Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis
Abstract
1. Introduction
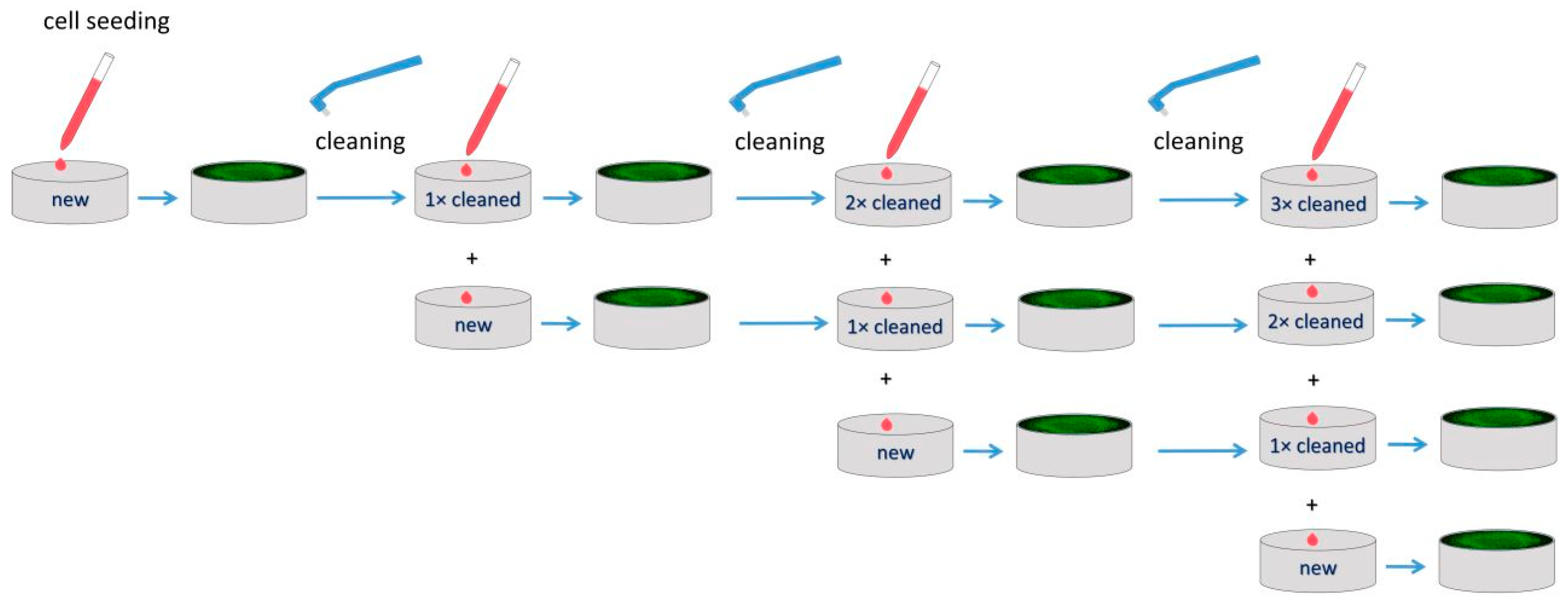
2. Materials and Methods
2.1. Material
2.2. Characterization of Surfaces
2.3. Cell Cultures
2.4. Cell Adhesion and Proliferation
2.5. Cell Staining
2.6. Statistical Analysis
3. Results
3.1. Roughness and Wettability
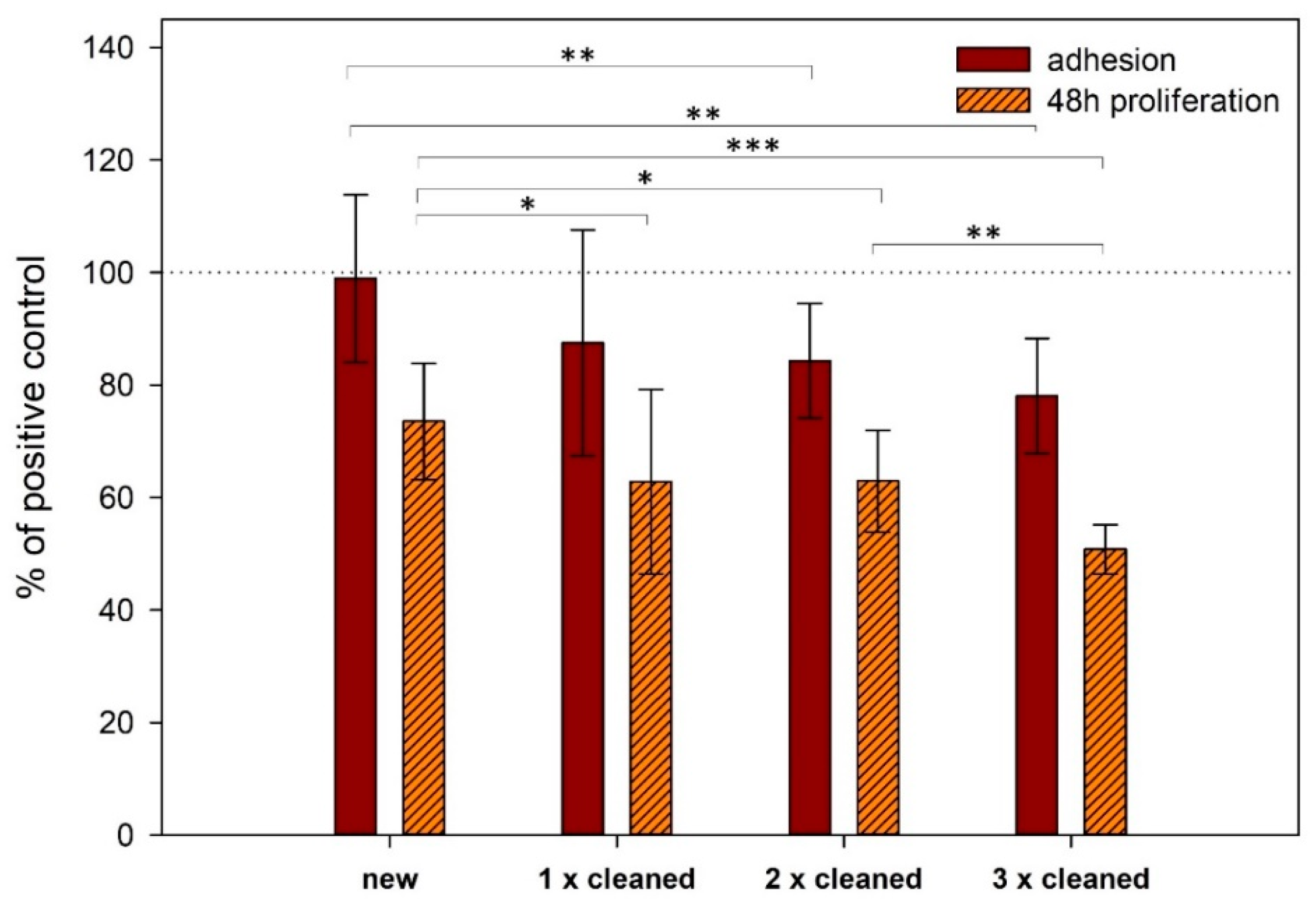
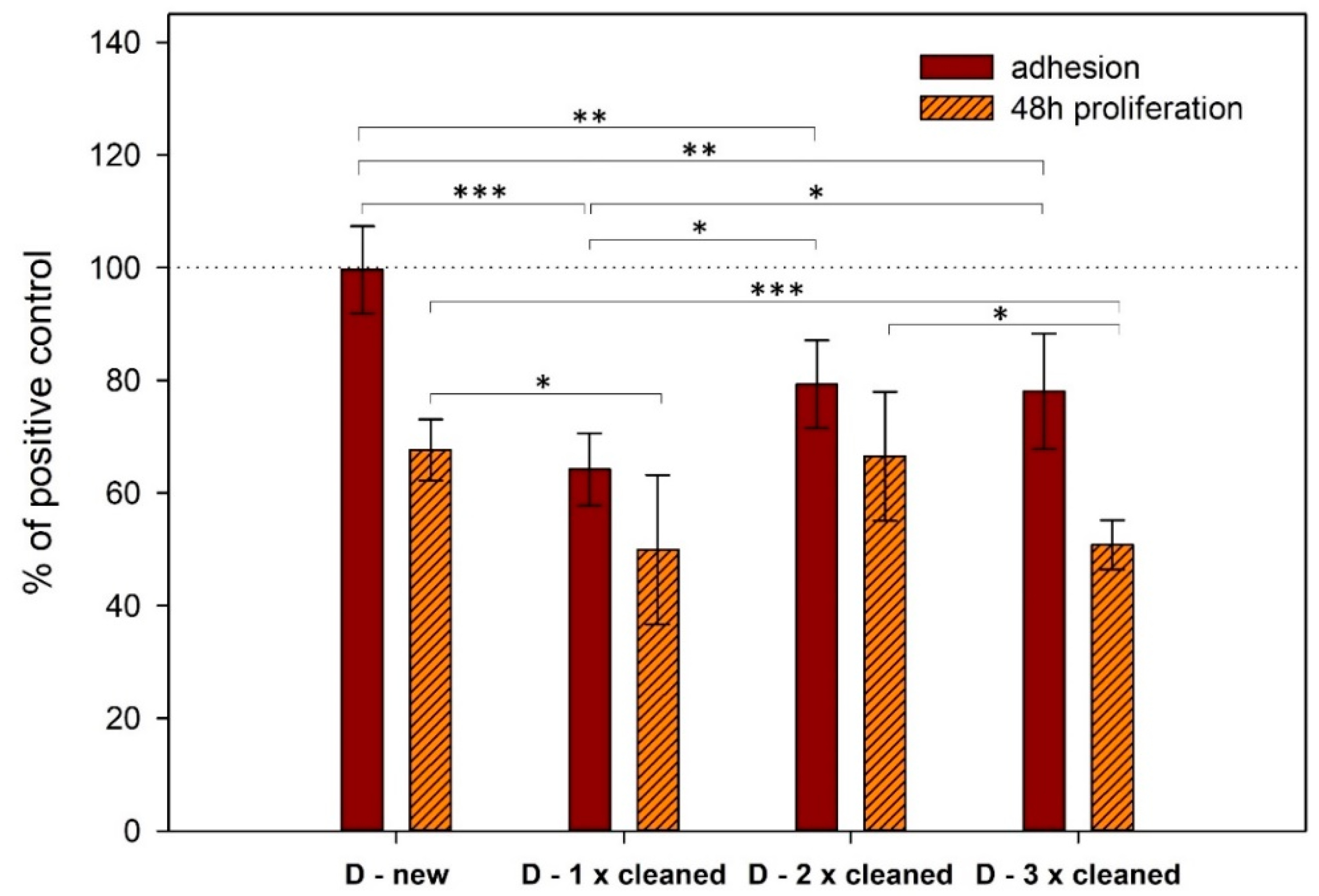
3.2. Cell Adhesion and Proliferation
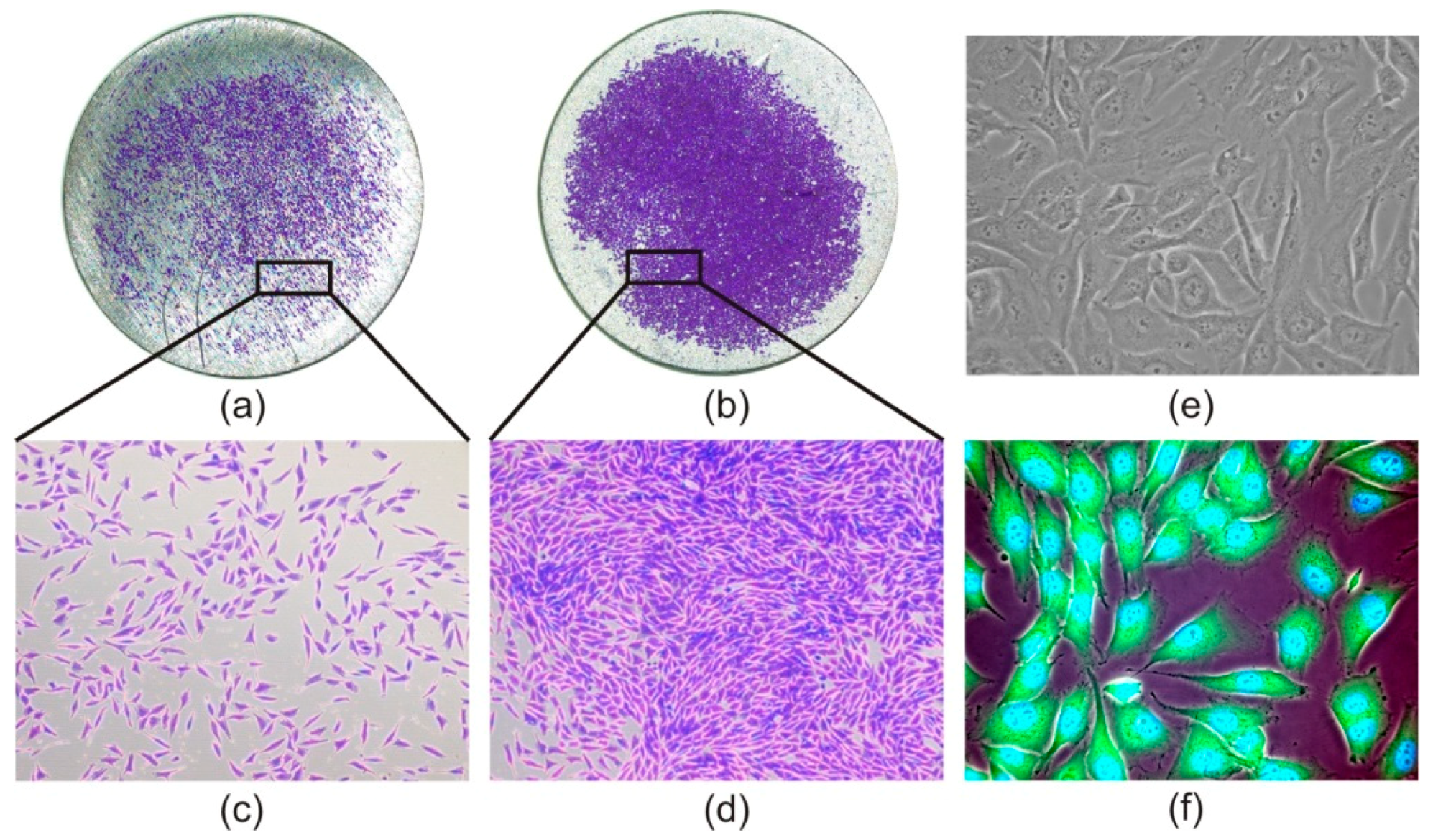
3.3. Cell Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Babuska, V.; Dobra, J.; Kulda, V.; Kripnerova, M.; Moztarzadeh, A.; Bolek, L.; Lahoda, J.; Hrusak, D. Comparison of fibroblast and osteoblast response to cultivation on titanium implants with different grain sizes. J. Nanomater. 2015, 2015, 920893. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C. Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Qarni, M.J.; Sivaswamy, G.; Rosochowski, A.; Boczkal, S. On the evolution of microstructure and texture in commercial purity titanium during multiple passes of incremental equal channel angular pressing (I-ECAP). Mater. Sci. Eng. A 2017, 699, 31–47. [Google Scholar] [CrossRef]
- Novak, Z. Periimplantitis, problems and solutions. Quintessenz 2004, 13, 1–4. [Google Scholar]
- Persson, G.R.; Renvert, S. Cluster of Bacteria Associated with Peri-Implantitis: Pathogens in Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- de Waal, Y.C.; Eijsbouts, H.V.; Winkel, E.G.; van Winkelhoff, A.J. Microbial Characteristics of Peri-Implantitis: A Case-Control Study. J. Periodontol. 2017, 88, 209–217. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group 4. Int. Dent. J. 2019, 69, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.T.; Khoury, F.; Keeve, P.L.; Schwarz, F.; Ramanauskaite, A.; Sculean, A.; Romanos, G. Implant Surface Decontamination by Surgical Treatment of Periimplantitis: A Literature Review. Implant Dent. 2019, 28, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Zigdon, H.; Coelho, P.G.; Suzuki, M.; Machtei, E.E. Reimplantation of Dental Implants following Ligature-Induced Peri-Implantitis: A Pilot Study in Dogs: Reimplantation following Ligature-Induced Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2013, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.G.; Ericsson, I.; Berglundh, T.; Lindhe, J. Osseintegration following treatment of peri-implantitis and replacement of implant components. An experimental study in the dog. J. Clin. Periodontol. 2001, 28, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, F.; Monje, A.; Elnayef, B.; Huang, W.; Wu, Y. Feasibility of Dental Implant Replacement in Failed Sites: A Systematic Review. Int. J. Oral Maxillofac. Implants 2016, 31, 535–545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I.; Maguire, R. Re-osseointegration on previously contaminated surfaces: A systematic review. Clin. Oral Implants Res. 2009, 20, 216–227. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Z.; Pan, W.L.; Wang, H.L. The effect of supportive care in preventing peri-implant diseases and implant loss: A systematic review and meta-analysis. Clin. Oral Implants Res. 2019, 30, 714–724. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y. Producing Bulk Ultrafine-Grained Materials by Severe Plastic Deformation: Ten Years Later. JOM 2016, 68, 1216–1226. [Google Scholar] [CrossRef]
- Nazarov, D.; Zemtsova, E.; Solokhin, A.; Valiev, R.; Smirnov, V. Modification of the Surface Topography and Composition of Ultrafine and Coarse Grained Titanium by Chemical Etching. Nanomaterials 2017, 7, 15. [Google Scholar] [CrossRef]
- Valente, N.A.; Andreana, S. Peri-implant disease: What we know and what we need to know. J. Periodontal Implant Sci. 2016, 46, 136–151. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglič, V.; Milosev, I.; Schmuki, P.; Iglic, A.; Mozetic, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Palan, J.; Kolaja Dobra, J.; Kulda, V.; Duchek, M.; Cerny, J.; Hrusak, D. Proliferation of Osteoblasts on Laser-Modified Nanostructured Titanium Surfaces. Materials 2018, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Pilet, P.; Guicheux, J.; Weiss, P.; Louarn, G.; Layrolle, P. Behaviour of mesenchymal stem cells, fibroblasts and osteoblasts on smooth surfaces. Acta Biomater. 2011, 7, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, L.; Munar, M.L.; Ishikawa, K. Hydrothermal treatment for TiN as abrasion resistant dental implant coating and its fibroblast response. Mater. Sci. Eng. C 2015, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74A, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Eichler, M.; Geis-Gerstorfer, J. Wetting behavior of dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 1256–1266. [Google Scholar] [PubMed]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. EPL 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and Roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Marmur, A. A guide to the equilibrium contact angles maze. In Contact Angle Wettability and Adhesion; CRC Press: Boca Raton, FL, USA, 2009; Volume 6, pp. 3–18. [Google Scholar]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral Implants Res. 2008, 19, 233–241. [Google Scholar] [CrossRef]
- Areid, N.; Peltola, A.; Kangasniemi, I.; Ballo, A.; Närhi, T.O. Effect of ultraviolet light treatment on surface hydrophilicity and human gingival fibroblast response on nanostructured titanium surfaces. Clin. Exp. Dent. Res. 2018, 4, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Balakrishnan, A.; Lee, B.C.; Kim, W.S.; Smetana, K.; Park, J.K.; Panigrahi, B.B. In vitro biocompatibility of equal channel angular processed (ECAP) titanium. Biomed. Mater. 2007, 2, S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Le Guehennec, L.; Lopez-Heredia, M.-A.; Enkel, B.; Weiss, P.; Amouriq, Y.; Layrolle, P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008, 4, 535–543. [Google Scholar] [CrossRef] [PubMed]
- van Wachem, P.B.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Babuska, V.; Moztarzadeh, O.; Kubikova, T.; Moztarzadeh, A.; Hrusak, D.; Tonar, Z. Evaluating the osseointegration of nanostructured titanium implants in animal models: Current experimental methods and perspectives (Review). Biointerphases 2016, 11, 030801. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]

| Implants | Group A | Group B | Group C | Group D | ||||
|---|---|---|---|---|---|---|---|---|
| New | 1× Cleaned | New | 2× Cleaned | New | 3× Cleaned | New | 4× Cleaned | |
| Ra (µm) | 0.358 | 0.316 | 0.359 | 0.322 | 0.393 | 0.342 | 0.400 | 0.346 |
| Rq (µm) | 0.504 | 0.462 | 0.506 | 0.443 | 0.565 | 0.473 | 0.521 | 0.517 |
| (°) | 75.9 | 71.3 | 77.9 | 63.6 | 81.1 | 68.8 | 72.5 | 65.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babuska, V.; Kolaja Dobra, J.; Dluhos, L.; Dvorakova, J.; Moztarzadeh, J.; Hrusak, D.; Kulda, V. Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis. Materials 2020, 13, 697. https://doi.org/10.3390/ma13030697
Babuska V, Kolaja Dobra J, Dluhos L, Dvorakova J, Moztarzadeh J, Hrusak D, Kulda V. Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis. Materials. 2020; 13(3):697. https://doi.org/10.3390/ma13030697
Chicago/Turabian StyleBabuska, Vaclav, Jana Kolaja Dobra, Ludek Dluhos, Jana Dvorakova, Jana Moztarzadeh, Daniel Hrusak, and Vlastimil Kulda. 2020. "Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis" Materials 13, no. 3: 697. https://doi.org/10.3390/ma13030697
APA StyleBabuska, V., Kolaja Dobra, J., Dluhos, L., Dvorakova, J., Moztarzadeh, J., Hrusak, D., & Kulda, V. (2020). Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis. Materials, 13(3), 697. https://doi.org/10.3390/ma13030697







