Electroactive γ-Phase, Enhanced Thermal and Mechanical Properties and High Ionic Conductivity Response of Poly (Vinylidene Fluoride)/Cellulose Nanocrystal Hybrid Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. CNCs Synthesis and their Transfer from Water to DMF
2.3. Nanocomposite Fabrication
2.4. Characterization
3. Results and Discussion
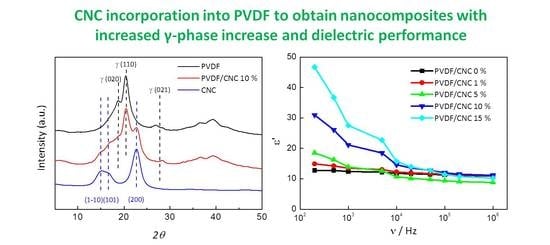
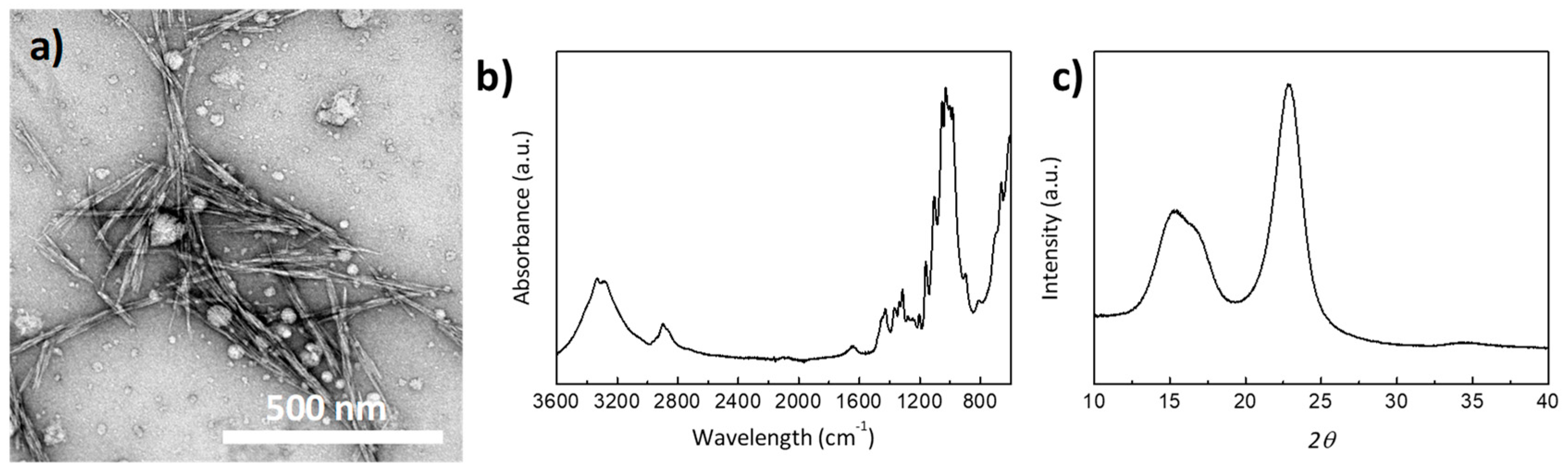
3.1. CNC Characterization
3.2. Morphology and Structure of PVDF/CNC Nanocomposites
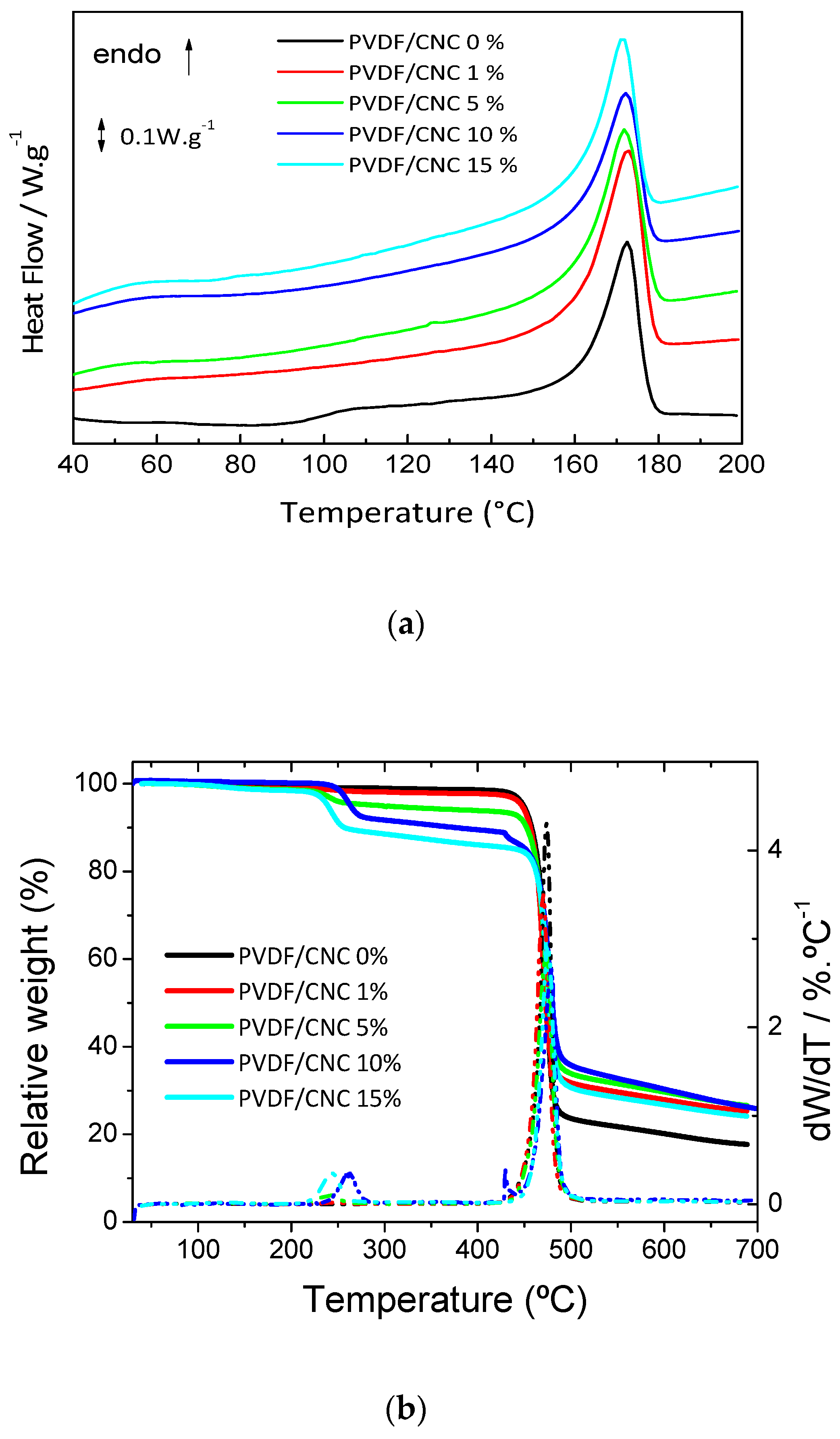
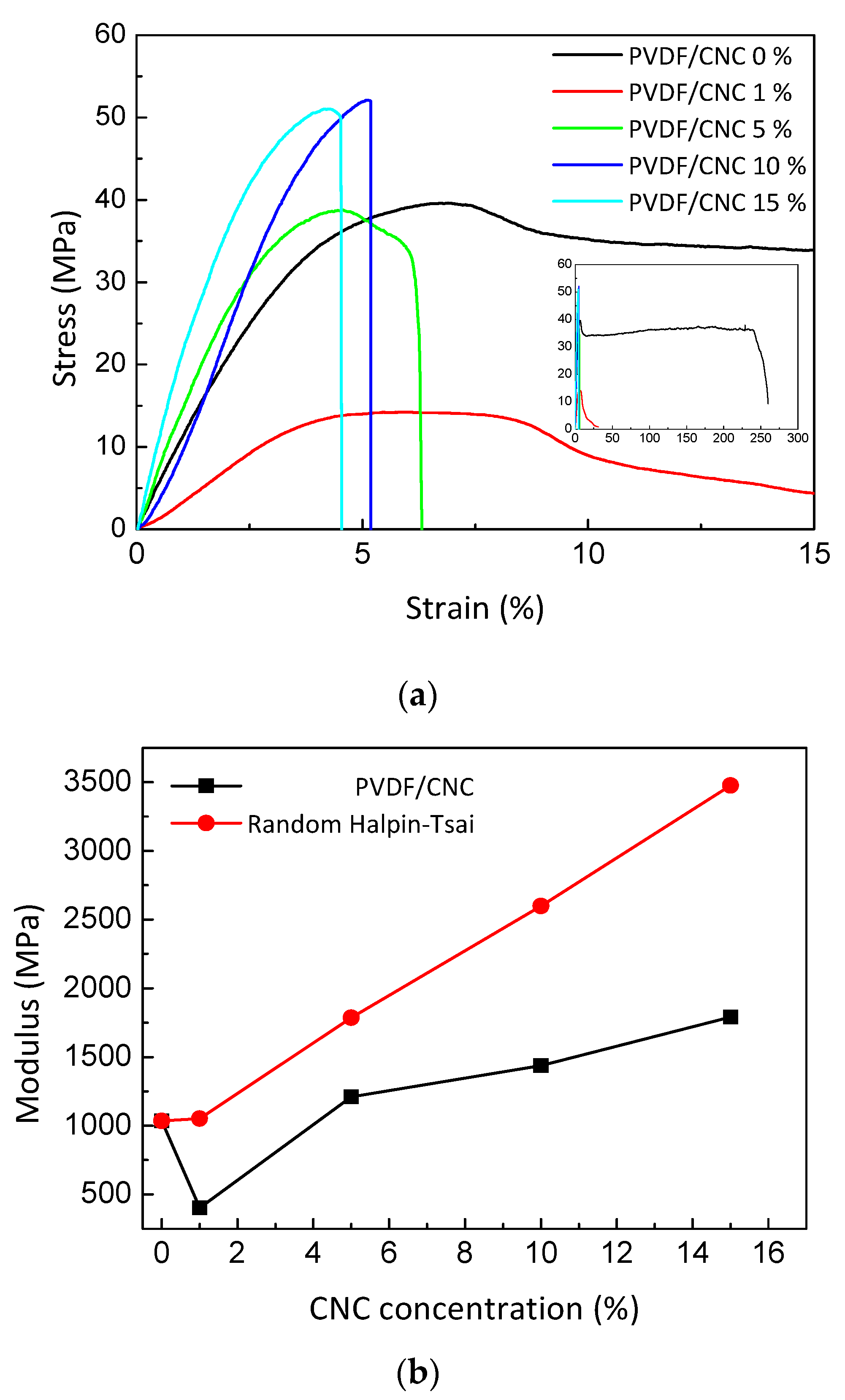
3.3. PVDF/CNC Thermal and Mechanical Properties
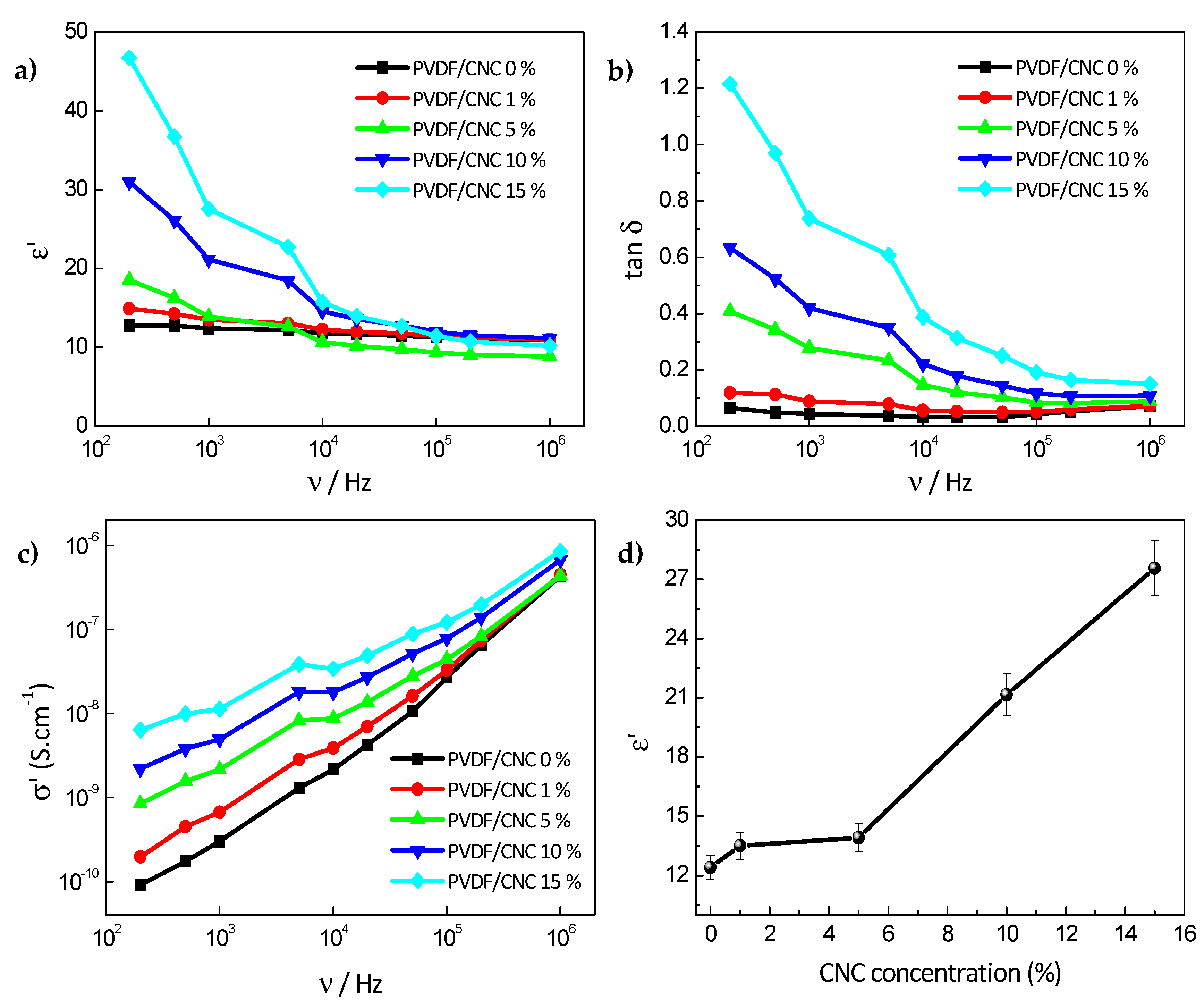
3.4. PVDF/CNC Dielectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, R.E. Crystallization and morphology of ultrathin films of homopolymers and polymer blends. Prog. Polym. Sci. 2016, 54–55, 214–231. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Kawai, H. The piezoelectricity of poly (vinylidene fluoride). Jpn. J. Appl. Phys. 1969, 8, 975–976. [Google Scholar] [CrossRef]
- Sencadas, V.; Gregorio, R.; Lanceros-Méndez, S. α to β phase transformation and microestructural changes of PVDF films induced by uniaxial stretch. J. Macromol. Sci. B 2009, 48, 514–525. [Google Scholar] [CrossRef]
- Satapathy, S.; Pawar, S.; Gupta, P.K.; Rvarma, K.B. Effect of annealing on phase transition in poly(vinylidene fluoride) films prepared using polar solvent. Bull. Mater. Sci. 2011, 34, 727–733. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, Y.; Yu, J.; Wu, Z.; Li, W.; Xu, J.; Xie, G. Study of thermally poled and corona charged poly(vinylidene fluoride) films. Polym. Eng. Sci. 2007, 47, 1344–1350. [Google Scholar] [CrossRef]
- Costa, P.; Silva, J.; Sencadas, V.; Costa, C.M.; van Hattum, F.W.J.; Rocha, J.G.; Lanceros-Mendez, S. The effect of fibre concentration on the α to β-phase transformation, degree of crystallinity and electrical properties of vapour grown carbon nanofibre/poly(vinylidene fluoride) composites. Carbon 2009, 47, 2590–2599. [Google Scholar] [CrossRef]
- Martins, P.; Costa, C.M.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S. On the origin of the electroactive poly(vinylidene fluoride) β-phase nucleation by ferrite nanoparticles via surface electrostatic interactions. CrystEngComm 2012, 14, 2807–2811. [Google Scholar] [CrossRef]
- Martín, J.; Zhao, D.; Lenz, T.; Katsouras, I.; de Leeuw, D.M.; Stingelin, N. Solid-state-processing of δ-PVDF. Mater. Horiz. 2017, 4, 408–414. [Google Scholar] [CrossRef]
- Lopes, A.C.; Costa, C.M.; Tavares, C.J.; Neves, I.C.; Lanceros-Mendez, S. Nucleation of the electroactive γ phase and enhancement of the optical transparency in low filler content poly(vinylidene)/clay nanocomposites. J. Phys. Chem. C 2011, 115, 18076–18082. [Google Scholar] [CrossRef]
- Ahn, Y.; Lim, J.Y.; Hong, S.M.; Lee, J.; Ha, J.; Choi, H.J.; Seo, Y. Enhanced piezoelectric properties of electrospun poly(vinylidene fluoride)/multiwalled carbon nanotube composites due to high β-phase formation in poly(vinylidene fluoride). J. Phys. Chem. C 2013, 117, 11791–11799. [Google Scholar] [CrossRef]
- Jia, N.; Xing, Q.; Xia, G.; Sun, J.; Song, R.; Huang, W. Enhanced β-crystalline phase in poly(vinylidene fluoride) films by polydopamine-coated BaTiO3 nanoparticles. Mater. Lett. 2015, 139, 212–215. [Google Scholar] [CrossRef]
- Xin, Y.; Qi, X.; Tian, H.; Guo, C.; Li, X.; Lin, J.; Wang, C. Full-fiber piezoelectric sensor by straight PVDF/nanoclay nanofibers. Mater. Lett. 2016, 164, 136–139. [Google Scholar] [CrossRef]
- Chen, J.; Wu, D.; Tam, K.C.; Pan, K.; Zheng, Z. Effect of surface modification of cellulose nanocrystal on nonisothermal crystallization of poly(β-hydroxybutyrate) composites. Carbohydr. Polym. 2017, 157, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.G. Transcrystallization of polypropylene at cellulose nanocrystal surfaces. Cellulose 2008, 15, 297–301. [Google Scholar] [CrossRef]
- Kashani Rahimi, S.; Aeinehvand, R.; Kim, K.; Otaigbe, J.U. Structure and biocompatibility of bioabsorbable nanocomposites of aliphatic-aromatic copolyester and cellulose nanocrystals. Biomacromolecules 2017, 18, 2179–2194. [Google Scholar] [CrossRef]
- Lizundia, E.; Vilas, J.L.; León, L.M. Crystallization, structural relaxation and thermal degradation in Poly(l-lactide)/cellulose nanocrystal renewable nanocomposites. Carbohydr. Polym. 2015, 123, 256–265. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Lizundia, E.; Goikuria, U.; Vilas, J.L.; Cristofaro, F.; Bruni, G.; Fortunati, E.; Armentano, I.; Visai, L.; Torre, L. Metal nanoparticles embedded in cellulose nanocrystal based films: Material properties and post-use analysis. Biomacromolecules 2018, 19, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chu, R.; Wu, R.; Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 2011, 12, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.G.; Sam, S.T.; Abdullah, M.F.B.; Omar, M.F. Thermal properties of nanocellulose-reinforced composites: A review. J. Appl. Polym. Sci. 2020, 137, 48544. [Google Scholar] [CrossRef]
- Myrian Silva, P.M.M.-B.; Aldás, V.V. Biopolymers based on polylactic acid and starch: An alternative for the preservation of the environment. DYNA 2017, 93, 581. [Google Scholar]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Kollmann, F.V.A. Bazhenov: Piezoelectric Properties of Wood; Berichte der Bunsengesellschaft für physikalische Chemie; Consultants Bureau: New York, NY, USA, 1961; Volume 66, pp. 522–523. [Google Scholar]
- Csoka, L.; Hoeger, I.C.; Rojas, O.J.; Peszlen, I.; Pawlak, J.J.; Peralta, P.N. Piezoelectric effect of cellulose nanocrystals thin films. ACS Macro Lett. 2012, 1, 867–870. [Google Scholar] [CrossRef]
- Bodkhe, S.; Rajesh, P.S.M.; Kamle, S.; Verma, V. Beta-phase enhancement in polyvinylidene fluoride through filler addition: Comparing cellulose with carbon nanotubes and clay. J. Polym. Res. 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Ejaz Ahmed, F.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen, E.; Arafat, H.A.; Hashaikeh, R. Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application. Desalination 2014, 332, 134–141. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Maadeed, M.; Luyt, A.S.; Mrlik, M.; Hassan, M.K. Investigation of the physico-mechanical properties of electrospun PVDF/cellulose (nano)fibers. J. Appl. Polym. Sci. 2016, 133, 43594. [Google Scholar] [CrossRef]
- Filpponen, I.; Argyropoulos, D.S. Regular Linking of Cellulose Nanocrystals via Click Chemistry: Synthesis and Formation of Cellulose Nanoplatelet Gels. Biomacromolecules 2010, 11, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, T.; Chang, P.R.; Li, K.; Wu, Q. Starch composites reinforced by bamboo cellulosic crystals. Bioresour. Technol. 2010, 101, 2529–2536. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Sencadas, V.; Gregorio Filho, R.; Lanceros-Mendez, S. Processing and characterization of a novel nonporous poly(vinilidene fluoride) films in the β phase. J. Non-Cryst. Solids 2006, 352, 2226–2229. [Google Scholar] [CrossRef]
- ASTM D882—18 Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2018; Available online: https://www.astm.org/Standards/D882 (accessed on 5 January 2020).
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Hamad, W.Y.; Hu, T.Q. Structure-process-yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; Leon, L.M.; Armentano, I.; Torre, L.; Kenny, J.M. PLLA-grafted cellulose nanocrystals: Role of the CNC content and grafting on the PLA bionanocomposite film properties. Carbohydr. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef]
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical, morphological and conducting properties of chiral nematic mesoporous cellulose/polypyrrole composite films. J. Mater. Chem. A 2017, 5, 19184–19194. [Google Scholar] [CrossRef]
- Kressler, J.; Schafer, R.; Thomann, R. Imaging of Semicrystalline Polymers and Polymer Blends by FT-IR Microspectroscopy. Appl. Spectrosc. 1998, 52, 1269–1273. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.N.; Huang, Z.G.; Weng, Y.-X. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Termonia, Y. Chain confinement in polymer nanocomposites and its effect on polymer bulk properties. J. Polym. Sci. Polym. Phys. 2010, 48, 687–692. [Google Scholar] [CrossRef]
- Lizundia, E.; Pérez-Álvarez, L.; Sáenz-Pérez, M.; Patrocionio, D.; Vilas, J.L.; Léon, L.M. Physical aging and mechanical performance of Poly (L-lactide)/ZnO nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43619. [Google Scholar] [CrossRef]
- Hietala, S.; Koel, M.; Skou, E.; Elomaa, M.; Sundholm, F. Thermal stability of styrene grafted and sulfonated proton conducting membranes based on poly(vinylidene fluoride). J. Mater. Chem. 1998, 8, 1127–1132. [Google Scholar] [CrossRef]
- Goikuria, U.; Larranaga, A.; Vilas, J.L.; Lizundia, E. Thermal stability increase in metallic nanoparticles-loaded cellulose nanocrystal nanocomposites. Carbohydr. Polym. 2017, 171, 193–201. [Google Scholar] [CrossRef]
- Ni, X.; Cheng, W.; Huan, S.; Wang, D.; Han, G. Electrospun cellulose nanocrystals/poly(methyl methacrylate) composite nanofibers: Morphology, thermal and mechanical properties. Carbohydr. Polym. 2019, 206, 29–37. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Progr. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Carfagna, C.; Ambrogi, V.; Avolio, R.; Cerruti, P.; Errico, M.E.; Gentile, G.; Nasti, G. Polymer nanocomposites: Functionalisation of the nanofiller and control of the interface. Adv. Mater. Proces. Technol. 2015, 1, 423–434. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Wang, X.; Wang, S. Effect of PMMA on crystallization behavior and hydrophilicity of poly(vinylidene fluoride)/poly(methyl methacrylate) blend prepared in semi-dilute solutions. Appl. Surf. Sci. 2007, 253, 8377–8388. [Google Scholar] [CrossRef]
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical luminescent nanostructured cellulose films. Mater. Chem. Front. 2017, 1, 979–987. [Google Scholar] [CrossRef]
- Aytomaki, Y.; Oksman, C. Reinforcing efficiency of nanocellulose in polymers. React. Funct. Polym. 2016, 85, 151–156. [Google Scholar] [CrossRef]
- Chi-Fung, L.V.; Kuang, X.; Mulyadi, A.; Hamel, C.M.; Deng, Y.; Jerry, Q.H. 3D printed cellulose nanocrystal composites through digital light processing. Cellulose 2019, 26, 3973–3985. [Google Scholar]
- Dunlop, M.J.; Acharya, B.; Bissessur, R. Study of plant and tunicate based nanocrystalline cellulose in hybrid polymeric nanocomposites. Cellulose 2020, 27, 249–261. [Google Scholar] [CrossRef]
- Affdl, J.C.H.; Kardos, J.L. The Halpin-Tsai equations: A review. Polym. Eng. Sci. 1976, 16, 344–352. [Google Scholar] [CrossRef]
- Lee, W.J.; Clancy, A.J.; Kontturi, E.; Bismarck, A.; Shaffer, M.S.P. Strong and stiff: High-performance cellulose nanocrystal/poly(vinyl alcohol) composite fibers. ACS Appl. Mater. Interfaces 2016, 8, 31500–31504. [Google Scholar] [CrossRef]
- Rusli, R.; Eichhorn, S.J. Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using Raman spectroscopy. Appl. Phys. Lett. 2008, 93, 033111. [Google Scholar] [CrossRef]
- Reddy, N.V.; Kumar, C.R.; Rao, V.V.R.N. Dielectric properties of amorphous cellulose acetate-butyrate polymer films. Polym. Int. 1993, 32, 381–384. [Google Scholar] [CrossRef]
- Lizundia, E.; Maceiras, A.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Magnetic cellulose nanocrystal nanocomposites for the development of green functional materials. Carbohydr. Polym. 2017, 175, 425–432. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.; Jia, C.; Liu, J.; Lv, Y.; Liu, Y.; Zhou, Y.; Liu, C.; Shao, Z. Cellulosic Biomass-Reinforced Polyvinylidene Fluoride Separators with Enhanced Dielectric Properties and Thermal Tolerance. ACS Appl. Mater. Interfaces 2017, 9, 20885–20894. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Liu, X.; Pei, Q.; Qian, J.; Zhuang, Q.; Han, Z. Preparation of solution-processable reduced graphene oxide/polybenzoxazole nanocomposites with improved dielectric properties. Macromolecules 2015, 48, 365–372. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, T.; Suk, J.W.; Chou, H.; Jang, J.H.; Lee, J.H.; Kholmanov, I.N.; Akinwande, D.; Ruoff, R.S. Enhanced dielectric performance in polymer composite films with carbon nanotube-reduced graphene oxide hybrid filler. Small 2014, 10, 3405–3411. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Shao, Z.; Fan, H.; Feng, R.; Wang, F.; Wang, W.; Wang, J.; Zhang, D.; Lv, Y. Barium titanate as a filler for improving the dielectric property of cyanoethyl cellulose/antimony tin oxide nanocomposite films. Compos. Part A Appl. Sci. Manuf. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘universal’ dielectric response. Nature 1977, 267, 673. [Google Scholar] [CrossRef]
- Ladhar, A.; Arous, M.; Kaddami, H.; Raihane, M.; Kallel, A.; Graça, M.P.F.; Costa, L.C. Ionic hopping conductivity in potential batteries separator based on natural rubber–nanocellulose green nanocomposites. J. Mol. Liq. 2015, 211, 792–802. [Google Scholar] [CrossRef]
- Abdel-karim, A.M.; Salama, A.H.; Hassan, M.L. Electrical conductivity and dielectric properties of nanofibrillated cellulose thin films from bagasse. J. Phys. Org. Chem. 2018, 31, e3851. [Google Scholar] [CrossRef]

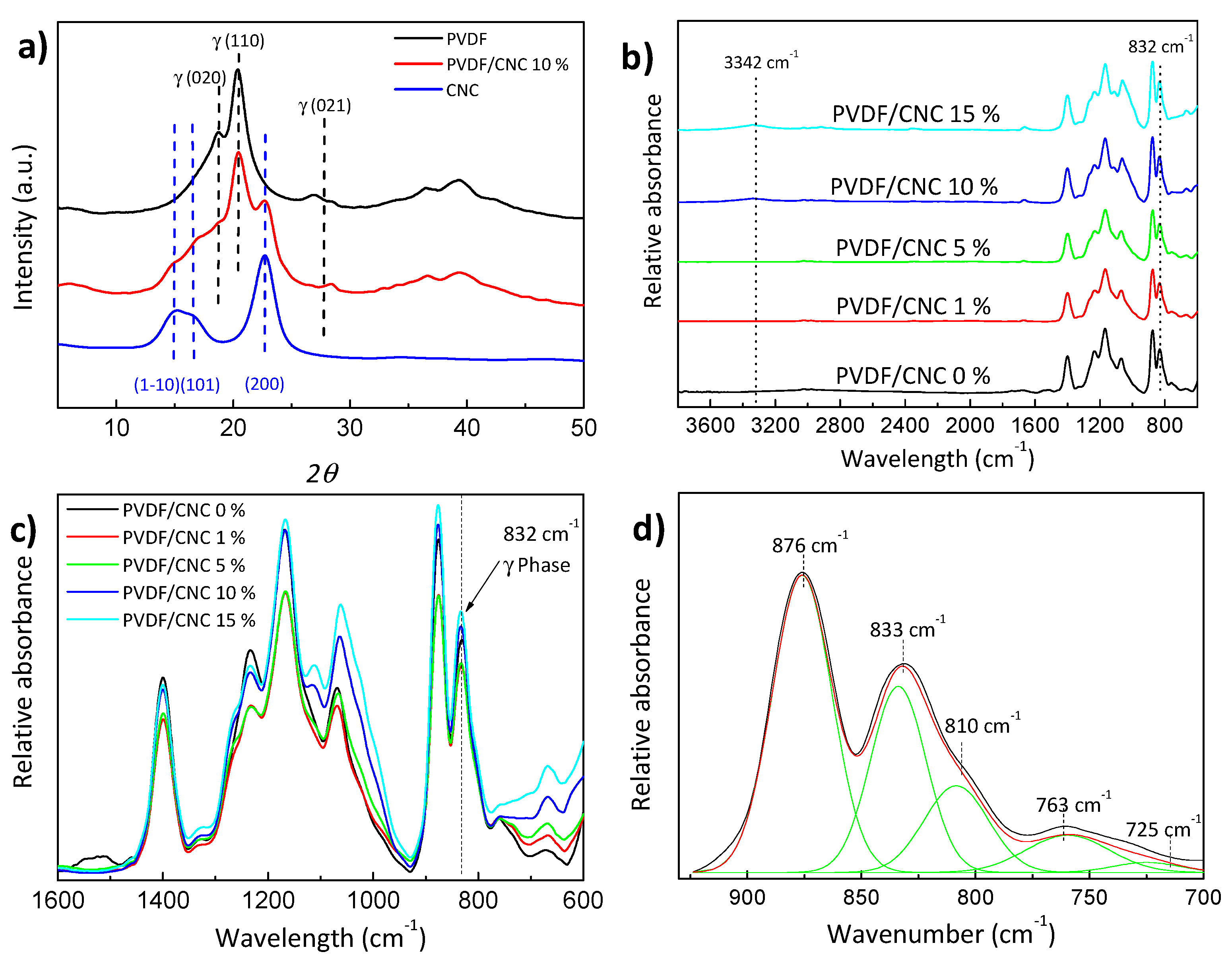
| Wavenumber (cm−1) | Material | Vibrational Mode |
|---|---|---|
| 897 | CNC | C–O–C asymmetric stretching |
| 1160 | CNC | C–O–C bending |
| 1337 | CNC | C–O–H bending |
| 3650–3200 | CNC | O–H stretching |
| 766 | PVDF | α-phase |
| 796 | PVDF | α-phase |
| 812 | PVDF | γ-phase |
| 833 | PVDF | γ-phase |
| 838 | PVDF | γ-phase |
| 840 | PVDF | β-phase |
| 855 | PVDF | α-phase |
| 976 | PVDF | α-phase |
| 1234 | PVDF | γ-phase |
| 1275 | PVDF | β-phase |
| Sample Name | Xc (%) | Tm (°C) | Tdpeak (°C) |
|---|---|---|---|
| PVDF/CNC 0 wt.% | 38 ± 2 | 173 | 473 |
| PVDF/CNC 1 wt.% | 53 ± 4 | 173 | 470 |
| PVDF/CNC 5 wt.% | 48 ± 2 | 172 | 471 |
| PVDF/CNC 10 wt.% | 43 ± 2 | 172 | 478 |
| PVDF/CNC 15 wt.% | 48 ± 3 | 171 | 470 |
| SampleN | E (MPa) | σy (MPa) | εb (%) |
|---|---|---|---|
| PVDF/CNC 0 wt.% | 1035 ± 170 | 39.6 | 250 |
| PVDF/CNC 1 wt.% | 427 ± 63 | 14.3 | 9.5 |
| PVDF/CNC 5 wt.% | 1249± 166 | 39.2 | 6.3 |
| PVDF/CNC 10 wt.% | 1410± 123 | 52.7 | 5.2 |
| PVDF/CNC 15 wt.% | 1662 ± 47 | 51.1 | 4.5 |
| Sample Name | n |
|---|---|
| PVDF/CNC 0 wt.% | 0.5 |
| PVDF/CNC 1 wt.% | 0.6 |
| PVDF/CNC 5 wt.% | 0.7 |
| PVDF/CNC 10 wt.% | 0.9 |
| PVDF/CNC 15 wt.% | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizundia, E.; Reizabal, A.; Costa, C.M.; Maceiras, A.; Lanceros-Méndez, S. Electroactive γ-Phase, Enhanced Thermal and Mechanical Properties and High Ionic Conductivity Response of Poly (Vinylidene Fluoride)/Cellulose Nanocrystal Hybrid Nanocomposites. Materials 2020, 13, 743. https://doi.org/10.3390/ma13030743
Lizundia E, Reizabal A, Costa CM, Maceiras A, Lanceros-Méndez S. Electroactive γ-Phase, Enhanced Thermal and Mechanical Properties and High Ionic Conductivity Response of Poly (Vinylidene Fluoride)/Cellulose Nanocrystal Hybrid Nanocomposites. Materials. 2020; 13(3):743. https://doi.org/10.3390/ma13030743
Chicago/Turabian StyleLizundia, Erlantz, Ander Reizabal, Carlos M. Costa, Alberto Maceiras, and Senentxu Lanceros-Méndez. 2020. "Electroactive γ-Phase, Enhanced Thermal and Mechanical Properties and High Ionic Conductivity Response of Poly (Vinylidene Fluoride)/Cellulose Nanocrystal Hybrid Nanocomposites" Materials 13, no. 3: 743. https://doi.org/10.3390/ma13030743
APA StyleLizundia, E., Reizabal, A., Costa, C. M., Maceiras, A., & Lanceros-Méndez, S. (2020). Electroactive γ-Phase, Enhanced Thermal and Mechanical Properties and High Ionic Conductivity Response of Poly (Vinylidene Fluoride)/Cellulose Nanocrystal Hybrid Nanocomposites. Materials, 13(3), 743. https://doi.org/10.3390/ma13030743