scholar] [CrossRef]
- Vignoletti, F.; de Sanctis, M.; Berglundh, T.; Abrahamsson, I.; Sanz, M. Early healing of implants placed into fresh extraction sockets: An experimental study in the beagle dog. II: Ridge alterations. J. Clin. Periodontol. 2009, 36, 688–697. [Google Scholar] [CrossRef]
- Botticelli, D.; Renzi, A.; Lindhe, J.; Berglundh, T. Implants in fresh extraction sockets: A prospective 5-year follow-up clinical study. Clin. Oral Implant. Res. 2008, 19, 1226–1232. [Google Scholar] [CrossRef]
- Rossi, F.; Lang, N.P.; De Santis, E.; Morelli, F.; Favero, G.; Botticelli, D. Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin. Oral Implant. Res. 2014, 25, 124–131. [Google Scholar] [CrossRef]
- Bateli, M.; Att, W.; Strub, J.R. Implant neck configurations for preservation of marginal bone level: A systematic review. Int. J. Oral Maxillofac. Implant. 2011, 26, 290–303. [Google Scholar]
- Choi, K.S.; Lozada, J.L.; Kan, J.Y.; Lee, S.H.; Kim, C.S.; Kwon, T.G. Study of an experimental microthreaded scalloped implant design: Proximal bone healing at different interimplant distances in a canine model. Int. J. Oral Maxillofac. Implant. 2010, 25, 681–689. [Google Scholar]
- Abrahamsson, I.; Berglundh, T. Tissue characteristics at microthreaded implants: An experimental study in dogs. Clin. Implant. Dent. Relat. Res. 2006, 8, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, L.; Kahnberg, K.E.; Tan, A. Effects of implant design and surface on bone regeneration and implant stability: An experimental study in the dog mandible. Clin. Implant. Dent. Relat. Res. 2001, 3, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Koodaryan, R.; Hafezeqoran, A. Evaluation of Implant Collar Surfaces for Marginal Bone Loss: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 4987526. [Google Scholar] [CrossRef] [PubMed]
- Messias, A.; Nicolau, P.; Guerra, F. Titanium dental implants with different collar design and surface modifications: A systematic review on survival rates and marginal bone levels. Clin. Oral Implant. Res. 2019, 30, 20–48. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Han, D.H. Influence of microgrooved collar design on soft and hard tissue healing of immediate implantation in fresh extraction sites in dogs. Clin. Oral Implant. Res. 2010, 21, 804–814. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Jiménez-Soto, R.; Pérez Albacete-Martinez, C.; Fernández-Domínguez, M.; Gehrke, S.A.; Maté Sánchez de Val, J.E. Influence of implant neck design on peri-implant tissue dimensions: A comparative study in dogs. Materials 2018, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- de Sanctis, M.; Vignoletti, F.; Discepoli, N.; Muñoz, F.; Sanz, M. Immediate implants at fresh extraction sockets: An experimental study in the beagle dog comparing four different implant systems. Soft tissue findings. J. Clin. Periodontol. 2010, 37, 769–776. [Google Scholar] [CrossRef]
- Weng, D.; Nagata, M.J.J.; Bell, M.; Bosco, A.F.; de Melo, L.G.; Richter, E.J. Influence of microgap location and configuration on the periimplant bone morphology in submerged implants. An experimental study in dogs. Clin. Oral Implant. Res. 2008, 19, 1141–1147. [Google Scholar] [CrossRef]
- Todescan, F.F.; Pustiglioni, F.E.; Imbronito, A.V.; Albrektsson, T.; Gioso, M. Influence of the microgap in the peri-implant hard and soft tissues: A histomorphometric study in dogs. Int. J. Oral Maxillofac. Implant. 2002, 17, 467–472. [Google Scholar]
- Rimondini, L.; Bruschi, G.B.; Scipioni, A.; Carrassi, A.; Nicoli-Aldini, N.; Giavaresi, G.; Fini, M.; Mortellaro, C.; Giardino, R. Tissue healing in implants immediately placed into postextraction sockets: A pilot study in a mini-pig model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 43–50. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T.; Moon, I.S.; Lindhe, J. Peri-implant tissues at submerged and non-submerged titanium implants. J. Clin. Periodontol. 1999, 26, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Mau, L.P.; Higginbottom, F.L.; Wilson, T.G.; Bosshardt, D.D.; Schoolfield, J.; Jones, A.A. Soft and hard tissue histologic dimensions around dental implants in the canine restored with smaller-diameter abutments: A paradigm shift in peri-implant biology. Int. J. Oral Maxillofac. Implant. 2013, 28, 494–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karring, T.; Cumming, B.R.; Oliver, R.C.; Löe, H. The origin of granulation tissue and its impact on postoperative results of mucogingival surgery. J. Periodontol. 1975, 46, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Lang, N.P.; Favero, G.; León, I.G.; Salata, L.A.; Botticelli, D. Role of teeth adjacent to implants installed immediately into extraction sockets: An experimental study in the dog. Clin. Oral Implant. Res. 2012, 23, 402–408. [Google Scholar] [CrossRef]
- Favero, G.; Botticelli, D.; Rea, M.; Pantani, F.; León, I.G.; Lang, N.P. Influence of presence or absence of teeth adjacent to implants installed immediately into extraction sockets on peri-implant hard tissue levels: An experimental study in the dog. Clin. Oral Implant. Res. 2013, 24, 262–269. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the periimplant mucosa. Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Bengazi, F.; Lang, N.P.; Canciani, E.; Viganò, P.; Velez, J.U.; Botticelli, D. Osseointegration of implants with dendrimers surface characteristics installed conventionally or with Piezosurgery®. A comparative study in the dog. Clin. Oral Implant. Res. 2014, 25, 10–15. [Google Scholar] [CrossRef]
- Bengazi, F.; Lang, N.P.; Caroprese, M.; Urbizo Velez, J.; Favero, V.; Botticelli, D. Dimensional changes in soft tissues around dental implants following free gingival grafting: An experimental study in dogs. Clin. Oral Implant. Res. 2015, 26, 176–182. [Google Scholar] [CrossRef]
- Blanco, J.; Nuñez, V.; Aracil, L.; Muñoz, F.; Ramos, I. Ridge alterations following immediate implant placement in the dog: Flap versus flapless surgery. J. Clin. Periodontol. 2008, 35, 640–648. [Google Scholar] [CrossRef]
- Yaffe, A.; Fine, N.; Binderman, I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J. Periodontol. 1994, 65, 79–83. [Google Scholar] [CrossRef] [PubMed]


| Experimental Group | Implant Design | Implant Size | Body Treatment | Platform Treatment | Ra Value Treated Surface | Ra Value Machined Surface |
|---|---|---|---|---|---|---|
| Straumann BL |  | 3.3 × 8 | SLA® Surface | Machined | 1.66 µm | 0.27 µm |
| IPX-Std |  | 3.5 × 8 | Nanoblast Plus® Surface | Machined | 1.69 µm | 0.28 µm |
| IPX-Half |  | 3.5 × 8 | Nanoblast Plus® Surface | Outer Half: Nanoblast Plus® Surface Inner Half: Machined | 1.69 µm | 0.28 µm |
| IPX-Full |  | 3.5 × 8 | Nanoblast Plus® Surface | Nanoblast Plus® Surface | 1.69 µm | Not Apply |
| IPX-Control |  | 3.5 × 8 | Machined | Machined | Not Apply | 0.28 µm |
| Biological Width | Immediate Implants (n = 40) | Delayed Implants (n = 40) | Comparisons by | |||
|---|---|---|---|---|---|---|
| Student T; p-Value | ||||||
| Buccal | Lingual | Buccal | Lingual | Buccal | Lingual | |
| Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | |||
| Strauman BL | 3.8(0.7) | 3.1(1.7) * | 3.2(0.6) | 1.8(0.2) *,a | T = 1.6 p = 0.13 | T = 2.2 p = 0.04 |
| IPX-Std | 4.4(2.3) | 3.9(1.0) * | 4.0(0.7) | 3.0(0.5) *,b | T = 0.4 p = 0.68 | T = 2.1 p = 0.05 |
| IPX-Half | 4.6(2.4) | 4.4(2.3) | 4.2(1.1) | 2.9(0.5) | T = 0.4 p = 0.72 | T = 1.8 p = 0.13 |
| IPX-Full | 4.5(1.8) | 3.9(1.1) * | 4.1(0.3) | 2.7(0.6) * | T = 0.6 p = 0.56 | T = 2.5 p = 0.03 |
| IPX-Control | 5.1(1.1) | 4.7(1.3) * | 4.3(0.6) | 2.9(1.1) *,b | T = 1.5 p = 0.18 | T = 2.8 p = 0.02 |
| Comparisons by ANOVA: p-value | F = 0.4 p = 0.79 | F = 1.1 p = 0.40 | F = 2.1 p = 0.11 | F = 3.3 p = 0.02 | ||
| Epithelial Width | Immediate Implants (n = 40) | Delayed Implants (n = 40) | Comparisons by | |||
|---|---|---|---|---|---|---|
| Student T; p-Value | ||||||
| Buccal | Lingual | Buccal | Lingual | Buccal | Lingual | |
| Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | |||
| Strauman BL | 2.0(0.6) | 2.1(1.7) | 2.2(0.5) | 0.9(0.3) | T = 0.5 p = 0.65 | T = 1.8 p = 0.11 |
| IPX-Std | 2.6(1.3) | 2.0(1.1) | 2.1(0.9) | 1.4(0.5) | T = 0.9 p = 0.39 | T = 1.3 p = 0.22 |
| IPX-Half | 2.7(1.3) | 2.1(1.1) | 2.2(0.5) | 1.2(0.7) | T = 1.1 p = 0.30 | T = 1.9 p = 0.08 |
| IPX-Full | 2.9(1.0) * | 1.9(0.6) | 2.1(0.5) * | 1.3(0.7) | T = 2.1 p = 0.05 | T = 1.8 p = 0.10 |
| IPX-Control | 2.4(0.9) | 1.8(0.5) | 2.3(0.6) | 1.3(0.5) | T = 0.2 p = 0.82 | T = 1.9 p = 0.08 |
| Comparisons by ANOVA; | F = 0.7 p = 0.60 | F = 0.1 p = 0.97 | F = 0.1 p = 0.97 | F = 0.8 p = 0.53 | ||
| p-value | ||||||
| Connective Width | Immediate Implants (n = 40) | Delayed Implants (n = 40) | Comparisons by | |||
|---|---|---|---|---|---|---|
| Student T; p-Value | ||||||
| Buccal | Lingual | Buccal | Lingual | Buccal | Lingual | |
| Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | |||
| Strauman BL | 1.8(0.6) * | 1.1(0.5) a | 1.1(0.5) *,a | 1.0(0.2) | T = 2.5 p = 0.03 | T = 0.3 p = 0.75 |
| IPX-Std | 2.1(1.5) | 2.0(1.2) | 1.9(0.5) | 1.6(0.5) | T = 0.3 p = 0.75 | T = 0.7 p = 0.51 |
| IPX-Half | 2.2(1.4) | 2.3(1.5) | 2.0(1.0) b | 1.6(0.5) | T = 0.3 p = 0.75 | T = 1.1 p = 0.30 |
| IPX-Full | 1.6(1.0) | 2.0(0.6) * | 2.0(0.6) | 1.5(0.2) * | T = 0.8 p = 0.45 | T = 2.2 p = 0.05 |
| IPX-Control | 2.7(1.2) | 3.0(1.3) *,b | 2.0(0.2) | 1.7(0.8) * | T = 1.6 p = 0.16 | T = 2.2 p = 0.05 |
| Comparisons by ANOVA; p-value | F = 0.8 p = 0.54 | F = 2.7 p = 0.05 | F = 2.6 p = 0.05 | F = 2.0 p = 0.13 | ||
| Marginal Bone Loss | Immediate Implants (n = 40) | Delayed Implants (n = 40) | Comparisons by | |||
|---|---|---|---|---|---|---|
| Student T; p-Value | ||||||
| Buccal | Lingual | Buccal | Lingual | Buccal | Lingual | |
| Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | |||
| Strauman BL | 1.3(0.8) * | 0.6(0.3) | 0.6(0.4) * | 0.4(0.3) | T = 2.5 p = 0.03 | T = 1.6 p = 0.13 |
| IPX-Std | 0.3(0.9) | 0.3(0.4) | 0.2(0.3) | 0.4(0.5) | T = 0.4 p = 0.70 | T = 0.2 p = 0.83 |
| IPX-Half | 0.8(0.6) | 0.5(0.4) * | 0.7(1.3) | 0.1(0.3) * | T = 0.2 p = 0.81 | T = 2.3 p = 0.04 |
| IPX-Full | 1.0(0.9) | 0.3(0.4) | 0.3(0.6) | 0.2(0.4) | T = 1.8 p = 0.10 | T = 0.4 p = 0.68 |
| IPX-Control | 0.8(1.4) | 0.9(0.8) | 1.6(2.2) | 0.9(1.1) | T = 0.9 p = 0.31 | T = 0.0 p = 0.99 |
| Comparisons by ANOVA; p-value | F = 1.2 p = 0.35 | F = 2.0 p = 0.13 | F = 1.9 p = 0.14 | F = 2.0 p = 0.12 | ||
| BIC | Immediate Implants (n = 40) | Delayed Implants (n = 40) | Comparisons by |
|---|---|---|---|
| Student T; p-value | |||
| Mean (sd) | Mean (sd) | ||
| Strauman BL | 54.8(22.9) | 64.6(19.1) | T = 0.9 p = 0.37 |
| IPX-Std | 62.3(16.3) | 68.6(21.3) | T = 0.7 p = 0.52 |
| IPX-Half | 72.5(12.8) a | 72.7(9.8) | T = 0.0 p = 0.97 |
| IPX-Full | 63.9(19.6) | 74.2(15.6) | T = 1.2 p = 0.26 |
| IPX-Control | 43.2(15.2) b | 52.9(21.3) | T = 1.1 p = 0.31 |
| Comparisons by ANOVA; p-value | F = 3.1 p = 0.03 | F = 1.8 p = 0.15 |
| Dependent Variable/Parameters | Standardized β | p-Value | β | CI-95% β | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Average Biological Width † | |||||
| Constant (mm) | <0.001 | 3.5 | 2.9 | 4.1 | |
| Time of placement (immediate as reference) | −0.43 | 0.001 | −1.0 | −0.5 | −1.6 |
| Grade of surface treatment (none/half/full) | 1.0 | 0.007 | 1.1 | 0.3 | 1.9 |
| Average Marginal Bone Loss ‡ | |||||
| Constant (mm) | <0.001 | 0.8 | 0.6 | 1.1 | |
| Time of placement (immediate as reference) | −0.24 | 0.04 | −0.3 | −0.1 | −0.5 |
| Grade of surface treatment (none/half/full) | −0.83 | 0.02 | −0.4 | −0.1 | −0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, J.; Fernández-Ruiz, A.; Pardal-Peláez, B.; Jiménez-Guerra, A.; Velasco-Ortega, E.; Nicolás-Silvente, A.I.; Monsalve-Guil, L. Effect of Rough Surface Platforms on the Mucosal Attachment and the Marginal Bone Loss of Implants: A Dog Study. Materials 2020, 13, 802. https://doi.org/10.3390/ma13030802
Montero J, Fernández-Ruiz A, Pardal-Peláez B, Jiménez-Guerra A, Velasco-Ortega E, Nicolás-Silvente AI, Monsalve-Guil L. Effect of Rough Surface Platforms on the Mucosal Attachment and the Marginal Bone Loss of Implants: A Dog Study. Materials. 2020; 13(3):802. https://doi.org/10.3390/ma13030802
Chicago/Turabian StyleMontero, Javier, Alberto Fernández-Ruiz, Beatriz Pardal-Peláez, Alvaro Jiménez-Guerra, Eugenio Velasco-Ortega, Ana I. Nicolás-Silvente, and Loreto Monsalve-Guil. 2020. "Effect of Rough Surface Platforms on the Mucosal Attachment and the Marginal Bone Loss of Implants: A Dog Study" Materials 13, no. 3: 802. https://doi.org/10.3390/ma13030802
APA StyleMontero, J., Fernández-Ruiz, A., Pardal-Peláez, B., Jiménez-Guerra, A., Velasco-Ortega, E., Nicolás-Silvente, A. I., & Monsalve-Guil, L. (2020). Effect of Rough Surface Platforms on the Mucosal Attachment and the Marginal Bone Loss of Implants: A Dog Study. Materials, 13(3), 802. https://doi.org/10.3390/ma13030802







