Inclusion Characteristics in 95CrMo Steels with Different Calcium and Sulfur Contents
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
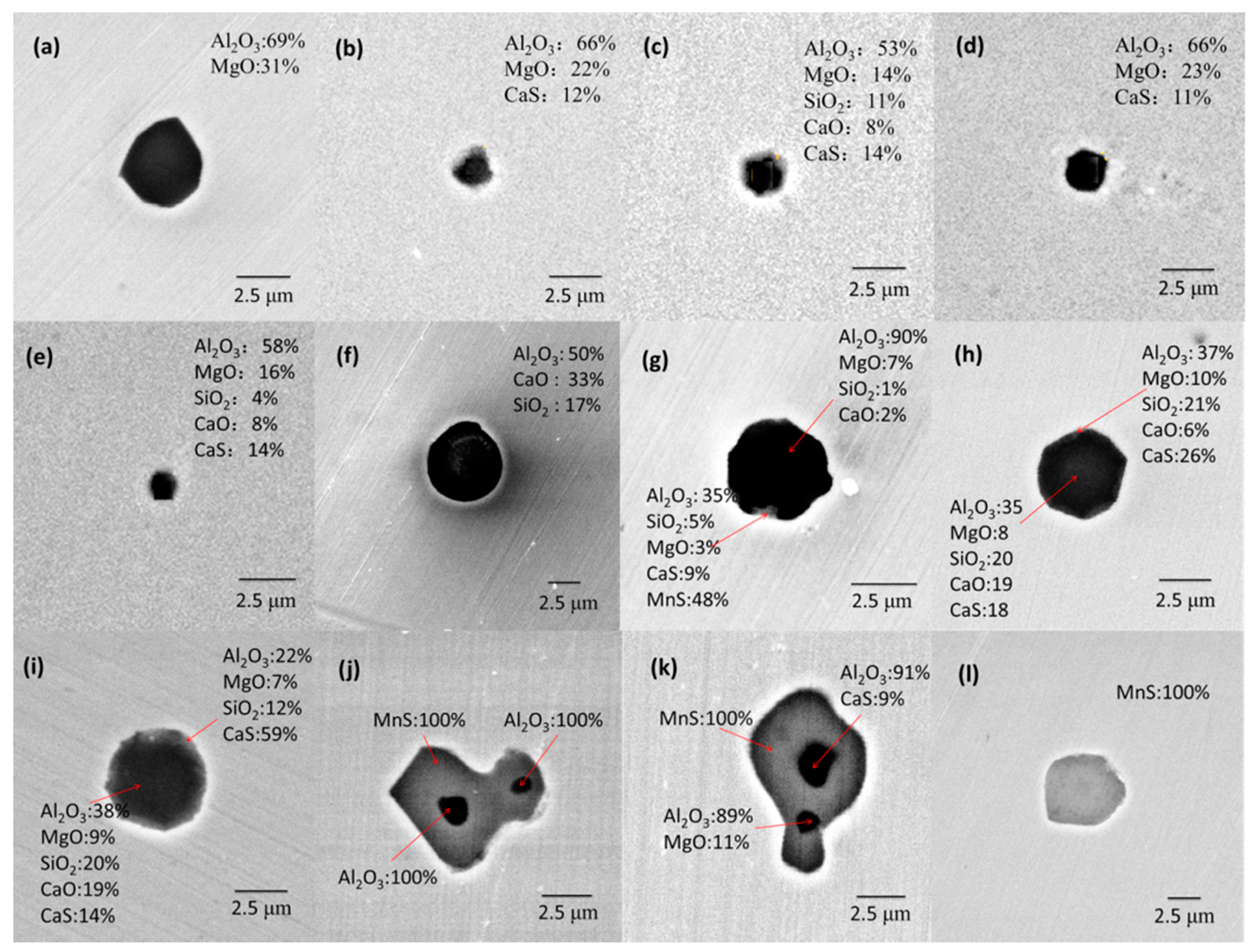
3.1. Morphologies and Compositions of Inclusion
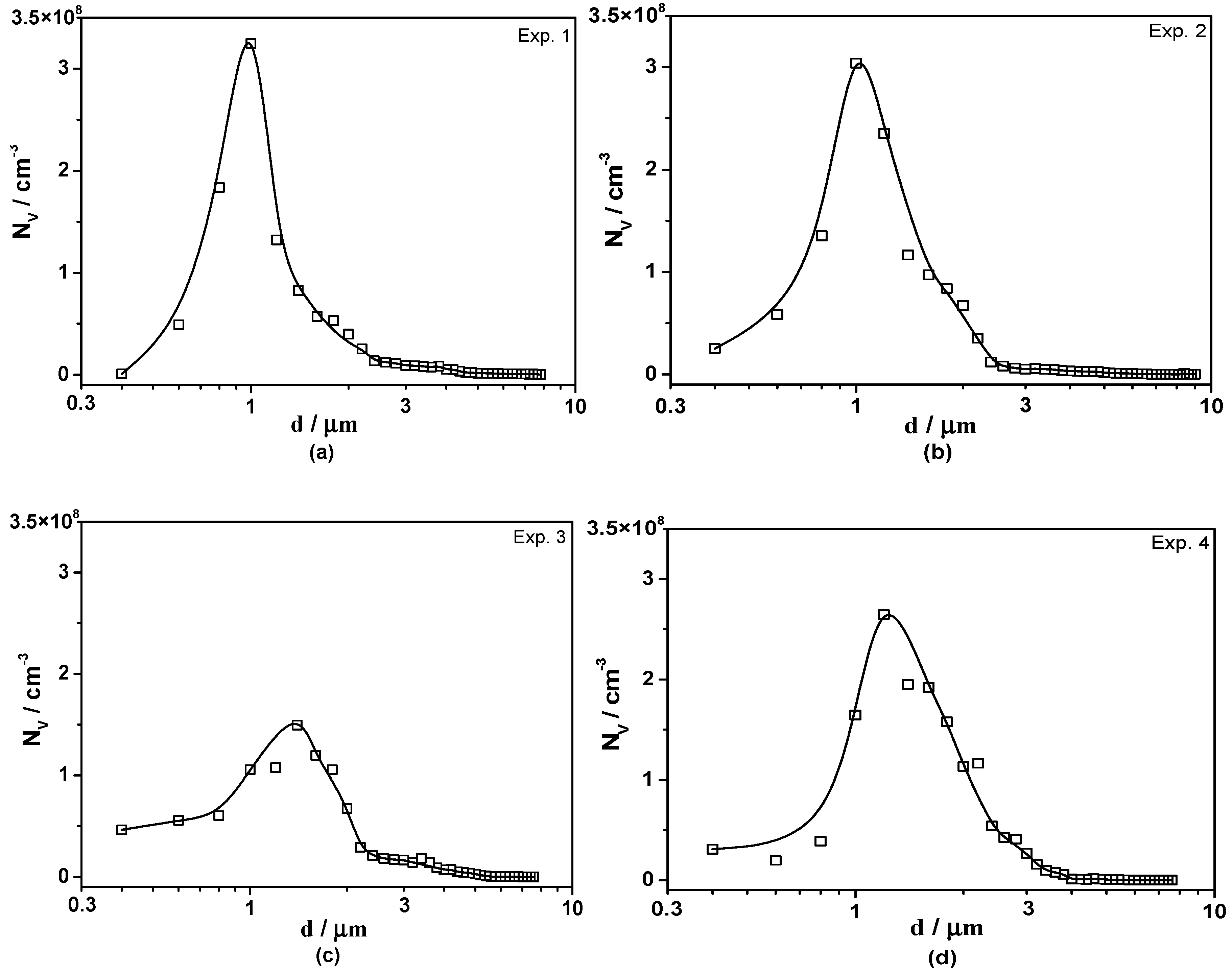
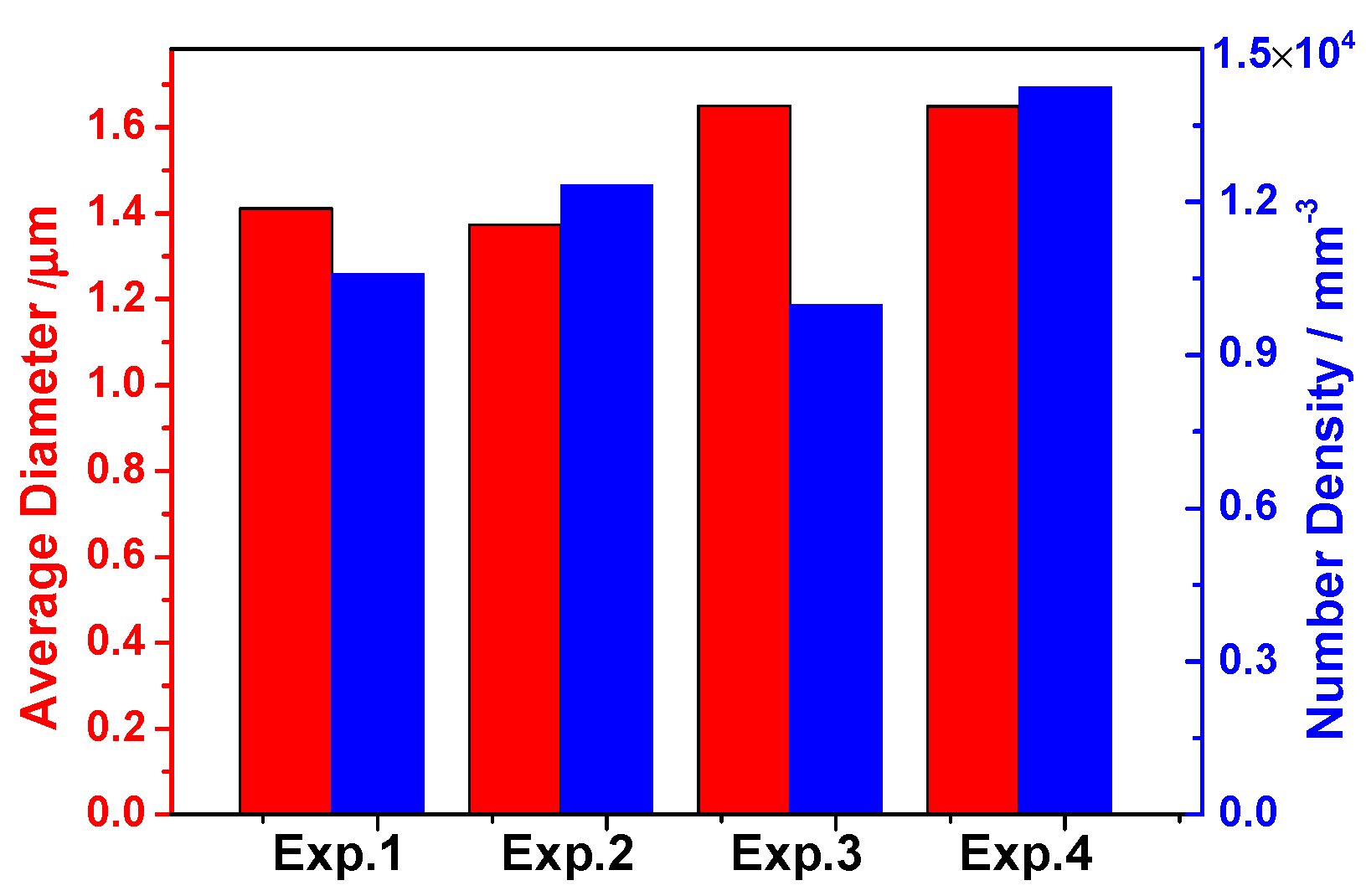
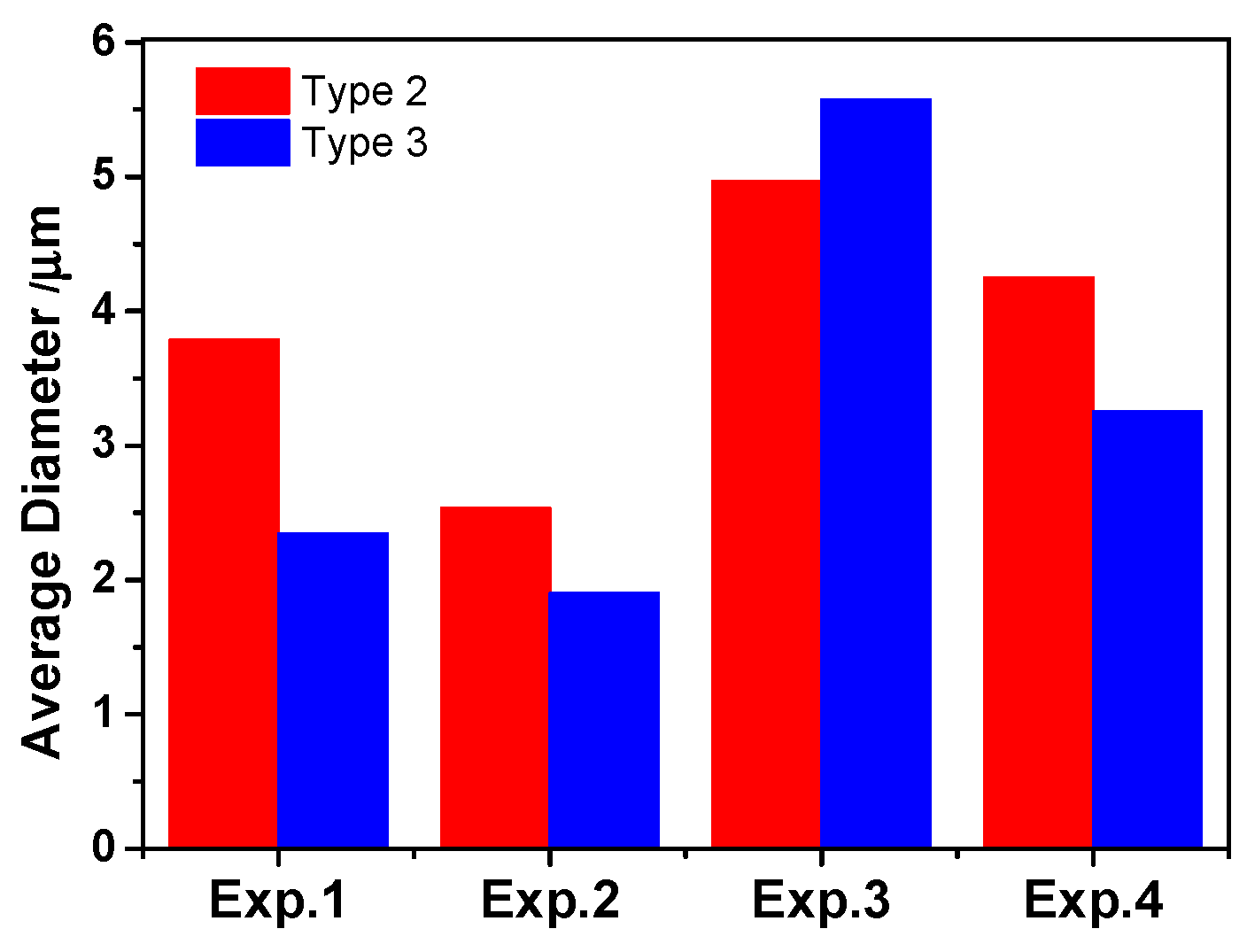
3.2. Size and Number Density of Inclusions
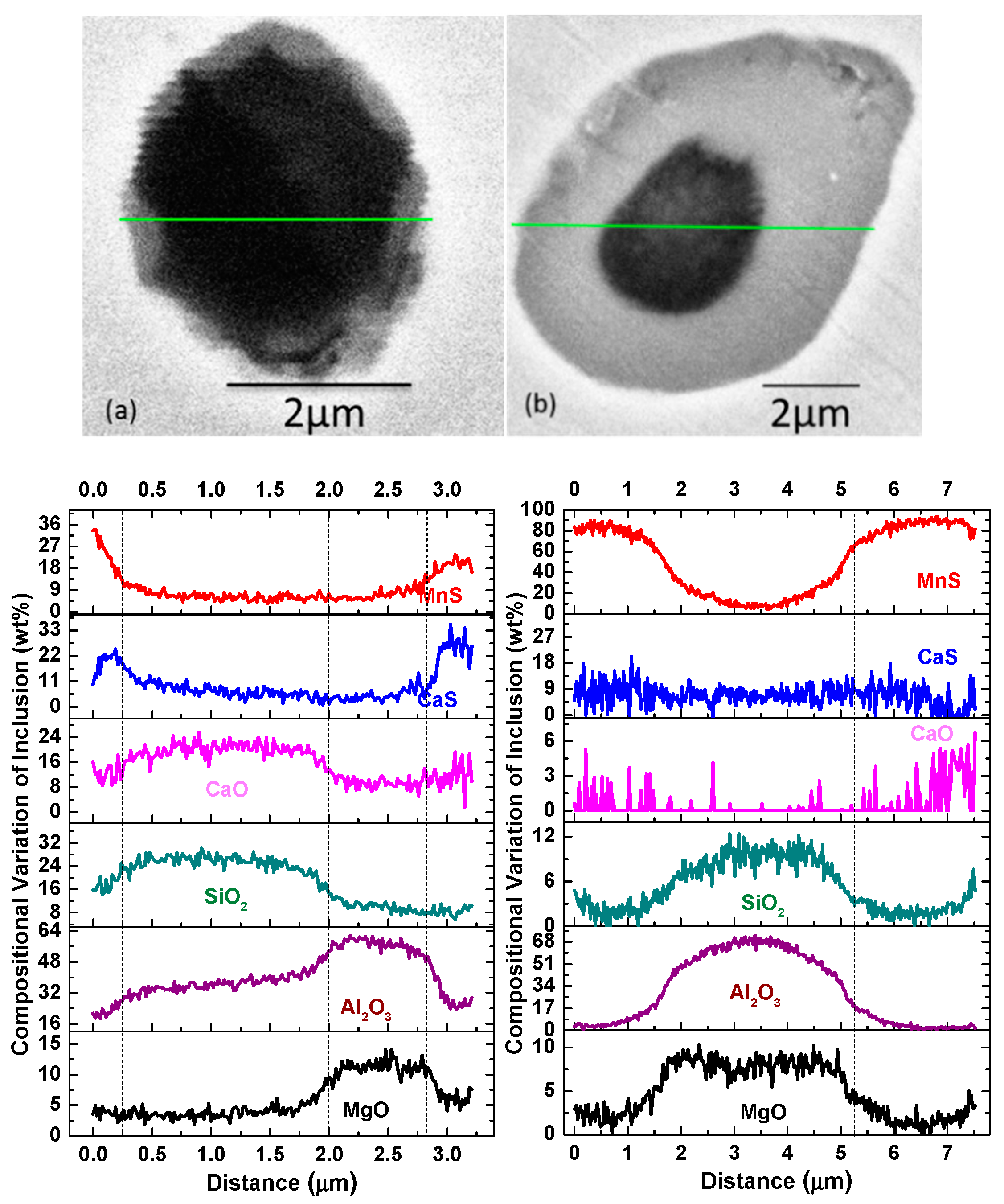
3.3. Inclusion Distribution
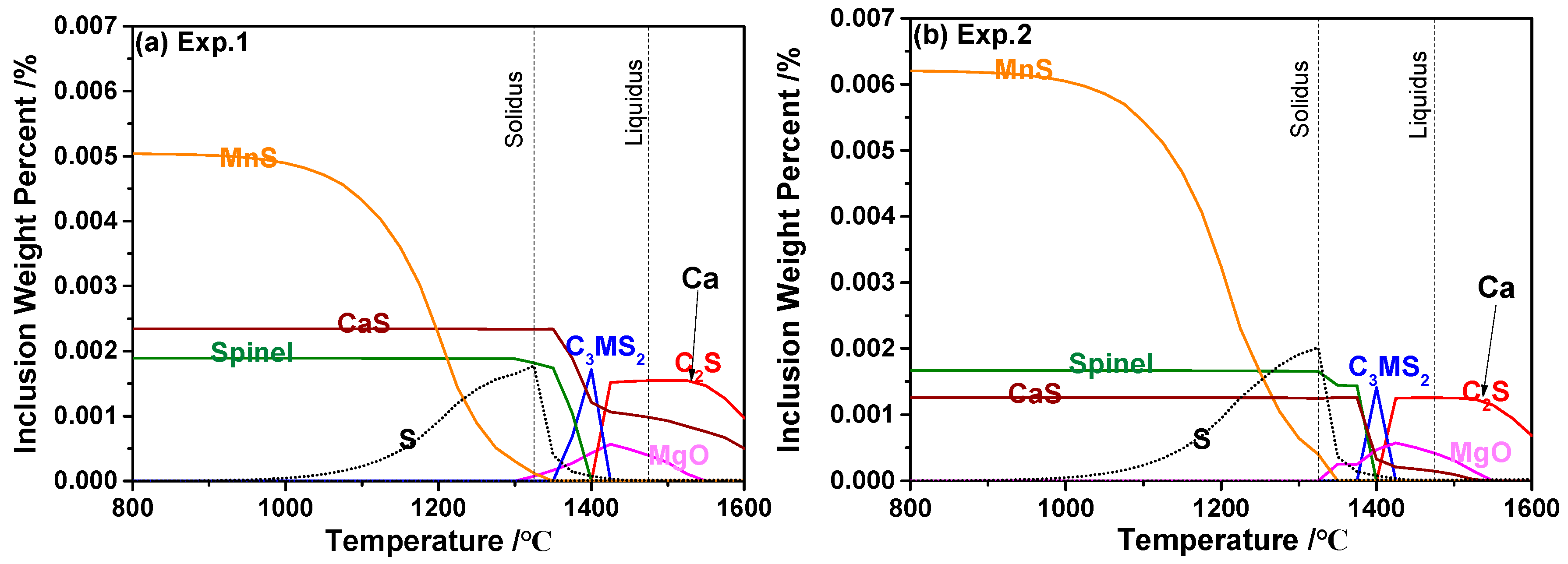
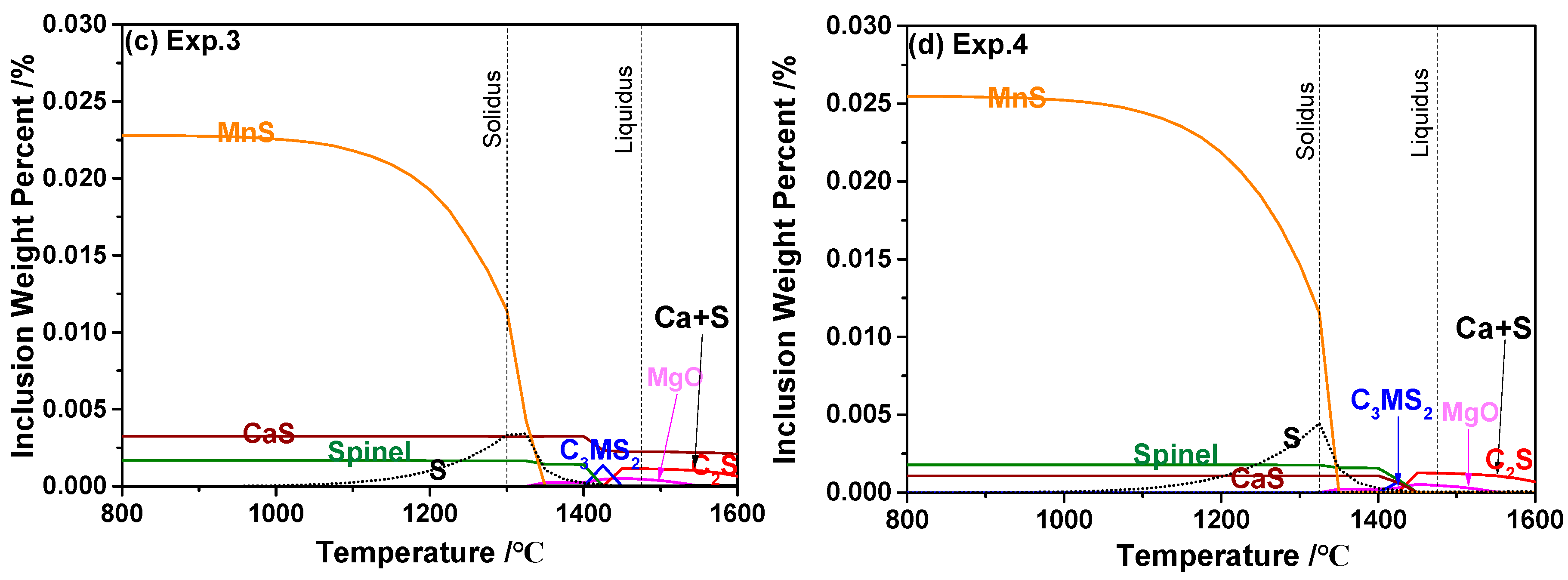
3.4. Formation Mechanism of Inclusions
- (1)
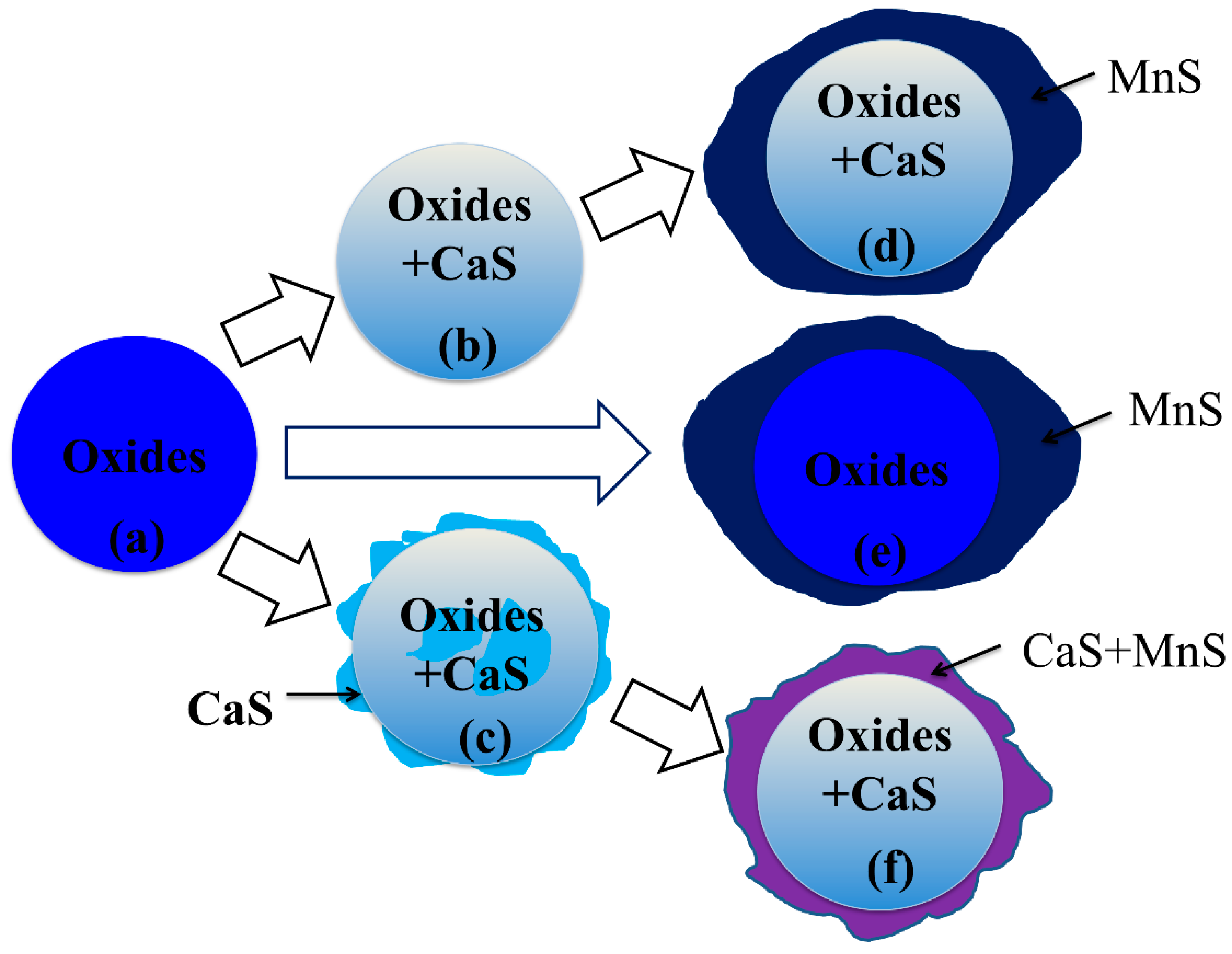
- Type-1 inclusions include pure oxides, as illustrated in Figure 10a, and the complex oxide–sulfide inclusions, as illustrated in Figure 10b. The pure oxides were formed during the refining process or precipitated during the cooling process. The inclusions in Figure 10b composed of oxides and CaS formed during the transformation process of inclusions as reaction [4]. The generated CaS dissolved in the oxides, and the solubility degree of CaS in calcium aluminate was high;
- (2)
- Type-2 inclusions are pure MnS which nucleated homogeneously from about 1350 °C;
- (3)
- Type-3 inclusions are inclusions with multi-phase, as illustrated in Figure 10c–f. The inclusions surrounded with a CaS shell always form when the amount of CaS increases to the extent that the CaS content is more than its supersaturation degree in oxide, as illustrated in Figure 10c. Some CaS detected in the core of inclusions may be due to the aggregation or uneven distribution of S during its dissolution in liquid steel. MnS nucleated around pure oxides and complex oxides–CaS inclusions heterogeneously after about 1350 °C, as illustrated in Figure 10d–f. The calculation of lattice disregistry based on Equation (5) [40] indicated that the disregistry between MgAl2O4 and MnS had the lowest value of 4.1 [15] and that for Al2O3 was 26.9, which facilitated the heterogeneous nucleation of MnS.where (hkl)s is a low-index plane of the substrate, [uvw]s is a low-index direction in (hkl)s plane, (hkl)n is a low-index plane of the nucleated solid, [uvw]n is a low-index direction in (hkl)n plane, d[uvw]s is the distance between a nonmetallic element along [uvw]s, d[hkl]s is the distance between a nonmetallic element along [hkl]n, and θ is the angle between [uvw]s and [uvw]n.
4. Conclusions
- (1)
- The three-dimensional inclusion size distribution suggests that there were more Type-1 inclusions with a small size in low S containing steels. The average diameter of all types of inclusions increased with increasing Ca or S content in 95CrMo steel, indicating that the formation of MnS and CaS coarsened their size;
- (2)
- The density distribution of inclusions indicates that the more inclusions there are, the more easily they aggregate and collide. Moreover, it is presumably concluded that the formation of sulfide in the outer layer of oxide inclusions weaken the attraction between oxide inclusions;
- (3)
- The thermodynamic calculation indicates that the equilibrated transformation trajectory of inclusions in 95CrMo steel during the cooling process is Ca2SiO4 + MgO → Ca3MgSi2O8 → Spinel + CaS. The precipitation temperature of the CaS phase was higher in the steel containing high Ca, and its amount increased with the increasing concentration of Ca and S. The segregation of S in γ-Fe promotes the formation of MnS, not CaS. Furthermore, the amount of MnS was larger in the experiments with higher S concentration;
- (4)
- The formation mechanism on three types of inclusions was discussed. The inclusions surrounded with a CaS shell always formed when the amount of CaS increased to the extent that CaS content was more than its supersaturation degree in oxide. The low disregistry for MnS–spinel and MnS–Al2O3 facilitated the heterogeneous nucleation of MnS.
Author Contributions
Funding
Conflicts of Interest
References
- Kusano, Y.; Kawauchi, Y.; Wajima, M.; Sugawara, K.; Yoshida, M.; Hayashi, H. Calcium Treatment Technologies for Special Steel Bars and Wire Rods. ISIJ Int. 1996, 36, S77–S80. [Google Scholar] [CrossRef] [Green Version]
- Janke, D.; Ma, Z.; Valentin, P.; Heinen, A. Improvement of Castability and Quality of Continuously Cast Steel. ISIJ Int. 2000, 40, 31–39. [Google Scholar] [CrossRef]
- Guo, Y.M.; Xu, Z.B.; Wang, H.T. Research on inclusions in Al-killed steel during LF refining. Iron Steel Vanadium Titan. 2007, 28, 14–18. [Google Scholar]
- Zheng, L.; Malfliet, A.; Wollants, P.; Blanpain, B.; Guo, M. Effect of Surfactant Te on the Formation of MnS Inclusions in Steel. Metall. Mater. Trans. B 2017, 48, 2447–2458. [Google Scholar] [CrossRef]
- Yashiki, H.; Kaneko, T. Effects of Mn and S on the grain growth and texture in cold rolled 0.5% Si steel. ISIJ Int. 1990, 30, 325–330. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, X.; Ren, Y.; Liu, X.; Shan, Q. Characteristics of Inclusions in Low Carbon Al-Killed Steel during Ladle Furnace Refining and Calcium Treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yang, S.; Li, J.; Ni, H.; Zhang, X. Solid-State Reaction Between Fe-Al-Ca Alloy and Al2O3-CaO-FeO Oxide During Heat Treatment at 1473K (1200 °C). Metall. Mater. Trans. B 2017, 48, 1348–1357. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhang, Y.; Yang, W.; Chen, W. Transient Evolution of Nonmetallic Inclusions during Calcium Treatment of Molten Steel. Metall. Mater. Trans. B 2018, 49, 1841–1859. [Google Scholar] [CrossRef]
- Liu, C.; Ni, H.; Yang, S.; Li, J.; Ye, F. Interfacial reaction mechanism between multi-component oxides and solid alloys deoxidised by Mn and Si during heat treatment. Ironmak. Steelmak. 2018, 45, 195–203. [Google Scholar] [CrossRef]
- Khurana, B.; Spooner, S.; Rao, M.B.V.; Roy, G.G.; Srirangam, P. In situ Observation of Calcium Oxide Treatment of Inclusions in Molten Steel by Confocal Microscopy. Metall. Mater. Trans. B 2017, 48, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, J.; Yang, S.; Chen, C.; Jin, H.; Li, X. Nucleation and Ostwald Growth of Particles in Fe-O-Al-Ca Melt. Sci. Rep. 2018, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Lind, M.; Holappa, L. Transformation of Alumina Inclusions by Calcium Treatment. Metall. Mater. Trans. B 2010, 41, 359–366. [Google Scholar] [CrossRef]
- Holappa, L.; Hämäläinen, M.; Liukkonen, M.; Lind, M. Thermodynamic examination of inclusion modification and precipitation from calcium treatment to solidified steel. Ironmak. Steelmak. 2003, 30, 111–115. [Google Scholar] [CrossRef]
- Guo, Y.-T.; He, S.-P.; Chen, G.-J.; Wang, Q. Thermodynamics of Complex Sulfide Inclusion Formation in Ca-Treated Al-Killed Structural Steel. Metall. Mater. Trans. B 2016, 47, 2549–2557. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Formation Mechanism of Oxide-Sulfide Complex Inclusions in High-Sulfur-Containing Steel Melts. Metall. Mater. Trans. B 2018, 49, 311–324. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S. Transient Inclusion Evolution during Modification of Alumina Inclusions by Calcium in Liquid Steel: Part I. Background, Experimental Techniques and Analysis Methods. Metall. Mater. Trans. B 2011, 42, 711–719. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S.R. Transient Inclusion Evolution During Modification of Alumina Inclusions by Calcium in Liquid Steel: Part II. Results and Discussion. Metall. Mater. Trans. B 2011, 42, 720–729. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhang, Y.; Duan, H.; Ren, Y.; Yang, W. Effect of Sulfur in Steel on Transient Evolution of Inclusions During Calcium Treatment. Metall. Mater. Trans. B 2018, 49, 610–626. [Google Scholar] [CrossRef]
- Yang, G.; Wang, X. Inclusion Evolution after Calcium Addition in Low Carbon Al-Killed Steel with Ultra Low Sulfur Content. ISIJ Int. 2015, 55, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sridhar, S.; Valdez, M. Formation of CaS on Al2O3-CaO inclusions during solidification of steels. Metall. Mater. Trans. B 2002, 33, 625–632. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Ghosh, A. Thermodynamic Evaluation of Formation of Oxide–Sulfide Duplex Inclusions in Steel. ISIJ Int. 2008, 48, 1552–1559. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, S.; Li, J.; Wu, T.; Liu, W.; Xiong, J. Improving Cleanliness of 95CrMo Drill Rod Steel by Slag Refining. Metall. Mater. Trans. B 2016, 47, 99–107. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, Q.; Huang, F.; Li, H.; Yang, J. Control of Stringer Shaped Non-Metallic Inclusions of CaO-Al2O3System in API X80 Linepipe Steel Plates. Steel Res. Int. 2013, 85, 155–163. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.Q.; Han, H.B.; Bai, R.J. Influence of calcium treatment on cleanness and fatigue life of 60Si2MnA spring steel. Ironmak. Steelmak. 2017, 44, 28–35. [Google Scholar] [CrossRef]
- Yiquan, W. Study of Characteristics of Inclusions and Property of High Heat Input Welding for Oxide Metallurgy Steel Developed by Ca Deoxidation; Central South University: Changsha, China, 2014. [Google Scholar]
- Sui, H.; Wang, L.; Wang, Q.; Wang, H.; Che, D.; Li, J.; Chou, K. The Formation and Growth of Sulfides in Free-Cutting Stainless Steel. Steel Res. Int. 2018, 89. [Google Scholar] [CrossRef]
- Lv, Z.-A.; Ni, H.-W.; Zhang, H.; Liu, C.-S. Evolution of MnS inclusions in Ti-bearing X80 pipeline steel. Iron Steel Res. Int. 2017, 24, 654–660. [Google Scholar] [CrossRef]
- Ren, Q.; Yang, W.; Cheng, L.; Zhang, L.; Conejo, A.N. Formation and Deformation Mechanism of Al2O3-CaS Inclusions in Ca-Treated Non-Oriented Electrical Steels. Metall. Mater. Trans. B 2019, 51, 200–212. [Google Scholar] [CrossRef]
- Blais, C.; L’Espérance, G.; Lehuy, H.; Forget, C. Development of an integrated method for fully characterizing multiphase inclusions and its application to calcium-treated steels. Mater. Charact. 1997, 38, 25–37. [Google Scholar] [CrossRef]
- Yu, W.; Xie, B.S.; Wang, B.; Cai, Q.W.; Xu, S.X. Effect of Rolling Process on Microstructure and Properties of 95CrMo Drill Steel. J. Iron Steel Res. Int. 2016, 23, 910–916. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Li, J.; Liu, W.; Zhou, Y. Fatigue Life Improving of Drill Rod by Inclusion Control. High Temp. Mater. Process. 2016, 35, 661–668. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Yang, S.; Chen, C.; Jin, H.; Li, X.; Zhuang, C.; Ju, J. Industrial experiment study on inclusion evolution in 95CrMo Steel. Met. Res. Technol. 2019, 116. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, S.; Cai, C.; Liu, Y.; Li, H.; Yang, J. The Evolution and Morphology of Sulfide Inclusions in 95CRMO Hollow Steel. In Proceedings of the 5th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 16–20 February 2014; pp. 201–207. [Google Scholar]
- Ohta, H.; Suito, H. Characteristics of Particle Size Distribution of Deoxidation Products with Mg, Zr, Al, Ca, Si/Mn and Mg/Al in Fe-10mass%Ni Alloy. ISIJ Int. 2006, 46, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Kanatani, K.-I.; Ishikawa, O. Error analysis for the stereological estimation of sphere size distribution: Abel type integral equation. J. Comput. Phys. 1985, 57, 229–250. [Google Scholar] [CrossRef]
- Li, T.; Shimasaki, S.-I.; Taniguchi, S.; Uesugi, K.; Narita, S. Stereological Analysis of Nonspherical Particles in Solid Metal. Metall. Mater. Trans. B 2013, 44, 750–761. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Li, J.; Zhang, S.; Ju, J. Effect of Mg Addition on the Refinement and Homogenized Distribution of Inclusions in Steel with Different Al Contents. Metall. Mater. Trans. B 2017, 48, 805–818. [Google Scholar] [CrossRef]
- Shi, C.; Park, J.H. Evolution of Oxide Inclusions in Si-Mn-Killed Steel during Protective Atmosphere Electroslag Remelting. Metall. Mater. Trans. B 2019, 50, 1139–1147. [Google Scholar] [CrossRef]
- Lyu, S.; Ma, X.; Huang, Z.; Yao, Z.; Lee, H.-G.; Jiang, Z.; Wang, G.; Zou, J.; Zhao, B. Formation Mechanism of Al2O3-Containing Inclusions in Al-Deoxidized Spring Steel. Metall. Mater. Trans. B 2019, 50, 2205–2220. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]



| Exp. | Remarks | C | Si | S | P | Mn | Mo | Cr | Alt | Als | Ca | Mg | O.T. | OInsol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight Percent (%) | Mass (ppm) | |||||||||||||
| 1 | High Ca Low S | 0.93 | 0.35 | 0.003 | 0.012 | 0.33 | 0.2 | 1.06 | 30 | 24 | 13 | <5 | 8–9 | 7.6 |
| 2 | Low Ca Low S | 0.97 | 0.28 | 0.003 | 0.010 | 0.32 | 0.2 | 1.04 | 30 | 26 | 7 | <5 | 6–9 | 7.3 |
| 3 | High Ca High S | 0.98 | 0.26 | 0.010 | 0.010 | 0.31 | 0.2 | 1.04 | 30 | 26 | 18 | <5 | 7–8 | 7.7 |
| 4 | Low Ca High S | 0.94 | 0.23 | 0.010 | 0.013 | 0.31 | 0.2 | 1.03 | 30 | 19 | 6 | <5 | 7–9 | 7.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Long, X.; Wang, L.; Tong, S.; Wang, X.; Zhang, Y.; Li, Y. Inclusion Characteristics in 95CrMo Steels with Different Calcium and Sulfur Contents. Materials 2020, 13, 619. https://doi.org/10.3390/ma13030619
Li X, Long X, Wang L, Tong S, Wang X, Zhang Y, Li Y. Inclusion Characteristics in 95CrMo Steels with Different Calcium and Sulfur Contents. Materials. 2020; 13(3):619. https://doi.org/10.3390/ma13030619
Chicago/Turabian StyleLi, Xiang, Xiao Long, Linzhu Wang, Shouhao Tong, Xiutao Wang, Yin Zhang, and Yutang Li. 2020. "Inclusion Characteristics in 95CrMo Steels with Different Calcium and Sulfur Contents" Materials 13, no. 3: 619. https://doi.org/10.3390/ma13030619
APA StyleLi, X., Long, X., Wang, L., Tong, S., Wang, X., Zhang, Y., & Li, Y. (2020). Inclusion Characteristics in 95CrMo Steels with Different Calcium and Sulfur Contents. Materials, 13(3), 619. https://doi.org/10.3390/ma13030619




